Prospective comparison of prophylactic antibiotic use between intravenous moxifloxacin and ceftriaxone for high-risk patients with post-ERCP cholangitis
2017-10-09NamHeeKimHongJooKimandKiBaeBang
Nam Hee Kim, Hong Joo Kim and Ki Bae Bang
Seoul, Korea
Prospective comparison of prophylactic antibiotic use between intravenous moxifloxacin and ceftriaxone for high-risk patients with post-ERCP cholangitis
Nam Hee Kim, Hong Joo Kim and Ki Bae Bang
Seoul, Korea
METHODS: From November 2013 to July 2015, 86 consecutive patients with biliary obstruction with one or more factors predicting benefits of antibiotic prophylaxis prior to ERCP were included in the current randomized open-label non-inferiority trial (ClinicalTrial.gov identifier NCT02098486). Intravenous moxifloxacin (400 mg/day) or ceriaxone (2 g/day)were given 90 minutes before ERCP, and were administered for more than 3 days if the patient developed symptoms and signs of cholangitis or septicemia. Recalcitrant cholangitis was defined as persistence of cholangitis for more than 5 days aer ERCP or recurrence of cholangitis within 30 days aer ERCP.
RESULTS: Recalcitrant cholangitis occurred in 1 (2.3%) and 2(4.8%) patients receiving intravenous moxifloxacin and ceriaxone group, respectively (P=0.612). Septicemia was noted in 1 (2.3%) and 1 (2.4%) patient in intravenous moxifloxacin andceriaxone group, respectively (P=1.0).e mean hospital stay was also not significantly different between the moxifloxacin and ceriaxone groups (8.8±7.2 vs 9.1±9.4 days,P=0.867).Antibiotic resistance of the isolated pathogens byin vitroactivity assay was noted in 1 (2.3%) and 2 (4.8%) patients in the moxifloxacin and ceriaxone group, respectively (P=0.612).
CONCLUSION: Intravenous moxifloxacin is not inferior to intravenous ceriaxone for the prophylactic treatment of post-ERCP cholangitis and cholangitis-associated morbidity.
(Hepatobiliary Pancreat Dis Int 2017;16:512-518)
endoscopic retrograde cholangiopancreatography;
cholangitis;
moxifloxacin;
recalcitrant cholangitis
Introduction
Endoscopic retrograde cholangiopancreatography(ERCP) is a standard diagnostic and therapeutic modality in pancreatobiliary disorders.[1,2]Bile duct obstruction caused by various disorders like choledocholithiasis, and benign or malignant stricture can lead to increased intra-biliary pressure with cholangio-venous reflux and bacteremia, which may progress to septicemia.[3]In the presence of an obstructed bile duct, biliary decompression by endoscopic sphincterotomy, stone extraction, stent insertion, and balloon dilatation are essential therapeutic techniques which can restore free biliary drainage.[4,5]e risk of precipitating cholangitis or septicemia during these procedures is much greater than in simple diagnostic ERCP.[6-8]
A recent guideline recommended that antibiotic prophylaxis could be considered before an ERCP only in patients with known or suspected biliary obstruction,where there is a possibility that complete drainage maynot be achieved at the ERCP, such as occurs in patients with a hilar stricture and primary sclerosing cholangitis.[9]However, the strength of evidence for this recommendation was weak. A recent systematic review and meta-analysis indicated that administration of antibiotics prior to elective ERCP can reduce the risk of bacteremia,cholangitis, septicemia, and pancreatitis.[1,10]us, the available data regarding the clinical significance of prophylactic antibiotics preceding ERCP are inconsistent.Another limitation in evaluating an optimal prophylactic antibiotic strategy is the variation in antibiotics used prior to ERCP procedures.e aforementioned-studies used various kinds of antibiotics in practice. Hence, it is not possible to determine which antibiotic may provide prophylactic effects. Although the optimum prophylactic antibiotics for ERCP are not yet determined, the recommendations of recent guidelines and its once-daily administration, has made ceriaxone the most frequently prescribed antibiotic in patients with bile duct obstruction prior to therapeutic ERCP.
