Delayed introduction of immunosuppressive regimens in critically ill patients after liver transplantation
2017-10-09YingLuoWenBinJiWeiDongDuanXianJieShiandZhiMingZhao
Ying Luo, Wen-Bin Ji, Wei-Dong Duan, Xian-Jie Shi and Zhi-Ming Zhao
Beijing, China
Delayed introduction of immunosuppressive regimens in critically ill patients after liver transplantation
Ying Luo, Wen-Bin Ji, Wei-Dong Duan, Xian-Jie Shi and Zhi-Ming Zhao
Beijing, China
METHODS: From March 2009 to February 2014, 225 consecutive liver recipients in our institute were included.e delayed administration of immunosuppressive regimens was attempted in 11 liver recipients with multiple severe comorbidities.
(Hepatobiliary Pancreat Dis Int 2017;16:487-492)
infection;
immune monitoring;
liver transplantation;
immunosuppression;
critical care;
Immuknow assay
Introduction
Due to the lack of donor organs, a large proportion of patients with advanced chronic liver disease or acute liver failure develop multiple organ dysfunction before liver transplantation.[1-3]With respect to the post-transplant critical care of these patients, we have to weigh in two different biological systems, the newly implanted allograand the critically ill patient. On the one hand, the immunosuppressive strategies are mandatory in order to protect the allografrom rejection. On the other hand, the numerous adverse events associated with immunosuppressive regimens, such as infections,renal toxicity, neurologic toxicity etc,[4-6]significantly increased the risks of morbidities in these critically ill patients. Since the early 2009, we have attempted to delay the administration of immunosuppressive regimens in the critically ill patients aer liver transplantation under immune monitoring with ImmuKnow assay. In the present study, we aimed to evaluate the outcomes of this strategy in liver transplantation.
Methods
Patients
From March 2009 to February 2014, 225 consecutive liver recipients in our institute were included. Eleven deceased donor liver recipients developed severe infection or multiple organ failure aer transplantation, including acute renal failure, severe encephalopathy, or respiratoryfailure. To avoid the adverse effects of immunosuppression, we attempted to delay the administration of immunosuppressive regimens in these critically ill patients.Demographics of the patients are shown in Table.ere were 7 males and 4 females, with an average age of 47.9 years (range 32-67). Two of them were alcoholic liver cirrhosis, 9 were acute-on-chronic liver failure (ACLF)rooted by hepatitis B virus (HBV) infection.e average score of model for end-stage liver disease (MELD) was 35 (range 18-45) before transplantation.e major reasons of delayed administration of immunosuppressive drugs are intra-abdominal infection, sepsis, acute renal failure and hepatic encephalopathy (n=1), sepsis and acute renal failure (n=1), acute renal failure and pulmonary infection (n=6), acute renal failure, encephalopathy,pulmonary infection and sepsis (n=3). Before transplantation, 3 patients developed hepatic encephalopathy of grade 3 (n=2) or grade 4 (n=1) requiring intubation and mechanical ventilation.e patient, who developed post-transplant intra-abdominal infection, sepsis, acute renal failure and encephalopathy, had been treated in another institute for decompensated alcoholic liver cirrhosis and primary peritonitis at 1 month before liver transplantation.e diagnosis of sepsis was based on the clinical manifestations, significantly increased levels of C-reactive protein and procalcitonin, and subsequent positive results of blood culture.e definitions and classifications of hepatic encephalopathy and acute renal failure were based on the West Haven criteria[7]and the risk, injury, failure, loss, and end-stage kidney disease criteria,[8]respectively.
