Thermal Decomposition Behavior of Terephthalate in Inert Gas
2017-11-01YuJingWangJiming
Yu Jing; Wang Jiming
(1. Key Laboratory of Advanced Control and Optimization for Chemical Process, Ministry of Education, East China University of Science and Technology, Shanghai 200237; 2. DuPont China Holding Co., Ltd, Shanghai 201203; 3. School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
Thermal Decomposition Behavior of Terephthalate in Inert Gas
Yu Jing1,2; Wang Jiming3
(1. Key Laboratory of Advanced Control and Optimization for Chemical Process, Ministry of Education, East China University of Science and Technology, Shanghai 200237; 2. DuPont China Holding Co., Ltd, Shanghai 201203; 3. School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
Several metal terephthalates were synthesized by hydrothermal solvent method. Firstly, the coordination type of metal ions and carboxylates in terephthalate was studied by FTIR spectroscopy. The results showed that the binding type of zinc terephthalate and aluminum terephthalate are mainly bridged coordination, while the chelating coordination mode dominated in the magnesium terephthalate and cerium terephthalate. Secondly, the thermal decomposition mechanism of zinc terephthalate in nitrogen atmosphere was studied by TG and Py-GC/MS techniques. Finally, the activation energy of the thermal decomposition process was obtained by the Friedman method and the Flynn-Wall-Ozawa (FWO) method, and the most probabilistic function was obtained by multiple linear fitting. The results showed that the decomposition process of zinc terephthalate was an one-step reaction and the activation energy was equal to 187.38 kJ / mol.
terephthalate; TG / DTG; thermal decomposition; mechanism; kinetics
1 Introduction
It has been 200 years since the industrial production of aromatics was started. Currently, as an important raw material for producing synthetic polyester fiber and resin, the aromatics production is the second largest only after the ethylene and propylene production. However,the aromatics related processes such as para-xylene (PX)and purified terephthalic acid (PTA) plants have been attracting more and more public environmental concerns,especially in China. As a result, the pollutant treatment for these processes becomes more and more important.A large number of terephthalic acid-based residues are discharged during PTA production. These residues are traditionally treated by incineration, burial or solvent extraction methods, which are likely wasting resources and causing secondary pollution. Such residue should be treated by catalytic decarboxylation over metal oxides to improve the resource utilization efficiency and environmental protection[1]. Zhuang, et al.[2]studied the decarboxylation of terephthalic acid catalyzed by ZnO and other oxides. Their results revealed that terephthalic acid first forms carboxylate complex with the catalyst,and then the complex is decomposed to obtain benzene,carbon dioxide and other products. As an intermediate,the metal terephthalate plays an important role in the catalytic decarboxylation of terephthalic acid, and the thermal decomposition behavior of the intermediates can directly affect the catalytic decarboxylation activity of terephthalic acid. In order to develop a new type of highly efficient aromatic acid deacidification catalyst,it is important to explore the thermal decomposition behavior of terephthalate. In addition, the aromatic carboxylic acid complexes can be applied to make the rare earth ion luminescent matrix materials, luminescent materials and other fields thanks to their special physical and chemical properties[3-4]. It is important to study the process for decomposition of aromatic carboxylic acid complex by the thermal analysis method, which can help understand the effect of the change of ligand and the change of its complex structure on the properties of the materials.
At present, the study of the thermal decompositionbehavior of aromatic carboxylic acid salts mainly focuses on the thermal decomposition products of various types of metal aromatic carboxylates, the crystalline water removal and the crystal structure, etc.[5-6]Brzyska, et al.[7]systematically studied the thermal decomposition behavior of rare earth carboxylate complexes such as phthalic acid in the air. The results show that phthalate complexes lose all of the crystalline water in three steps by heating in the air.The product is generally a rare-earth metal oxide, and the coordination relationship between rare earth metal ions and carboxylate groups, the ligand polarization and bond energy differences will have an impact on the thermal stability. However, the study did not reveal the thermal decomposition mechanism. Zhang, et al.[8]studied the thermal decomposition mechanism of rare earth benzoate in nitrogen. RE(C6H5COO)3(RE = La, Nd, Sm, Gd, Dy, Er) salts were thermally decomposed, where the main gas-phase components were benzophenone, 9,10-anthraquinone, etc. During the thermal decomposition process, the benzoates were first converted to RE2O(CO3)2, and then were decomposed into rare earth metal oxides. However,to our best knowledge, the systematic research of the thermal decomposition behavior of terephthalates has not been reported so far. The thermodynamic properties of terephthalates including the apparent activation energy (E), the pre-exponential factor(A) and the decomposition mechanism functions of thermal decomposition reaction are crucial properties that determine the structure and thermal stability of terephthalates but are rarely reported. Based on the above research status, several terephthalates had been synthesized by hydrothermal solvent method in this paper. The terephthalate structure, the coordination type and the thermal decomposition process had been characterized by XRD, FTIR, TG and Py-GC/MS techniques. The thermal decomposition reaction mechanism was investigated in the inert gas atmosphere. The kinetics of thermal decomposition of zinc terephthalate were also discussed. It should be noted that the thermal decomposition process of terephthalates was in line with the catalytic decarboxylation reaction of terephthalic acid over metal
oxides. This study not only extends the understanding of the thermal decomposition of terephthalates, but also provides guidance for the development of PTA residue decarboxylation catalysts.
