Antimicrobial Degradation Performance of Novel Polyacrylamide Derivatives by Microbial Consortia for Enhanced Oil Recovery
2017-11-01WuGangYuLiangminJiangXiaohuiBaoMutai
Wu Gang; Yu Liangmin; Jiang Xiaohui; Bao Mutai
(Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China,Qingdao 266100)
Antimicrobial Degradation Performance of Novel Polyacrylamide Derivatives by Microbial Consortia for Enhanced Oil Recovery
Wu Gang; Yu Liangmin; Jiang Xiaohui; Bao Mutai
(Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China,Qingdao 266100)
The pursuit of high oil recovery rate has been a persistent objective for oil industry. Pseudomonas sp. LP-7 and Bacillus sp. PAH-2 were isolated from oil-contaminated surface soil samples of an oil field. The antimicrobial degradation rates (ADRs) of polymers achieved by LP-7 and PAH-2 were evaluated at a temperature of 35 °C in the mineral salt media during the shaken flask trial. The ADRs of copolymer synthesized by using a surfactant with a concentration of 5% could reach 8.4% for PAH-2 and 15.3% for LP-7. The ADRs of copolymer could reach 10.4% for PAH-2 and 21.3% for LP-7,when the polymer concentration was 2 g/L. All results con firmed that the ADRs of copolymers increased with an increasing content of HDDE (capsaicin derivative monomer) in the polymer. The copolymers also manifested excellent antimicrobial degradation performance in the presence of Cu2+, Zn2+, and Pb2+ions, respectively, which had great potential for applications in enhanced oil recovery.
polymer flooding; enhanced oil recovery; capsaicin derivative; antimicrobial degradation; heavy metal ions
1 Introduction
Enhanced oil recovery (EOR) technology is a good alternative to meet the impending energy demand.Approximately two thirds of crude oil remain trapped in reservoirs after the primary and secondary extractions[1-2](Figure 1a). Polymer flooding is frequently used in EOR and has been attracting much attention. This strategy is one of the well-documented EOR techniques in oil fields and has been extensively used for several decades[3-5].Thus, various new classes of polymers have been prepared and studied in the past few years. However, the high salinity, the high temperature, and especially high bacterial content in underground environment can cause the degradation of polymer, molecular chain curling,and loss of solution viscosity. Moreover, heavy metal contamination (Pb2+, Zn2+, and Cu2+ions) is a ubiquitous phenomenon in petroleum and its derivatives[6]. Therefore,traditional polymer flooding is not recommended for oil reservoir by only having a higher displacing fluid viscosity. According to the screening criteria[7](Figure 1b), more viscoelastic, chemically robust, and anti-
degrading polymer systems are being developed for EOR application.
A novel hydrophobically associating polyacrylamide derivative P(AM-MAA-AMPS-HDDE-SA) (PAMAHS)(reported, DOI 10.1002/pen.24337) has been synthesized by micellar copolymerization technique and characterized by FTIR spectroscopy, UV-Vis fluorescence spectroscopy,and1H nuclear magnetic resonance[8]techniques.The morphology and microstructure of PAMAHS copolymer show the formation of multilayer network structure by scanning electron microscope and atomic force microscope, and thus PAMAHS exhibits unique solution behavior. The dissolution and thermogravimetric analysis of the copolymer show that PAMAHS has a good solubility and an enhanced thermal stability.Accordingly, all results reveal that PAMAHS can be used as an excellent polymer flooding agent. Moreover,PAMAHS incorporating the capsaicin-like monomer withbiological activity is provided with excellent antibacterial performance and can reduce the viscosity loss caused by microbial degradation[9-10].This work was primarily conducted to examine the anti-microbial degradation performance of PAMAHS copolymer under different conditions. The effects of the surfactant, capsaicin derivative monomer, polymer concentration (c), and heavy metal ions were investigated.To the best of our knowledge, very few information is available on the assessment of the antimicrobial degradation of hydrophobic polymers. This investigation was primarily focused on the mechanism of polymer resistance to microbial degradation in crude oil recovery.The crude oil degradation efficiency of Pseudomonas sp.LP-7 and Bacillus sp. PAH-2 consortia was also explored.
Figure 1 (a) The relationship between reserves and resources; (b) Requirements for EOR polymers
2 Experimental
2.1 Materials
Acrylamide (AM) (Jiangxi Jiunongke Chem. Co.) was recrystallized from acetone and stored in dark until required. N,N'-((2-Hydroxy-4,5-dimethylbenzene-1,3-diyl) dimethanediyl) bisprop-2-enamide (HDDE)[11]was synthesized in our own laboratories. 2,2'-Azobis(2-methylpropionitrile) (AIBN), and N-methylol acrylamide (N-MA) were obtained from the Tianjin Damao Chem. Co. and used as received. 2-Acrylamido-2-methylpropane-sulfonic acid (AMPS), and stearyl acrylate(SA) were obtained from the Shouguang Yuyuan Chem.Co. Methacrylic acid (MAA) (Tianjin Kwangfu Fine Chemical Industry Research Institute), sodium dodecyl sulfate (SDS) and petroleum ether (60—90 °C) were obtained from the Sinopharm Chemical Reagent Co. and used as received, and other reagents were of analytical reagent grade and used without further purification.
