Photocatalytic Oxidative Desulfurization of Dibenzothiophene on TiO2 modified Bimodal Mesoporous Silica
2017-11-01XuMeizhenYangLinaLiJian
Xu Meizhen; Yang Lina; Li Jian
(School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
Photocatalytic Oxidative Desulfurization of Dibenzothiophene on TiO2modified Bimodal Mesoporous Silica
Xu Meizhen; Yang Lina; Li Jian
(School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
By using the bimodal mesoporous silica(BMMS) as the carrier and butyl titanate as the titanium source, the TiO2/BMMS catalyst was prepared. The samples were characterized by XRD, XRF, N2adsorption and desorption, FTIR, UV-vis, SEM, EDS, and TEM techniques. The test results showed that TiO2was amorphous, the TiO2/BMMS catalyst had an ordered bimodal mesoporous structure, and the chemical interaction existed between BMMS and TiO2. Since the TiO2/BMMS had a lower band gap, its photocatalytic activity was better than TiO2. Under UV irradiation a one-pot PODS system was set up, using TiO2/BMMS as the catalyst, H2O2as the oxidant, and methanol as the solvent. The TiO2/BMMS catalyst showed better photocatalytic activity than the mono-modal mesoporous TiO2/SBA-15 catalyst, and the desulfurization rate of dibenzothiophene (DBT) over TiO2/BMMS catalyst could reach 99.2%. The TiO2/BMMS catalyst also had so good stability that the desulfurization rate of DBT did not drop apparently after 8 cycles of reusing, and could still be close to 90%.
bimodal mesoporous silica; amorphous TiO2; photocatalytic activity; UV irradiation; dibenzothiophene
1 Introduction
Aliphatic sulfides and thiols contained in liquid fuel can be easily removed by hydrodesulfurization (HDS), however, to remove benzothiophene (BT),dibenzothiophene (DBT) and their derivates, the high temperature and pressure conditions are often necessary,which make HDS process very expensive[1-3]. As an alternative energy-efficient technology, oxidative desulfurization (ODS) is considered as one of the most attractive methods to obtain ultra-low sulfur fuels under facile reaction conditions without consumption of expensive hydrogen[4-5]. In this process, the organic sulfides are oxidized to the corresponding polar sulfoxide (DBTO) and sulfone (DBTO2), which can be removed by conventional separation processes like extraction, adsorption, distillation or decomposition[6-8].Compared with ODS, the photocatalytic oxidative desulfurization (PODS) process is more competent as a green pathway, since it can convert organic sulfur compounds with high product selectivity, and can be very efficient without heating while using the ultraviolet or visible light as the energy source[9-12].
It was reported that the PODS system using H2O2as the oxidant under ultraviolet (UV) irradiation with TiO2as the photocatalyst showed excellent activity[13-14]. The TiO2-based photocatalyst has been widely used for degradation of organic pollutants thanks to its superb photocatalytic activity, nontoxicity, widespread availability, abundance,stability and low cost under UV irradiation[15-16]. However,it has some disadvantages such as the fast recombination of the photogenerated electron-hole pairs, the broad band gap and the low specific surface area, which has limited its photocatalytic efficiency to some extent[17-19].Thus many studies now focus on loading TiO2on some supports, such as molecular sieve materials[20-22],porous glass[23]and carbon materials[24-25]to improve its photocatalytic activity.
Bimodal mesoporous silica (BMMS) is a type of hierarchical porous material with high surface area, as compared with the mono-modal mesoporous materials,and it owns a double-peak pore size distribution in the mesoporous range. The large mesopores allow the dispersion of the large molecules such as BT and DBT,and its small mesopores can provide high specific surface area[26-27]. BMMS should be a good selection as PODS catalyst support, since it can provide more opportunities for sulfur compounds to approach the catalytic sites and increases the reaction rate.
Nowadays, bimodal mesoporous materials are applied in the heterogeneous catalysis, medicine, biology and other fields[28-30]. The CTA-PMO/SBA-15 and CTAPMO/BMMS catalysts were prepared with the monomodal mesoporous and bimodal mesoporous silica serving as the carrier, respectively[31]. The results showed that the bimodal mesoporous silica has better performance than the mono-modal mesoporous silica in the oxidative desulfurization reaction. Herein, we report a photocatalytic oxidation system for DBT based on the bimodal mesoporous silica containing TiO2serving as the catalyst. Results of photocatalytic oxidation of DBT proved that the TiO2/BMMS catalyst demonstrated excellent catalytic activity.The related properties of the TiO2/BMMS catalyst were characterized and discussed. Then, a possible mechanism was proposed for the removal of DBT to elucidate the reaction process. The recovered TiO2/BMMS sample can be reused directly.
2 Experimental
2.1 Materials
All chemicals were used directly in our experiments without further purification. Tetraethylorthosilicate (TEOS, AR), tetrabutyl titanate (TBT, AR), ethanol(AR), methanol (AR), hydrogen peroxide (H2O2,30%), dibenzothiophene (DBT, 98%), hydrochloric acid (2.0 mol/L), dodecane (AR) and Na2SO4·10 H2O(AR) were all purchased from the China National Pharmaceutical Group. In addition, P123 (AR) and F127 (AR) were purchased from the Sigma-Aldrich Corporation.
2.2 Synthesis of SBA-15
The SBA-15 zeolite was prepared by the sol-gel method.As regards its detailed synthesis process, please refer to the literature[32].
2.3 Synthesis of bimodal mesoporous silica
As referred to in the published literature[31], BMMS was prepared by using P123 and F127 as the template and TEOS as the silica source.