2 Experimental
2.1 Experimental materials
Mg(NO3)2·6H2O, Al(NO3)3·6H2O, Ce(NO3)3·6H2O,Zn(NO3)2·6H2O, pyridine, and terephthalic acid were all purchased from the Sinopharm Chemical Reagent Co.,Ltd. (Shanghai, China), and all reagents were of analytical reagent grade.
2.2 Sample preparation
We chose a common synthesis method using organic solvent to prepare the samples. The synthesis process of zinc terephthalate is taken as an example. A certain amount of terephthalic acid and zinc nitrate calculated by the stoichiometric reaction coefficients was dissolved in pyridine under stirring to form a homogeneous solution.This solution was then refluxed for 4 h by heating at 100 °C. The solid was collected by filtration and washed with aqueous ethanol. The solid after washing was dried to a constant weight under vacuum, and then was calcined for 2 hours at 280 °C. Finally, the as-prepared zinc terephthalate was obtained. The rest of the three samples were obtained using the same process. The corresponding terephthalate was denoted as TP-Mg, TP-Al, TP-Ce, and TP-Zn.
2.3 Sample characterization
The X-ray diffraction patterns were obtained with a Shimadzu XRD-600 model X-ray diffractometer using Ni- filtered Cu Kα radiation (λ =0.154 06).
The infrared spectra of the samples in KBr pellets were measured on a Nicolet 5700 FT-IR spectrometer in the range of 400—4 000 cm-1.
The TG-DTA curves of the as-prepared samples were recorded with a Mettler TGA/SDTA thermal analyzer in nitrogen or air (30 mL/min), when the samples were heated from room temperature to 900 °C at a temperature increase rate of 15 °C/min. The non-isothermal decomposition kinetics of zinc terephthalate was measured in a 30 mL/min flow of N2at a heating rate of 5,10, 15, 20, and 25 °C/min, respectively.
Analytical pyrolysis was performed using a CDS5200/DSQ II model pyrolysis GC/MS. The sample (typically 100 μg) was placed in a quartz tube. The pyrolysis was carried out at 700 °C for 10 s. The pyrolysis chamber was kept at 250 °C and purged with helium in order to transfer the pyrolysis products as quickly as possible to the GC column. The gas chromatograph equipped with an oncolumn injector and a capillary column (DB-WAX, 30 m × 0.25 mm ID, with 0.25 μm of film thickness). The chromatograph was programmed from 50 °C (4 min) to 260 °C at a rate of 20 °C/min. The final temperature was held for 15 min. The mass spectra were recorded under an electron impact ionization energy of 70 eV. The MS detector scanned from m/z 25 to 500.
3 Results and Discussion
3.1 Infrared spectra of terephthalate
Figure 1 shows the infrared spectra of the terephthalates.It can be seen that no stretching vibration peaks for-OH at 2 500—3 000 cm-1or -C=O at 1 678 cm-1of terephthalate can be found. This indicates that the carboxyl groups in the terephthalic acid are all bound to the metal cations to form carboxylates.The two C-O bonds in the -COO-radical have similar vibrational frequency and strong vibrational coupling interaction, resulting in two absorption peaks. As shown in Figure 1, the carboxyl group stretching vibration peaks of TP-Zn are γas(O-C-O)(1 591 cm-1, 1 542 cm-1) and γs(O-C-O) (1 386 cm-1,1 448 cm-1), respectively. According to the frequency and the difference (Δasy-sym) of antisymmetric stretching vibration and symmetrical stretching vibration of -COO-in all salts, the coordination mode of metal ions and carboxylate can be determined[9-10]. The characteristic bands of carboxylate are strongly affected by the combination of metal ions and carboxyl groups. The carboxylic acid groups can coordinate with metal ions in monodentate, chelated bidentate, bridged bidentate,and bridged triclinic mode. For the monodentate coordination mode, with the increase in strength of the M-O bond, the anti-symmetrical vibration peak of the -COO-will shift to the high wavenumber region,and the symmetrical stretching vibration peak will move to the opposite direction. Therefore, the interval of the two absorption bands is larger than the free radical of the carboxyl group[11]. Since the chelating coordination mode and the bridging coordination mode are symmetrical structures, the Δasy-symof these two modes is closer to the one of free ions.It can be seen from Figure 1 that Δasy-symof four terephthalates decreases in the following order: Al(227 cm-1)> Zn (205 cm-1) > Mg (181 cm-1) > Ce (157 cm-1).In general, the Δasy-symvalue of carboxylate sites in the bridging coordination mode is greater than the other modes[11]. Therefore, the binding type of terephthalic acid zinc salt and aluminum salt is mainly bridging coordination, while the binding type of magnesium salts and cerium salts is chelating coordination.