2.2 Microorganisms and culture media
Pseudomonas sp. LP-7 and Bacillus sp. PAH-2 consortia were isolated from petroleum contaminated seawater and mud in the Qingdao port. The sequence results about Pseudomonas sp. LP-7 and Bacillus sp. PAH-2 strains were compared with other 16S rDNA sequences of lactobacilli in GenBank, and therefore these strains could be differentiated exactly. The enrichment media contained 3.0 g/L of beef extract, 10.0 g/L of peptone, and 5.0 g/L of NaCl. The crude oil degradation experiments were conducted in a mineral salt medium (MSM), which contained the following components: 5.0 g/L of NaCl,10.0 g/L of K2HPO4, 4.0 g/L of KH2PO4, 1.0 g/L of(NH4)2SO4, and 0.25 g/L of MgSO4·7H2O. All liquid media were sterilized in the autoclave at 121 °C for 20 min before use.
2.3 Synthesis of copolymer PAMAHS
A series of PAMAHS compounds were prepared by free radical micellar copolymerization[12-13]using sodium dodecyl sulfate (SDS) as the surfactant and AIBN as the initiator. A three-neck round-bottom 500-mL flask was equipped with a mechanical stirrer and a nitrogen inlet and outlet. AM, MAA, SA, AMPS, and HDDE were dissolved in a mixed solvent (anhydrous ethanol and distilled water (1:10, v/v)). The solution was then placed in a beaker. NaOH was used to control the pH value at between 6 and 7[14]. The mixture was stirred under N2until a clear homogeneous mixture was observed.Copolymerization proceeded under continuous stirring for 5 h. The copolymer was precipitated and dried under vacuum at 50 °C for 24 h and stored in a desiccator.The seven copolymers used in the experiment contained different concentration of SDS (0, 4%, 5%, and 6%)and different concentration of hydrophobic monomer(HDDE: 0.5 mol%, 1.0 mol%, and 1.5 mol%) to elucidate the influence of the addition of SDS and HDDE on the antimicrobial degradation performance of copolymers.The characteristics of the prepared copolymers are shown in Table 1.
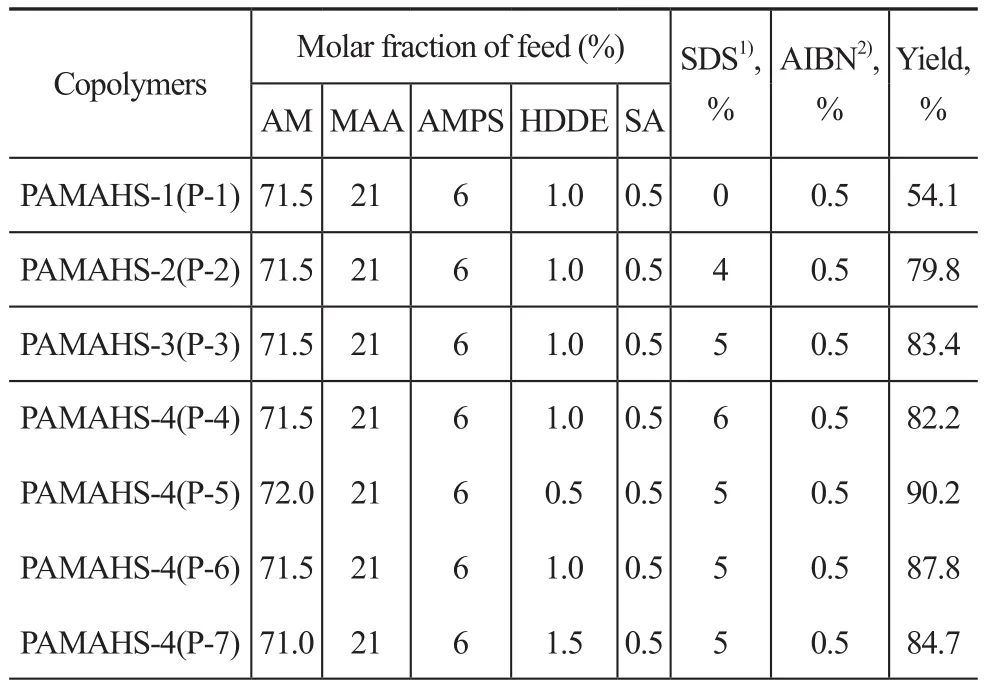
Table 1 The characteristics of the prepared copolymers
2.4 Standard curve of crude oil
tandard curve should be prepared for the assay to generate quantitative data. The standard curve of crude oil can be used to quantify the microbial degradation of crude oil[15]. The initial crude sample was dissolved in petroleum ether and filtered to remove impurities. The sample was allowed to stand for 24 h to remove water by adding anhydrous sodium sulfate. The dissolved petroleum ether was removed using a rotary evaporator to obtain the crude standard samples.
2.5 Antimicrobial degradation assessment
2.5.1 Strain screening
1) Enrichment culture and isolation
The oil-contaminated sludge was inoculated in the enrichment media at 30 °C under shaking at a rate of 120 r/min for 7 days. The enrichment liquid was continuously transferred to the fresh enrichment media. The cultures were repeated thrice under the same conditions as those used for glycerol and were sealed for the follow-up study.The above enrichment solution was diluted into gradient solutions, which were coated onto a solid tablet suitable for incubating the constant temperature culture for 2 days at 35 °C. Then, the tablet with different single colonies was picked, and three line separation methods were used successively for further purification, until only a single colony was found on the tablet, which was stored for the subsequent use.