ImmuKnow assay
For the early detection of acute allograrejection,continuous intensive monitoring of hepatic biochemistry was mandatory during the non-immunosuppression period, including serum aminotransferase, alkaline phosphatase, glutamyltranspeptidase, and bilirubin levels. CD4 T lymphocyte function assay (ImmuKnow, Catalogue No. 4400, Cylex Inc., Columbia, MD, USA) was routinely performed every three days, in addition to any occasion when there was a significant increase in liver enzyme levels.e assay's ability to discern between immune profiles of overimmunosuppression and underimmunosuppression has been reported in previous studies.[9-12]Briefly,100 μL whole blood (1:4 dilution) was added into wells of a 96-well microtiter plate in quadruplicate, and was incubated overnight (15-18 hours) in a 5% CO2incubator at 37 ℃ with or without phytohemagglutinin (PHA, 2.5 μg/mL) as a stimulant. Anti-human CD4 monoclonal antibody coated magnetic particles (Dynal, Oslo, Norway)were added to immunoselect CD4 cells from both the stimulated and nonstimulated wells. Aer washing the selected CD4 cells, a lysing reagent was added to release intracellular adenosine triphosphate (ATP). A luciferin/luciferase mixture was then added to the cell lysate.Within 10 minutes aer addition of enzyme, the bioluminescent product was measured in a luminometer.e amount of light emitted was compared with a calibration curve generated with ATP calibrators (0, 1, 10, 100 and 1000 ng/mL).e concentration of ATP (ng/mL) in each sample was then calculated from the calibration curve. According to the instruction of Cylex Immu-Know assay, the cut-offlevels are ≤225 ng/mL for low immune cell response, 226-524 ng/mL for moderate immune cell response, and ≥525 ng/mL for strong immune cell response, respectively. Twenty liver recipients with stable clinical status and 20 healthy people were included in the study as control groups.e median time aer liver transplantation was 22.3 months (range 18-56) in the liver recipient with stable status.
Perioperative management
Statistical analysis
Nonparametric tests were used for statistical analysis.e Mann-WhitneyUtest and Wilcoxon test were used to compare continuous variables, and proportionswere compared by Chi-square test (SPSS/PC+; SPSS, Inc.,Chicago, IL, USA). APvalue of less than 0.05 was considered statistically significant.
Results
Clinical outcomes
All transplants were performed with ABO-compatible whole gras.ere was 1 perioperative death.e patient died of multiple organ failure with concurrent confirmed sepsis 14 days aer liver transplantation, and the remaining patients were in good status and had normal grafunction during a median follow-up period of 43 months (range 8-64).e median length of intensive care unit (ICU) and hospital stay were 17 days (range 11-32) and 27 days (range 14-65), respectively (Table).e prolonged mechanical ventilation was required in all patients due to unstable mental status and/or respiratory failure secondary to infection or adult respiratory distress syndrome.e median duration of mechanical ventilation was 8 days (range 2-28), and tracheostomy was performed in 2 patients. Of the 11 patients, 10 received continuous renal replacement therapy (CRRT) due to acute renal failure.e median length of CRRT was 9 days (range 2-26).
All of the 11 patients had culture-proven infections during the early period aer liver transplantation.e major morbidity was pulmonary infection (81.8%, 9/11),which may be resulted from liver failure, prolonged mechanical ventilation, and the complexity and urgent status of surgical procedure. Five patients developed systemic sepsis withStaphylococcus aureus(n=2),Enterococcus(n=1),Enterobacter(n=1), andEnterobacterandAspergillusspecies (n=1). Antimicrobial treatment was adjusted in 4 patients based on susceptibility testing,including vancomycin, tigecycline, carbapenems with or without trimethoprim sulfamethoxazole. In those patients receiving CRRT, the daily doses of antibiotics were based on local practice guidelines and published recommendations,[13]and each antibiotic was administered as a prolonged infusion of 4-6 hours. One patient developed intra-abdominal abscess, and was treated with percutaneous drainage guided by ultrasonography.
Table.e demographic data of liver transplant recipients

Table.e demographic data of liver transplant recipients
LT: liver transplantation; HBV: hepatitis B virus; MELD: model for end-stage liver disease; ICU: intensive care unit; CRRT: continuous renal replacement therapy.