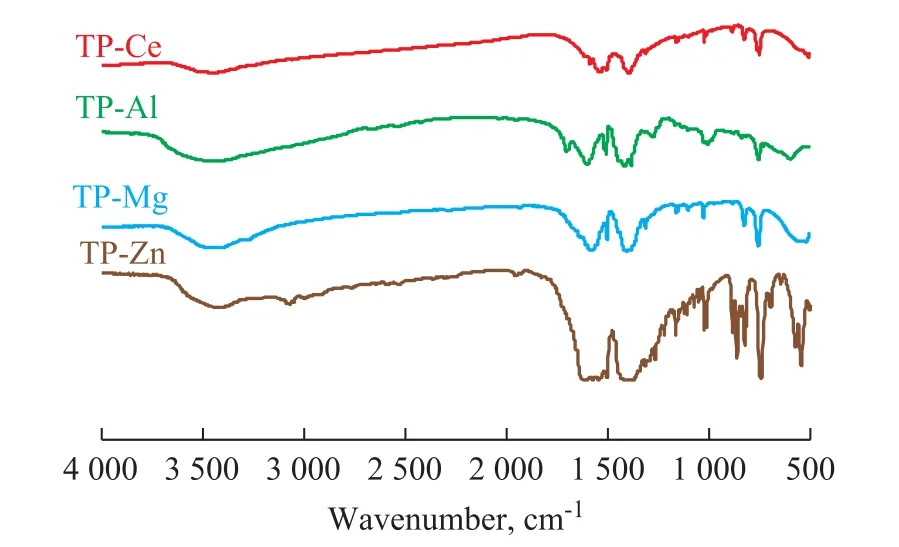
Figure 1 FTIR spectra of terephthalates
3.2 Thermal analysis of terephthalate
The TG / DTA curves of terephthalate in a nitrogen atmosphere are shown in Figure 2. All the carboxylates contained a small amount of crystalline water and adsorbed solvent, so they displayed an endothermic peak in the low temperature range of the TG / DTA curves. The weight loss of the anhydrous salt occurred mainly after 400 °C. The XRD analysis of the solid residue after thermal decomposition of the four terephthalates manifested that the final solid residue of thermal decomposition was the corresponding metal oxide. Figure 3 shows the DTG curves of the four terephthalates. It can be seen that the maximum thermal decomposition temperature of various terephthalates decreased in the following order: TP-Mg> TPAl> TP-Ce> TP-Zn, and the maximum weight loss temperature of zinc terephthalate was 492 °C which that zinc terephthalate was most prone to thermal decomposition into zinc oxide products in the nitrogen atmosphere.
