Bone metastases from hepatocellular carcinoma:clinical features and prognostic factors
2017-10-09YangLuJinGenHuXiangJinLinandXiGongLi
Yang Lu, Jin-Gen Hu, Xiang-Jin Lin and Xi-Gong Li
Hangzhou, China
Bone metastases from hepatocellular carcinoma:clinical features and prognostic factors
Yang Lu, Jin-Gen Hu, Xiang-Jin Lin and Xi-Gong Li
Hangzhou, China
BACKGROUND: Bone metastases (BMs) from hepatocellular carcinoma (HCC) is an increasingly common disease in Asia. We assessed the clinical features, prognostic factors, and differences in outcomes related to BMs among patients with different treatments for HCC.
METHODS: Forty-three consecutive patients who were diagnosed with BMs from HCC between January 2010 and December 2014 were retrospectively enrolled.e clinical features were identified, the impacts of prognostic factors on survival were statistically analyzed, and clinical data were compared.RESULTS:e median patient age was 54 years; 38 patients were male and 5 female.e most common site for BMs was the trunk (69.3%). BMs with extension to the sotissue were found in 14 patients (32.5%). Most (90.7%) of the lesions were mixed osteolytic and osteoblastic, and most (69.8%) patients presented with multiple BMs.e median survival aer BMs diagnosis was 11 months. In multivariate analyses, survival aer BM diagnosis was correlated with Karnofsky performance status (P=0.008) and the Child-Pugh classification(P<0.001); BM-free survival was correlated with progression beyond the University of California San Francisco criteria(P<0.001) and treatment of primary tumors (P<0.001). BMs with extension to sotissue were less common in liver transplantation patients. During metastasis, the control of intrahepatic tumors was improved in liver transplantation and hepatectomy patients, compared to conservatively treated patients.
(Hepatobiliary Pancreat Dis Int 2017;16:499-505)
hepatocellular carcinoma;
bone metastases;
survival;
prognostic factors
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer worldwide and the third leading cause of cancer-related death. HCC has a heterogeneous geographical distribution, with most cases (>80%) occurring in eastern Asia and sub-Saharan Africa.[1]Bone metastases (BMs) from HCC are highly vascularized lesions and cause severe deterioration of the patients' quality of life.e reported incidence of BM from HCC was relatively low before the 20th century.However, bone is now the second major site and accounts for approximately 25% of extrahepatic metastases from HCC.[2-4]is increase is due to the advances of bone imaging technique and the prolonged survival of HCC patients.[5-8]Hepatectomy, radiofrequency ablation, transhepatic arterial chemotherapy and embolization, and tyrosine kinase inhibitors such as sorafenib are effective treatment modalities for HCC.[9]Liver transplantation(LT) has also been established as a promising treatment for malignant liver diseases.[10-12]However, whether these treatments contribute towards the survival of patients with BMs from HCC, and whether they are related to the condition of patients during metastasis, remain unclear.In this study, the clinical features and prognostic factors were investigated in patients with BMs from HCC, and the clinical data were compared among patients with different treatments for intrahepatic tumors.
Methods
Study design
We reviewed the data of 43 consecutive patients diagnosed with BMs from HCC in the First Affiliated Hospital, Zhejiang University School of Medicine betweenJanuary 2010 and December 2014. Sixteen patients underwent LT as treatment for HCC and subsequently developed BMs.e final follow-up date was June 30, 2015,and the median follow-up time was 11 months. None of the patients were enrolled in any clinical trials or experimental protocols.e Institutional Research Ethics Committee approved this retrospective observational study.
All patients diagnosed with BMs from HCC were provided with histological confirmation of the disease via bone biopsy or surgical pathology.99mTc bone scintigraphy was used to locate all possible sites of BM.Magnetic resonance imaging (MRI) or computed tomography (CT) was performed to either confirm or provide detailed information on symptomatic sites.[13]Additional pretreatment evaluation included assessment of the patient's medical history, as well as a physical examination,complete blood routine, serum chemistries, blood gas analysis, alpha-fetoprotein (AFP) level, γ-glutamyl transpeptidase (γ-GT) level, chest X-ray, and abdominal and cardiac ultrasonography.
