Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
2017-11-01SongLinhuaWangZiYanYixuanBaoxiuWuPingpingZhaoXiaodongLiuDong
Song Linhua; Wang Zi; Yan Yixuan; Lü Baoxiu; Wu Pingping; Zhao Xiaodong; Liu Dong
(1. College of Science, China University of Petroleum (East China), Qingdao 266580;2. School of Pharmacy, Lanzhou University, Lanzhou 730030;3. State Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580)
Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
Song Linhua1; Wang Zi1; Yan Yixuan2; Lü Baoxiu1; Wu Pingping3; Zhao Xiaodong1; Liu Dong3
(1. College of Science, China University of Petroleum(East China), Qingdao 266580;2. School of Pharmacy, Lanzhou University, Lanzhou 730030;3. State Key Laboratory of Heavy Oil Processing, China University of Petroleum(East China), Qingdao 266580)
A series of Cu (II)-, Zn (II)-, Co (II)-loaded zeolites were successfully prepared by liquid phase ion-exchange method and characterized by X-ray diffraction (XRD), N2-adsorption specific surface area measurement (BET), X-ray fluorescence (XRF), and scanning electron microscopy (SEM) techniques. The adsorptive desulfurization performance of different adsorbents for treating Saudi Arabian medium crude oil was investigated, and the results showed that desulfurization efficiency declined in the following order: Zn(II)X > Cu(II)Y > Co(II)X > Cu(II)X > Co(II)Y> Zn(II)Y. The best desulfurization efficiency of 41.01% was achieved by using Zn(II)X as the adsorbent under the optimized conditions.The desulfurization performance of Zn(II)X in different distillates showed that a better performance was obtained in heavy petroleum fractions. Furthermore, the distribution of sulfur compounds in distillates after desulfurization was analyzed and the results showed that the adsorbent Zn(II)X could preferably adsorb aromatic sulfides such as thiophenes,benzothiophenes, and dibenzothiophenes. These results suggested that the π-complexation between zinc ions and sulfides would be crucial to the excellent desulfurization efficiency of Zn(II)X. In addition, the used adsorbent could be regenerated by heating at 700 °C in air, and around 84% of desulfurization capacity was recovered after the first cycle of regeneration.
desulfurization; zeolites; adsorption; crude oil; sulfur compounds
1 Introduction
At present, particular attention has been focused on adsorptive desulfurization (ADS) because of its convenient operation, small investment cost and high efficiency[1-3]. A large variety of adsorbents such as zeolites[4-8], MOFs[9-10], porous silica[11], and activated carbon (AC)[12-13]have been developed and investigated to improve their performance for desulfurization of model fuels, gasoline, and diesel. However, fewer of them have been directly applied in desulfurization of crude oil. In our previous study[14], a series of acid/baseimpregnated AC desulfurizers have been synthesized and applied in the removal of sulfur compounds in crude oil,but the desulfurization efficiency (7.9%—32.4%) still needs to be improved. Compared with AC, the structure of zeolite is more regular and well-defined, in which the silicon/aluminium-oxygen constitutional units can be efficiently modified by many transition metal ions.Modified zeolites such as Ni(II)Y, Ce(III)Y, Cu(I)Y,Zn(II)X, as well as some bimetal-exchanged zeolites,have high capacity for selective adsorption of aromatic sulfur compounds, especially the sterically hindered BDT because of π-complexation between metal ions and sulfur compounds[2,4,15-16]. In this paper, Cu(II)-,Zn(II)-, Co(II)-zeolites were prepared to investigate the adsorptive desulfurization performance for treating Saudi Arabian medium crude oil, and the relationship between desulfurization efficiency and the type of metal ions as well as the structure of zeolites was also discussed.The composition and morphology of synthesized adsorbents have been characterized by N2adsorption/desorption method, X-ray diffraction, scanning electron microscopy, and X-ray fluorescence techniques. Theadsorptive desulfurization performance of different adsorbents for treating Saudi Arabian medium crude oil was investigated. Furthermore, the preparation and desulfurization conditions of Zn(II)X adsorbent were optimized, including the ion-exchange concentration,the impregnation temperature, the desulfurization time,and the desulfurization temperature. The mechanism for desulfurization of crude oil by Zn(II)X was also discussed and its regeneration performance was studied.
2 Experimental
2.1 Experimental materials
Saudi Arabian medium crude oil containing 2.63% of sulfur was obtained from a local petroleum refinery in Saudi Arabia, with the typical properties of this crude oil summarized in Table 1[14]. The NaY and 13X zeolites(with Si/Al=2.56 and 1.25, respectively) were purchased from the Sinopharm Chemical Reagent Co., Ltd., and Cu(NO3)2·3H2O, Zn(NO3)2·6H2O and Co(NO3)2·6H2O were purchased from the J&K Chemical Ltd., China. All solvents used in experiments were purchased from the Xilong Chemical Co., Ltd. and used without further purification.