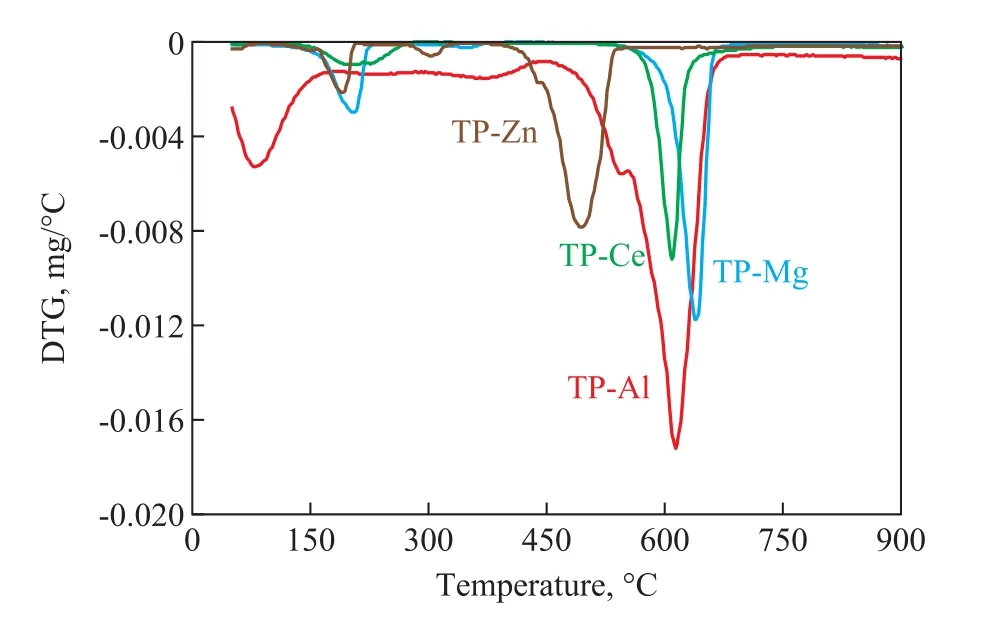
Figure 3 DTG curves of terephthalates under N2 atmosphere
3.3 Thermal decomposition mechanism of terephthalate
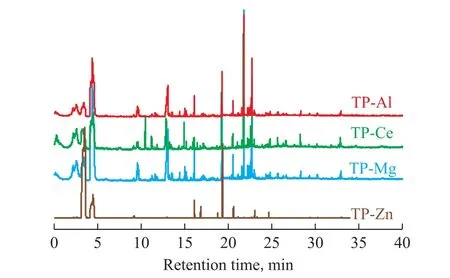
Figure 4 Py-GC/MS pro file for terephthalates
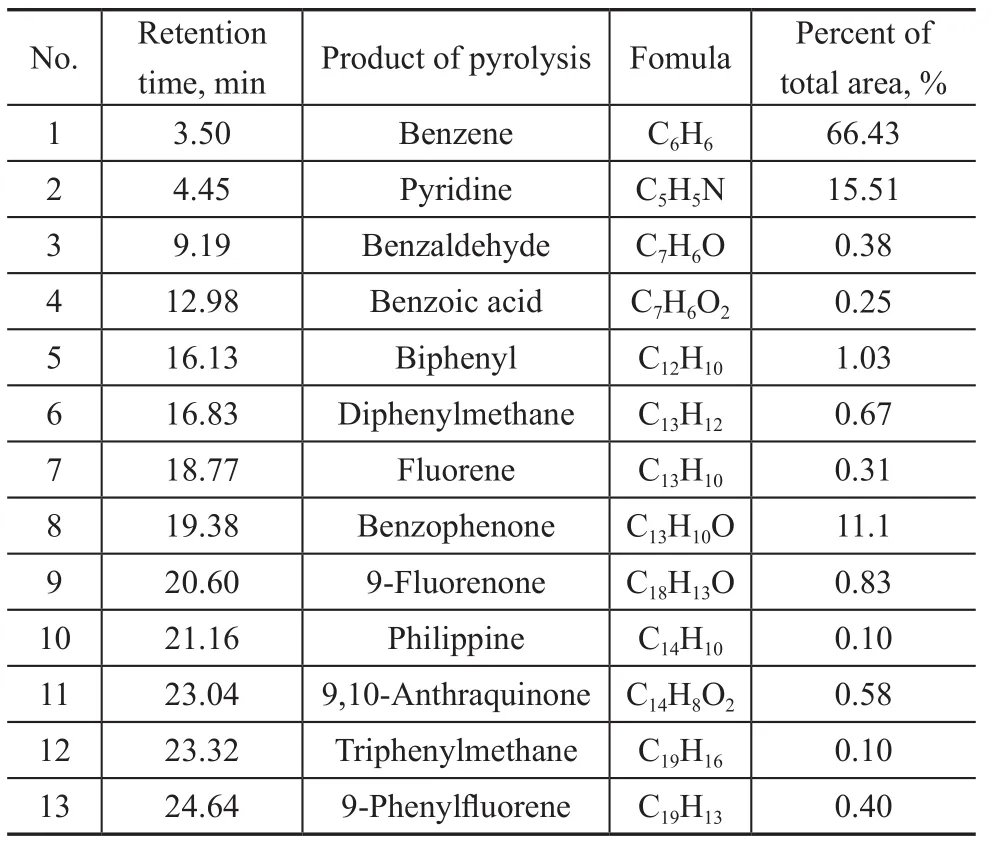
Table 1 Compounds identi fied in pyrograms from Py-GC/MS analysis of terephthalates
The GC-MS analysis results of pyrolysis products are shown in Figure 4. It can be seen from Figure 4 that the components of thermal decomposition products were nearly the same among different terephthalates, while their content of different components was quite different.For example, the main thermal decomposition products of zinc terephthalate covered benzene, benzophenone,biphenyl, phenanthrene, fluorene and other substances(Table 1), while the decomposition products of other terephthalates mainly contained benzophenone,9-fluorenone, phenanthrene, and 9,10-anthraquinone.The benzene product selectivity of these terephthalates was relatively low. Consequently, it can be found that zinc terephthalate could be readily subjected to direct decarboxylation during the thermal decomposition process.was much lower than other salts. It can be deduced Based on the distribution of the decomposition products,the proposed thermal decomposition pathways of terephthalate are shown in Figure 5. First, the fracture of C-C and C-O bonds of aromatic carboxylic acid groups can occur directly with the formation of benzoyl and benzene radicals. These free radicals can combine with each other to produce benzophenone, biphenyl and other substances,while the free radicals can also undergo hydrogenation or dehydrogenation reaction on the benzene rings. The manner in which the metal ions bind to the aromatic carboxylate group and the strength of the coordination bond can affect the location of the chemical bond breakage. For the reaction of