Statistical analysis
Clinical features were assessed based on descriptive statistics.e impact of prognostic factors on survival was analyzed by the Kaplan-Meier method and tested using the log-rank test. Median survivals were also calculated. Variables were entered using the Backward-Wald method in a Cox regression analysis (the level of statistical significance was set at aPvalue <0.05).e features of BMs in patients given different treatments were compared using Fisher's exact test (atP<0.017). All analyses and calculations were performed using the SPSS statistical soware (version 17.0; SPSS Inc., Chicago, IL, USA).
Results
Clinical features
Of the 43 patients, 38 were male and 5 female, with a median age of 54 years (range 26-83). For the first BM-related event, 28 patients had pain (65.1%), 5 had dysneuria(11.6%), and 4 had a continuous rise in AFP levels (9.3%).Eight patients experienced no clinical symptoms, and their BMs were discovered by follow-up imaging studies of the HCC (18.6%). Five patients were diagnosed with BMs immediately aer HCC diagnosis (11.6%), and 38 developed BMs later on (88.4%).irty patients had multiple BMs (69.8%), 39 presented with mixed osteolytic and osteoblastic lesions (90.7%), 4 presented with purely osteolytic lesions (9.3%), and no patient had a purely osteoblastic lesion. BMs with extension to the sotissue were discovered in 14 patients (32.6%). Of the 101 BM sites, 69.3% were located on the trunk.e lumbar vertebrae (35.3%) were the most common responsible site, followed by the thoracic vertebrae (29.4%) (Table 1).