CharacteristicsData (n=11)Average age (yr)47.9 (32-67)Gender (male/female) 7/4 Underlying liver diseases before LT Alcoholic liver cirrhosis 2 Acute liver failure on chronic HBV infection 9 Average MELD score before LT35 (18-45)Comorbidities aer LT Intra-abdominal infection 1 Sepsis 5 Pulmonary infection 9 Acute renal failure11 Encephalopathy 4 Median ICU stay (d)17 (11-32)Median hospital stay (d)27 (14-65)Median duration of mechanical ventilation (d) 8 (2-28)Median duration of CRRT (d) 9 (2-26)Median duration of non-immunosuppression (d)12 (5-58)
T cell-mediated immune response
Before transplantation, these critically ill patients presented a significantly lower ATP level of 286±101 ng/ mL as compared with healthy people with ATP level of 421±145 ng/mL in ImmuKnow assay (P<0.05), and then, the ATP level decreased to 64±35 ng/mL at day 3 post-transplant when all of the patients showed symptoms of infection (Fig. 1).e sequential tests showed that there was a distinct increase in the ATP level aer the system infections were controlled and the general status of patients were improved (Figs. 2 and 3). More importantly, the increase of ATP level was followed by the development of acute rejection against the allogras(Fig. 3).e average ATP level measured just before the development of acute rejection was 271±115 ng/mL(Fig. 1). A continuous distinct elevation of both ATP and glutamyltranspeptidase levels was a strong indication of the occurrence of acute rejection.e highest Immu-Know value was 510±173 ng/mL during the episodes of acute rejection, and was significantly higher than thatof the post-transplant recipients with clinical stability(247±123 ng/mL,P<0.05).e median non-immunosuppressed duration was 12 days (range 5-58) in the 11 patients aer transplantation (Table). Grade I or II acute grarejection was identified in all of these patients by pathological analysis based on BanffSchema. Aer the administration of immunosuppressive agents, the acute rejection was completely reversed in the 10 patients who survived perioperatively.
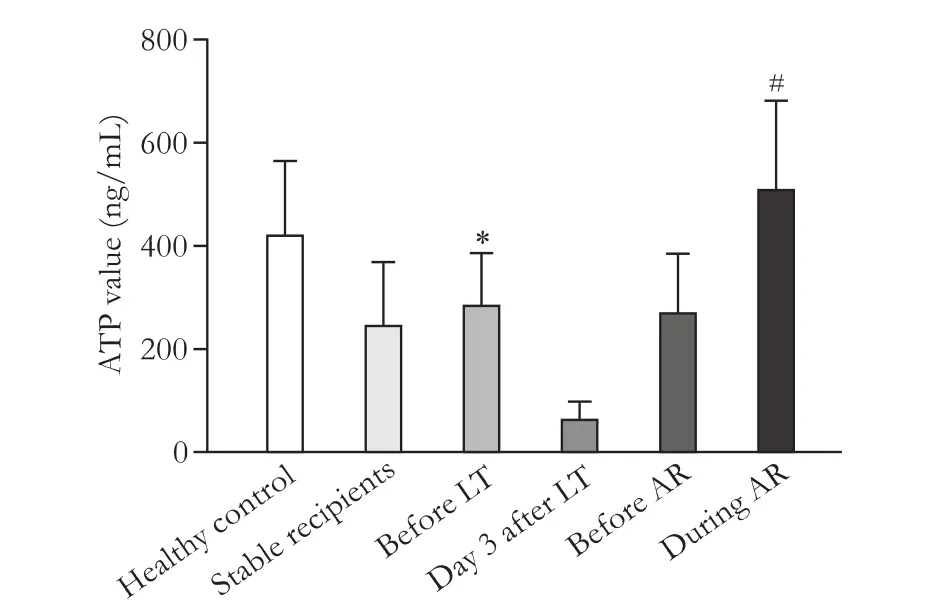
Fig. 1.ImmuKnow measurements in healthy people, liver recipients with clinical stability, and critically ill patients at different time points. LT: liver transplantation; AR: acute rejection. *:P<0.05, compared with healthy control; #:P<0.05, compared with stable recipients.