Table 1 Typical properties of Saudi Arabian medium crude oil
2.2 Synthesis of metal ion-zeolites
Liquid-phase ion-exchange process was used to prepare the metal-zeolites. For the typical synthesis of Zn(II)X desulfurizer, 13X zeolite (1 g) was at first treated with 10 mL of Zn(NO3)2solution with a concentration of 0.4 M,0.5 M, 0.6 M, 0.7 M, and 0.8 M, respectively, for 16 h at a definite temperature, followed by filtration, washing with deionized water and drying in a vacuum oven at 110oC for 2 h. In the process of ion-exchange, the pH value of solutions was maintained at 6 to prevent hydrolysis of Zn(NO3)2. Afterwards, the final adsorbent samples were obtained after calcination at 450oC in air for 2 h.Following the same synthesis procedure, the Cu(II)X,Co(II)X, Cu(II)Y, Zn(II)Y, and Co(II)Y adsorbents were also prepared.
2.3 Characterization of adsorbents
The Brunauer-Emmett-Teller (BET) surface area, pore diameter and pore volume of the desulfurizer samples were recorded and analyzed on a Micromeritics ASAP 2020-M porosity analyzer by physical adsorption of N2with relative pressure (p/p0) varying from 0.01 to 0.99.The Density Functional Theory (DFT) method was used to calculate the mean pore volume and pore diameter.Scanning electron microscopy (SEM) observation of the morphology of samples was carried out on a Hitachi S4800 scanning electron microscope. X-ray diffraction(XRD) analysis was performed on a Rigaku D/Max-RB diffractometer with CuKα radiation, operated at 40 kV and 40 mA. The content of metals in desulfurizers was determined by a MDX1000 (Varian) X-ray fluorescence(XRF) spectrometer.
2.4 Desulfurization test
Desulfurization performance of the zeolites was investigated by bench method under a definite desulfurization temperature and duration. The Saudi Arabian medium crude oil and modified zeolite were mixed at a mass ratio of 45:1 in a 500 mL flask. The mixture was maintained under vigorous shaking condition at 30-70oC for 1-4 h, followed by the separation of adsorbent from crude oil. Through high-temperature steam purging recovery, the yield of all recovered liquid oil samples after being treated by different adsorbents reached more than 90% of the mass of original crude oil.The total sulfur content in the crude oil before and after desulfurization was analyzed according to the test method GB/T 387-1990 (Chinese national standard) as described in our previous work[14].
The efficiency of desulfurization (φs) is expressed by the following equation.

where w0and wsrespectively represent the mass fraction of sulfur in the crude oil before and after desulfurization(g/g).
The distillate fractions obtained from crude oil in the boiling range of 30—150oC, 150—250oC, and 250—350oC, respectively, were analyzed by a Varian CP-3800 equipment to determine the content of different sulfur compounds in fractions. The total sulfur content analysis of these fractions was performed by a Vario EL III elemental analyzer.
3 Results and Discussion
3.1 Desulfurization performance of modified zeolites
Desulfurization performance of the original and metal modified zeolites is shown in Figure 1. Compared with the NaY and 13X zeolites, the modified zeolites, namely Zn2+, Co2+, and Cu2+ion-exchanged zeolites, exhibited much higher desulfurization efficiency. The ZnX adsorbent had a best desulfurization performance of 35%,higher than that of the original zeolite 13X by 29.1%. By contrast, the copper ion exchanged zeolite had improved the desulfurization performance of virgin NaY zeolite by 28.1%. These results suggested that the modification of zeolites by using transition metal ions had a positive influence on the sulfur removal from crude oil, which was possibly ascribed to the interaction between metal ions and sulfur-containing molecules, especially BT, DBT, and other aromatic sulfur compounds[15,17].

Figure 1 Desulfurization efficiency of different modified desulfurizers
3.2 Adsorbents characterization
3.2.1 XRD analysis
The XRD patterns of original and modified desulfurizers are shown in Figure 2. The similarity of the XRD patterns of CuY, ZnX and CoX zeolites to that of respective original zeolites indicated that the structure of NaY and 13X zeolites was well retained after ion exchange. No new diffraction peaks that were found in the XRD patterns of prepared adsorbents suggested that the metal ions (Cu2+, Zn2+and Co2+) were well dispersed in the relevant zeolites, and few or no metallic oxides were generated. This result indicated that the structure of original zeolites could hardly be affected during the solution impregnation and the following calcination in the synthesis process.