2) Plotting of growth curves
After a certain amount of culture media was incubated overnight, 10% (v/v) of inoculation amount were inoculated in a series of liquid media at 35 °C under shaking at a rate of 120 r/min. The absorbance wavelength of bacterial suspension was determined every 0.5—2 h with UV spectrophotometry at 600 nm, in which the liquid culture media without bacteria were used as the blank control. The bacterial growth change was monitored by the turbidity change of culture media.
2.5.2 Antimicrobial degradation trial
Antimicrobial degradation of polymer was tested using different strains over a prolonged time. Experiments were conducted in three groups of parallel tests. The trials were performed in MSM inoculated with 5.0% (v/v) of target strain and 0.2% (v/v) of crude oil after the media were sterilized at 120 °C for 20 min. Batches of media sufficient for each component were prepared and dispensed into 250 mL Erlenmeyer flasks prior to sterilization. The pH value was adjusted to 7.0—7.2.The cells were incubated at 35 °C under shaking at a rate of 120 r/min for 2, 4, 6, 8, 10, 12, and 14 days,respectively.
All crude oil samples in liquid media using the “liquidliquid” method were extracted with petroleum ether as the extraction agent. Extraction was consecutively performed thrice, and the crude oil was diluted to a certain concentration with the extractant. The optimum absorption wavelength of 225 nm was determined by measuring the absorbance of the crude oil. During the experiment, approximately 50 mL of petroleum ether were added into the Erlenmeyer flasks, followed by separation using a separatory funnel. Then, the absorbance of the upper liquid was measured with a UV spectrophotometer at a wavelength of 225 nm with petroleum ether serving as the reference. The pending test crude oil concentration was calculated by means of the standard curve[16], and the antimicrobial degradation rates (ADRs) of the polymer were obtained. All treatments, except the sterile control,were performed in triplicate. The formulas for calculating the antimicrobial degradation rate and the degradation rates of crude oil are shown in formulas (1) and (2).Figure 2 shows the parallel experiments designed to calculate the antimicrobial degradation rate (ΔR1) and the microbial degradation rate (ΔR2).

Figure 2 The characteristics of parallel tests
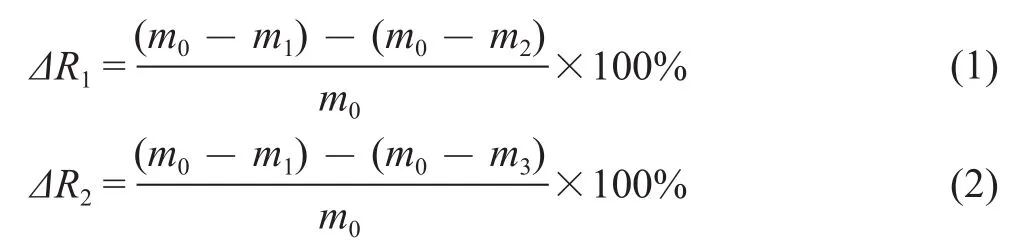
where m0is the mass of initial crude oil, g; m1is the mass of remaining crude oil in Figure 2a, g; m2is the mass of remaining crude oil in Figure 2b; m3is the mass of remaining crude oil in Figure 2c; ΔR1is the antimicrobial degradation rate of polymer, %; and ΔR2is the microbial degradation rate, %.
3 Results and Discussion
3.1 Determination of standard curve of crude oil
The crude oil/ petroleum ether solution (1 g/L) is accurately prepared, and the solution is diluted to prepare the following solutions (mg/L): 6, 8, 10, 12, 14, 16, 18,20, 22, 24, 26, 28, and 30, respectively. The absorbance values at 200 nm-300 nm are measured using a UV-vis spectrometer with petroleum ether serving as the reference to determine the maximum absorbance wavelength of λmax= 225 nm. The standard curve of crude oil is presented in Figure 3a.
3.2 Growth curves in bacterial cultivation
Figure 3b shows the growth curves in bacterial cultivation for Pseudomonas sp. LP-7 and Bacillus sp.PAH-2. The initial lag phase is a period of slow growth during which the bacteria are adapting to the conditions in the fresh media. The duration of a lag phase is determined by the type, strain, temperature, and media.This phase is followed by a logarithmic phase which is characterized by exponential growth, doubling every replication cycle. Stationary phase occurs when the nutrients become limiting and the rate of multiplication is equal to the rate of death. Figure 3b shows that Pseudomonas sp. LP-7 reaches a stationary state of growth after 25 h, while Bacillus sp. PAH-2 attains a stationary state after 17 hours. Bacteria normally display a characteristic four-phase pattern of growth in liquid culture. A logarithmic decline phase occurs when cells die faster than they are replaced (with the latter occurring over a much longer period of time than the previous three)[17]. Therefore, the following experiments were performed after the culture was inoculated for 25 h for Pseudomonas sp. LP-7 and 17 h for Bacillus sp.PAH-2 after reaching the stationary state of bacterial growth.