2.4 Synthesis of catalysts
4 mL of distilled water were added into a beaker containing a mixture of 15 mL of ethanol and 4 mL of tetrabutyl titanate under vigorous stirring, while the white flocs were formed immediately. Aging occurred for 24 h at room temperature after continuous stirring for 1.0 h, and then the beaker was placed in a water bath at 40 °C for 6 h under constant stirring to evaporate ethanol, and the white powder was dried at 100 °C to obtain the amorphous TiO2particles.The TiO2/BMMS and TiO2/SBA-15 catalysts were prepared by adding 1.0 g of BMMS or 1.0 g of SBA-15, respectively, in 4 mL of the ethanol solution of tetrabutyl titanate at the beginning of the synthesis of TiO2followed by the same treatment steps as mentioned above. Finally, the TiO2/BMMS and TiO2/SBA-15 catalysts were obtained, respectively, after being dried at 100 °C for 12 h.
2.5 Characterization of catalysts
X-ray diffraction (XRD) patterns were obtained on a D/max-IIIA X-ray diffractometer (Rigaku, Japan)with Cu-Kα radiation operating at 35 kV and 30 mA.The composition of the catalyst was identified by a ZSX-100 (Rigaku, Japan) X-ray fluorescence spectrometer (XRF). The specific surface areas,the pore volume and the pore diameter of all the samples were measured by N2adsorption-desorption isotherms acquired at 77 K with an ASAP 2420(Micromeritics, USA) system. The Fourier-transform infrared spectra (FTIR) were recorded on a Nicolet 380 spectrometer (Thermo Fisher Scientific) in the range of 4 000—400 cm-1at a resolution of 4 cm-1.The UV-vis diffuse reflection spectra (UV-vis)were recorded on a UV-vis spectrometer (UV-2450, Shimadzu) in the range of 200—800 nm.The surface morphology was studied by a scanning electron microscope (SEM) (Hitachi SU 8010)operated at an acceleration voltage of 15 kV and then the elements of the samples were analyzed by an energy dispersive spectrometer (EDS). The transmission electron microscope (TEM) images of catalyst samples were obtained on a JEM-2010CX(JEOL, Japan) electron microscope operating at 200 kV.
2.6 Photocatalytic oxidative desulfurization
The model fuel with a sulfur concentration of 200 μg/g was prepared by dissolving DBT in dodecane. A 25-W UV lamp was used as the source of irradiation for PODS reaction at room temperature and the distance from the source to the beaker was 5 cm, while the catalyst (0.4%,calculated according to the mass ratio of TiO2to model fuel), hydrogen peroxide (O/S mole ratio = 10:1), 10 mL of model fuel, and 10 mL of methanol were put into in a beaker under stirring. This mixture was stirred for 2 h, and then the reaction mixture was separated with centrifugation, and the upper clear solution was subjected to sulfur measurement by a WK-2D type microcoulomb comprehensive analyzer. The catalyst at the bottom of the beaker was recovered with filtration and reused after drying at 100 °C.
3 Results and Discussion
3.1 XRD analysis
The XRD patterns of TiO2, TiO2/SBA-15 and TiO2/BMMS are shown in Figure 1. In Figure 1a, the small angle XRD patterns of TiO2/SBA-15 (2θ = 0.90°, 1.54°and 1.77°) reveal the long range ordered arrangement of 2D hexagonal channels. As for TiO2/BMMS,the distinct double-peaks are found in the range of 2θ = 0.89°-1.0°, indicating that there are two kinds of ordered mesoporous structures. It can be seen from Figure 1b that no significant diffraction peaks can be identified as TiO2. Therefore, we have successfully prepared amorphous TiO2[33]. Only one obvious broad peak appears in the TiO2/SBA-15 and TiO2/BMMS samples, which is assigned to the amorphous structure of silica.
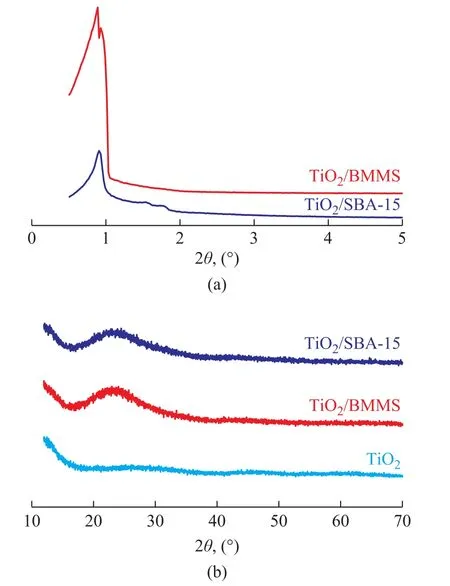
Figure 1 (a) Small angle XRD patterns and (b) wide angle XRD patterns of TiO2, TiO2/SBA-15 and TiO2/BMMS
3.2 N2 adsorption and desorption analysis
The N2adsorption and desorption isotherms results are given in Figure 2. The isotherm of TiO2/SBA-15 catalyst has a single typical H1 hysteresis loop,indicating to the existence of mono-modal cylindrical mesopores in this sample. A typical H1 hysteresis loop and a H2 hysteresis loop are both found in the isotherms of BMMS and TiO2/BMMS. The H1 hysteresis loops are attributed to the cylindrical mesopore, while H2 hysteresis loops is ascribed to the bottle mesopore. These results indicate that the loading of TiO2does not change the bimodal mesoporous structure of BMMS. The pore size distribution of the samples is listed in Figure 2b, in which the TiO2/SBA-15 catalyst displays the characteristic single peak, indicating to the existence of the monomodal mesopores. The double peaks in the pore size distribution of BMMS and TiO2/BMMS can further verify the bimodal mesoporous structure. Table 1 lists the physical structure parameters and composition of these samples. The TiO2contents in TiO2/BMMS and TiO2/SBA-15 are almost the same. The specific surface area and pore volume of the TiO2/BMMS catalyst are much greater than TiO2. Compared with the TiO2/SBA-15 catalyst, the specific surface area of the TiO2/BMMS catalyst has increased but is not particularly obvious,while its pore volume has decreased.