zinc terephthalate, the C-C fracture dominates during the thermal decomposition process, which shows the highest content of benzene in the products. Based on the above analysis, the thermal decomposition process of terephthalate follows the free radical reaction mechanism,while the decarboxylation, deoxidation, dehydrogenation,coupling, and other reactions exist at the same time.
3.1 Thermal decomposition kinetics of zinc phthalate TP-Zn
Figure 6 shows the TG curve of zinc terephthalate in the nitrogen atmosphere, and the thermogravimetric curves at different heating rates are similar, indicating that the thermal decomposition behaviors are basically the same. However, there is a difference in the TG weight loss rate of TP-Zn at different heating rates, indicating that the heating rate has a significant effect on the weight loss of the sample. This is due to the fact that the dehydrogenation and decarboxylation reactions occur during the thermal decomposition of zinc terephthalate in an inert atmosphere, resulting in the formation of a solid residue of carbon and a metal oxide. When the heating rate increases, more solid residue is formed.
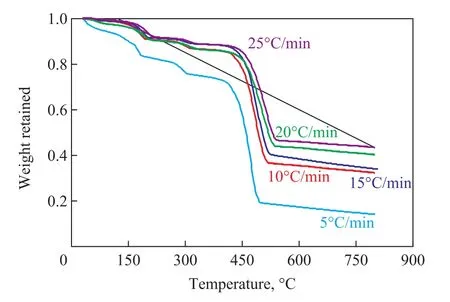
Figure 6 Thermal decomposition of zinc terephthalate at different heating rate
We have employed the most common method, viz. the isoconversional method, for thermal decomposition study. This method is also called the model-free method because no kinetic model was set before the study of kinetic activation energy. Based on the isothermal kinetic theory, the thermal decomposition kinetic equation can be expressed as follows[12]:
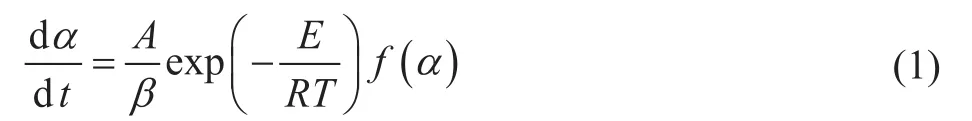
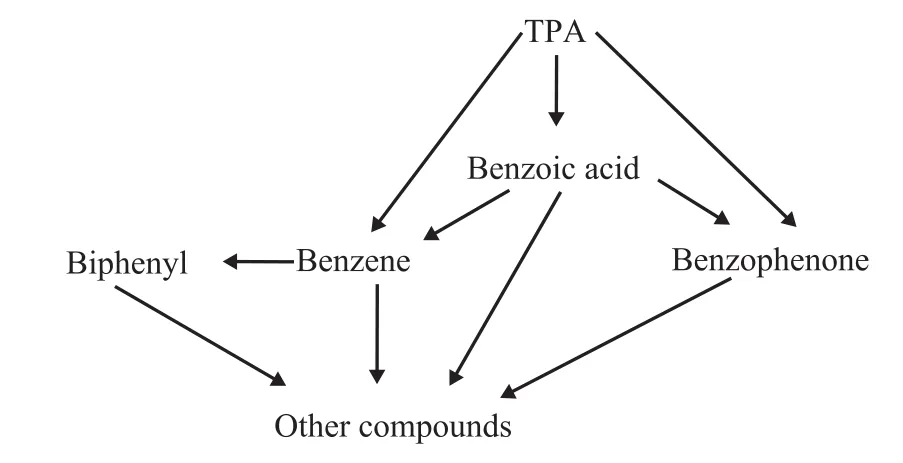
Figure 5 Proposed decomposition pathway for the terephthalate
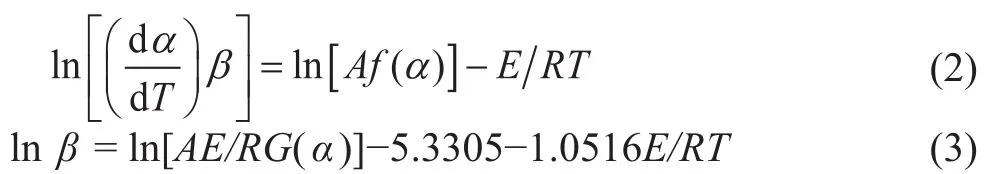