Moxifloxacin is a newly marketed fourth-generation fluoroquinolone antibiotic with a broad spectrum of antibacterial activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria.[11-13]It has excellent microbiological activity against common pathogens found in biliary tract infection.[12-14]Moxifloxacin displays significant biliary excretion and sufficient bactericidal concentrations above minimal inhibitory concentrations for most expected bacteria in biliary tract infection even in patients with an obstructed bile duct.[12,15,16]Based on these reasons, we speculated that moxifloxacin might be an effective prophylactic antibiotic prior to therapeutic ERCP to reduce the occurrence rate of post-procedural complications aer endoscopic biliary drainage.
Methods
Study population
Among the 109 patients, 12 with previous antibiotic treatment because of other definite infections before ERCP were excluded.e remaining 97 patients were randomly allocated to either moxifloxacin or ceriaxone group. For these 97 initially randomized patients,11 were excluded from the final analysis because of the following reasons: an attending endoscopist failed in cannulation of bile duct and in obtaining cholangiogram(n=6), only a simple diagnostic procedure was performed(n=2), and relevant clinical data were incomplete (n=3).e final numbers of eligible subjects were 44 in the moxilf oxacin group and 42 in the ceriaxone group (Fig.).Randomization was done using consecutively numbered computer-generated cards containing treatment assignment (https://www.randomizer.org/). We adopted“simple randomization” for random allocation of our enrolled patients to each antibiotic group. “Randomization”database was password-protected and accessible only by principal investigator.
Before participation, patients were fully informed of the objective and methods of this clinical trial, and potential benefits and risks of participation. Only then they were provided written informed consent for participation.e study protocol was approved by the EthicsCommittee of Kangbuk Samsung Hospital (KBC13141;approval date, 2013-09-01).

Fig.Flow diagram illustrating the selection of study subjects.
Study design and definitions
ERCP was performed in the standard manner using a side-viewing duodenoscope (TJF-260, Olympus Optical Co. Ltd., Tokyo, Japan). Extrahepatic choledocholithiasis was removed by a stone basket and retrieval balloon aer endoscopic sphincterotomy using a standard pull type papillotome with a mechanical lithotripter used for large stones. In case of malignant bile duct strictures, biliary drainage was performed by endoscopic retrograde biliary drainage aer tissue acquisition using Geenen brushing and/or endobiliary forcep biopsy.
Vital signs (body temperature, blood pressure, respiratory rate, and pulse rate) were monitored at least every 6 hours.e levels of leukocytes, erythrocyte sedimentation rate, C-reactive protein (CRP), procalcitonin, hemoglobin, total bilirubin, aspartate aminotransferase (AST),alanine aminotransferase (ALT), alkaline phosphatase(ALP), amylase, and lipase were reported before and 1, 3,and 7 days aer the procedure. Blood and bile cultures were obtained upon the following circumstances: (1)clinical diagnosis of acute cholangitis, (2) elevated infection parameters (leukocytes >12×109/L, CRP >3 mg/dL)or presence offever (>38.5 ℃).
Acute cholangitis was diagnosed if: (1) Right upper quadrant pain and fever (more than one episode, >38.0 ℃), (2)leukocytes count >12×109/L, and CRP >3 mg/dL, and (3)ALT >upper limit of normal (ULN), and total bilirubin>ULN. Recalcitrant cholangitis aer ERCP was defined as the persistent presence of the aforementioned-acute cholangitis for longer than 5 days or transient resolution with subsequent recurrence of acute cholangitis within 30 days aer ERCP. Septicemia was defined as follows:(1) systemic inflammatory response syndrome (conform with same or more than two of the followings; tachypnea>20/min, leukocytes <4×109/L or >12×109/L, pulse rate>90/min, fever >38.0 ℃ or hypothermia <36.0 ℃, and end-organ dysfunction evident as renal failure, hepatic failure, change in consciousness, or increase in blood lactate level); and (2) persistent hypotension despite continuous fluid resuscitation. Post-ERCP pancreatitis was defined as typical abdominal pain, raised serum amylase level more than 3-times of ULN, and typical findings on contrast-enhanced computed tomography (CT) imaging>24 hours aer the procedure.[17]
Statistical analysis
For the sample size calculation, a method of Pearson's Chi-square test was used. Delta, the difference in the occurrence rate of post-ERCP complications between the intravenous moxifloxacin and ceriaxone of 10%, type I error rate of 0.05 and power of 80% were presumed and estimated sample sizes were 40 for each antibiotic group,respectively. Data are expressed as mean±standard deviation (SD) or frequency (%).e primary aim of our study was to demonstrate that intravenous moxifloxacin is not inferior to ceriaxone with regard to the frequency of recalcitrant cholangitis, bacteremia and septicemia. As a secondary objective, we also examined the effectiveness of intravenous moxifloxacin and ceriaxone with regard to the length of hospital stay (day), frequency of bacterial resistance, and drug-related adverse effects. Baseline characteristics according to the administered antibiotics were compared by Chi-square analysis or Fisher's exact test for categorical variables and Student'sttest or Mann WhitneyUtest for continuous variables.Pvalues <0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS statistics 18.0(IBM, Armonk, NY, USA).