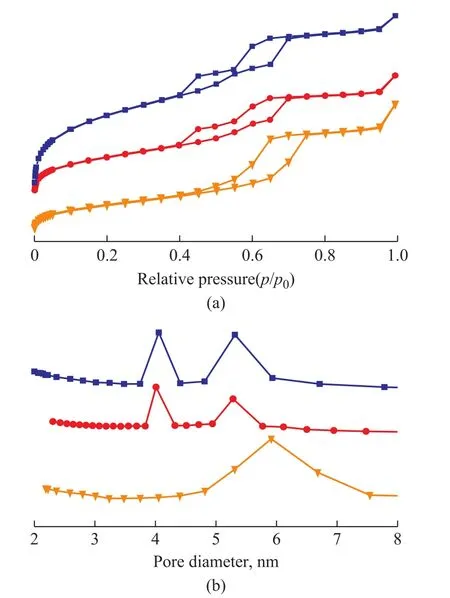
Figure 2 (a) N2 adsorption-desorption isotherms and (b)pore diameter distribution of BMMS, TiO2/SBA-15 and TiO2/BMMS
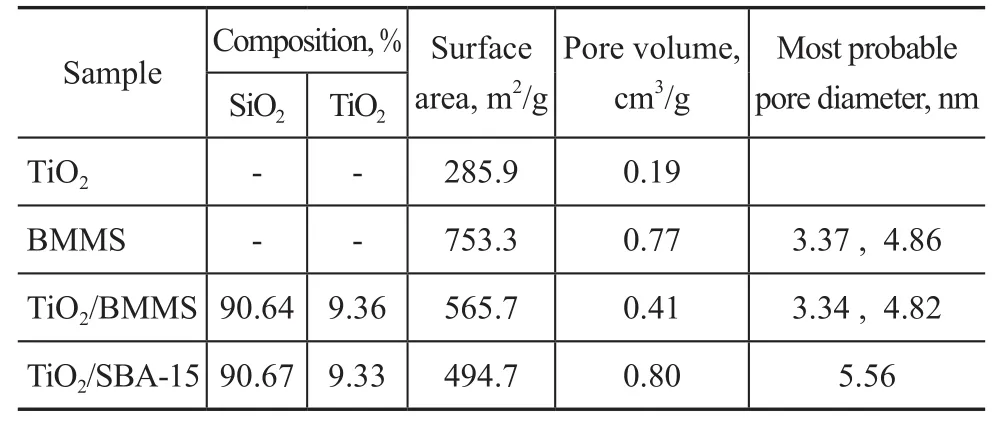
Table 1 Physical parameters of pore structure and composition of samples
3.3 FTIR spectrometric analysis
The FIIR spectra of the samples are shown in Figure 3.The peak around 800 cm-1—500 cm-1is assigned to the O-Ti-O lattice[34]. The characteristic peaks of TiO2at 1 463 cm-1and 1 377 cm-1are assigned to the stretching vibration of Ti-O, and the characteristic peak at 1 235 cm-1—1 050cm-1can be attributed to the Ti-OH[35].The strong and broad peaks around 1 077 cm-1and 810 cm-1of TiO2/SBA-15 and TiO2/BMMS are assigned to the antisymmetric and symmetric stretching vibrations of Si-O-Si, respectively. The band near 463 cm-1is assigned to the symmetric vibration of Si-OH forming hydrogen bond. The band at around 968 cm-1can be contributed to the stretching vibration of Si-O bond formed by Si and Ti in Si-O-Ti[36]. Based on the above FTIR results it can be deduced here that TiO2was successfully introduced in BMMS and the chemical interaction existed between TiO2and the silica support.
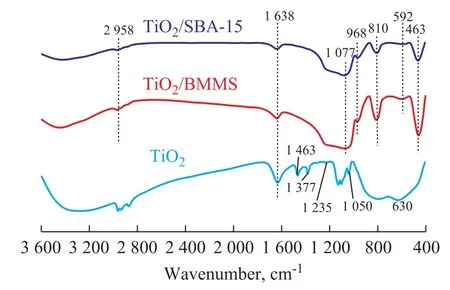
Figure 3 FTIR of TiO2, TiO2/SBA-15 and TiO2/BMMS
3.4 UV-vis spectrometric analysis
Figure 4 (a) displays the diffuse reflectance spectra of TiO2and TiO2/BMMS. It is shown that both samples have sharp absorption bands at 200 nm—400 nm.Compared with the pure TiO2, the intensity of the absorption peak of TiO2/BMMS is higher, suggesting that the rate of electron-hole pair formation increases when TiO2is supported on the surface of BMMS,resulting in an enhanced photocatalytic activity[8]. The forbidden band width of the sample can be calculated with the formula αhv= A (hv-Eg)1/2, where α, hv, Eg and A are the optical absorption coefficient, the photon energy, the forbidden band width, and a constant,respectively. Figure 4 (b) shows the energy intercept of a plot of (αhv)2vs. hv for a direct transition. After calculation, the band gap energy (hv) of TiO2and TiO2/BMMS is obtained, which is equal to 3.23 eV and 3.03 eV, respectively. The results indicate that the UV light absorption of TiO2is clearly improved after being loaded on BMMS and hence the photocatalytic activity is enhanced under UV-light irradiation[37].