Equation (1) can be deformed by differential and integral.After several manipulations, we can obtain kinetic equations corresponding to the Friedman method[13]and the Flynn-Wall-Ozawa (FWO) method[14-15]:where α is the solid matter decomposition rate (%), A is the pre-exponential factor, E is the activation energy,T is the temperature, β is the linear heating rate, R is the universal gas constant, and f(α) and G(α) stand for the differential and integral mechanism functions of the decomposition reaction, respectively.
The above two equations can be used to solve the activation energy in the thermal decomposition reaction.The decomposition of terephthalate is the key to the decomposition process of the anhydrous salt, so only the high temperature thermal decomposition reaction process is investigated here. The experimental data at temperatures higher than 300 °C were used to calculate the activation energy by linear fitting. Figure 7(1) shows that the curves of lnβ versus 1 000/T used the FWO method, and the curves were parallel to each other. The change in the calculated activation is shown in Figure 7(2) and the activation energy varied slightly with the conversion rate. It is speculated that the thermal decomposition process is a simple one-step process. The corresponding E values for the different conversion rates α obtained by the two methods are listed in Table 4. It can be seen that the activation energy obtained by the Friedman method is lower than that of the FWO method,and the E value obtained by the FWO method fluctuates in a wide range, which may be ascribed to its high sensitivity to experiment noise[16].In addition, because the Kissinger method is simple and effective for calculating the activation energy, it is often used by researchers[17]. The method is determined by Equation (2) at the maximum decomposition rate, where
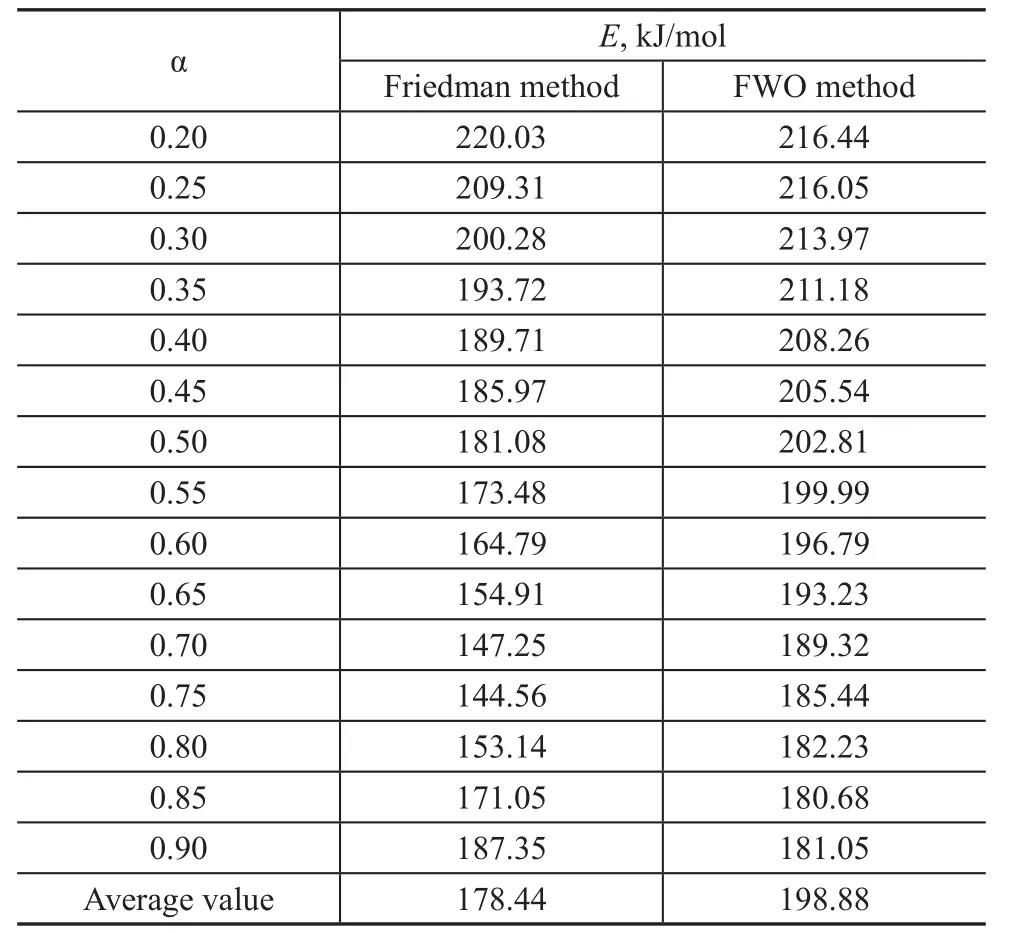
Table 4 Results of E for the decomposition of TP-Zn obtained by two methods