Results
Baseline characteristics of study population
Eighty-six consecutive patients with biliary obstruction and one or more factors predicting benefits of antibiotic prophylaxis prior to ERCP were included (Fig.). Mean age was 62.5 years and the proportion of participants aged 50-59, 60-69, and ≥70 years was 20.9%, 36.0%, and 43.0%, respectively.
ERCP procedure
ERCP procedures were performed by three endoscopists. ERCP procedures included endoscopic sphincterotomy, stone extraction, removal of biliary sludge, insertion of stents and biopsy or brush cytology in the biliary and pancreatic ductal systems (Table 2). Bile duct stones were extracted by basket or balloon sweeping in 81.4% of the patients and biopsy and/or brush cytology for ductal system were performed in 10.5% of patients.ere were no significant differences in type and number of endoscopic procedures with ERCP between the study groups(Table 2).
Comparisons of overall procedure-related complications
Table 1.Comparison of baseline characteristics between the intravenous moxifloxacin and ceriaxone groups (n, %)
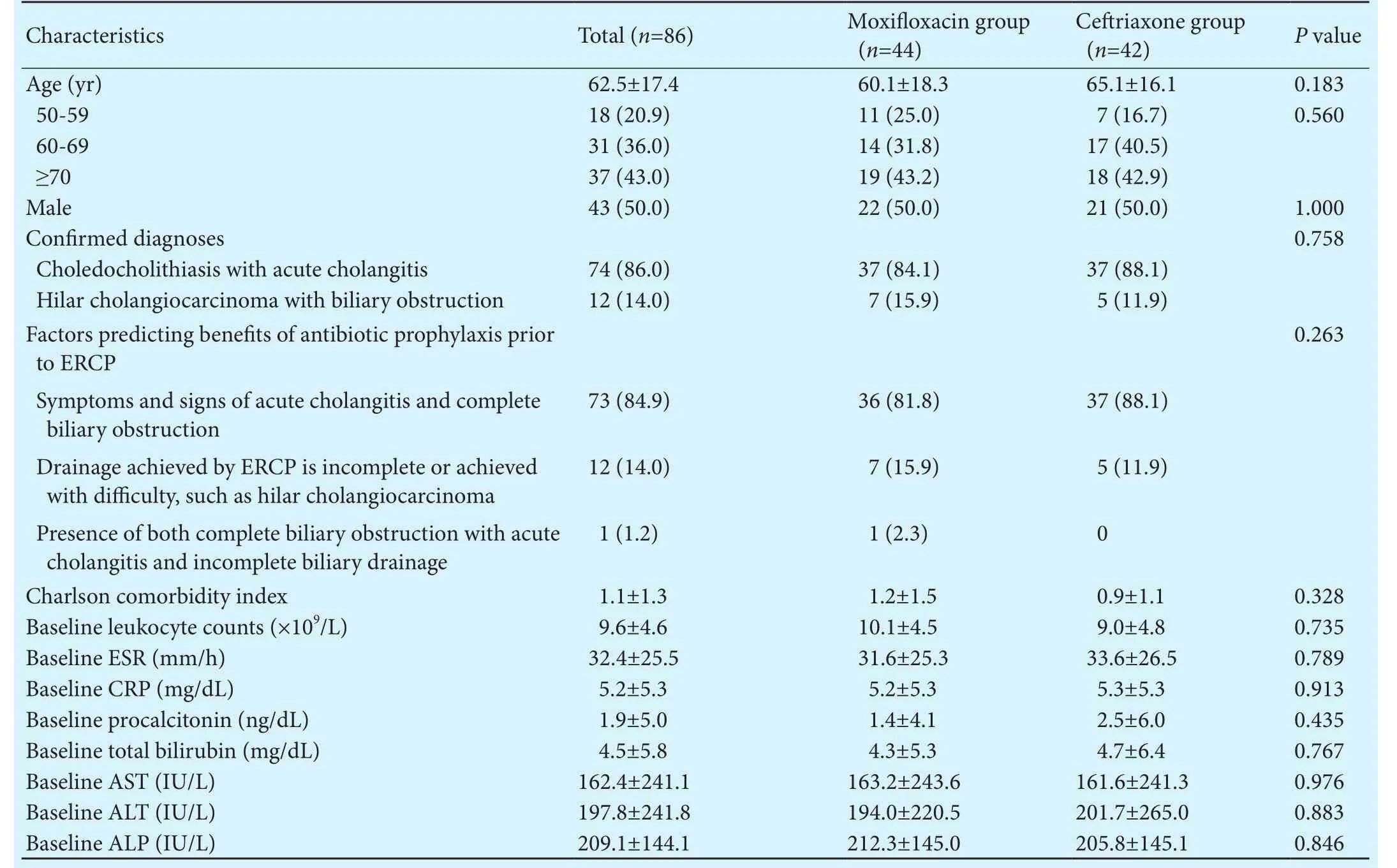
Table 1.Comparison of baseline characteristics between the intravenous moxifloxacin and ceriaxone groups (n, %)
ERCP: endoscopic retrograde cholangiopancreatography; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase.