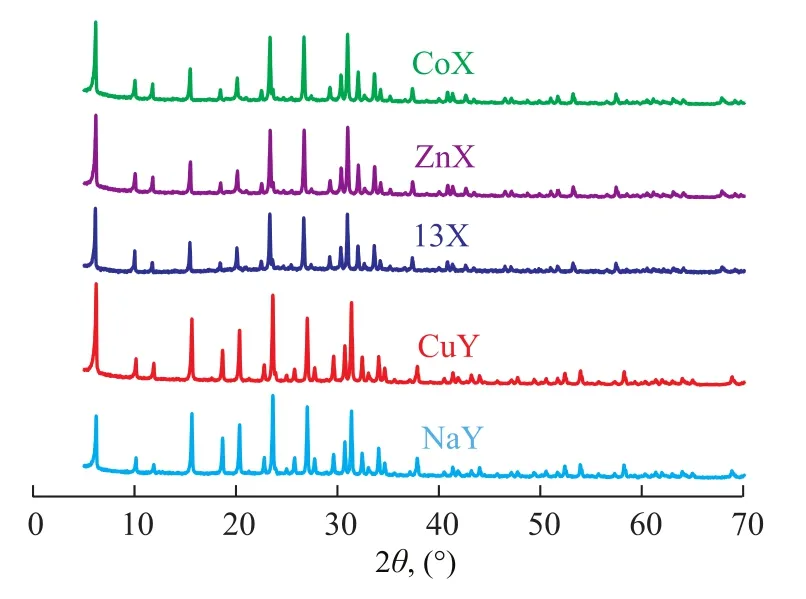
Figure 2 XRD patterns of zeolite adsorbents.
3.2.2 BET and XRF analyses
The chemical composition of NaY and 13X zeolites,and related ion-exchanged zeolites determined by XRF spectrometry is presented in Table 2. For the CuY zeolite,if a divalent metal ion can compensate for two aluminum tetrahedrals after the ion-exchange process, the double molar ratio of copper to aluminum (2Cu/Al), which denotes the cation-exchange capacity, can be described as follows:
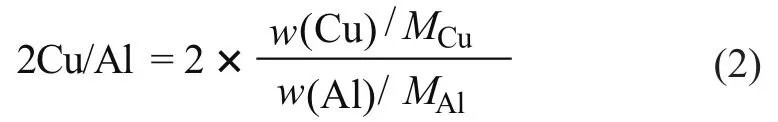
where w(Cu) and w(Al) represent the mass fraction (g/g)of copper and aluminum ions in the CuY zeolite shown in Table 2, respectively. MCuand MAlare the molar mass(g/mol) of copper and aluminum, respectively. The calculation result showed that the 2Cu/Al ratio was 1.050,which was close to the original Na/Al ratio of 1.057,indicating that the replacement of Na+by Cu2+during the synthesis process was almost stoichiometric. For the CoX and ZnX zeolites, the Co and Zn ion-exchange ratios were 67.0% and 85.2%, respectively (2Co/Al = 0.670 and 2Zn/Al = 0.852), suggesting that the ZnX zeolite had a larger ion-exchange degree than CoX.
As illustrated in Figure 3, the N2-physisorption isotherm of ZnX was of type I, which indicated that this zeolite was a microporous solid. Furthermore, there were no mesopores in ZnX zeolite because no hysteresis loop was found in the isotherm. Table 2 also shows the textural properties of the desulfurizers obtained from BET analysis. When Cu2+, Zn2+, and Co2+ions were loaded on the relevant zeolites, the BET surface and average pore volume of the zeolites decreased.This could be attributed to the partial collapse of pore structure in zeolites arising from the introduction of metal ions or the calcination process. However, the mean pore diameter of CuY, CoX and ZnX zeolites was larger than that of their parental zeolites. As for the modified zeolites, the BET surface area of ZnX and CoX zeolites was larger than that of CuY zeolite, while the average pore diameter of ZnX and CoX zeolites was smaller than that of CuY zeolite. In addition, the mean pore diameter of CoX and ZnX adsorbents was almost equal,which suggested that they had the similar pore structure.Since the pore size of all modified zeolites (0.671—0.811 nm) was close to the size of large sulfur compounds (i.e. alkylbenzothiophene) in crude oil[11], the high sulfurremoving capacity of ZnX desulfurizer might be partly caused by its large BET surface area.

Figure 3 Nitrogen adsorption-desorption isotherm plot for ZnX zeolite

Table 2 Physical properties and chemical composition of pure zeolites and modified zeolites
3.2.3 Morphological evolution of modified zeolites
The representative morphology of the desulfurizers is shown in Figure 4. The comparison between the morphology of NaY zeolite and CuY zeolite (Figure 4a and 4b) demonstrated that the ion-exchange process had little impact on the morphology of original zeolite. As demonstrated in Figure 4b, the shape of the CuY zeolite particles varied from a polygon to a short prism with some of them aggregating together. The impregnation of Zn or Co ions followed by heating could hardly change the shape of 13X zeolite, because the 13X, ZnX and CoX zeolites showed almost identical near-spherical shapes(4—6 μm of average diameter) with rough surface as demonstrated in Figure 4c, 4e and 4d.
