Comparative study of the effects of terlipressin versus splenectomy on liver regeneration after partial hepatectomy in rats
2017-10-09TomFlorianUlmerAnneWeilandGeorgLurjeChristianKlinkAnneAndertHamidAlizaiChristophHeidenhainandUlfNeumann
Tom Florian Ulmer, Anne Weiland, Georg Lurje, Christian Klink, Anne Andert,Hamid Alizai, Christoph Heidenhain and Ulf Neumann
Aachen, Germany
Comparative study of the effects of terlipressin versus splenectomy on liver regeneration after partial hepatectomy in rats
Tom Florian Ulmer, Anne Weiland, Georg Lurje, Christian Klink, Anne Andert,Hamid Alizai, Christoph Heidenhain and Ulf Neumann
Aachen, Germany
BACKGROUND: Post-hepatectomy liver failure as a result of insufficient liver remnant is a feared complication in liver surgery. Efforts have been made to find new strategies to support liver regeneration.e aim of this study was to investigate the effects of terlipressin versus splenectomy on postoperative liver function and liver regeneration in rats undergoing 70%partial hepatectomy.
METHODS: Seventy-two male Wistar rats were randomly assigned into three groups (n=24 in each group): 70% partial hepatectomy as control (PHC), 70% partial hepatectomy with splenectomy (PHS) or 70% partial hepatectomy with a micropump for terlipressin administration (PHT). Eight rats in each group were sacrificed on postoperative day (POD) 1,3 and 7. To assess liver regeneration, immunohistochemical analysis of liver tissue using bromodeoxyuridine (BrdU) and Ki-67 labeling was performed. Portal venous pressure, serum concentrations of creatinine, urea, albumin, bilirubin and prothrombin time as well as liver-, body-weight and their ratio were determined on POD 1, 3 and 7.
CONCLUSIONS: Neither the administration of terlipressin nor splenectomy improved liver regeneration aer 70% partial hepatectomy in rats. Further studies assessing the regulation of portal venous pressure as well as extended hepatectomy animal models and liver function tests will help to further investigate mechanisms of liver regeneration.
(Hepatobiliary Pancreat Dis Int 2017;16:506-511)
liver regeneration;
liver failure;
terlipressin;
splenectomy;
hepatectomy
Introduction
Surgical resection still offers the best option for prolonged survival in patients with primary and secondary liver tumors. Innovations in surgical technique and modern intensive care treatment allow increasingly extensive liver resections. Nevertheless, insufficient future liver remnant (FLR) as determined by post-hepatectomy liver failure remains a major problem and impacts postoperative morbidity and mortality in these patients.[1,2]erefore, pre-, peri-, and postoperative strategies to support liver regeneration have been the subject of numerous recent studies.[3-5]Even though the mechanisms of portal venous pressure (PVP)-modulation and its effect on liver regeneration are not well understood, it is well-known that extensive liver resection is associated with a significant andimmediate rise in PVP.[6-8]In addition, the reduction of PVP by splenectomy or ligation of the splenic artery in“small-for-size” patients aer major liver resection (20%-30% FLR) or orthotopic liver transplantation and associated portal hypertension has been shown to improve outcome in these patients.[9-12]Furthermore, terlipressin, the synthetic analogue of the peptide hormone vasopressin,has similar effects in this setting.[13-15]It lowers the PVP by splanchnic vasoconstriction and is used primarily for the treatment of acute gastrointestinal variceal bleeding or hepatorenal syndrome in cases of high PVP.
However, the impact of splenectomy and terlipressin administration on liver regeneration and PVP aer minor liver resections (FLR≥30%) remains unclear.[16-18]To the best of our knowledge, this is the first animal study investigating the effects of terlipressin versus splenectomy on postoperative liver function and liver regeneration in rats undergoing 70% partial hepatectomy.
Methods
Experimental design
At eight weeks of age, 72 male Wistar rats were randomly assigned into three groups (n=24 in each group): 70%partial hepatectomy as control (PHC), 70% partial hepatectomy with splenectomy (PHS) or 70% partial hepatectomy with a micropump for terlipressin administration(PHT). A web-based randomization service was used for animal allocation (www.randomizer.at).e allocation ratio was 1:1 for three groups. All procedures were performed by one surgeon. Eight rats from each group were sacrificed on postoperative day (POD) 1, 3, and 7.