CharacteristicsTotal (n=86)Moxifloxacin group(n=44)Ceriaxone group(n=42)Pvalue Age (yr) 62.5±17.4 60.1±18.3 65.1±16.10.183 50-59 18 (20.9) 11 (25.0) 7 (16.7)0.560 60-69 31 (36.0) 14 (31.8) 17 (40.5)≥70 37 (43.0) 19 (43.2) 18 (42.9)Male 43 (50.0) 22 (50.0) 21 (50.0)1.000 Confirmed diagnoses 0.758 Choledocholithiasis with acute cholangitis 74 (86.0) 37 (84.1) 37 (88.1)Hilar cholangiocarcinoma with biliary obstruction 12 (14.0) 7 (15.9) 5 (11.9)Factors predicting benefits of antibiotic prophylaxis prior to ERCP 0.263 Symptoms and signs of acute cholangitis and complete biliary obstruction 73 (84.9) 36 (81.8) 37 (88.1)Drainage achieved by ERCP is incomplete or achieved with difficulty, such as hilar cholangiocarcinoma 12 (14.0) 7 (15.9) 5 (11.9)1 (1.2) 1 (2.3) 0 Charlson comorbidity index 1.1±1.3 1.2±1.5 0.9±1.10.328 Baseline leukocyte counts (×109/L) 9.6±4.6 10.1±4.5 9.0±4.80.735 Baseline ESR (mm/h) 32.4±25.5 31.6±25.3 33.6±26.50.789 Baseline CRP (mg/dL) 5.2±5.3 5.2±5.3 5.3±5.30.913 Baseline procalcitonin (ng/dL) 1.9±5.0 1.4±4.1 2.5±6.00.435 Baseline total bilirubin (mg/dL) 4.5±5.8 4.3±5.3 4.7±6.40.767 Baseline AST (IU/L)162.4±241.1 163.2±243.6161.6±241.30.976 Baseline ALT (IU/L)197.8±241.8 194.0±220.5201.7±265.00.883 Baseline ALP (IU/L)209.1±144.1212.3±145.0205.8±145.10.846 Presence of both complete biliary obstruction with acute cholangitis and incomplete biliary drainage
Table 2.ERCP procedures performed in patients with intravenous moxifloxacin and ceriaxone groups (n, %)
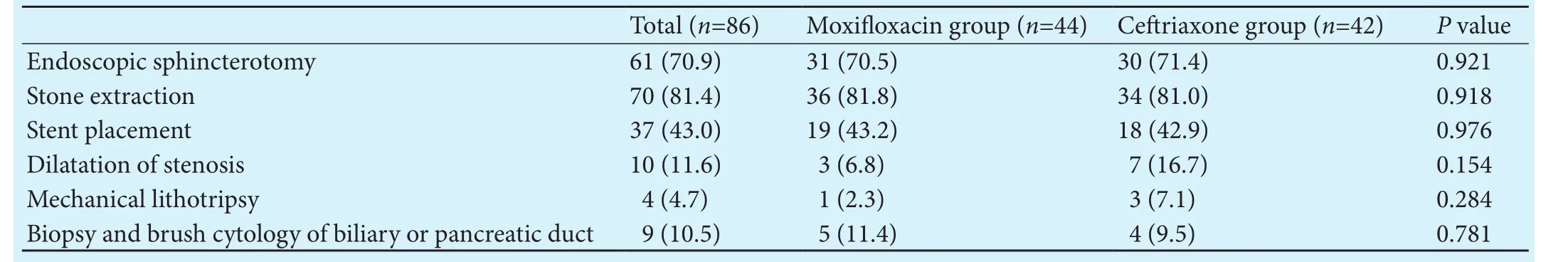
Table 2.ERCP procedures performed in patients with intravenous moxifloxacin and ceriaxone groups (n, %)
ERCP: endoscopic retrograde cholangiopancreatography.