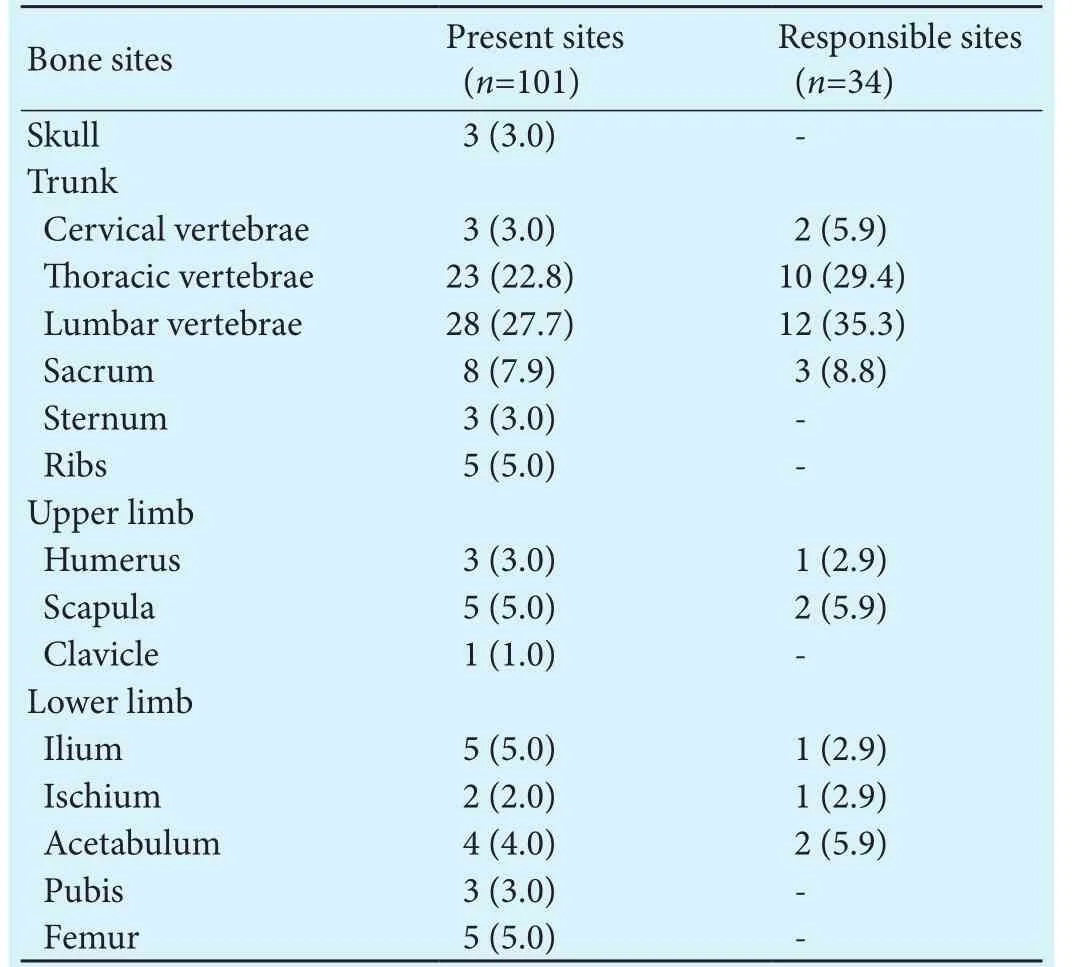
Table 1.BM sites in the 43 HCC patients (n, %)
All 43 patients were prescribed zoledronic acid.Twenty-two patients underwent palliative EBRT, 3 had curettage, 9 had curettage following EBRT, and 6 underwent wide resection following EBRT.e 1-year survivals for patients with EBRT, curettage, and wide resection were 40.9%, 50.0%, and 33.3%, respectively. Intraosseous recurrence occurred in one patient who had a wide resection.
Outcomes
Seven patients survived until the end offollow-up.Five of them had undergone LT and two had a hepatectomy for the treatment of the primary tumors, and their intrahepatic tumors remained under control since then.Five of these patients were treated with EBRT for BMs and two were treated with curettage. Causes of death included liver failure, tumor progression, or both in 33 patients (91.7%), pulmonary infection in 2 patients (5.6%),and stroke in 1 patient (2.8%).
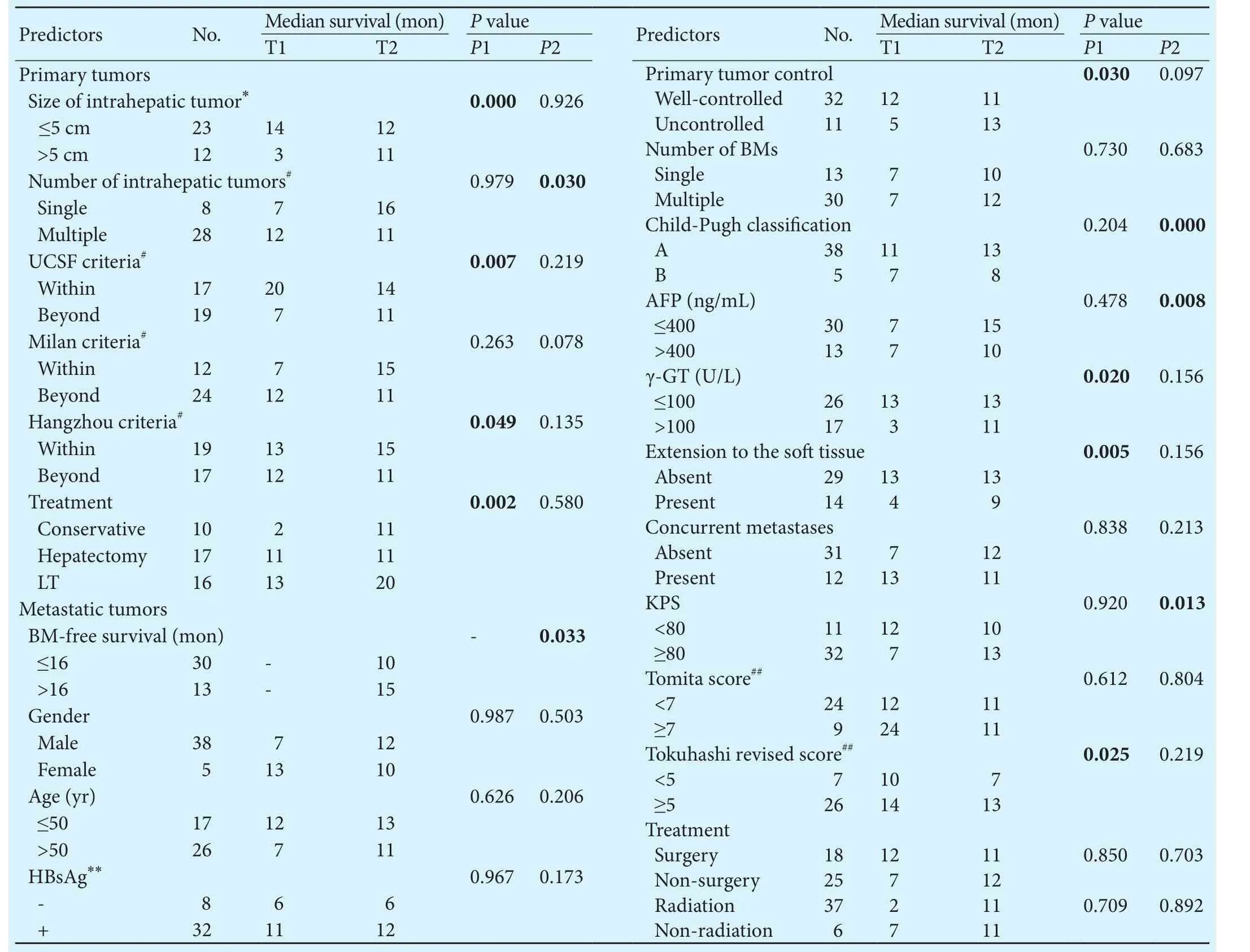
Table 2.Univariate analysis of predictors in 43 patients

Fig. 1.Survival curves of different surgical modalities for BM.The survival after BM diagnosis was not significantly different(P=0.989).

Fig. 2.Survival curves of different treatments for intrahepatic tumors.e treatment of the primary tumors significantly influenced the BM-free survival (P=0.002).
BM-free survival
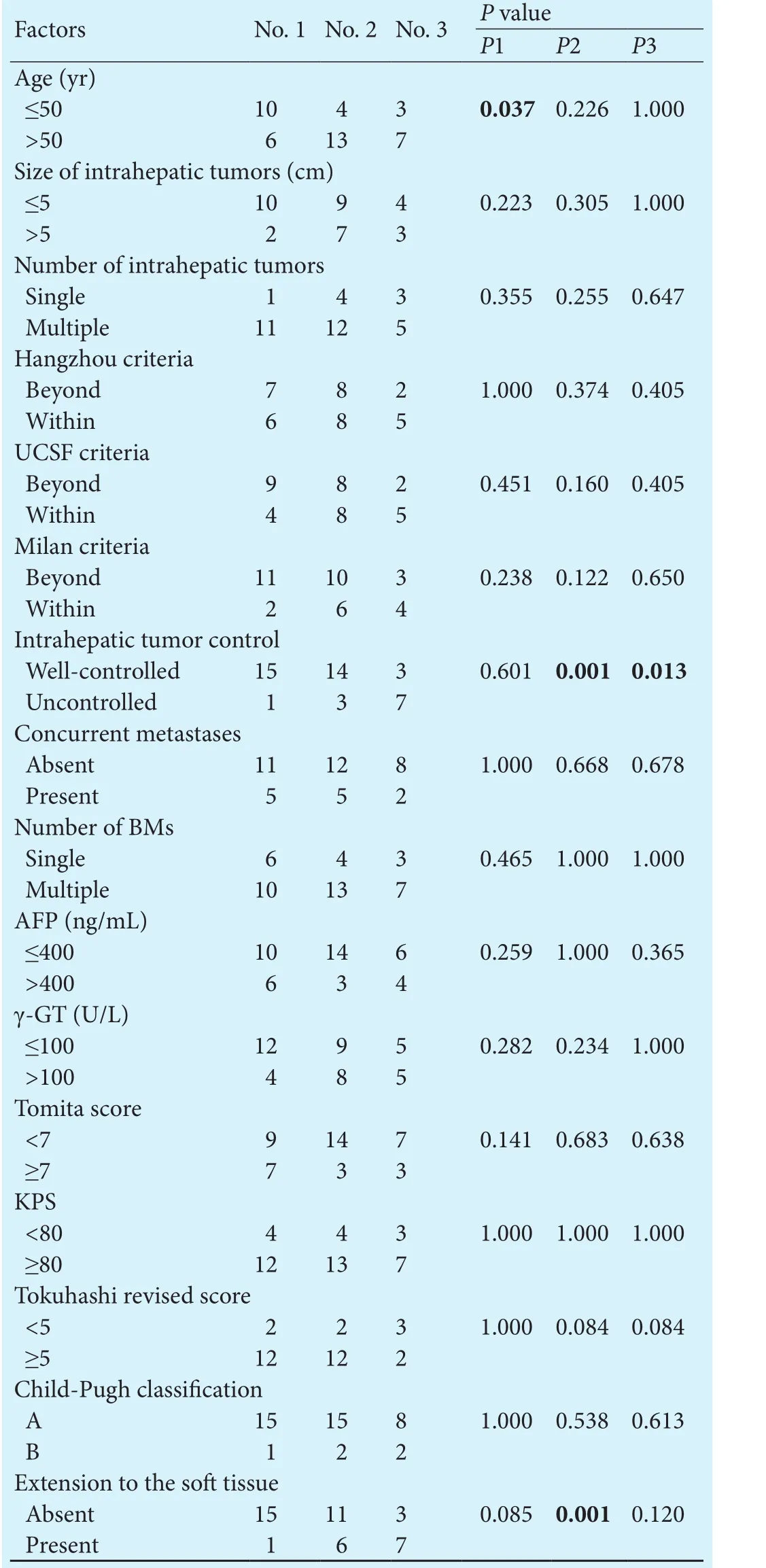