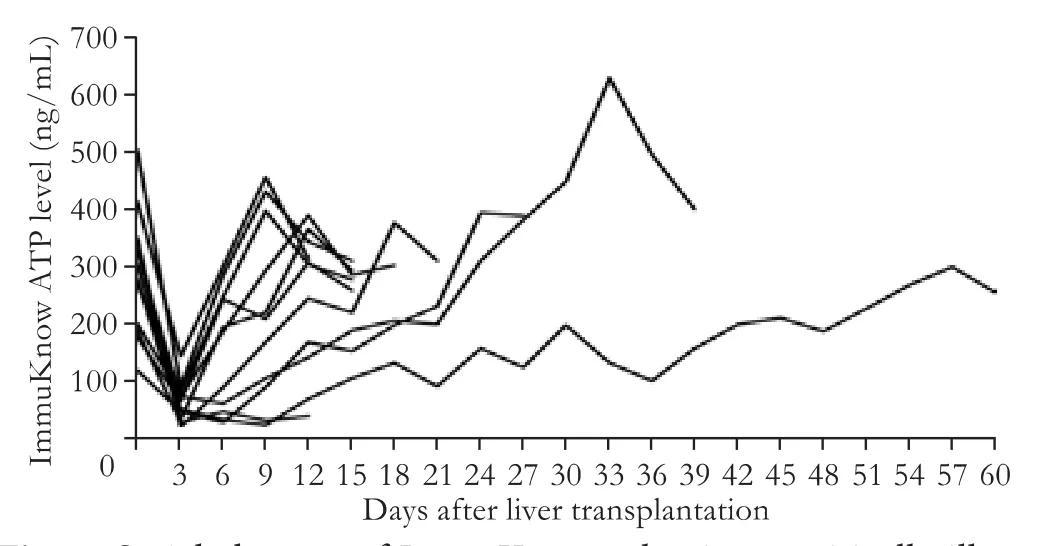
Fig. 2.Serial changes of ImmuKnow value in 11 critically ill patients aer liver transplantation.

Fig. 3.A 60-year-old female patient, who underwent liver transplantation for acute liver failure on chronic HBV infection, developed pulmonary infection, sepsis, encephalopathy and acute renal failure at the early stage after transplantation. Blood and sputum culture tests showedEnterobacter,AspergillusandStreptococcusspecies infection. She was mechanically ventilated because of hepatic encephalopathy before transplantation, and received continuous renal replacement therapy (CRRT) immediately after transplantation. The duration of mechanical ventilation and CRRT were 28 and 17 days, respectively.e immunosuppressive regimens including cyclosporine and mycophenolate mofetil were administered at day 30 aer transplantation.A: Serial changes of ImmuKnow and hepatic biochemistry levels aer liver transplantation;B: Chest X-ray at different time points aer liver transplantation. ALT: alanine aminotransferase; γ-GT: glutamyltranspeptidase; ATP: adenosine triphosphate.
Discussion
Despite the fact that the graand patient survival aer liver transplantation has significantly improved during the past decades, the perioperative care of recipients with multiple comorbidities and organ dysfunction remains challenging.[14-17]Infectious complications are leading causes of morbidity and mortality in these recipients. Effective critical care during the immediate posttransplant recovery period results in good outcomes. A better understanding and application of immunosuppressive strategies plays an important role in the posttransplant intensive care of the critically ill patients. As these critically ill patients are vulnerable to infections and organ injury,the delayed administration or dose reduction of immunosuppressive drugs is oen required in clinical practice.[18]However, the duration of non-immunosuppression or low-dose immunosuppressives remains empirical.e transplant specialists have to balance between comorbidities and immunosuppressive therapy, especially in recipients with infectious complications. For minimizing the adverse effects of immunosuppressive drugs, criteria are needed to treat the critically ill patients individually according to their own immune status.