Total (n=86)Moxifloxacin group (n=44)Ceriaxone group (n=42)Pvalue Endoscopic sphincterotomy61 (70.9)31 (70.5)30 (71.4)0.921 Stone extraction70 (81.4)36 (81.8)34 (81.0)0.918 Stent placement 37 (43.0)19 (43.2)18 (42.9)0.976 Dilatation of stenosis 10 (11.6) 3 (6.8) 7 (16.7)0.154 Mechanical lithotripsy 4 (4.7) 1 (2.3) 3 (7.1)0.284 Biopsy and brush cytology of biliary or pancreatic duct 9 (10.5) 5 (11.4) 4 (9.5)0.781
Table 3.Clinical outcomes in patients with intravenous moxifloxacin and ceriaxone groups (n, %)
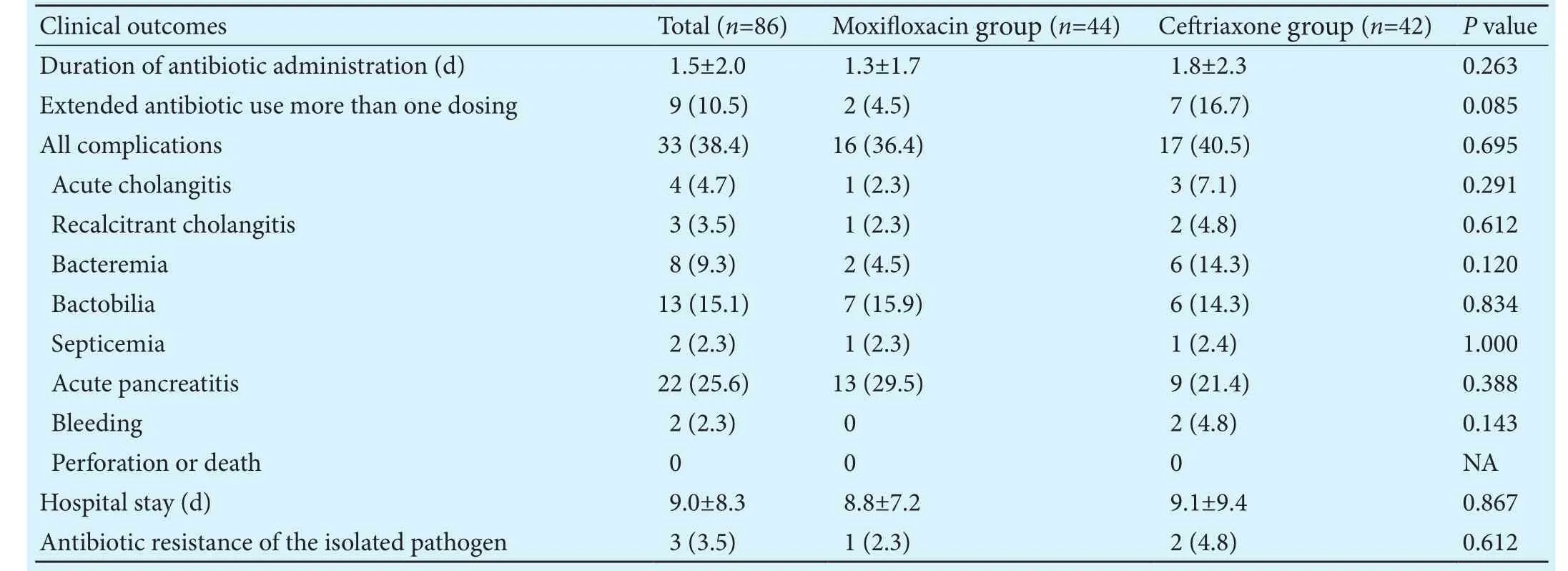
Table 3.Clinical outcomes in patients with intravenous moxifloxacin and ceriaxone groups (n, %)
NA: not available.