3.3 Optimization of desulfurization performance
Owing to the highest desulfurization efficiency (35.0%)achieved by ZnX zeolite among the investigated zeolite samples (Figure 1), its synthesis conditions and desulfurizing conditions were optimized to further improve the desulfurization performance.
3.3.1 Effects of synthesis conditions on desulfurization efficiency

Figure 4 SEM photographs of virgin NaY zeolite: (a), CuY zeolite (b), virgin 13X zeolite (c), CoX zeolite (d), ZnX zeolite(e), and ZnX zeolite (f) after four cycles of regeneration
The impregnation solution concentration and impregnation temperature adopted in the synthetic procedure can significantly affect the loading of transition metal ions, and sequentially influence the efficiency of modified desulfurizers. In these experiments, the effect of the Zn(NO3)2impregnation solution concentration was firstly investigated under the same conditions covering a impregnation temperature of 40oC, a desulfurization temperature of 40oC, and a desulfurization time of 2 h. The results are shown in Figure 5a in which the desulfurization efficiency of ZnX zeolite increased with an increasing concentration of Zn(NO3)2solution,which reached a peak value of 38.32% at a Zn(NO3)2solution concentration of 0.6 mol/L. After that, probably because of the plugging effect caused by excess zinc ions in pores and channels, the desulfurization efficiency decreased with a growing Zn(NO3)2concentration. Then the impregnation temperature was studied at an optimal impregnation solution concentration of 0.6 mol/L. In Figure 5b, the desulfurization efficiency of ZnX zeolite initially increased with the increase of impregnation temperature, which reached a maximum desulfurization efficiency (41.01%) at 45oC, and then decreased.
As a result, the ZnX zeolite synthesized under the conditions covering an impregnation solution concentration of 0.6 mol/L and an impregnation temperature of 45oC showed an optimal desulfurization efficiency.
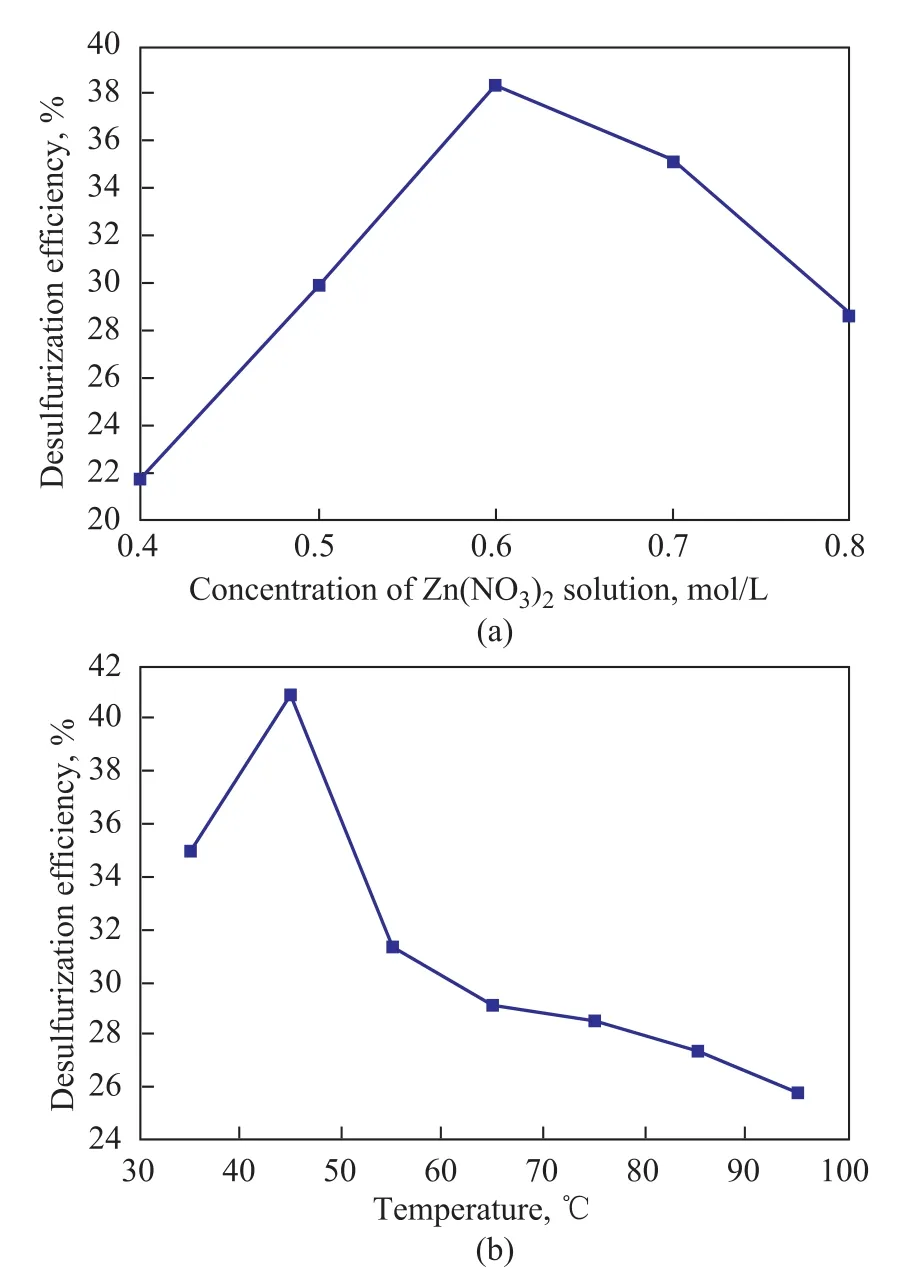
Figure 5 Effects of concentration of the impregnation liquid (a) and impregnation temperature (b) on desulfurization efficiency
3.3.2 Effects of desulfurization conditions on desulfurization efficiency
In the desulfurizing process, the adsorption temperature and adsorption time have a significant influence on the diffusion rate of sulfur compounds in porous channels, the adsorption kinetics and the equilibrium consequently can affect the desulfurization efficiency.The desulfurization time was firstly investigated at a desulfurization temperature of 40oC, indicating that a best desulfurization efficiency of 41.01% was achieved in a reaction duration of 2 h (Figure 6a). Then the investigation of desulfurization temperature was performed in an optimal desulfurization time of 2 h. The results showed that the desulfurization efficiency reached a maximum value of 41.01% when the desulfurization temperature was 40oC(Figure 6b). Thus, a temperature of 40oC and a reaction duration of 2 h are the optimized operating conditions for desulfurization of oil samples over the ZnX zeolite.