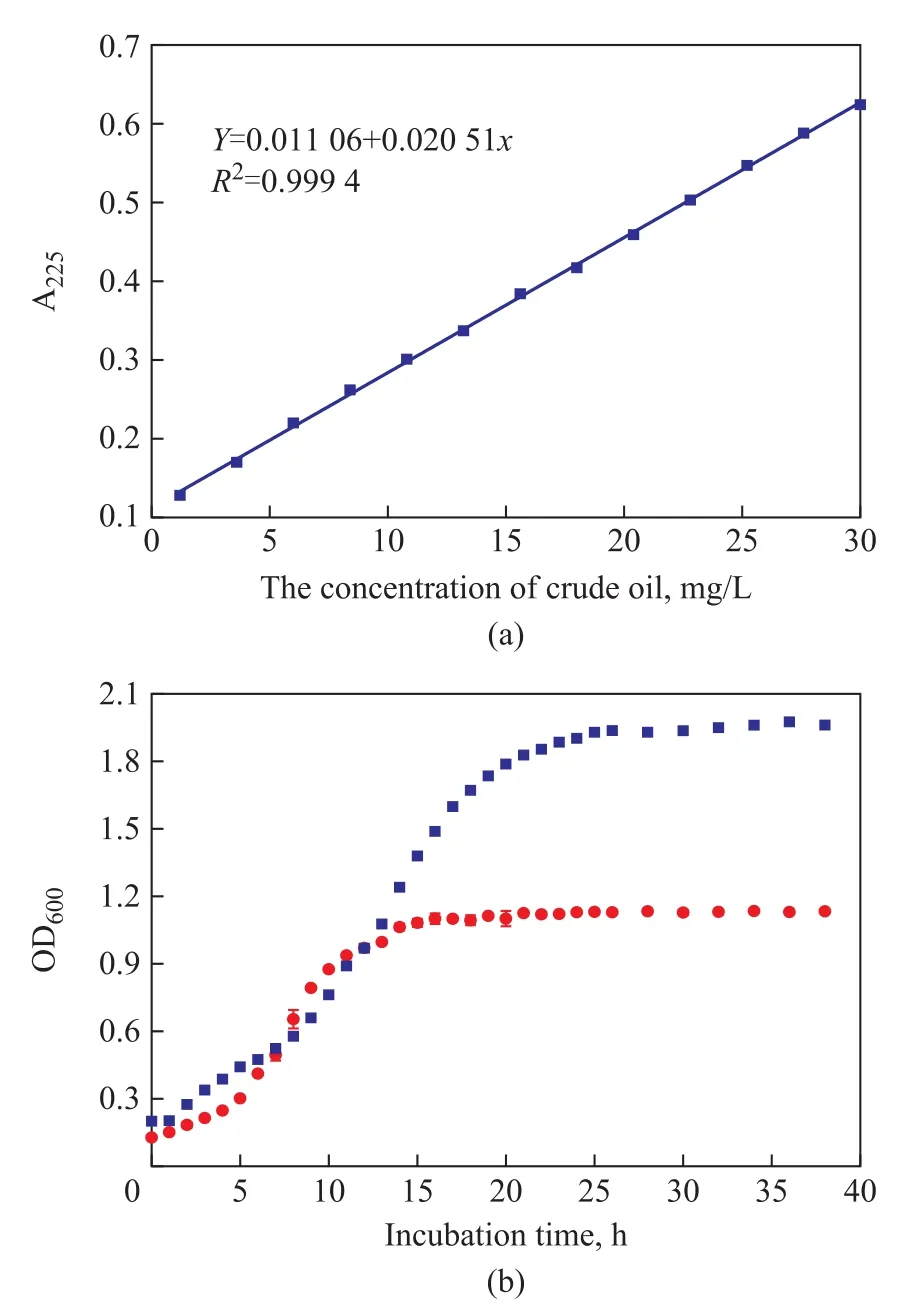
Figure 3 (a) The standard curve of crude oil; (b) Growth curves in bacterial cultivation for LP-7 and PAH-2
3.3 Change in the number of viable cells over incubation time
Several bacterial characteristics are considerably affected by the phase of the growth of organism, including the space, the pH value, the temperature, the susceptibility to environmental changes, and the moisture content of the media. Figure 4a shows the variation curves of viable cells over incubation time in MSM. Figure 4a shows the bacterial populations with an initial abrupt increase at the beginning of the incubation followed by a steady decrease towards the end of the experiment. This phenomenon can occur mainly because the cells can gradually adjust before they begin to divide actively in the early stage. Moreover,the depletion of nutrients and subsequent accumulation of metabolic waste products and other toxic materials in the media can facilitate the phase of the bacteria death with an increasing incubation time.
Antimicrobial agents can kill bacteria through different methods depending on the type of bacteria. Most antimicrobial agents can kill bacteria immediately on contact by causing the bacterial cell to burst or by depleting the source offood for the bacteria to prevent the bacterial reproduction, which is also known as bacterial conjugation[18]. Antimicrobial polymers commonly kill bacteria through the first method, which is accomplished through a series of steps. As shown in Figure 4c, the antimicrobial polymer must first be adsorbed onto the bacterial cell wall and then diffuse through the cell wall in order to be adsorbed onto the cytoplasmic membrane.Finally, the disruption of the cytoplasmic membrane leads to the death of the cell[19]. Meanwhile, the bacteria nearly lose its ability to reproduce because of the unfavorable conditions. In consequence, the number of dead cells exceeds the number of live cells. However, some organisms which can resist the unfavorable conditions can survive in the environment by producing endospores.