Animal model
Male Wistar rats (Harlan-Winkelmann, Borchen,Germany) were housed at room temperature (21±2 ℃)under a 12/12-hour light-dark cycle with access to standard rat diet and waterad libitum. Body-weight varied between 155 and 235 g (mean 196±15).e animals were acclimatized to laboratory conditions for 1 week before the experiments were initiated. All experiments were performed in accordance with the German Animal Welfare Act (Senator for Health and Welfare, Berlin, Germany, G 0166/04).
Partial hepatectomy
Partial hepatectomy (70%) was performed as previously described.[19]All surgical procedures were performed under inhalation anesthesia with isoflurane(concentration 1.5% to 2%, Forene, Abbott, Wiesbaden,Germany), oxygen (flow rate 0.2 L/min), and nitrous oxide (flow rate 0.1 L/min). Aer midline incision, the interlobular ligaments were dissected. For rats undergoing 70% partial hepatectomy, the leand median lobes were removed aer ligation with Vicryl 5-0 (Ethicon, Norderstedt, Germany). Subsequently, either a splenectomy was performed (PHS group) by placing 6-0 silk ligatures around the splenic hilar vessels or a micropump was implanted subcutaneously in the inguinal region (PHT group) for the administration of terlipressin. Aer irrigating the abdominal cavity with warm saline solution,the abdomen was closed in two layers with running silk sutures 4-0 (Ethicon, Norderstedt, Germany).
Postoperative management
Once the rats were awake and mobile, postoperative analgesia was instituted by administering subcutaneous novaminsulfone (0.2 mg/g body-weight). Immediately aer surgery all animals also received 5 mL subcutaneous injections of 10% glucose.e rats had free access to food and tap water.ey were sacrificed under anesthesia by exsanguination on POD 1, 3 and 7 aer partial hepatectomy.
Measurement of PVP
PVP (cmH2O) was measured at specific times aer partial hepatectomy (POD 1, 3 and 7) through a laparotomy under anesthesia. First, the portal vein was directly cannulated about one cm distal to the liver hilum. PVP was measured directly and recorded on a scale in cm of water (cmH2O).
Hepatocyte proliferation (BrdU and Ki-67)
Bromodeoxyuridine (BrdU) and Ki-67 were used to evaluate hepatocyte proliferation. BrdU was injected(5 mg/100 g body-weight) intraperitoneally 0.5 hours before sacrifice to determine hepatocyte proliferation.Blood was drawn by aortic puncture. Serum and coagulation parameters were determined immediately by routine biochemical workup. Serum levels of creatinine, urea,albumin, direct and total bilirubin, as well as prothrombin time were determined by standard technique (SRL,Inc., Tokyo, Japan). At harvesting time, the weight of the liver and body were measured, and the liver- to bodyweight ratio was calculated in percent [(liver-weight/body-weight)×100]. Liver sections were then fixed in 4%buffered formalin and processed for staining with hematoxylin eosin or immunohistochemical dyes.
Micropump for the administration of terlipressin
Immunohistochemistry
For BrdU nuclear and Ki-67 staining, 4 μm paraffin embedded sections were fixed, depara ffinized and antigens were retrieved by microwave treatment. BrdU incorporation was detected using a commercial detection kit with slight modi fications of the manufacturer's instructions (Bromo-Deoxy-Uridine Labeling and Detection Kit II; Roche Diagnostics, Ltd., Rotkreuz, Switzerland).e analysis of the ubiquitous cell cycle marker Ki-67 was performed using a combination of a primary monoclonal mouse antibody (1:10, Dako, Glostrup, Denmark) and a secondary biotinylated rabbit-anti-mouse antibody (1:300, Dako, Glostrup, Denmark).e visualization was performed with DAB (Sigma-Aldrich, Steinheim, Germany). Nuclei counterstaining was performed using hematoxylin (Sigma-Aldrich, Steinheim, Germany). BrdU and Ki-67 positive hepatocytes for each liver specimen were scored semi-quantitatively in 20 randomly selected fields (40×) as the ratio of nuclear-positive hepatocytes to the total number of hepatocytes. All samples were independently evaluated by two experienced pathologists, who were blinded to both the intervention groups and related clinical data.
Statistical analysis
Data were expressed as mean±standard deviation(SD).e criterion for statistical significance wasP<0.05.e Kolmogorov-Smirnov test was used to analyze the distribution of the quantitative variables.e ANOVA test with Bonferroni correction for normally distributed variables and the Mann-Whitney test for variables with a non-normal distribution were applied for differences in the quantitative variables. Data management and analysis were performed using SPSS 20.0 (SPSS Inc., Chicago, IL).