Clinical outcomesTotal (n=86)Moxifloxacin group (n=44)Ceriaxone group (n=42)Pvalue Duration of antibiotic administration (d) 1.5±2.0 1.3±1.7 1.8±2.30.263 Extended antibiotic use more than one dosing 9 (10.5) 2 (4.5) 7 (16.7)0.085 All complications 33 (38.4)16 (36.4)17 (40.5)0.695 Acute cholangitis 4 (4.7) 1 (2.3) 3 (7.1)0.291 Recalcitrant cholangitis 3 (3.5) 1 (2.3) 2 (4.8)0.612 Bacteremia 8 (9.3) 2 (4.5) 6 (14.3)0.120 Bactobilia13 (15.1) 7 (15.9) 6 (14.3)0.834 Septicemia 2 (2.3) 1 (2.3) 1 (2.4)1.000 Acute pancreatitis22 (25.6)13 (29.5) 9 (21.4)0.388 Bleeding 2 (2.3) 0 2 (4.8)0.143 Perforation or death 0 0 0 NA Hospital stay (d) 9.0±8.3 8.8±7.2 9.1±9.40.867 Antibiotic resistance of the isolated pathogen 3 (3.5) 1 (2.3) 2 (4.8)0.612
Discussion
In this randomized open-label non-inferiority study, the occurrence rate of post-procedural complications of cholangitis, bacteremia, and septicemia in patients with prophylactic intravenous moxifloxacin use was not significantly higher than those with intravenous ceriaxone who were scheduled to therapeutic ERCP. In addition,the bacterial species isolated from blood or bile cultures was similar in two groups; antibiotic resistance of the isolated pathogens, length of hospital stay, and drug-induced complications between the two groups were also comparable. Only 4 cases of cholangitis and 2 of septicemia were identified and no mortality in the 86 patients in 30 days.erefore, use of moxifloxacin as a prophylactic antibiotic in the setting of therapeutic ERCP seems to be not inferior to ceriaxone concerning the variables of our primary and secondary outcomes.
Post-procedural recalcitrant cholangitis, bacteremia,and septicemia are important complications of ERCP.[9,18,19]Transient bacteremia has been documented in up to 27%of patients undergoing ERCP and most ERCP-related cholangitis and septicemia occurs in the setting of biliary obstruction.[8,20-22]Risk of post-procedural cholangitis and/or septicemia during therapeutic ERCP is much greater than that in simple diagnostic ERCP.[6-8]ere are several expected mechanisms for this association. First,biliary stasis promotes bacterial colonization with a high prevalence of mixed infections. Second, damage to the epithelium during contrast injection or other procedures such as stone extraction using basket or balloon sweeping, stent insertion, and passage of dilators may provide a portal of bacteria entry.[3]e most commonly isolated bacteria in this setting areEnterobacteriaceaesuch asEsche-richia coliandKlebsiella spp.,Enterococcus spp., andStreptococcus spp., and our results echo the historic data.[3,20,21]
Two meta-analyses assessing the role of prophylactic antibiotics in the setting of diagnostic and therapeutic ERCP failed to show a decrease in the incidence of post-ERCP related complications.[23,24]However, some of the trials included in these meta-analyses dealt with both diagnostic and therapeutic ERCP procedures and had used a variable number of antibiotics. Hence, it is not possible to determine which antibiotic provides prophylactic effects. Meanwhile, several studies reported that prophylactic antibiotics reduced the incidences of cholangitis, bacteremia, and septicemia in patients undergoing therapeutic or complicated diagnostic ERCP.[25,26]A recent Cochrane systematic review and meta-analysis that included 9 randomized clinical trials and a total of 1573 patients documented that administration of antibiotics prior to the procedure reduces the risk of bacteremia, cholangitis, and septicemia in patients undergoing elective ERCP.[10]e optimum prophylactic antimicrobial agent prior to therapeutic ERCP remains unclear.