Initially, as the adsorption temperature increased, the movement rate of the sulfur-containing molecules grew,denoting that more molecules would enter the interior pores and channels and interact with adsorbed molecules,which could result in the growth of desulfurization efficiency. However, with the temperature continuously increasing, some adsorbed sulfur compounds began to dissociate from the adsorption sites owing to the high temperature, leading to the decrease of desulfurization efficiency. Similarly, the initial increase of adsorption time also resulted in the growth of desulfurization efficiency because of the effect of diffusion kinetics.When the time was long enough, other oil components,such as polycyclic aromatic hydrocarbons and nitrogenous heterocyclic compounds, which showed stronger interaction with Zn2+ions and could occupy more adsorption sites, gradually reached the adsorption sites and competed for the sites with sulfur-containing molecules. As a result, the desulfurization efficiency decreased with a further growing time.
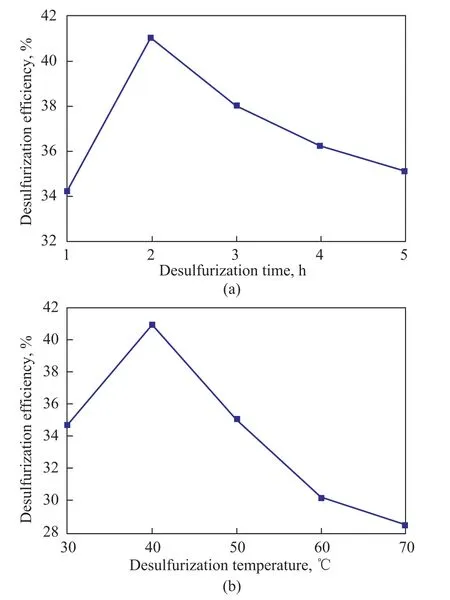
Figure 6 Effects of desulfurization time(a) and temperature(b) on desulfurization performance
3.4 Discussion on desulfurization mechanism via analysis of distillates
The sulfur content in different distillates before and after desulfurization is presented in Table 3 in order to analyze the performance of ZnX desulfurizer in different fractions.The results suggested that the desulfurization efficiency increased with an increasing boiling range of fractions,indicating that the sulfur compounds in high-boiling range fractions could be more easily adsorbed by the ZnX desulfurizer than the fractions with lower boiling range.

Table 3 Sulfur concentration in different distillates
As the high-boiling petroleum fractions mainly contain sulfur compounds with high boiling point such as TP, BT,and DBT[18], it could be concluded that these aromatic sulfur compounds were more preferably adsorbed by ZnX zeolite as compared to the sulfur compounds with low boiling point.
The above view was further confirmed by the sulfur compounds distribution in fractions before and after desulfurization, which is shown in Table 4. In the fraction with a distillation range of 30—150oC, the content of mercaptan, a saturated sulfur compound, slightly decreased after desulfurization, while the content of aromatic alkylthiophenes was significantly reduced. The similar trend also could be found in the fraction of 150—250oC, showing that the efficiency for removing aromatic alkylthiophenes and benzothiophenes by zeolites was obviously higher than the figure for removing saturated thioether. Furthermore, the sulfur removal efficiency for benzothiophenes and dibenzothiophenes in the fraction of 250—350oC was moderate.