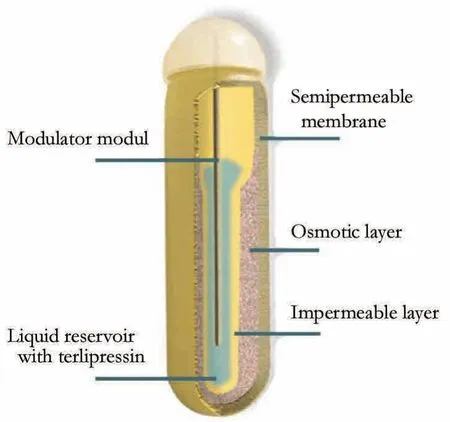
Fig. 1.Osmotic pump. The fluid chamber of the osmotic pump was filled with 200 μL (0.04 mg) terlipressin. It was implanted into a subcutaneous pocket.e delivery rate of the pump was 0.2 μg terlipressin per hour.
Results
All 72 male Wistar rats resumed normal activity the day aer surgical intervention. None of the animals died from intervention-related complications.
Body- and liver-weight
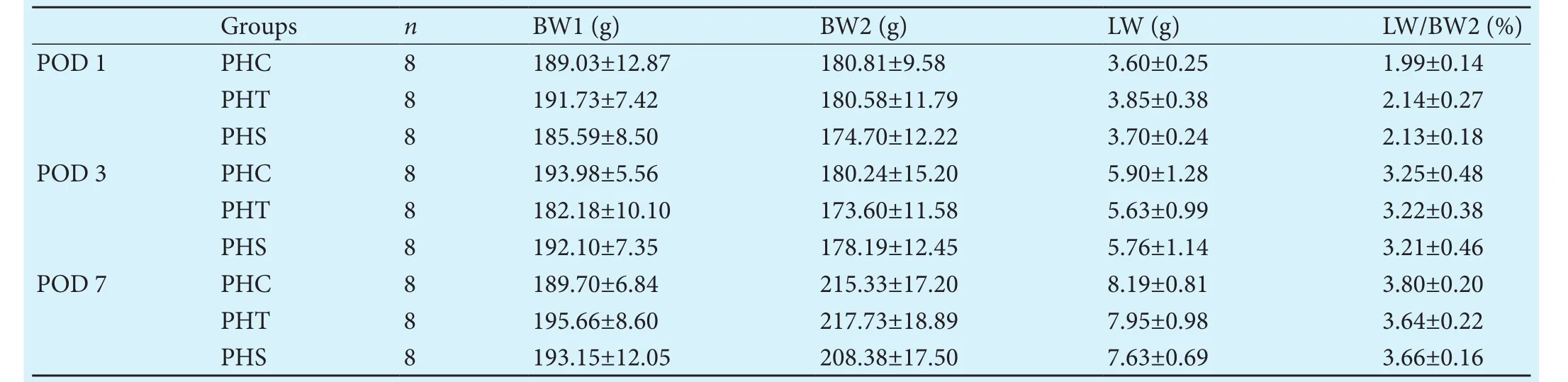
Table 1.Body-, liver-weights and liver- to body-weight ratio for the different groups and time points
PVP
Compared to PHC, PVP in the intervention groups(PHT and PHS) was lower on POD 1, 3 and 7 (Table 2 and Fig. 2); PVP was the lowest in PHS rats. However,none of the differences reached statistical significance(P=0.13,P=0.26,P=0.68; respectively for POD 1, 3 and 7)
BrdU and Ki-67
Laboratory parameters (creatinine, urea, albumin,direct and total bilirubin, prothrombin time)
At the three dates of analysis, creatinine, urea, direct bilirubin, and total bilirubin did not differ significantly among the groups (data not shown). On POD 3, the animals treated with terlipressin displayed significantly higher prothrombin time than the control or splenectomy groups (PHT 61.73±2.67 seconds vs PHC 57.0±2.14 seconds and PHS 57.25±3.06 seconds;P=0.001 andP=0.002). A similar effect was observed for albumin,where animals treated with terlipressin showed significantly lower levels (2.90±0.18 g/dL;P=0.001) than in the other two groups (PHC 3.25±0.48 g/dL and PHS 3.26±0.18 g/dL) on POD 3. Comparisons at POD 1 and 7 revealed no significant differences in prothrombin time or serum albumin levels among the three groups.