In conclusion, the clinical efficacy of intravenous moxifloxacin prophylaxis before therapeutic ERCP in reducing the occurrence rate of recalcitrant cholangitis,bacteremia, and septicemia was not inferior to that of intravenous ceriaxone.ese findings suggest that moxifloxacin is an appropriate prophylactic agent for patients with biliary obstruction who will undergo therapeutic ERCP procedure.
Contributors:KNH, KHJ, and BKB had the original idea for the study, and shared responsibility equally for data collection, data processing, statistical analyses, writing, and reviewing the manuscript. KHJ is the guarantor.
Funding:is study was financially supported by Chongkundang Pharmaceutical (Seoul, Korea).
Ethical approval:e study protocol was approved by the Ethics Committee of Kangbuk Samsung Hospital (KBC13141; approval date, 2013-09-01).
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Adler DG, Baron TH, Davila RE, Egan J, Hirota WK, Leighton JA, et al. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005;62:1-8.
2 Carr-Locke DL. Overview of the role of ERCP in the management of diseases of the biliary tract and the pancreas. Gastrointest Endosc 2002;56:S157-160.
3 Leung JW, Ling TK, Chan RC, Cheung SW, Lai CW, Sung JJ, et al. Antibiotics, biliary sepsis, and bile duct stones. Gastrointest Endosc 1994;40:716-721.
4 Cotton PB. Duodenoscopic placement of biliary prostheses to relieve malignant obstructive jaundice. Br J Surg 1982;69:501-503.
5 Classen M, Safrany L. Endoscopic papillotomy and removal of gall stones. Br Med J 1975;4:371-374.
6 Bilbao MK, Dotter CT, Lee TG, Katon RM. Complications of endoscopic retrograde cholangiopancreatography (ERCP). A study of 10,000 cases. Gastroenterology 1976;70:314-320.
7 Zimmon DS, Falkenstein DB, Riccobono C, Aaron B. Complications of endoscopic retrograde cholangiopancreatography. Analysis of 300 consecutive cases. Gastroenterology 1975;69:303-309.
8 Mollison LC, Desmond PV, Stockman KA, Andrew JH, Watson K, Shaw G, et al. A prospective study of septic complications of endoscopic retrograde cholangiopancreatography. J Gastroenterol Hepatol 1994;9:55-59.
9 ASGE Standards of Practice Committee, Khashab MA,Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015;81:81-89.
11 Ober MC, Hoppe-Tichy T, Köninger J, Schunter O, Sonntag HG, Weigand MA, et al. Tissue penetration of moxifloxacin into human gallbladder wall in patients with biliary tract infections. J Antimicrob Chemother 2009;64:1091-1095.
12 Weber A, Huber W, Kamereck K, Winkle P, Voland P, Weidenbach H, et al. In vitro activity of moxifloxacin and piperacillin/sulbactam against pathogens of acute cholangitis. World J Gastroenterol 2008;14:3174-3178.
13 Edmiston CE, Krepel CJ, Seabrook GR, Somberg LR, Nakeeb A, Cambria RA, et al. In vitro activities of moxifloxacin against 900 aerobic and anaerobic surgical isolates from patients with intra-abdominal and diabetic foot infections. Antimicrob Agents Chemother 2004;48:1012-1016.
14 Wirtz M, KleeffJ, Swoboda S, Halaceli I, Geiss HK, Hoppe-Tichy T, et al. Moxifloxacin penetration into human gastrointestinal tissues. J Antimicrob Chemother 2004;53:875-877.
15 Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med 1992;326:1582-1586.
16 Schwab D, Grauer M, Hahn EG, Mühldorfer S. Biliary secretion of moxifloxacin in obstructive cholangitis and the non-obstructed biliary tract. Aliment Pharmacoler 2005;22:417-422.
17 Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383-393.
18 Colton JB, Curran CC. Quality indicators, including complications, of ERCP in a community setting: a prospective study.Gastrointest Endosc 2009;70:457-467.
19 Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR,Spirito F, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781-1788.
20 Parker HW, Geenen JE, Bjork JT, Stewart ET. A prospective analysis offever and bacteremia following ERCP. Gastrointest Endosc 1979;25:102-103.
21 Kullman E, Borch K, Lindström E, Anséhn S, Ihse I, Anderberg B. Bacteremia following diagnostic and therapeutic ERCP.Gastrointest Endosc 1992;38:444-449.