Considering the above analysis and the strong π-complexation ability of aromatic sulfur compounds to many metal ions[15,17,19], it was speculated that the π-complexation between Zn2+ions and aromatic sulfur compounds, such as alkylthiophenes, BT and DBT, mainly contributed to the good desulfurization performance of ZnX zeolite. Compared with Cu2+ions and Co2+ions, the d-orbital of Zn2+ions possesses more electrons which can be supplied to the antibonding orbitals of aromatic compounds, and can consequently improve the stability of the coordinate bond. Therefore, the desulfurization performance of the modified 13X zeolite decreased in the following order: ZnX > CoX > CuX (Figure 1). The desulfurization performance of the modified Y zeolite decreased in the following order: CuY > CoY > ZnY.This phenomenon was identified in modified Y zeolite samples probably due to the different concentration of metal ions supported on Y zeolites, in which the content of Cu2+ions was 12.488%, while the content of Zn2+ions was only 10.571%. In addition, as shown in Table 2, the large surface area of ZnX could be beneficial to the adsorption via providing many anchoring sites for physical adsorption and opportunities for the access of sulfur compounds to metal ions. Owing to these reasons,the ZnX desulfurizer had a better performance than other modified zeolites for removal of sulfur compounds from Saudi Arabian medium crude oil.
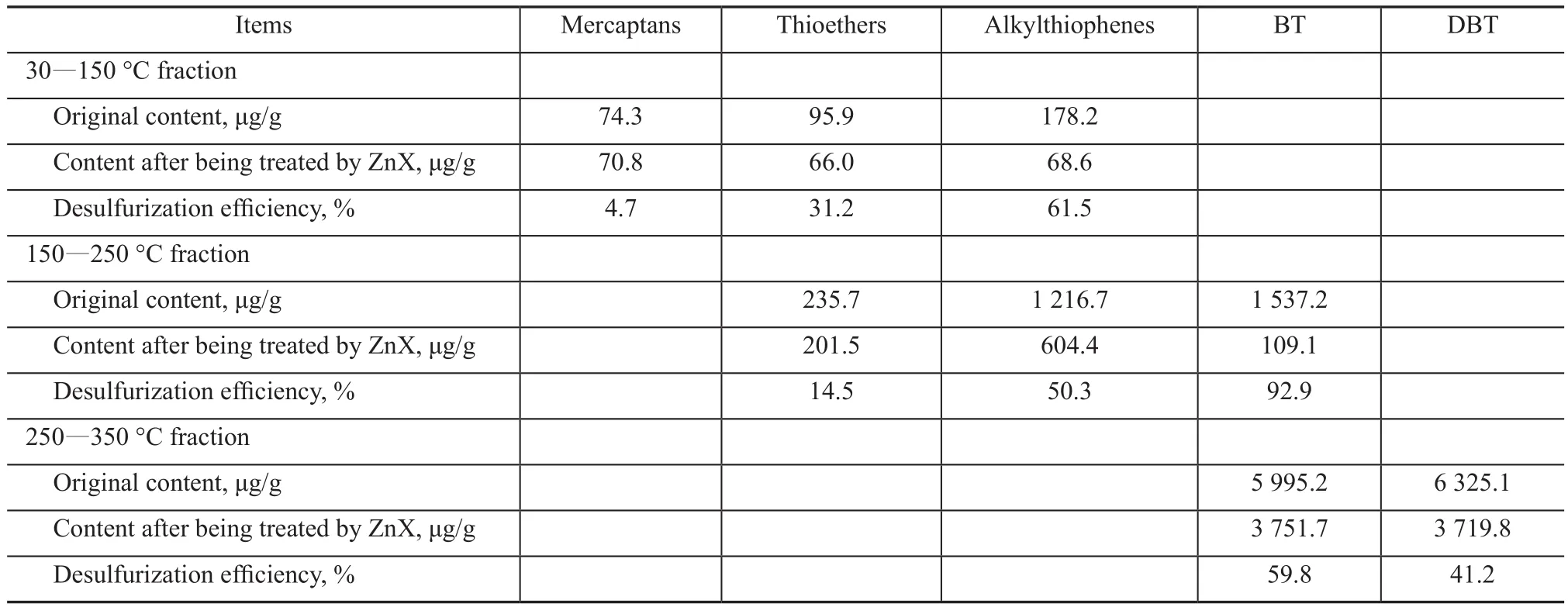
Table 4 The desulfurization efficiency for different sulfides in different fractions
3.5 Regeneration of ZnX zeolite after desulfurization
After adsorbing sulfur compounds in oil, the regeneration of ZnX was performed via heating at 700oC in a flowing air for 4 h. Figure 7 shows the desulfurization efficiency of ZnX obtained after being subject to different regeneration cycles.