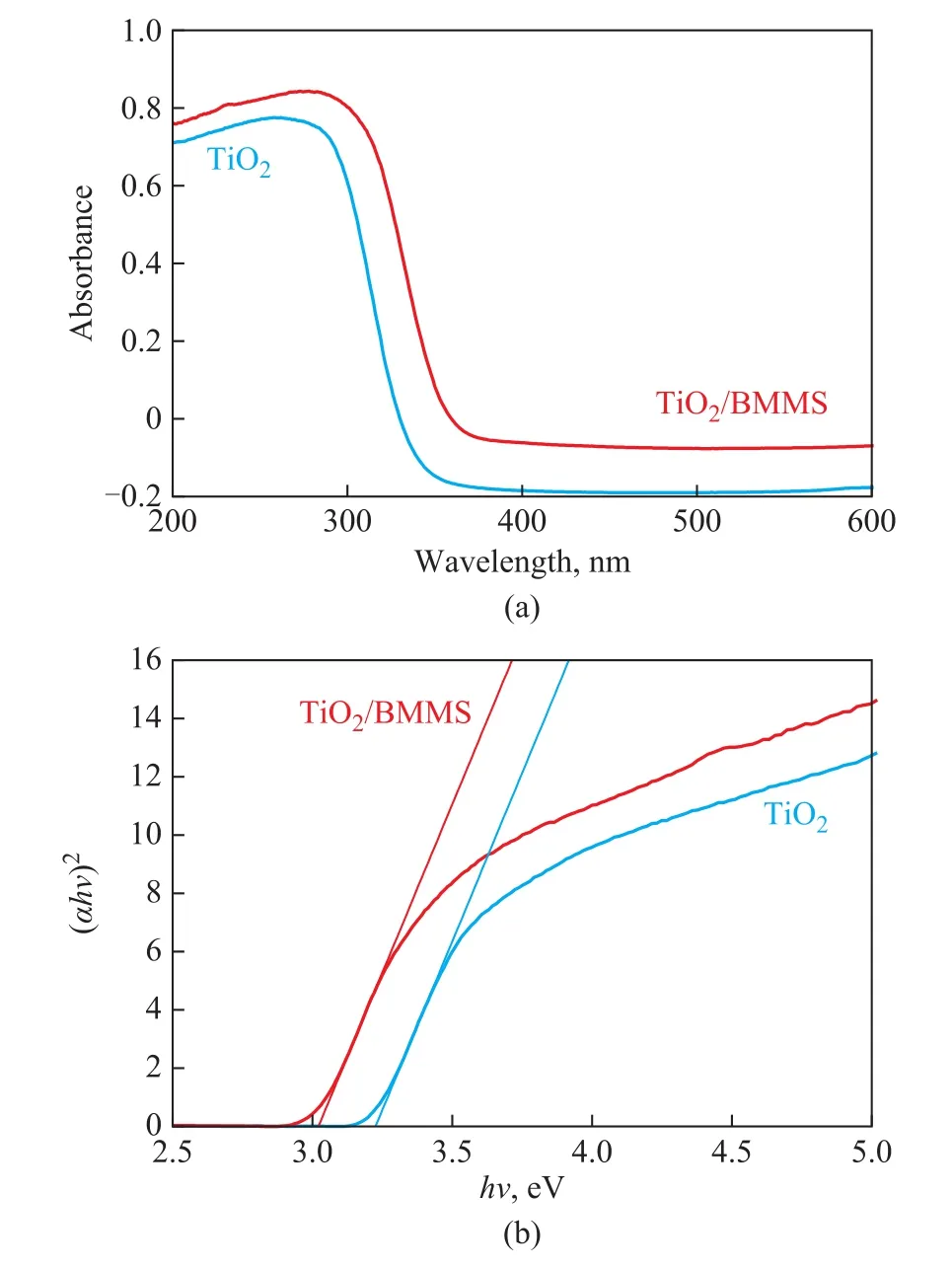
Figure 4 (a) UV-vis spectra and (b) (αhν)2 vs hν curves of TiO2 and TiO2/BMMS
3.5 SEM and EDS analyses
Figure 5 shows the SEM images of TiO2and TiO2/BMMS. It can be seen from Figure 5 (a) that the amorphous TiO2shows many particles, and the particle agglomeration is obvious. It can be seen from Figure 5 (b)that the TiO2/BMMS sample shows a grain shape, which is a typical appearance of BMMS, indicating that the addition of TiO2still cannot change the morphology of BMMS. It can be seen from Figure 6 that TiO2/BMMS is composed of Ti, Si and O elements, indicating that TiO2has been successfully supported on BMMS.
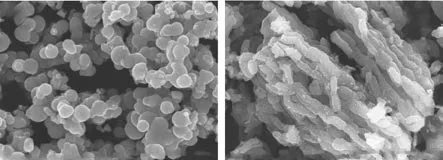
Figure 5 SEM images of (a) TiO2 and (b) TiO2/BMMS
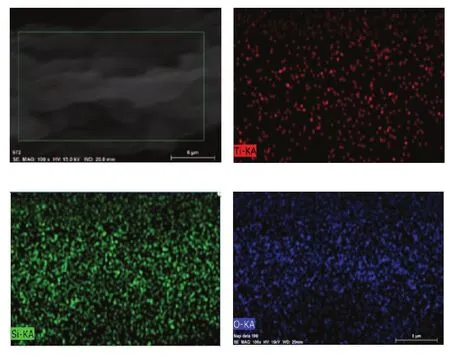
Figure 6 EDS photographs of TiO2/BMMS
3.6 TEM analysis
In order to further clarify the pore structure of TiO2/BMMS and the dispersion status of TiO2in BMMS, the TEM images of TiO2/BMMS are listed in Figure 7. It can be seen from the graph that A is a typical hexagonal pore structure and B is a cubic structure, and the sample shows the orderly hexagonal-cubic symbiotic structure of the long range ordered arrangement. And the different pore size of TiO2/BMMS is 3.0 nm and 5.0 nm, respectively,which is consistent with the N2adsorption-desorption and XRD results. The darker part in the field can be attributed to the existence of TiO2in the channels.