Table 3.Comparison offactors among patients who underwent different treatments
Comparison of patients with different treatments for intrahepatic tumors
Discussion
Due to an increased understanding of the interactions between metastatic tumor cells and the bone marrow microenvironment,[29]bone-targeted therapies such as zoledronic acid and denosumab have been used for the prevention of skeletal morbidities associated with malignant bone diseases, particularly in advanced-stage solid tumors arising from the breast, prostate, and multiple myeloma.[30-32]However, the efficiency of these treatments in patients with BMs from HCC has only been reported by several authors.[23,33]Sorafenib may improve patients' survival and affect bone metastasization,[34]but due to financial reasons, only two patients in the present study were treated with it and the treatment time was no longer than 3 months. We hope to recruit more patients treated with sorafenib and provide more clinical data in the future.
Hematogenous spread through the pulmonary circulation or vertebral venous plexus is the paramount approach for the metastasization of HCC to bone.[36,37]Our work demonstrates that larger intrahepatic tumors (>5 cm in diameter) are a strong predictor for faster metastasization to bone. Furthermore, patients with multiple intrahepatic tumors had a poorer survival aer the diagnosis of BMs.e UCSF criteria and Hangzhou criteria are efficient methods for predicting survival in patients treated with LT,[15]factors that also predicted BM-free survival in this study. In fact, the death of patients with BMs from HCC is mainly due to liver failure or tumor progression.[38]erefore, follow-up examination and treatment of intrahepatic tumors should be thoroughly implemented.
For patients with resectable HCC, surgery is the only proven potentially curative therapy.[9,39]Our work shows that the surgical removal of intrahepatic tumors helps to limit tumor metastasis. With the extensive use of LT in China, the number of LT patients with BMs is increasing in clinical practice. LT patients generally receive immunosuppressants, which will accelerate the recurrence or metastasization of tumors.[40,41]Previous studies have revealed that the presence of BMs is a contraindication for LT as well as an independent unfavorable prognostic factor for LT patients.[42,43]However, in this study, we found that LT patients had a longer BM-free survival and fol-lowed a trend for increased survival aer BM diagnosis,which means that LT is a favorable prognostic factor for BMs from HCC. We compared patients who underwent different treatments for HCC and found that LT patients had better intrahepatic tumor control and that fewer LT patients experienced extension to sotissue. We believe that the smaller tumor burden aer LT may have contributed towards this. Overall, the characteristics of HCC and the treatment of primary tumors were related to the speed of bone metastasization and the patient's prognosis.