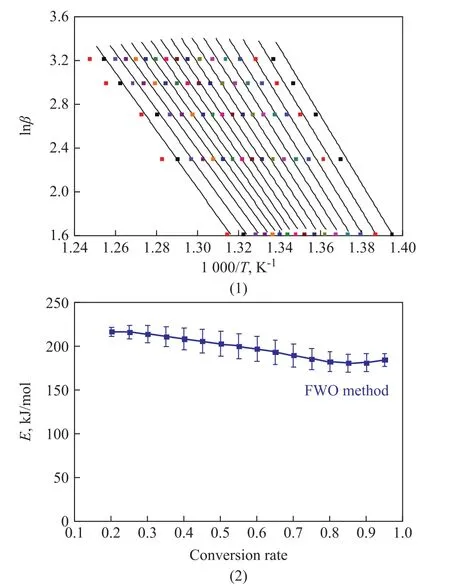
Figure 7 The curves of lnβ vs 1 000/T (1) and apparent activation energy (2) calculated by the FWO method
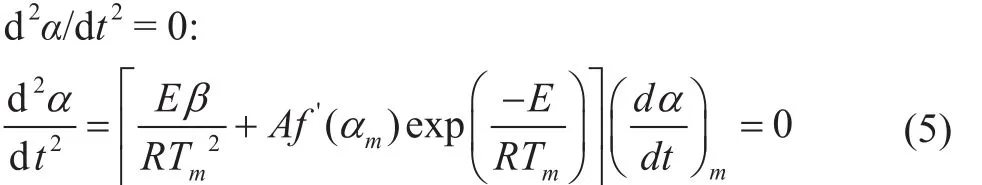
in which Tmis the temperature at the maximum reaction rate with different heating rates. Equation (5) can be transformed to obtain the Kissinger equation:
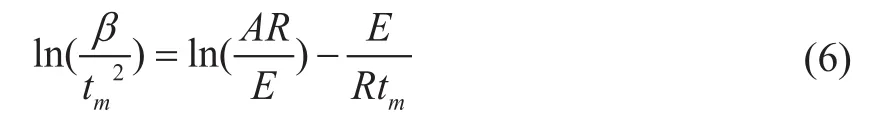
EKissingerand lnAKissingercan be obtained by using the corresponding TG curves at different heating rates, and the slope of the ln(β/tm2) ~1/tmline is -E/R, which can be used as a reference for judging the most likely thermal decomposition reaction mechanism function. It can be seen from Figure 6 that Tmat different heating rates can be obtained and substituted into Equation (4) and plotted to find EKissinger=185.86 kJ/mol, and lnAKissinger= 28.26. This is in close proximity to the activation energy obtained by the Friedman and FWO methods, and the accuracy of the two methods is also verified.
The transformed TG data are substituted into the common kinetic function model[18], and multiple linear regression is performed respectively. When the calculated activation energy is closest to the one calculated by the nonisothermal conversion method, the reaction model can be regarded as the most probable kinetic model for the decomposition reaction. The most probable kinetic model of terephthalate thermal decomposition process belongs to the Antype (n-dimensional nucleation reaction described by the Avrami-Erofeev equation[19-21]), and the final fitting model equation is f (α)=3/2(1-α)[-ln(1-α)]1/3. The activation energy E is equal to 187.38 kJ/mol, and logA is 23.1. The fitting result is close to that of the two previous conversion rates, and the difference is close to that of EKissinger, while the fitting correlation coefficient is more than 0.97, which indicates that the fitting kinetic model is credible.