Figure 7 TEM images of TiO2/BMMS
3.7 Comparison of catalytic performance between catalyst samples
The desulfurization performance of TiO2, BMMS, SBA-15, TiO2/BMMS and TiO2/SBA-15 samples was studied during PDOS of DBT, with the reaction results and the calculated dynamic data listed in Figure 8. According to Figure 8, for the catalyst systems with BMMS or SBA-15 the desulfurization rate was basically around 40%,showing that the supported catalysts had much higher activity than the bulk TiO2, BMMS and SBA-15, which occurred because the formation rate of conduction band electron (e-) and the valence band hole (h+) increased when TiO2was supported on the surface of BMMS under irradiation. The result was consistent with the outcome of the UV-vis analysis. The TiO2/BMMS catalyst had better catalytic performance than the TiO2/SBA-15 catalyst and its highest desulfurization rate could reach 99.2% thanks to its special bimodal mesoporous structure.
The kinetic data were obtained after linear fitting of -lnCA/C mapping to the reaction time (T), where C and CAare the sulfur content of the feed and the product after PODS respectively. It can be seen that all the samples showed a linear increase with the reaction time, and consequently the PODS of DBT over these catalysts complied with the pseudo- first-order kinetics equation, and the slope of the straight lines was the reaction rate constant, and the reaction rate reduced in the following order: TiO2/BMMS> TiO2/SBA-15 > TiO2.
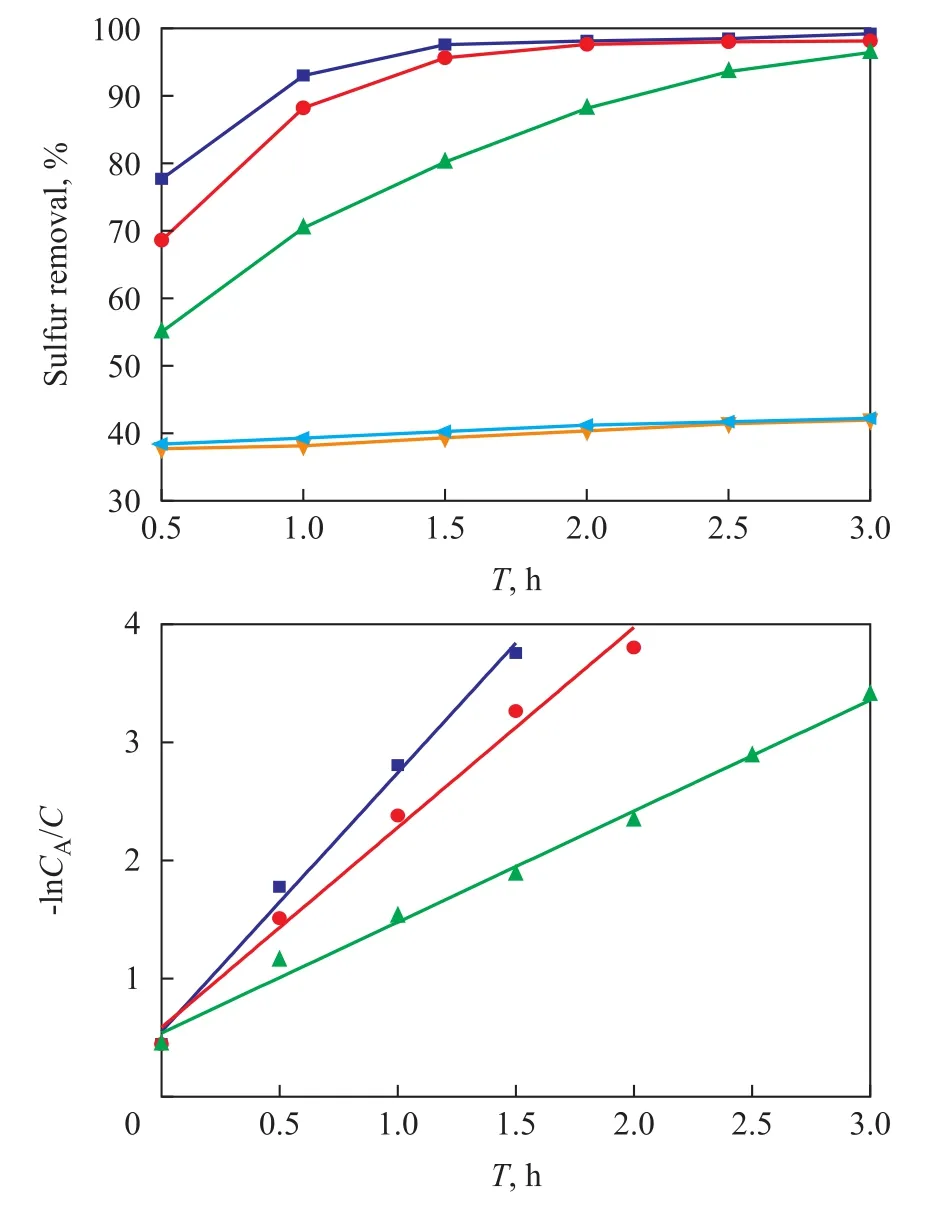
Figure 8 Comparison of catalytic activity and pseudo- firstorder kinetics for catalysts
3.8 Recycled use of TiO2/BMMS catalyst
In order to investigate the stability of the TiO2/BMMS catalyst, the catalyst was reused for 8 runs, the results of sulfur removal on the reused catalyst in Figure 9 showed that the desulfurization rate achieved by the TiO2/BMMS catalyst did not significantly decrease and the desulfurization rate could still reach about 90.0% in the 8th reaction cycle.