It has been reported that the Cylex ImmuKnow assay,which was approved by the USA Food and Drug Administration in 2002, has the potential to serve as an index of the immune status of organ transplant recipients receiving immunosuppressive therapy.[9,19-22]e assay quantifies the cell-mediated immunity by measuring the concentration of ATP from circulating CD4 T cells aerin vitrostimulation with PHA.e present study assessed the clinical relevance and reliability of the ImmuKnow assay as an immune monitoring tool in the critically ill patients aer liver transplantation. We found that the ImmuKnow ATP values can represent the level offunctional immunity of the patients. At the early stage aer transplantation, all of these critically ill recipients showed a significantly low degree of global immune response.is phenomenon may be associated with the development of infectious diseases. Importantly, there were no any signs of allograrejection in these patients.erefore, the immunosuppressive regimens were not administered in all of them during the early period aer transplantation in order to avoid the drug toxicity and the exacerbation of infectious diseases due to the immunosuppression.e median duration of non-immunosuppression was 12 days (5-58).is gives the ICU physicians very valuabletime to treat the recipients with multiple comorbidities and organ dysfunction.
In addition, the longitudinal ImmunKnow assay provided a reliable depiction of the dynamics offunctional immunity throughout the clinical course of the patients.We found that a continuous distinct elevation of both ATP and glutamyltranspeptidase levels was a strong indication of the development of acute rejection.is is very helpful in optimizing a favorable timing of introduction of the immunosuppressive therapy. Fig. 3 described a 60-year-old female patient who suffered from pulmonary infection, sepsis, encephalopathy and acute renal failure at the early stage aer transplantation.e longitudinal immune monitoring with ImmuKnow assay showed a significant correlation with the clinical episodes of the patient. Both the amelioration of comorbidities and the development of acute rejection were parallel to the distinct changes of the ImmuKnow value. Another 32-yearold male patient with alcoholic cirrhosis, who developed intra-abdominal infection, sepsis, acute renal failure and encephalopathy aer liver transplantation, had significantly low ImmuKnow ATP levels and nearly normal hepatic biochemistry during the early period aer transplantation. His ATP level started to increase significantly aer 50 days of liver transplantation.e immunosuppressive regimens were administered at day 58 aer transplantation when the acute allograrejection was identified (data not shown).ese results are comparable with the other reports, suggesting that the ImmuKnow assay could serve as an immune monitoring tool in organ transplant recipients.[9,10,19,20]In previous studies, the investigators have attempted to stratify patient's immune status using ImmuKnow assay.[9,19]In our experiences,however, both the inter-individual and the intra-individual deviations of ImmuKnow measurements were large.e serial longitudinal ImmuKnow measurements and analyses of the changes of ATP levels over time were more informative in identification of clinical adverse events including infectious diseases or acute rejection than a single measurement.
In conclusion, the present study shows that the delayed introduction of immunosuppressive regimens is safe and effective in management of critically ill patients aer liver transplantation.e longitudinal immune monitoring with ImmuKnow assay may be helpful in optimizing the timing of introduction of immunosuppression by providing the status of the functional immunity of a given critically ill recipient.
Contributors:LY proposed and performed the study, and wrote the dra. JWB performed the study and revised the paper. DWD performed the study and collected the data. SXJ performed the study. ZZM collected the data. All authors contributed to the design and interpretation of the study and to further dras. LY is the guarantor.
Funding:None.
Ethical approval:e study was conducted according to theDeclaration of Helsinkiand was approved by the Ethics Committee of Chinese PLA General Hospital, Beijing, China.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Findlay JY, Fix OK, Paugam-Burtz C, Liu L, Sood P, Tomlanovich SJ, et al. Critical care of the end-stage liver disease patient awaiting liver transplantation. Liver Transpl 2011;17:496-510.
2 Keegan MT, Kramer DJ. Perioperative care of the liver transplant patient. Crit Care Clin 2016;32:453-473.
4 Umbro I, Tinti F, Scalera I, Evison F, Gunson B, Sharif A, et al.Acute kidney injury and post-reperfusion syndrome in liver transplantation. World J Gastroenterol 2016;22:9314-9323.
5 Pedersen M, Seetharam A. Infections aer orthotopic liver transplantation. J Clin Exp Hepatol 2014;4:347-360.
6 Derle E, Kibaroğlu S, Öcal R, Kırnap M, Can U, Benli S, et al.Neurologic complications aer liver transplant: experience at a single center. Exp Clin Transplant 2015;13:327-330.
7 Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K,Blei AT. Hepatic encephalopathy--definition, nomenclature,diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna,1998. Hepatology 2002;35:716-721.