22 Nelson DB. Infectious disease complications of GI endoscopy:Part I, endogenous infections. Gastrointest Endosc 2003;57:546-556.
23 Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: a meta-analysis. Pancreas 2009;38:126-130.
24 Harris A, Chan AC, Torres-Viera C, Hammett R, Carr-Locke D. Meta-analysis of antibiotic prophylaxis in endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy 1999;31:718-724.
25 Niederau C, Pohlmann U, Lübke H,omas L. Prophylactic antibiotic treatment in therapeutic or complicated diagnostic ERCP: results of a randomized controlled clinical study. Gastrointest Endosc 1994;40:533-537.
26 Werth B, Stalder GA, Marbet U, Gyr K. ERCP under ceriaxone antibiotic cover in patients with obstructive jaundice. Schweiz Rundsch Med Prax 1990;79:387-388.
27 Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA,Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis.Gastrointest Endosc 2002;56:885-889.
28 Sartelli M, Catena F, Coccolini F, Pinna AD. Antimicrobial management of intra-abdominal infections: literature's guidelines. World J Gastroenterol 2012;18:865-871.
Accepted after revision June 6, 2017
one pro-phylactic dose of moxifloxacin or ceriaxone 90 minutes before ERCP; 9 (10.5%)
more than one dose of moxifloxacin or ceriaxone, and median (range) duration of moxifloxacin or ceriaxone medication for these patients was 4 (3-14) days.ere were no significant differences in duration of antibiotic administration between the two groups.e proportion of patients who received more than one dose of antibiotic displayed the tendency of more extended antibiotic use in the ceriaxone group. However, it did not reach statistical significance(Table 3).ere were 33 (38.4%) complications following the procedures.e incidence of procedure-related complications including acute cholangitis, recalcitrant cholangitis, bacteremia without clinical sepsis, septicemia,acute pancreatitis, and bleeding was not significantly different between the two groups (Table 3). Recalcitrant cholangitis occurred in 1 (2.3%) patient who received moxifloxacin and 2 (4.8%) patients who received ceriaxone (P=0.612). Septicemia occurred in 1 (2.3%) and 1(2.4%) patient in each groups (P=1.0).e incidence of bacteremia and bactobilia were 4.5% (n=2) and 15.9%(n=7) in the moxifloxacin group, and 14.3% (n=6) and 14.3% (n=6) in the ceriaxone group (P>0.05 for both).Gram-negative bacteria such asEscherichia coli,Klebsiella pneumoniae, andEnterococcus spp. were isolated most commonly from blood and bile cultures of both groups.Antibiotic resistance of the isolated pathogens was in 1(2.3%) in moxifloxacin group and 2 (4.8%) in ceriaxone group (P=0.612). One patient (2.3%) had diarrhea in the moxifloxacin group and 2 (4.8%) had allergic reaction(skin rashes) in the ceriaxone group.e mean hospital stay was also not significantly different between the two groups (8.8±7.2 vs 9.1±9.4 days,P=0.867).
December 12, 2016
Author Affiliations: Department of Internal Medicine, Sungkyunkwan University Kangbuk Samsung Hospital, Seoul 03181, Korea (Kim NH and Kim HJ); Departm
e
nt of Internal Medicine, Dankook University Hospital, Cheonan, Korea (Bang KB)
Prof. Hong Joo Kim, MD, Division of Gastroenterology, Department of Internal Medicine, Kangbuk Samsung Hospital,Sungkyunkwan University School of Medicine, 29, Saemunan-Ro, Jongno-Gu, Seoul 03181, Korea (Tel: +82-2-2001-8330; Fax: +82-2-2001-8340;Email: hongjoo3.kim@samsung.com)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60056-0
Published online September 4, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Letters to the Editor
- The “Colonial Wig” pancreaticojejunostomy:zero leaks with a novel technique for reconstruction after pancreaticoduodenectomy
- Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy
- Tailored pancreatic reconstruction after pancreaticoduodenectomy: a single-center experience of 892 cases
- Helicobacter pyloriand 17β-estradiol induce human intrahepatic biliary epithelial cell abnormal proliferation and oxidative DNA damage
- Comparative study of the effects of terlipressin versus splenectomy on liver regeneration after partial hepatectomy in rats