Figure 7 Effect of regeneration cycle on desulfurization efficiency
It indicated that the sulfur removal efficiency offresh ZnX decreased from 41.01% to 34.46% after the first cycle of regeneration, recovering about 84% of the sulfur adsorption capacity. After that, the desulfurization efficiency consistently decreased with the increase in regeneration frequency, reaching 6.48% of desulfurization efficiency after the fourth regeneration cycle. The SEM photo of the ZnX reused for four times (Figure 4f)showed that the structure of zeolite particles was mostly destroyed, and surface of the remaining particles was full of corroded holes and ravines formed during the calcination of zeolite for regeneration or caused by the corrosive action of Saudi crude oil. As a result, the use of ZnX desulfurizer for 2—3 times in desulfurization of crude oil may be a good choice.
4 Conclusions
Desulfurization of Saudi Arabian medium crude oil was investigated via adsorption using zeolites modified by Cu2+, Co2+, and Zn2+ions. Based on static adsorption experiments, it was found that the introduction of transition metal ions into zeolites by the aqueous ionexchange method could significantly improve their desulfurization performance. The zeolite capacity for removing sulfur compounds from crude oil decreased in the following order: ZnX > CuY > CoX > CuX > CoY>ZnY. The characteristics of these zeolites determined by XRD, XRF, BET, and SEM techniques suggested that a good cation-exchange capacity could be achieved, and the framework structure of relevant zeolites was not damaged by the ion exchange and the following heating procedure. After optimizing the synthesis conditions and desulfurization conditions, the desulfurization efficiency of ZnX zeolite could reach 41.01%. This desulfurizer had a good adsorptive selectivity of aromatic sulfur compounds such as thiophenes, benzothiophenes and dibenzothiophenes contained in the oil fractions. In addition, the investigation of desulfurization mechanism showed that the π-complexation between Zn2+ions and aromatic sulfur compounds and the large surface area of ZnX zeolite might be the main reason for its good desulfurization performance. The used ZnX zeolite could be regenerated by calcination in air, but four regeneration cycles would almost totally deactivate this desulfurizer.
Acknowledgments: The authors sincerely acknowledge the financial support of the King Abdulaziz City for Science and Technology (KACST) (Grant N1204068) and the Natural Science Foundation of Shandong province of China(ZR2016BM29).
[1] Shen C, Wang Y J, Xu J H, et al. Porous glass beads as a new adsorbent to remove sulfur-containing compounds[J].Green Chemistry, 2012, 14(4): 1009-1015
[2] Liao J J, Bao W R, Chang L P. An approach to study the desulfurization mechanism and the competitive behavior from aromatics: A case study on CeY zeolite[J]. Fuel Processing Technology, 2015, 140: 104-112
[3] Li H, Han X, Huang H, et al. Competitive adsorption desulfurization performance over K - doped NiY zeolite[J].Journal of Colloid and Interface Science, 2016, 483: 102-108
[4] Song H, Cui X H, Song H L, et al. Characteristic and adsorption desulfurization performance of Ag-Ce bimetal ion-exchanged Y zeolite[J]. Industrial & Engineering Chemistry Research, 2014, 53(37): 14552-14557
[5] Song H, Wan X, Dai M, et al. Deep desulfurization of model gasoline by selective adsorption over Cu-Ce bimetal ion-exchanged Y zeolite[J]. Fuel Processing Technology,2013, 116: 52-62
[6] Xue M, Chitrakar R, Sakane K, et al. Preparation of cerium-loaded Y-zeolites for removal of organic sulfur compounds from hydrodesulfurized gasoline and diesel oil[J]. Journal of Colloid and Interface Science, 2006,298(2): 535-542
[7] Lee K X, Valla J A. Investigation of metal-exchanged mesoporous Y zeolites for the adsorptive desulfurization of liquid fuels[J]. Applied Catalysis B: Environmental, 2017,201: 359-369
[8] Song H, Gao H, Song H, et al. Effects of Si/Al ratio on adsorptive removal of thiophene and benzothiophene over ion-exchanged AgCeY zeolites[J]. Industrial &Engineering Chemistry Research, 2016, 55(13): 3813-3822[9] Khan N A, Hasan Z, Jhung S H. Ionic Liquids Supported on Metal-Organic Frameworks: Remarkable Adsorbents for Adsorptive Desulfurization[J]. Chemistry - A European Journal, 2014, 20(2): 376-380
[10] Khan N A, Yoon J W, Chang J S, et al. Enhanced adsorptive desulfurization with flexible metal-organic frameworks in the presence of diethyl ether and water[J].Chemical Communications, 2016, 52(56): 8667-8670
[11] Chen H, Wang Y, Yang F H, et al. Desulfurization of high-sulfur jet fuel by mesoporous π-complexation adsorbents[J]. Chemical Engineering Science, 2009,64(24): 5240-5246.