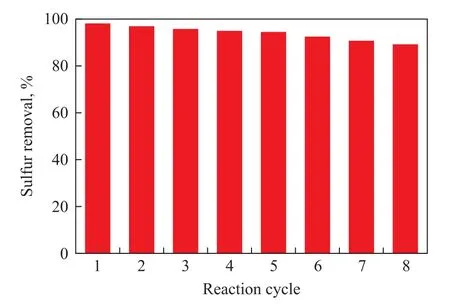
Figure 9 Desulfurization rates over TiO2/BMMS catalyst reused for different cycles
3.9 Mechanism of PODS on TiO2/BMMS catalyst
Since DBT can be converted effectively under UV irritation on the TiO2/BMMS catalyst, it is necessary to conduct a research on this PODS mechanism. The research on the functions of the constituents of the PODS system was carried out, with the desulfurization rates in different situations shown in Table 2. All the experiments were carried out under ultraviolet irradiation. The data in Table 2 show that when only H2O2and DBT were used, the desulfurization rate was only 12.2%. When the TiO2/BMMS catalyst and H2O2were added to the DBT, the desulfurization rate was improved to reach 34.6% but the effect was not very good. When CH3OH was added into the above system, the desulfurization rate increased sharply to reach 98.9%, which indicated that TiO2/BMMS, H2O2and CH3OH had a good synergistic effect to improve the photocatalytic oxidative desulfurization. Here H2O2acted as an oxidant, TiO2/BMMS was a catalyst and CH3OH functioned as a solvent.The mechanism of photocatalytic oxidation of DBT on TiO2/BMMS is proposed in Figure 10. The TiO2/BMMS catalyst produces photogenerated electrons and holes under the irradiation of ultraviolet light, while electrons can quickly move and react with H2O2to form hydroxyl radicals ·OH and the holes on the surface can react with the adsorbed H2O or OH-on catalyst surface to form ·OH radicals[38-39]. The ·OH radicals have a strong oxidizing ability to further oxidize the DBT molecules adsorbed on the surface of TiO2/BMMS catalyst. At the water and oil interface, DBT can be easily oxidized to the corresponding sulfone or sulfoxide, which is then extracted by methanol to achieve the purpose of deep desulfurization. In this process, when TiO2is loaded on BMMS, the specific surface area and pore volume are increased, and the large pores of BMMS can provide a transport channel for the DBT and transfer the product to the inside and outside of the pores. The small mesopores have higher specific surface area which is good for the fine dispersion of TiO2particles that can be more conductive to the formation of photogenerated electrons and holes, and consequently this method can effectively increase the effective conversion of DBT.
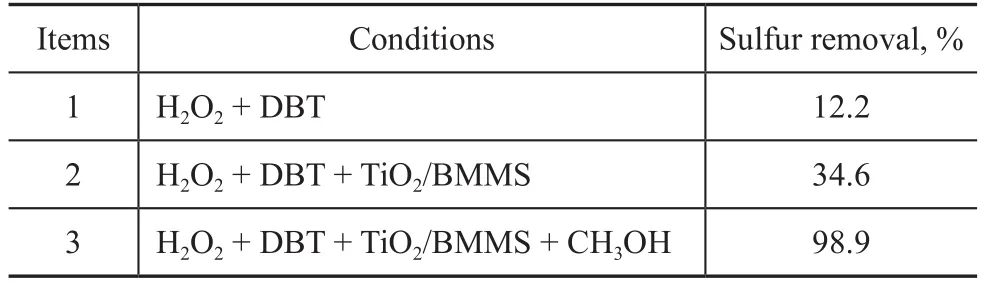
Table 2 Effect of different systems on the sulfur removal of DBT
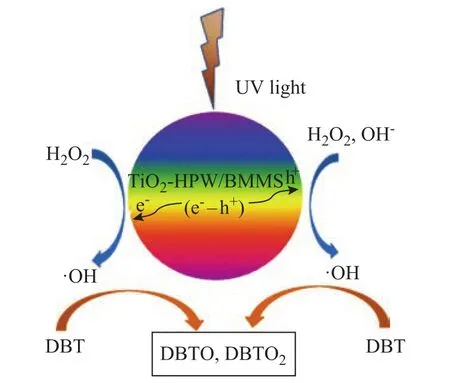
Figure 10 Proposed mechanism of photocatalytic oxidation of DBT on TiO2/BMMS catalyst
4 Conclusions
In summary, TiO2was successfully supported on the BMMS and its photocatalytic performance was better than pure TiO2and TiO2/SBA-15. The large mesopores could afford the passageway for the dispersion of DBT and its products in the reaction system, in the meantime because of the high surface area of small mesopores the distribution of TiO2became more homogeneous on the pore wall surface. The TiO2/BMMS catalyst could effectively inhibit the recombination of photogenerated electrons and holes and had a lower band gap to improve its photocatalytic performance. The PODS of DBT over the TiO2/BMMS catalyst could comply with the firstorder kinetic equation and the reaction rate constant was obviously higher than the monomodal mesoporous materials. The desulfurization rate could reach 99.2%,along with the good reusability of the catalyst.
Acknowledgment: The work was financially supported by the Program for Liaoning Excellent Talents in University,abbreviated as “LNET”(LJQ2015062), Program for Science and Technology Agency of Liaoning Province (20170540585),General Scientific Research Project of Liaoning Provincial Department of Education(L2015296, L2016018) and Science and Technology Planning project of fushun (FSKJHT201376).