Table 2.Portal venous pressure and values of BrdU and Ki-67 positive cells
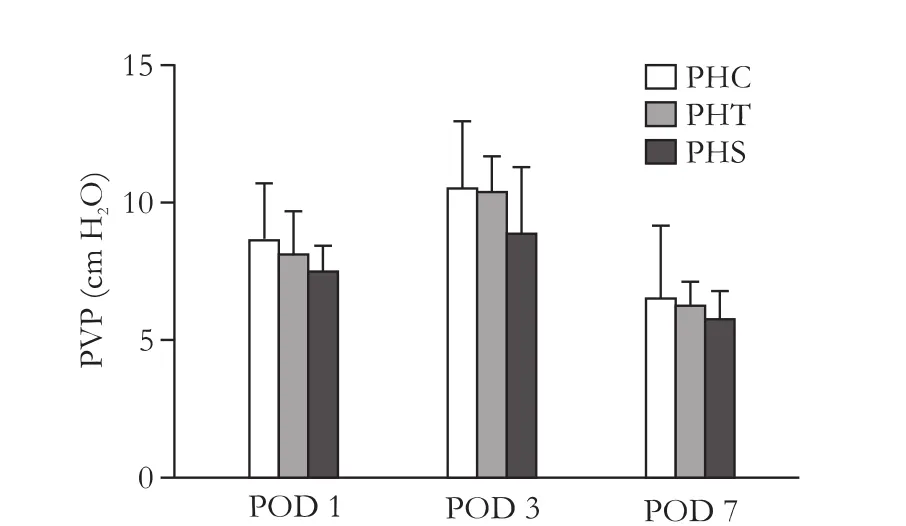
Fig. 2.Portal venous pressure.e maximum PVP was measured on POD 3.e differences between the intervention groups (PHT and PHS) and the control group (PHC) were not significant at any time.

Fig. 3.Proliferation of hepatocytes (BrdU and Ki-67).e highest proliferation was seen on POD 1. In all groups the positively stained cells regressed over time. None of the differences detected reached statistical significance.
Discussion
Nevertheless, neither splenectomy nor administration of terlipressin have been established as therapeutic standards. On the one hand, this is due to the risks of the procedure itself, while on the other hand there is an ongoing discussion regarding the indication and pathogenesis and - to some extent - the therapeutic effect.
Whether a postoperative reduction in PVP significantly improves liver regeneration may well depend on the extent of liver resection and consequently on the postoperative PVP level. Compared to major liver resection (FLR≤20%), the impact of less extended liver resection (FLR≥30%) combined with splenectomy or administration of terlipressin on PVP and liver regeneration remains unclear.[15,17]Moreover, it was found that a moderately elevated PVP and the accompanying barotrauma may play a role in triggering and regulating regeneration,whereas decreased or delayed regeneration was observed in lower pressure levels.[27-30]ese findings are consistent with our results, where we demonstrated only a slightly decreased PVP and a delayed cellular proliferation aer a 70% partial hepatectomy in our intervention groups compared to the control group. In addition, we did not observe a statistically significant difference between liver resection alone or combined with splenectomy or the administration of terlipressin.
Apart from the extent of liver resection, another explanation for our statistically non-significant PVP changes in the present study might be the selected timeframe. One study[18]demonstrated changes in PVP after a very short-time span of just seconds and minutes.Furthermore, the observed mild reduction in PVP aer the administration of terlipressin might be the result of splanchnic hyposensitivity, caused by vasodilation and the ensuing activation of nitrite oxide synthase and the bradykinin system following massive blood loss.[31-33]is may be encountered during small animal surgery despite all precautions taken.
In any case, our findings must be interpreted with caution. Firstly, animal experiments have their natural limitations and results cannot be directly extrapolated into humans. Secondly, we primarily examined the structural morphology of liver regeneration. By assessing laboratory values alone, it is difficult to comprehensively investigate the extent to which functional liver regeneration is affected. Even though only feasible in large animal models, it would be interesting to add a liver function test, such as the LiMAx[34]in future studies. Lastly, a follow-up study with extended hepatectomy (FLR≤15%)might have possibly found significant differences in liver regeneration following splenectomy or the administration of terlipressin. However, the 70% partial hepatectomy is a standard animal model in the literature. Furthermore, 70% also matches current clinical practice in liver surgery. Nevertheless, we concur that further research is warranted to address the timing, concentration of terlipressin application and the extent of hepatectomy.