3.4 Effect of surfactants on the antimicrobial degradation of copolymers
3.4.1 Effect of surfactant on the viscosity of copolymer

Figure 4 (a) The variation curves of viable cells over incubation time; (b) Effect of surfactant content on the apparent viscosity of polymer at a shear rate of 0.01 s-1(with the polymer concentration equating to 0.8 g/dL at a temperature of 25 °C); (c) Schematic diagram of the action of antibacterial polymers on bacterial cells
Surfactants with some fixed hydrophilic and lipophilic groups are substances that can change the interface state of the solution system and can be aligned in the surface of the solution. Surfactants can play an important role in free radical micellar polymerization. On the one hand, these compounds have the solubilization and dispersion effect.On the other hand, these compounds can also activate the reaction of monomer to facilitate the polymerization between monomers. The effect of surfactant contents on the apparent viscosity of copolymer is presented in Figure 4b.
The investigated range of surfactant content covers from 0% to 6.0%. Surfactants have different influences on the viscosity of the polymer solution depending on the amount of added surfactant. This phenomenon is mainly ascribed to the influence of the surfactants on various microstructured water-soluble polymers[20]. Figure 4b shows that the initial increase in the viscosity at a sufficiently low surfactant concentration is attributed to the formation of mixed micelles and increased hydrophobic association. All surfactant molecules can associate with polymer, and no free micelles exist.However, a further addition of surfactant will result in the formation of free micelles because no site is available for surfactant-polymer association at high concentration. Therefore, the viscosity decreases because of the disruption of intermolecular association. The apparent viscosity reaches a maximum value when the SDS content increases to 5% of the solvent, which was reported by Kientz, et al.[21]The experimental results show that an appropriate content of surfactant can maximize the apparent viscosity of the hydrophobic copolymer PAMAHS because of the change in the internal structure of the polymers, as expected.
3.4.2 Antimicrobial degradation test
Surfactants are very important components in synthesized copolymers and can interact with copolymers according to various mechanisms[22]. An increase in viscosity will improve the macroscopic efficiency, while an increase in antimicrobial degradation will help increase the microscopic efficiency in EOR. Figure 5a shows that all copolymers display different degree of antimicrobial degradation ability (ADA), and the ADA of copolymers for Pseudomonas sp. LP-7 is significantly higher than that of Bacillus sp. PAH-2, which is related to the physiological structures of the two bacteria, as reflected by the varied amounts in viable cells. Moreover, an important characteristic of Bacillus sp. PAH-2 is its ability to produce spores with special resistance to adverse conditions. Thus,the ADA of copolymer P-3 for two bacteria achieves a highest value when the surfactant content is 5%, whereas the copolymer P-1 obtains the worst value as compared with other copolymers. These results are consistent with the findings from the apparent viscosity of the copolymers. This phenomenon is primarily ascribed to the decreased reaction amount of HDDE monomer without surfactant. The ADRs of the copolymers generally remains stable over eight days,although sporadic fluctuations appear occasionally, and the difference is not statistically significant. This phenomenon illustrates that copolymers (P-1, P-2, P-3, and P-4) show more stable ADA. However, a large variation in growth is observed with increased incubation time in the later stage,which is in accordance with the reduction of the number of viable cells with prolonged contact time in Figure 4a, which exhibits the increase in ADA. These results indicate that the addition of a proper content of surfactant(5%) is beneficial to enhancing the amount of HDDE taking part in the synthesis reaction, suggesting to achieve an excellent ADA. Similar results were also found by Zou, et al.[23]
3.5 Effect of HDDE on the antimicrobial degradation of copolymers
Capsaicin derivatives exhibit the properties similar to those of capsaicin which are effective in antibacterial dephlogistication[24-26]. A higher capsaicin derivative content shows a better antibacterial effect. The curves of variation in ADRs on different contents of HDDE over incubation time are shown in Figure 5b. The ADA of copolymers (P-5, P-6, and P-7) for Pseudomonas sp. LP-7 is slightly higher than that of Bacillus sp. PAH-2, which is caused by the facile paralysis of the Pseudomonas sp.LP-7 cells that can interfere with the neurotransmitter,thereby affecting their normal physiological activity.Moreover, the cell structure of Pseudomonas sp. LP-7 as a Gram-negative bacterium is relatively simple, and the cell wall is only composed of 2—3 layers of the peptidoglycan and lipopolysaccharide (10 nm). These characteristics allow for other molecules that can enter the cells easily to destroy their physiological structure. By contrast, the cell structure of Bacillus sp. PAH-2 as a Gram-positive bacterium is more complex. Its cell wall consists of 15—50 layers of peptidoglycan (20—80 nm), which comprise 20%—40%of the structurally stable polymer-teichoic acid. These characteristics hinder the entrance and destruction of other molecules. The results indicate that the ADRs of copolymers rise with an increasing HDDE content, which is consistent with our expectations. The ADRs gradually display an evident increment trend for the two bacteria with an increasing incubation time. This phenomenon can occur mainly because the two bacteria can gradually adapt to the new environment and produce resistance with the increased incubation time. The ADRs of copolymers increase greatly after 10 days because of the logarithmic decline phase suited probably to the two bacteria.