[1] Bokare A D, Choi W. Bicarbonate-induced activation of H2O2for metal-free oxidative desulfurization[J]. Journal of Hazardous Materials, 2016, 304: 313-319
[2] Zaid H F M, Chong F K, Mutalib M I A. Photooxidativeextractive deep desulfurization of diesel using Cu-Fe/TiO2and eutectic ionic liquid[J]. Fuel, 2015, 156: 54-62
[3] Xun S, Zhu W, Chang Y, et al. Synthesis of supported SiW12O40-based ionic liquid catalyst induced solventfree oxidative deep-desulfurization of fuels[J]. Chemical Engineering Journal, 2016, 288: 608-617
[4] Li S W, Gao R M, Zhang R L, et al. Template method for a hybrid catalyst material POM@ MOF-199 anchored on MCM-41: Highly oxidative desulfurization of DBT under molecular oxygen[J]. Fuel, 2016, 184: 18-27
[5] Li S, Mominou N, Wang Z, et al. Ultra-deep desulfurization of gasoline with CuW/TiO2- GO through photocatalytic oxidation[J]. Energy & Fuels, 2016, 30(2): 962-967
[6] Li L, Zhang J, Shen C, et al. Oxidative desulfurization of model fuels with pure nano-TiO2as catalyst directly without UV irradiation[J]. Fuel, 2016, 167: 9-16
[7] Choi A E S, Roces S, Dugos N, et al. Oxidation by H2O2of bezothiophene and dibenzothiophene over different polyoxometalate catalysts in the frame of ultrasound and mixing assisted oxidative desulfurization[J]. Fuel, 2016,180: 127-136
[8] Lu X, Li X, Qian J, et al. Synthesis and characterization of CeO2/TiO2nanotube arrays and enhanced photocatalytic oxidative desulfurization performance[J]. Journal of Alloys and Compounds, 2016, 661: 363-371
[9] Miao G, Huang D, Ren X, et al. Visible-light induced photocatalytic oxidative desulfurization using BiVO4/C3N4@SiO2with air/cumene hydroperoxide under ambient conditions[J]. Applied Catalysis B: Environmental, 2016,192: 72-79
[10] Lei W, Wenya W, Mominou N, et al. Ultra-deep desulfurization of gasoline through aqueous phase in-situ hydrogenation and photocatalytic oxidation[J]. Applied Catalysis B: Environmental, 2016, 193: 180-188
[11] Lin F, Jiang Z, Tang N, et al. Photocatalytic oxidation of thiophene on RuO2/SO42--TiO2: Insights for cocatalyst and solid-acid[J]. Applied Catalysis B: Environmental, 2016,188: 253-258
[12] Hitam C N C, Jalil A A, Triwahyono S, et al. Synergistic interactions of Cu and N on surface altered amorphous TiO2nanoparticles for enhanced photocatalytic oxidative desulfurization of dibenzothiophene[J]. RSC Advances,2016, 6(80): 76259-76268
[13] Zhu W, Xu Y, Li H, et al. Photocatalytic oxidative desulfurization of dibenzothiophene catalyzed by amorphous TiO2in ionic liquid[J]. Korean Journal of Chemical Engineering, 2014, 31(2): 211-217
[14] Matsuzawa S, Tanaka J, Sato S, et al. Photocatalytic oxidation of dibenzothiophenes in acetonitrile using TiO2:Effect of hydrogen peroxide and ultrasound irradiation[J].Journal of Photochemistry and Photobiology A: Chemistry,2002, 149(1): 183-189
[15] Xun S, Zhu W, Zheng D, et al. Supported ionic liquid[Bmim] FeCl4/Am TiO2as an efficient catalyst for the catalytic oxidative desulfurization of fuels[J]. RSC Advances, 2015, 5(54): 43528-43536
[16] Zarrabi M, Entezari M H, Goharshadi E K. Photocatalytic oxidative desulfurization of dibenzothiophene by C/TiO2@MCM-41 nanoparticles under visible light and mild conditions[J]. RSC Advances, 2015, 5(44): 34652-34662
[17] Vu T H T, Nguyen T T T, Nguyen P H T, et al. Fabrication of photocatalytic composite of multi-walled carbon nanotubes/TiO2and its application for desulfurization of diesel[J]. Materials Research Bulletin, 2012, 47(2): 308-314
[18] Lin F, Zhang Y, Wang L, et al. Highly efficient photocatalytic oxidation of sulfur-containing organic compounds and dyes on TiO2with dual cocatalysts Pt and RuO2[J]. Applied Catalysis B: Environmental, 2012, 127:363-370
[19] Moradi S, Vossoughi M, Feilizadeh M, et al. Photocatalytic degradation of dibenzothiophene using La/PEG-modified TiO2under visible light irradiation[J]. Research on Chemical Intermediates, 2015, 41: 4151-4167
[20] Zhang J, Zhao D, Ma Z, et al. Phase-boundary photocatalytic oxidation of dibenzothiophene over amphiphilic Ti-MCM-41 molecular sieve[J]. Catalysis Letters, 2010, 138(1/2): 111-115
[21] Wang L, Cai H, Li S, et al. Ultra-deep removal of thiophene compounds in diesel oil over catalyst TiO2/Ni-ZSM-5 assisted by ultraviolet irradiation[J]. Fuel, 2013,105: 752-756
[22] Ren X, Miao G, Xiao Z, et al. Catalytic adsorptive desulfurization of model diesel fuel using TiO2/SBA-15 under mild conditions[J]. Fuel, 2016, 174: 118-125
[23] Shen C, Wang Y J, Xu J H, et al. Oxidative desulfurization of DBT with H2O2catalysed by TiO2/porous glass[J].Green Chemistry, 2016, 18(3): 771-781
[24] Zhang J, Zhao D, Wang J, et al. Photocatalytic oxidation of dibenzothiophene using TiO2/bamboo charcoal[J]. Journal of Materials Science, 2009, 44(12): 3112-3117