4 Conclusions
We have characterized several synthesized terephthalates by infrared spectrometry. The thermal decomposition mechanism and decomposition kinetics of these terephthalates have also been studied. The main conclusions are as follows:
(1) The binding type of zinc and aluminum terephthalate was mainly bridged coordination, while the chelating coordination mode dominated in the magnesium salt and cerium salt of terephthalic acid. As for the decomposition process, terephthalates first lost the crystalline water and adsorption solvent, and then the anhydrous salt was decomposed to the corresponding metal oxide as the final solid residue.
(2) The fracture of C-C and C-O bonds of aromatic carboxylic acid groups in terephthalates could occur directly with the formation of benzoyl and benzene radicals and these radicals could further combine with each other to produce benzophenone, biphenyl and other products. Notably, zinc terephthalate was most prone to thermal decomposition, and showed the highest selectivity of benzene products during thermal decomposition,which gave the evidence that ZnO should be one of the most promising catalysts for catalytic decarboxylation of terephthalic acid.
(3) The activation energy E was obtained by the Friedman method and the Flynn-Wall-Ozawa (FWO) method, and the kinetics of the thermal decomposition kinetics of zinc terephthalate was f (α)=3/2(1-α)[-ln(1-α)]1/3, with E = 187.38 kJ/mol.
[1] Kumagai S, Grause G, Kameda T, et al. Decomposition of gaseous terephthalic acid in the presence of CaO[J].Industrial & Engineering Chemistry Research, 2011, 50(4):1831-1836
[2] Zhuang Y, Jiang B, Wang J, et al. Catalytic decarboxylation mechanism of terephthalic acid to benzene over ZnO catalyst [J]. Acta Petrolei Sinica (Petroleum Procession Section), 2015, 31(3): 698-704 (in Chinese)
[3] Sun J, Xie W, Yuan L, et al. Preparation and luminescence properties of Tb3+-doped zinc salicylates [J]. Materials Science and Engineering: B, 1999, 64(3): 157-160
[4] Wang Y, Wang D, Ren M, et al. Tb3+-doped zinc phthalate luminescent material [J]. Journal of Inorganic Material,1998(2): 152-156 (in Chinese)
[5] Panasyuk G P, Azarova L A, Khaddaj M, et al. Preparation and properties of sodium, potassium, magnesium, calcium,and aluminum terephthalates [J]. Inorganic Materials,2003, 39(12): 1292-1297
[6] Sun J, Yuan L, Zhang K, et al. Synthesis and thermal decomposition of zinc phthalate [J]. Thermochimica Acta,2000, 343(1): 105-109
[7] Brzyska W, Wołodkiewicz W. Thermal decomposition of rare earth element complexes with o-phthalic acid in air atmosphere [J]. Thermochimica Acta, 1992, 197(1): 1-7
[8] Zhang K, Yuan J, Yuan L, et al. Synthesis and thermal decomposition mechanism of RE benzoates [J]. Journal of the Chinese Rare Earth Society, 1999(3): 203-207 (in Chinese)
[9] Deacon G B, Huber F, Phillips R J. Diagnosis of the nature of carboxylate coordination from the direction of shifts of carbon oxygen stretching frequencies [J]. Inorganica Chimica Acta, 1985, 104(1): 41-45
[10] Li X, Hu K, Huang Y, et al. IR spectra of dicarboxylate of alkali-earth metal [J]. Spectroscopy and Spectral Analysis,2002, 22(3): 392-395
[11] Nakamoto K. Infrared and Raman Spectra of Inorganic and
date: 2017-03-24; Accepted date: 2017-05-06.
Professor Wang Jiming, E-mail:liujun@sinopec.com.

杂志排行
中国炼油与石油化工的其它文章
- Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
- Characterization and Apparent Kinetics of Polymerization of 1-Decene Catalyzed by Boron Trifluoride/Alcohol System
- Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
- Study on Con fined Impinging Jet Mixer and Mechanism offlash Nanoprecipitation
- Antimicrobial Degradation Performance of Novel Polyacrylamide Derivatives by Microbial Consortia for Enhanced Oil Recovery
- Hydrophobic and Magnetic Reduced Graphene Oxide Nanocomposite for Emulsified Oil Removal