8 Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure -definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative(ADQI) Group. Crit Care 2004;8:R204-212.
9 Israeli M, Klein T, Sredni B, Avitzur Y, Mor E, Bar-Nathen N,et al. ImmuKnow: a new parameter in immune monitoring of pediatric liver transplantation recipients. Liver Transpl 2008;14:893-898.
10 Xue F, Zhang J, Han L, Li Q, Xu N, Zhou T, et al. Immune cell functional assay in monitoring of adult liver transplantation recipients with infection. Transplantation 2010;89:620-626.
11 Ravaioli M, Neri F, Lazzarotto T, Bertuzzo VR, Di Gioia P,Stacchini G, et al. Immunosuppression modifications based on an immune response assay: results of a randomized, controlled trial. Transplantation 2015;99:1625-1632.
12 Sugiyama K, Tsukaguchi M, Toyama A, Satoh H, Saito K, Nakagawa Y, et al. Immune monitoring with a lymphocyte adenosine triphosphate assay in kidney transplant recipients treated with a calcineurin inhibitor. Exp Clin Transplant 2014;12:195-199.
13 Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 2005;41:1159-1166.
14 Niemann CU, Kramer DJ. Transplant critical care: standards for intensive care of the patient with liver failure before and aer transplantation. Liver Transpl 2011;17:485-487.
15 Razonable RR, Findlay JY, O'Riordan A, Burroughs SG, Ghobrial RM, Agarwal B, et al. Critical care issues in patients aer liver transplantation. Liver Transpl 2011;17:511-527.
16 Carton EG, Plevak DJ, Kranner PW, Rettke SR, Geiger HJ,Coursin DB. Perioperative care of the liver transplant patient:Part 2. Anesth Analg 1994;78:382-399.
17 Feltracco P, Barbieri S, Galligioni H, Michieletto E, Carollo C, Ori C. Intensive care management of liver transplanted patients. World J Hepatol 2011;3:61-71.
18 Sood S, Testro AG. Immune monitoring post liver transplant.World J Transplant 2014;4:30-39.
19 Israeli M, Ben-Gal T, Yaari V, Valdman A, Matz I, Medalion B,et al. Individualized immune monitoring of cardiac transplant recipients by noninvasive longitudinal cellular immunity tests.Transplantation 2010;89:968-976.
20 Uemura T, Riley TR, Khan A, Hollenbeak C, Schreibman I,Ghahramani N, et al. Immune functional assay for immunosuppressive management in post-transplant malignancy. Clin Transplant 2011;25:E32-37.
21 Mendler M, Kwok H, Franco E, Baron P, Weissman J, Ojogho O. Monitoring peripheral blood CD4+ adenosine triphosphate activity in a liver transplant cohort: insight into the interplay between hepatitis C virus infection and cellular immunity. Liver Transpl 2008;14:1313-1322.
22 Hooper E, Hawkins DM, Kowalski RJ, Post DR, Britz JA,Brooks KC, et al. Establishing pediatric immune response zones using the Cylex ImmuKnow assay. Clin Transplant 2005;19:834-839.
September 7, 2016
Accepted after revision May 25, 2017
Author Affiliations: Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China (Luo Y, Ji WB, Duan WD, Shi XJ and Zhao ZM)
Ying Luo, MD, Department of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing 100853, China (Tel: +86-10-66938334; Fax: +86-10-68241383; Email: luoyingly@hotmail.com)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60050-X
Published online August 8, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy
- Prospective comparison of prophylactic antibiotic use between intravenous moxifloxacin and ceftriaxone for high-risk patients with post-ERCP cholangitis
- Helicobacter pyloriand 17β-estradiol induce human intrahepatic biliary epithelial cell abnormal proliferation and oxidative DNA damage
- Tailored pancreatic reconstruction after pancreaticoduodenectomy: a single-center experience of 892 cases
- Comparative study of the effects of terlipressin versus splenectomy on liver regeneration after partial hepatectomy in rats
- The “Colonial Wig” pancreaticojejunostomy:zero leaks with a novel technique for reconstruction after pancreaticoduodenectomy