A limitation of this study was the small sample size and retrospective nature. Analyses of data on patients with different first BM-related events may introduce bias into the calculated survival intervals because there is a time lapse in diagnosis for patients who present to the hospital due to clinical symptoms compared to those whose BMs are discovered by periodic examination.Meanwhile, the retrospective nature makes it difficult to control the homogeneity of the extent of disease between patients undergoing different treatments.
In conclusion, the independent prognostic factors of survival aer BM diagnosis were the KPS and Child-Pugh classification. HCC patients who went on to develop BMs may also benefit from LT or hepatectomy. A future study with a larger sample size, or a laboratory study into sotissue masses, should be conducted to better prove or illustrate the findings of this study and to explore potential therapeutic targets.
Acknowledgements:We thank Division of Hepatobiliary Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine for providing clinical data of primary tumors. We also thank Dr. Hong-Xun Zhou from University of Western Australia for advice on this article.
Contributors:LXJ proposed the study. LY and HJG performed research and wrote the first dra. LY and HJG collected and analyzed the data. All authors contributed to the design and interpretation of the study to further dras. LY and HJG contributed equally to this study. LXJ is the guarantor.
Funding:e study was supported by grants from Zhejiang Provincial Clinical Scientific Research Foundation of China(2013ZYC-A17), Ministry of Health of China (WKJ-ZJ-12) and Health Bureau of Zhejiang Province (2013KYB098).
Ethical approval:is study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-1273.
2 Fukutomi M, Yokota M, Chuman H, Harada H, Zaitsu Y,Funakoshi A, et al. Increased incidence of bone metastases in hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2001;13:1083-1088.
3 Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis:clinical features and prognostic factors. Cancer 2011;117:4475-4483.
4 Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K,Sato T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 2005;20:1781-1787.
5 Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma.J Nucl Med 2007;48:902-909.
6 Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Changes in the characteristics and survival rate of hepatocellular carcinoma from 1976 to 2000: analysis of 1365 patients in a single institution in Japan. Cancer 2004;100:2415-2421.
7 Yang BH, Xia JL, Huang LW, Tang ZY, Chen MS, Li JQ, et al. Changed clinical aspects of primary liver cancer in China during the past 30 years. Hepatobiliary Pancreat Dis Int 2004;3:194-198.
8 Capocaccia R, Sant M, Berrino F, Simonetti A, Santi V, Trevisani F, et al. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol 2007;102:1661-1670.
9 Ryder SD; British Society of Gastroenterology. Guidelines for the diagnosis and treatment of hepatocellular carcinoma(HCC) in adults. Gut 2003;52:iii1-8.
10 Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L.Survival aer liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl 2004;10:886-897.
11 Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV,Goldstein LI, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg 2005;241:905-918.
12 Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut 2016;65:1035-1041.
13 Groves AM, Beadsmoore CJ, Cheow HK, Balan KK, Courtney HM, Kaptoge S, et al. Can 16-detector multislice CT exclude skeletal lesions during tumour staging? Implications for the cancer patient. Eur Radiol 2006;16:1066-1073.
14 Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A,Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-699.
15 Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG,Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 2007;246:502-511.
16 Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-1732.
17 Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1-85.
18 Karnofsky DA. Nitrogen mustards in the treatment of neoplastic disease. Adv Intern Med 1950;4:1-75.
19 Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A re-vised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-2191.
20 Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H,Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298-306.
21 He J, Zeng ZC, Fan J, Zhou J, Sun J, Chen B, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma aer liver transplantation.BMC Cancer 2011;11:492.