Figure 5 (a) The curves of variation in ADRs of surfactant on polymers over incubation time for two bacteria; (b) The curves of variation in ADRs of different contents of HDDE over incubation time; (c) The curves of variation in ADRs of different polymer (P-6) concentrations (1 g/L, 2 g/L, and 3 g/L) over incubation time; (d) The curves of variation in ADRs of copolymer P-6 over incubation time for two bacteria in the presence of heavy metals ions
3.6 Effect of values of c on the antimicrobial degradation of copolymers (P-6)
The choice of P-6 copolymer with excellent hydrophobically associating and antibacterial properties[8]is representative and of practical value. Figure 5c shows the ADRs of different c over the incubation time. The results demonstrate that the ADRs with different values of c for two bacteria slightly increased over the incubation time, and ADRs of the same c for Pseudomonas sp. LP-7 were higher as compared with those of Bacillus sp. PAH-2.This phenomenon is ascribed to the decreased growth space and the change in living conditions (including the pH value and nutrient elements). The highest ADR of the copolymers was achieved at 2 g/L for the two bacteria,which might occur because the copolymer exerted no effect on microbial degradation at low concentration. Thus, the content of HDDE was very little in the copolymer chains.After bacteria have yielded resistance to copolymer, the copolymer can be considered as the nutrient material at high concentration. No positive correlation exists between the sensitivity of bacteria and polymer concentration.Moreover, competition may exist between the antibacterial activity and the promotion of bacterial growth. However,the ADRs of copolymers at 3 g/L are still higher than the case of 1 g/L, which is consistent with the general findings for normal antimicrobial polymers. The ADR of polymer P-6 for Bacillus sp. PAH-2 displays a relatively unstable characteristic than that for Pseudomonas sp. LP-7 because of the different structural features among the two bacteria.Therefore, an appropriate c can significantly improve the performance of the antimicrobial degradation.
3.7 Curves of variation in ADR of copolymer P-6 in the presence of different heavy metal ions over incubation time
Heavy metal contamination, especially Cu2+, Zn2+,and Pb2+ions, is often found in many rivers, oceans,or groundwater[27-28]. Given the dual pollution in the environment, previous studies are limited to the degradation of crude oil while ignoring the existence of other adverse conditions, such as the presence of heavy metal ions. Therefore, investigating the effect of copolymer on the degradation of crude oil in the presence of heavy metal ions and the resistance of microorganisms to heavy metal ions and their sensitivity is important.
The choice of P-6 copolymer with excellent hydrophobically associating and antibacterial properties[8]is representative and of practical value. Figure 6 shows the maximum tolerated concentration of heavy metal ions by the microbial consortia (Pseudomonas sp. LP-7 and Bacillus sp. PAH-2). Microbial consortia are highly resistant to heavy metal ions. Figure 6 shows that the two bacteria are tolerant of heavy metal compounds(including CuSO4·5H2O, ZnSO4·7H2O, and Pb(NO3)2)with their maximum concentration less than 1.56 g/L of Pb2+(4.71 mmol/L), 0.78 g/L of Cu2+(3.12 mmol/L),and 0.39 g/L of Zn2+(1.36 mmol/L), respectively. The results showed higher tolerance ability compared with the results obtained by Máthé, et al.[6], who reported the tolerable concentration data of Cu2+(3 mmol/L) and Pb2+(3.5 mmol/L), which were slightly lower than the tolerable concentration of Zn2+(4 mmol/L). Moreover,the maximum ability of the two bacteria to tolerate heavy metal ions is higher than the normal concentration of heavy metals in seawater, viz.: 0.01—0.3 mg/L of Pb2+, ~5mg/L of Zn2+, and 1—10 mg/L of Cu2+[29]. The results indicate that the two bacteria can tolerate higher concentration of Cu2+, Zn2+, and Pb2+ions.