In conclusion, neither the administration of terlipressin nor splenectomy improved liver regeneration or significantly reduced PVP in our study setting. Further studies assessing the regulation of PVP as well as extended hepatectomy animal models and liver function tests will help to investigate mechanisms of liver regeneration in more detail.
Contributors:UTF performed the statistical analysis, and prepared and draed the manuscript. WA performed the surgical procedures, collected the data, and contributed to the analysis and interpretation of the results. LG and AA participated in the design of the study, data analysis and helped to drathe manuscript. KC and AH participated in the coordination of the study and conducted an extensive literature search. HC and NU planned the study, and participated in the design, coordination and draing of the manuscript. UTF is the guarantor.
Funding:None.
Ethical approval:All experiments were performed in accordance with the German Animal Welfare Act (Senator for Health and Welfare, Berlin, Germany, G 0166/04).
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kenjo A, Miyata H, Gotoh M, Kitagawa Y, Shimada M, Baba H, et al. Risk stratification of 7732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg 2014;218:412-422.
2 Asencio JM, García Sabrido JL, Olmedilla L. How to expand the safe limits in hepatic resections? J Hepatobiliary Pancreat Sci 2014;21:399-404.
3 van Lienden KP, van den Esschert JW, de Graaf W, Bipat S,Lameris JS, van Gulik TM, et al. Portal vein embolization be-fore liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34.
4 Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S,Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid lelateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-414.
5 Schmeding M, Boas-Knoop S, Lippert S, Ruehl M, Somasundaram R, Dagdelen T, et al. Erythropoietin promotes hepatic regeneration aer extended liver resection in rats. J Gastroenterol Hepatol 2008;23:1125-1131.
6 Jiang SM, Zhou GW, Zhang R, Peng CH, Yan JQ, Wan L, et al.Role of splanchnic hemodynamics in liver regeneration aer living donor liver transplantation. Liver Transpl 2009;15:1043-1049.
7 Michalopoulos GK. Liver regeneration aer partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2-13.
8 Doignon I, Julien B, Serrière-Lanneau V, Garcin I, Alonso G, Nicou A, et al. Immediate neuroendocrine signaling aer partial hepatectomy through acute portal hyperpressure and cholestasis. J Hepatol 2011;54:481-488.
9 Ren YS, Qian NS, Tang Y, Liao YH, Liu WH, Raut V, et al.Beneficial effects of splenectomy on liver regeneration in a rat model of massive hepatectomy. Hepatobiliary Pancreat Dis Int 2012;11:60-65.
10 Arakawa Y, Shimada M, Uchiyama H, Ikegami T, Yoshizumi T, Imura S, et al. Beneficial effects of splenectomy on massive hepatectomy model in rats. Hepatol Res 2009;39:391-397.
11 Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients.Liver Transpl 2003;9:S36-41.
12 Kuriyama N, Isaji S, Kishiwada M, Ohsawa I, Hamada T,Mizuno S, et al. Dual cytoprotective effects of splenectomy for small-for-size liver transplantation in rats. Liver Transpl 2012;18:1361-1370.
13 Ren Z, Xu Y, Zhu S.e effect of terlipressin on hepatic hemodynamics in small-for-size livers. Hepatogastroenterology 2012;59:208-211.
14 Mukhtar A, Salah M, Aboulfetouh F, Obayah G, Samy M, Hassanien A, et al.e use of terlipressin during living donor liver transplantation: effects on systemic and splanchnic hemodynamics and renal function. Crit Care Med 2011;39:1329-1334.
15 Fahrner R, Patsenker E, de Gottardi A, Stickel F, Montani M, Stroka D, et al. Elevated liver regeneration in response to pharmacological reduction of elevated portal venous pressure by terlipressin aer partial hepatectomy. Transplantation 2014;97:892-900.
16 Darnis B, Mohkam K, Schmitt Z, Ledochowski S, Vial JP, Duperret S, et al. Subtotal hepatectomy in swine for studying smallfor-size syndrome and portal inflow modulation: is it reliable?HPB (Oxford) 2015;17:881-888.
17 Kim J, Kim CJ, Ko IG, Joo SH, Ahn HJ. Splenectomy affects the balance between hepatic growth factor and transforming growth factor-β and its effect on liver regeneration is dependent on the amount of liver resection in rats. J Korean Surg Soc 2012;82:238-245.