22 He J, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer 2009;115:2710-2720.
23 Santini D, Pantano F, Riccardi F, Di Costanzo GG, Addeo R,Guida FM, et al. Natural history of malignant bone disease in hepatocellular carcinoma: final results of a multicenter bone metastasis survey. PLoS One 2014;9:e105268.
24 Cho HS, Oh JH, Han I, Kim HS. Survival of patients with skeletal metastases from hepatocellular carcinoma aer surgical management. J Bone Joint Surg Br 2009;91:1505-1512.
25 Hayashi S, Tanaka H, Hoshi H. Palliative external-beam radiotherapy for bone metastases from hepatocellular carcinoma.World J Hepatol 2014;6:923-929.
26 Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int 2005;25:261-265.
27 Kim SU, Kim DY, Park JY, Ahn SH, Nah HJ, Chon CY, et al.Hepatocellular carcinoma presenting with bone metastasis:clinical characteristics and prognostic factors. J Cancer Res Clin Oncol 2008;134:1377-1384.
28 Manabe J, Kawaguchi N, Matsumoto S, Tanizawa T. Surgical treatment of bone metastasis: indications and outcomes. Int J Clin Oncol 2005;10:103-111.
29 Buijs JT, van der Pluijm G. Osteotropic cancers: from primary tumor to bone. Cancer Lett 2009;273:177-193.
30 Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L,et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-822.
31 Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011;29:1125-1132.
32 Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132-5139.
33 Montella L, Addeo R, Palmieri G, Caraglia M, Cennamo G,Vincenzi B, et al. Zoledronic acid in the treatment of bone metastases by hepatocellular carcinoma: a case series. Cancer Chemother Pharmacol 2010;65:1137-1143.
34 Alemán JO, Farooki A, Girotra M. Effects of tyrosine kinase inhibition on bone metabolism: untargeted consequences of targeted therapies. Endocr Relat Cancer 2014;21:R247-259.
35 Liaw CC, Ng KT, Chen TJ, Liaw YF. Hepatocellular carcinoma presenting as bone metastasis. Cancer 1989;64:1753-1757.
36 Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis:mechanisms and therapeutic opportunities. Nat Rev Endocrinol 2011;7:208-218.
37 Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655-1664.
38 Okusaka T, Okada S, Ishii H, Nose H, Nagahama H, Nakasuka H, et al. Prognosis of hepatocellular carcinoma patients with extrahepatic metastases. Hepatogastroenterology 1997;44:251-257.
39 Coleman RE. Bone cancer in 2011: Prevention and treatment of bone metastases. Nat Rev Clin Oncol 2011;9:76-78.
40 Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012;18:1277-1289.
41 Zhang CX, Wen PH, Sun YL. Withdrawal of immunosuppression in liver transplantation and the mechanism of tolerance.Hepatobiliary Pancreat Dis Int 2015;14:470-476.
42 Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM,Gondolesi GE, et al. Recurrence of hepatocellular carcinoma aer liver transplant: patterns and prognosis. Liver Transpl 2004;10:534-540.
43 Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, et al. Prognostic factors affecting survival aer recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2010;16:678-684.
December 21, 2015
Accepted after revision September 30, 2016
Author Affiliations: Department of Orthopedics, First Affiliated Hospital,Zhejiang University School of Medicine, Hangzhou 310003, China (Lu Y,Hu JG, Lin XJ and Li XG)
Xiang-Jin Lin, MD, Department of Orthopedics,First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel/Fax: +86-571-87236848; Email: doclinxj@zju.edu.cn)© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60173-X
Published online January 25, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy
- Prospective comparison of prophylactic antibiotic use between intravenous moxifloxacin and ceftriaxone for high-risk patients with post-ERCP cholangitis
- Helicobacter pyloriand 17β-estradiol induce human intrahepatic biliary epithelial cell abnormal proliferation and oxidative DNA damage
- Tailored pancreatic reconstruction after pancreaticoduodenectomy: a single-center experience of 892 cases
- Comparative study of the effects of terlipressin versus splenectomy on liver regeneration after partial hepatectomy in rats
- The “Colonial Wig” pancreaticojejunostomy:zero leaks with a novel technique for reconstruction after pancreaticoduodenectomy