[25] Wang C, Zhu W, Xu Y, et al. Preparation of TiO2/g-C3N4composites and their application in photocatalytic oxidative desulfurization[J]. Ceramics International, 2014, 40(8):11627-11635
[26] Zhang Y, Koike M, Tsubaki N. Preparation of aluminasilica bimodal pore catalysts for Fischer-Tropsch synthesis[J]. Catalysis Letters, 2005, 99(3/4): 193-198
[27] Sato S, Takahashi R, Sodesawa T, et al. Bimodal porous Pd-silica for liquid-phase hydrogenation[J]. Applied Catalysis A: General, 2005, 284(1): 247-251
[28] Xiu T, Wang J, Liu Q. Ordered bimodal mesoporous boria-alumina composite: One-step synthesis,structural characterization, active catalysis for methanol dehydration[J]. Microporous and Mesoporous Materials,2011, 143(2): 362-367
[29] Gao L, Sun J, Ren B, et al. Structural characterization and surface heterogeneity of bimodal mesoporous silicas functionalized with aminopropyl groups and loaded aspirin[J]. Materials Research Bulletin, 2011, 46(10):1540-1545
[30] Zhang Y, Shi X, Kim J M, et al. Synthesis and catalysis of nanometer-sized bimodal mesoporous aluminosilicate materials[J]. Catalysis Today, 2004, 93: 615-618
[31] Li Jian, Wang Haishun, Yang Lina, et al. An oxidative desulfurization catalyst based on bimodal mesoporous silica containing quaternary ammonium heteropolyphosphamolybdenum[J]. China Petroleum Processing & Petrochemical Technology, 2016, 18(2):33-42
[32] Zhao D, Feng J, Huo Q, et al. Triblock copolymer synthesis of mesoporous silica with periodic 50 to 300 Angstrom pores[J]. Science, 1998, 279(5350): 548-552
[33] Zheng D, Zhu W, Xun S, et al. Deep oxidative desulfurization of dibenzothiophene using low-temperature-mediated titanium dioxide catalyst in ionic liquids[J]. Fuel, 2015,159: 446-453
[34] Zou J, Gao J, Xie F. An amorphous TiO2sol sensitized with H2O2with the enhancement of photocatalytic activity[J].Journal of Alloys and Compounds, 2010, 497(1): 420-427
[35] Liao M H, Hsu C H, Chen D H. Preparation and properties of amorphous titania-coated zinc oxide nanoparticles[J]. Journal of Solid State Chemistry, 2006,179(7): 2020-2026
[36] Palcheva R, Spojakina A, Dimitrov L, et al.12-Tungstophosphoric heteropolyacid supported on modified SBA-15 as catalyst in HDS of thiophene[J].Microporous and Mesoporous Materials, 2009, 122(1):128-134
[37] Aazam E S. Visible light photocatalytic degradation of thiophene using Ag-TiO2/multi-walled carbon nanotubes nanocomposite[J]. Ceramics International, 2014, 40(5):6705-6711
[38] Zhu W, Wang C, Li H, et al. One-pot extraction combined with metal-free photochemical aerobic oxidative desulfurization in deep eutectic solvent[J]. Green Chemistry, 2015, 17(4): 2464-2472
[39] Bo S U N, Xue Y U, Liang W, et al. Enhanced visible light photocatalytic oxidative desulfurization by BiOBrgraphene composite[J]. Journal of fuel Chemistry and Technology, 2016, 44(9): 1074-1081
The Methanol-to-Propylene Fluidized-bed Process Developed by Dalian Institute of Chemical Physics Passed the Assessment of Research Achievement
The research project “The methanol to propylene (DMTP)fluidized-bed process”, on which the CAS Dalian Institute of Chemical Physics (DICP) owns the independent intellectual property rights, has passed the assessment of Scientific achievement organized by China Petroleum and Chemical Industry Federation (CPCIF).
The Dalian Institute of Chemical Physics (DICP) on the basis of successful development of the DMTO technology has again been innovatively engaging in the development of technical process for manufacturing propylene from methanol in the fluidized bed. DICP after having developed the special DMTP catalyst with outstanding performance has successfully integrated three reactions covering methanol conversion, ethylene alkylation and conversion ofhydrocarbons with the advanced performance and process technology indicators. The 100 t/a DMTP scaleup tests have been completed, with the DMTP fluidized process being verified and optimized to harvest the basic data required for compiling the process design package (PDP). The calibration test conducted in 72 hours had revealed that the propylene selectivity reached 75.0%, and the ethylene selectivity reached 10.4%, with the consumption of methanol was equal to 3.01 tons/ ton of propylene produced. The propylene selectivity can be further increased by changing the ethylene/methanol ratio.
date: 2017-03-30; Accepted date: 2017-05-09.
Professor Li Jian, E-mail:yanglnzg@163.com.
杂志排行
中国炼油与石油化工的其它文章
- Thermal Decomposition Behavior of Terephthalate in Inert Gas
- Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
- Characterization and Apparent Kinetics of Polymerization of 1-Decene Catalyzed by Boron Trifluoride/Alcohol System
- Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
- Study on Con fined Impinging Jet Mixer and Mechanism offlash Nanoprecipitation
- Antimicrobial Degradation Performance of Novel Polyacrylamide Derivatives by Microbial Consortia for Enhanced Oil Recovery