18 Lin PW, Shan YS. Effects of splenectomy and splenic artery ligation on the portal pressure in portal hypertensive rats. J Surg Res 1992;53:621-624.
19 Higgins GM, Anderson RM. Experimental pathology of liver:restoration of liver of white rat following partial surgical removal. Arch Pathol 1931;12:186-202.
20 Glanemann M, Eipel C, Nussler AK, Vollmar B, Neuhaus P.Hyperperfusion syndrome in small-for-size livers. Eur Surg Res 2005;37:335-341.
21 Onoe T, Tanaka Y, Ide K, Ishiyama K, Oshita A, Kobayashi T,et al. Attenuation of portal hypertension by continuous portal infusion of PGE1 and immunologic impact in adult-to-adult living-donor liver transplantation. Transplantation 2013;95:1521-1527.
22 Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, et al. Changes in portal venous pressure in the early phase aer living donor liver transplantation: pathogenesis and clinical implications. Transplantation 2003;75:1313-1317.
23 Man K, Fan ST, Lo CM, Liu CL, Fung PC, Liang TB, et al. Grainjury in relation to grasize in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragragene expression.Ann Surg 2003;237:256-264.
24 Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome aer partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant 2005;5:2605-2610.
25 Ho H, Sorrell K, Bartlett A, Hunter P. Modeling the hepatic arterial buffer response in the liver. Med Eng Phys 2013;35:1053-1058.
26 Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol 2010;16:6046-6057.
27 Mori S, Kim H, Park MS, Choi Y, Hong G, Yi NJ, et al. Graregeneration rate and small-for-size syndrome in living donor liver transplantation. Hepatogastroenterology 2013;60:1463-1468.
28 Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D,de Riva N, et al. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl 2010;16:364-374.
29 Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration aer a partial hepatectomy. Surg Today 1999;29:1-9.
30 Nobuoka T, Mizuguchi T, Oshima H, Shibata T, Kimura Y, Mitaka T, et al. Portal blood flow regulates volume recovery of the rat liver aer partial hepatectomy: molecular evaluation. Eur Surg Res 2006;38:522-532.
31 Chen CT, Chu CJ, Lee FY, Chang FY, Wang SS, Lin HC, et al. Splanchnic hyposensitivity to glypressin in a hemorrhage-transfused common bile duct-ligated rat model of portal hypertension: role of nitric oxide and bradykinin. Hepatogastroenterology 2009;56:1261-1267.
32 Huang HC, Chu CJ, Lee FY, Chang FY, Wang SS, Lin HC, et al.Chronic inhibition of nitric oxide ameliorates splanchnic hyposensitivity to glypressin in a hemorrhage-transfused rat model of portal hypertension. Scand J Gastroenterol 2000;35:1308-1313.
33 Valla D, Girod C, Lee SS, Braillon A, Lebrec D. Lack of vasopressin action on splanchnic hemodynamics during bleeding:a study in conscious, portal hypertensive rats. Hepatology 1988;8:10-15.
34 Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M,et al. Prediction of postoperative outcome aer hepatectomy with a new bedside test for maximal liver function capacity.Ann Surg 2009;250:119-125.
September 10, 2016
Accepted after revision March 14, 2017
Author Affiliations: Department of General, Visceral and Transplantation Surgery, RWTH Aachen University Hospital, Aachen, Germany(Ulmer TF, Lurje G, Klink C, Andert A, Alizai H, Heidenhain C and Neumann U); Department of Urology, Helios Hospital, Berlin-Buch, Germany(Weiland A)
Dr. Tom Florian Ulmer, Department of General,Visceral and Transplantation Surgery, RWTH Aachen University Hospital, Aachen 52064, Germany (Tel: +49-241-89500; Fax: +49-241-82417;Email: fulmer@ukaachen.de)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60036-5
Published online July 13, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Letters to the Editor
- The “Colonial Wig” pancreaticojejunostomy:zero leaks with a novel technique for reconstruction after pancreaticoduodenectomy
- Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy
- Tailored pancreatic reconstruction after pancreaticoduodenectomy: a single-center experience of 892 cases
- Helicobacter pyloriand 17β-estradiol induce human intrahepatic biliary epithelial cell abnormal proliferation and oxidative DNA damage
- Prospective comparison of prophylactic antibiotic use between intravenous moxifloxacin and ceftriaxone for high-risk patients with post-ERCP cholangitis