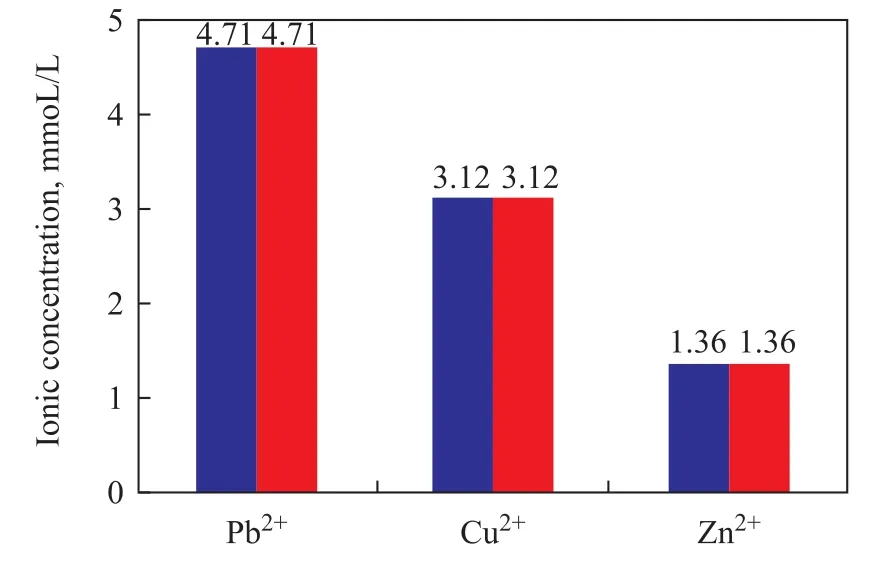
Figure 6 Maximum tolerable concentration values of heavy metal ions by the microbial consortia
The effect of copolymer on the degradation of crude oil at the heavy metal ions concentration of 1 mmol/L(Cu2+, Zn2+, and Pb2+) was studied to explore the effect of oil degradation in the presence of heavy metal ions on the copolymer resisting microbial community.The ADRs of copolymer for two bacteria decreased as compared with those without heavy metal ions.Figure 5d shows that the ADR of polymer P-6 for Pseudomonas sp. LP-7 was apparently higher than that of Bacillus sp. PAH-2 in the presence of heavy metal ions. The ADRs of polymer P-6 were the most remarkable in the presence of Zn2+ion in comparison to other metal ions, especially Pb2+ion. This phenomenon is ascribed to the worse resistance to Zn2+ion by the two bacteria so that the efficiency of crude oil degradation is reduced to show a high ADR. The ADRs of polymer P-6 slightly increased after incubation for eight days in the presence of heavy metal ions. The result showed the increased competition for nutrients and living space, which could lead to the death of some bacteria. Moreover, the network structure of copolymer was destroyed by heavy metal ions and led to reduced viscosity, and eventually the productivity of oil recovery was decreased. Compared with the suitable conditions, the results demonstrated that the two strains showed high resistance to microbial degradation in the presence of both heavy metal ions and copolymers.
4 Conclusions
A series of novel copolymers have been synthesized. The results indicate that the apparent viscosity of copolymer reached 680 mPa·s at a surfactant content of 5 wt%,and the ADRs of copolymer P-3 reached up to 8.4%for Bacillus sp. PAH-2 and 15.3% for Pseudomonas sp.LP-7. The ADRs of copolymer P-6 reached 10.4% for Bacillus sp. PAH-2 and 21.3% for Pseudomonas sp. LP-7 when c was 2 g/L. All results con firmed that the ADRs of copolymers increased with the content of HDDE in the polymer. Furthermore, the copolymers also manifested excellent antimicrobial degradation performance in the presence of Cu2+, Zn2+, and Pb2+ions, respectively, in which Zn2+ion had the biggest influence on ADRs.
Acknowledgments: The authors would like to acknowledge financial supports from the Natural Science Foundation of China(50673085, 41576077), the National High-Tech Research and Development Programme of China (2010AA09Z203), and the Fundamental Research Funds for the Central Universities of China (201562026).
[1] Tong F Y, Yang Q H, Li D D, et al. Study on the effect of catalyst properties on residue hydroconversion[J]. China Petroleum Processing and Petrochemical Technology,2016, 18(1): 1-7
[2] Elraies K A, Tan I M, Awang M, et al. The synthesis and performance of sodium methyl ester sulfonate for enhanced oil recovery[J]. Petrol Sci Technol, 2010, 28: 1799-1806
[3] Maitin B K. Performance analysis of several polyacrylamide floods in North German oil fields, in paper SPE 24118 presented SPE/DOE Enhanced Oil Recovery Symposium[M], Tulas, Oklahoma, 1992.
[4] Moffitt P D, Zornes D R, Moradi-Araghi A, et al.Application of freshwater and brine polymer flooding in the North Burbank Unit, Osage County, Oklahoma[J]. SPE Reserv Eval Eng, 1993, 8: 128-134
[5] Wassmuth F R, Arnold W, Green K, et al. Polymer flood application to improve heavy oil recovery at East Bodo[J].J Can Petrol Technol, 2009, 48: 55-61
[6] Máthé I, Benedek T, Táncsics A. Diversity, activity, antibiotic and heavy metal resistance of bacteria from petroleum hydrocarbon contaminated soils located in Harghita County(Romania)[J]. Int Biodeter Biodegr, 2012, 73: 41-49
[7] Taber J J, Martin F D, Seright R S. EOR screening criteria revisited. 1. Introduction to screening criteria and enhanced recovery field projects[J]. SPE Reserv Eval Eng, 1997, 12:189-198
[8] Wu G, Jiang X H, Yu L M, et al. Hydrophobically associating polyacrylamide derivatives with double bond for enhanced solution properties[J]. Polym Eng Sci, 2016,56(11): 1203-1212
[9] Bock J, Pace S J, Schulz D N. Enhanced oil recovery with hydrophobically associating polymer containing N-vinyl pyrrolidone functionality, US 4709759[P]. 1987
[10] Taylor K C, Nasr-El-Din H A. Water-soluble hydrophobically associating polymers for improved oil recovery: A literature review[J]. J Petrol Sci Eng, 1998, 19:265-280
[11] Yu X L, Yu L M, Jiang X H. Synthesis of acrylamide derivatives containing capsaicin structure and antibacterial and antifouling properties[J]. Applied Chemistry, 2014, 31:594-599 (in Chinese)
[12] Turner S R, Siano D B, Bock J. Acrylamidealkylacrylamide copolymers: The United States, US 4520182[P]. 1985