[12] Triantafyllidis K S, Deliyanni E A. Desulfurization of diesel fuels: Adsorption of 4,6-DMDBT on different origin and surface chemistry nanoporous activated carbons[J].Chemical Engineering Journal, 2014, 236: 406-414
[13] Jeon H-J, Ko C H, Kim S H, et al. Removal of refractory sulfur compounds in diesel using activated carbon with controlled porosity[J]. Energy & Fuels, 2009, 23(5): 2537-2543
[14] Al-Otaibi RL, He F, Yu T, et al. Alkaline-impregnated activated carbon: Synthesis and application in crude oil desulfurization[J]. Energy & Fuels, 2015, 29(11): 7456-7464
[15] Hernández-Maldonado A J, Yang F H, Qi G, et al.Desulfurization of transportation fuels by π-complexation sorbents: Cu(I)-, Ni(II)-, and Zn(II)-zeolites[J]. Applied Catalysis B: Environmental, 2005, 56(1/2): 111-126
[16] Tian F, Shen Q, Fu Z, et al. Enhanced adsorption desulfurization performance over hierarchically structured zeolite Y[J]. Fuel Processing Technology, 2014, 128: 176-182
[17] He C, Men G, Xu B, et al. Phenolic resin-derived activated carbon-supported divalent metal as efficient adsorbents(M-C, M=Zn, Ni, or Cu) for dibenzothiophene removal[J].Environmental Science and Pollution Research, 2017,24(1): 782-794
[18] Javadli R, de Klerk A. Desulfurization of heavy oil[J].Applied Petrochemical Research, 2012, 1(1): 3-19
[19] Hernández-Maldonado A J, Yang R T. Desulfurization of liquid fuels by adsorption via π-complexation with Cu(I)-Y and Ag-Y zeolites[J]. Industrial & Engineering Chemistry Research, 2003, 42(1): 123-129
Technology for Manufacture of Steam Cracking Feed through Hydrotreating of Coker LPG/Coker Gasoline Mixture Innovatively Developed by FRIPP
“The manufacture of steam cracking feed through hydrotreating of coker LPG/coker gasoline mixture”jointly developed by the SINOPEC Fushun Research Institute of Petroleum and Petrochemicals (FRIPP) and the SINOPEC Zhenhai Branch Company (ZBC) has passed the technical appraisal organized by the Science and Technology Division of the Sinopec Corp. The experts attending the appraisal meeting have recognized that the development and application of the said technology can provide an effective technical route for broadening and optimizing the source of steam cracking feedstocks in China.
It is learned that this technology is the first in China based on hydrotreating of a mixture of coker gasoline and coker LPG. This technology adopts a designated catalyst with good hydrotreating performance at low temperature used in the hydrotreating process scheme which is composed of a guard reactor for removal of diolefins via prehydrotreating of the feedstock and a connected in series main hydrotreating reactor. The process flow diagram can cleverly make use of the specific features relating to a low initial temperature for hydrotreating of LPG, which is capable of significantly decrease the reaction temperature in the guard prehydrotreating reactor to effectively overcome the shortcomings associated with
high coke forming tendency of diolefins contained in the coker gasoline and quick increase of pressure drop in the reactor. Furthermore, this technology can utilize the specific features of coker gasoline such as its high latent vaporization heat and high heat capacity to unravel the difficulty arising from hydrotreating of only LPG such as the high heat evolution rate, the concentrated heat release,the uncontrollable reaction rate, the formation of heat peaks in the top of reactor, and the quick coke formation in the upper part of the catalyst bed. The proposed process flow scheme is simple and easy in operation to ensure a stabilized product quality.
At present this technology has been applied in the first in China 600 kt/a unit for hydrotreating the coker gasoline/LPG mixture, which has been operating continuously for more than two years. The results of commercial application of this technology have revealed that adoption of this hydrotreating technology can produce the LPG product containing less than 1% of olefins and can provide qualified feedstock to the steam cracking unit.The calculation shows that this technology can increase the profit by more than 95 million RMB a year, with this scenario being reproducible among the integrated re fining/chemical enterprises to promise extensive market application prospects.
date: 2017-02-15; Accepted date: 2017-03-20.
Professor Song Linhua, E-mail:yanzfs@upc.edu.cn, Tel: +86-532- 86981571.
杂志排行
中国炼油与石油化工的其它文章
- A Study on Tribological Properties of Polypropylene Nanocomposites Reinforced with Pretreated HNTs
- Study on Quantitative Relationship between Surface Wettability and Frictional Coefficient of Liquid Flowing in a Turbulent Horizontal Pipe
- Study on the Hydrodynamic Characteristics of Venturi-Rod Deck Tray
- Behavior and Kinetics of Non-isothermal Pyrolysis of Coal at Different Heating Rates
- Characteristics of Hydrotreating Reaction in VRDS Units Located along the Yangtze River and Overall Solution for Long-cycle Running
- Synthesis of MIL-53(Fe)/MWCNTs Hybrid Material with Enhanced Efficiency for Photocatalytic Degradation of Rhodamine B