[13] Umar Y, Al-Muallem H A, Abu-Sharkh B F. Synthesis and solution properties of hydrophobically associating ionic polymers made from diallylammonium salts/sulfur dioxide cyclocopolymerization[J]. Polymer, 2004, 45(11): 3651-3656
[14] Jia L N, Yu L M, Li R, et al. Synthesis and solution behavior of hydrophobically associating polyacrylamide containing capsaicin-like moieties[J]. J Appl Polym Sci,2013, 130: 1794-1804
[15] Natarajan S, Remick D G. ELISA rescue protocol:Recovery of sample concentrations from an assay with an unsuccessful standard curve[J]. Methods, 2013, 61: 69-72
[16] Zhao X, Wang Y, Ye Z. Oil field wastewater treatment in biological aerated filter by immobilized microorganisms[J].Process Biochem, 2006, 41: 1475-1483
[17] Huang L H. Simulation and evaluation of different statistical functions for describing lag time distributions of a bacterial growth curve[J]. Microbial Risk Analysis, 2016, 1: 47-55
[18] Nonaka T, Li H, Ogata T, et al. Synthesis of watersoluble thermosensitive polymers having phosphonium groups from methacryloyloxyethyl trialkyl phosphonium chlorides-N-isopropylacrylamide copolymers and their functions[J]. J Appl Polym Sci, 2003, 87: 386-393
[19] Kenaway E R, Worley S D, Broughton R. The chemistry and applications of antimicrobial polymers: A state of the art review[J]. BioMacromolecules, 2007, 8: 1359-1384
[20] Robert J, Laurer J H, Spontak R J, et al. Hydrophobically modified associative polymer solutions: rheology and microstructure in the presence of nonionic surfactants[J].Ind Eng Chem Res, 2002, 41: 6425-6435
[21] Kientz E, Holl Y. Interactions in solution between a hydrophobic polymer and various kinds of surfactants[J].Colloid Polym Sci, 1994, 272: 141-150
[22] Goddard E D. Polymer/surfactant interaction: interfacial aspects[J]. J Colloid Interface Sci, 2002, 256: 228-235
[23] Zou C J, Wang M, Yu X, et al. Characterization and optimization of biosurfactants produced by Acinetobacter baylyi ZJ2 isolated from crude oil-contaminated soil sample toward microbial enhanced oil recovery applications[J]. Biochem Eng J, 2014, 90: 49-58
[24] Fischer K J. Marine organism repellent covering for protection of underwater objects and method of applying same, US 5226380[P]. 1993
[25] Watts J L. Anti-fouling coating composition containing capsaicin: The United States, US 5397385[P]. 1995
[26] Bullat D M, Vasishtha N. Biorepellent matrix coating: The United States, US 5925370[P]. 1999.
[27] Meybeck M, Lestel L, Bonté P. Historical perspective of heavy metals contamination (Cd, Cr, Cu, Hg, Pb, Zn) in the Seine River basin (France) following a DPSIR approach(1950-2005)[J]. Sci Total Environ, 2007, 375: 204-231
[28] Kwon J S, Yun S T, Lee J H. Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic (III) from aqueous solutions using scoria: Kinetics and equilibria of sorption[J]. J Hazard Mater, 2010, 174: 307-313
[29] Zhang Z B. Marine chemistry: Trace elements in sea water and heavy metal pollution in the sea (chapter 6)[D].Qingdao: Ocean University of China, 2004: 155-183
Successful Application of Catalyst for Removing Butadiene through Selective Hydrotreating of C4 Hydrocarbons Developed by Shanghai Research Institute of Petrochemical Technology
The 54 kt/a C4fraction hydrotreating unit at the aromatics production plant of the Shanghai Petrochemical Company(SPC) is aimed at separating the polymerization-grade butene-1 after removing butadiene through selective hydrotreating of C4fraction emanating from the MTBE unit, and the hydrotreating catalyst is always imported from the overseas since the startup of the hydrotreating unit. The novel SHB-01 catalyst for selective hydrotreating of C4fraction developed by the SINOPEC Shanghai Research Institute of Petrochemical Technology has been used in the hydrotreating section of MTBE unit at SPC with the relevant facilities being started up successfully at the first attempt. Till now this unit has been operating smoothly for 850 hours and the temperature in catalyst beds of hydrotreating reactors is distributed reasonably to meet the expected test targets.
date: 2017-02-20; Accepted date: 2017-03-28.
Professor Yu Liangmin, Tel/Fax: +86-532-66781845; E-mail: yuyan@ouc.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Thermal Decomposition Behavior of Terephthalate in Inert Gas
- Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
- Characterization and Apparent Kinetics of Polymerization of 1-Decene Catalyzed by Boron Trifluoride/Alcohol System
- Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
- Study on Con fined Impinging Jet Mixer and Mechanism offlash Nanoprecipitation
- Hydrophobic and Magnetic Reduced Graphene Oxide Nanocomposite for Emulsified Oil Removal
