Surgical resection versus liver transplantation for hepatocellular carcinoma within the Hangzhou criteria: a preoperative nomogram-guided treatment strategy
2017-10-09YangLiDanYunRuanChangChangJiaHuiZhaoGuoYingWangYangYangandNanJiang
Yang Li, Dan-Yun Ruan, Chang-Chang Jia, Hui Zhao, Guo-Ying Wang, Yang Yang and Nan Jiang
Guangzhou, China
Surgical resection versus liver transplantation for hepatocellular carcinoma within the Hangzhou criteria: a preoperative nomogram-guided treatment strategy
Yang Li, Dan-Yun Ruan, Chang-Chang Jia, Hui Zhao, Guo-Ying Wang, Yang Yang and Nan Jiang
Guangzhou, China
BACKGROUND: With the expansion of surgical criteria, the comparative efficacy between surgical resection (SR) and liver transplantation (LT) for hepatocellular carcinoma is inconclusive.is study aimed to develop a prognostic nomogram for predicting recurrence-free survival of hepatocellular carcinoma patients aer resection and explored the possibility of using nomogram as treatment algorithm reference.
METHODS: From 2003 to 2012, 310 hepatocellular carcinoma patients within Hangzhou criteria undergoing resection or liver transplantation were included. Total tumor volume,albumin level, HBV DNA copies and portal hypertension were included for constructing the nomogram.e resection patients were stratified into low- and high-risk groups by the median nomogram score of 116. Independent risk factors were identified and a visually orientated nomogram was constructed using a Cox proportional hazards model to predict the recurrence risk for SR patients.
(Hepatobiliary Pancreat Dis Int 2017;16:480-486)
hepatocellular carcinoma;
surgical resection;
liver transplantation;
nomogram;
recurrence-free survival
Introduction
Globally, hepatocellular carcinoma (HCC) is one of the most common cancers and causes 695 900 deaths annually.[1]Liver transplantation (LT) and surgical resection (SR) are the conventional treatment modalities that can offer long-term survival for patients with HCC.[2,3]However, recurrence is observed in approximately 70% of HCC patients within 5 years and is the primary cause of poor survival aer a surgical curative treatment.
According to the Barcelona Clinic Liver Cancer system, patients with solitary lesions (less than 5 cm in diameter) and well-preserved liver function are good candidates for surgical resection.[8]However, with the improvement in radiological assessment, patient selection, and perioperative management, the resectability and safety of surgical resections has greatly improved.Even in cirrhotic patients, the perioperative mortality of 2%-3% is similar to that reported with LT.[9]It has been shown that resection of HCC is possible in 60% of patients in Asia, and 25%-40% in Western countries.[3,10]
It has been shown that 20%-25% of the surgical population with HCC are both transplantable and resectable.[11]With the criteria expansion, the debate on selecting a first-line treatment for HCC remains open, and the choice is more difficult.[12]Currently, robust evidence to guide decision-making is still lacking.
Nomograms are graphical representations of statistical predictive models that generate numerical probabilities of an event and have been applied to various malignancies.[13-16]e ability of nomograms to generate personalized predictions allows their use in patient counseling and risk stratification. Nomograms have been applied to predict recurrence, survival, and distant metastasis aer various treatments for HCC.[17-20]To our knowledge, no study has been previously reported about a predictive nomogram for HCC recurrence within Hangzhou criteria aer SR. We aimed to construct a simple and clinically relevant nomogram to predict tumor recurrence in patients within the Hangzhou criteria who are undergoing curative SR. We also explored the possibilities of using the nomogram for risk stratification and treatment guidance by comparing recurrence-free survival (RFS) and overall survival (OS) in HCC patients undergoing SR or LT.
Methods
Patient selection and data collection
We retrospectively reviewed the medical records of patients with pathologically proven HCC who underwent SR or LT between September 2003 and December 2012 at our institution.e selection criteria were as follows:1) no extrahepatic tumor or lymph node metastasis; 2)no positive surgical margin upon SR; 3) no perioperative period death; 4) newly diagnosed HCC without any neoadjuvant therapy; and 5) regular follow-up. A total of 525 HCC patients were included in this study. Of 353 SR patients and 172 LT patients, 256 SR and 101 LT were within the Hangzhou criteria. Forty-seven LT patients were excluded with a Child-Pugh class B or C, and 310 patients with Child-Pugh class A were finally selected.All patients were potentially eligible for both SR and LT based on each patient personal choice and financial situation.e study protocol was designed in accordance with the guidelines outlined in theDeclaration of Helsinki.
Development, validation, and risk stratification of the nomogram
A 3-step validation of the nomogram was employed.First, a Harrell concordance index was used to determine its discriminating ability.[22]An internal validation with 100 sets offull bootstrap samples was performed to evaluate the ability of the nomogram to predict RFS for the SR patients. Second, aer grouping patients within quartiles of nomogram scores, a calibration plot was drawn to compare the predicted probabilities of survival and observed survival at 1- and 3-year intervals aer SR.ird, the area under the receiver operating characteristic (ROC) curve of the nomogram in assessing a 3-year RFS aer SR was calculated.e median value of the nomogram scores was used to stratify patients into two groups (low- and high-risk) for further analysis.
Statistical analysis
Categorical data were evaluated by using a Chisquare test and Fisher's exact test. For continuous variables, a Mann-WhitneyUtest was employed.e RFS and OS were examined using the Kaplan-Meier method.A receiver operating characteristic (ROC) analysis was performed to determine the cut-offvalue. All statistical analyses were performed using SPSS 16.0 for Windows(SPSS Inc., Chicago, IL), MedCalc version 11.4 (e MedCalc soware, Mariakerke, Belgium), R 3.1.0 (Lucent Technologies, New Jersey, USA) and SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC). Statistical significance was set atP<0.05.
Results
Characteristics of the patients in the LT and SR groups
A total of 310 HCC patients within the Hangzhou criteria were included in this study. Of these, 256 (82.6%)underwent SR and 54 (17.4%) underwent LT.e characteristics of the patients are summarized in Table 1.e vast majority of the patients were male (n=290, 93.5%),and 86.8% (n=269) were positive for HBsAg. Hepatic cirrhosis was present in 76.1% (n=236) of the patients.e patients in the SR group and LT group were comparable in demographic and baseline characteristics.
Construction and validation of the nomogram
In the patients undergoing SR, candidate predictors that may be linked with tumor recurrence were included in survival analysis.ese factors included age, gender,drinking history, smoking history, HCC family history,diabetes, positive HBsAg status, HBV DNA copy number, hepatic cirrhosis, portal hypertension, ascites, serum biochemistry, serum AFP level, tumor number and total tumor volume (TTV). Serum biochemistries were dichotomized by the normal range and were handled as categorical variables.e optimal cut-offvalue for TTV was determined using an ROC analysis and was 11.5 cm3.Decisions regarding the grouping of the variables were made before the actual modeling.e factors that were significant in predicting RFS aer SR in the final Cox model were the serum albumin level (≤35 g/L and >35 g/L), number of HBV DNA (>1×104copies and ≤1×104copies), TTV (>11.5 cm3and ≤11.5 cm3) and portal hypertension (Table 2).
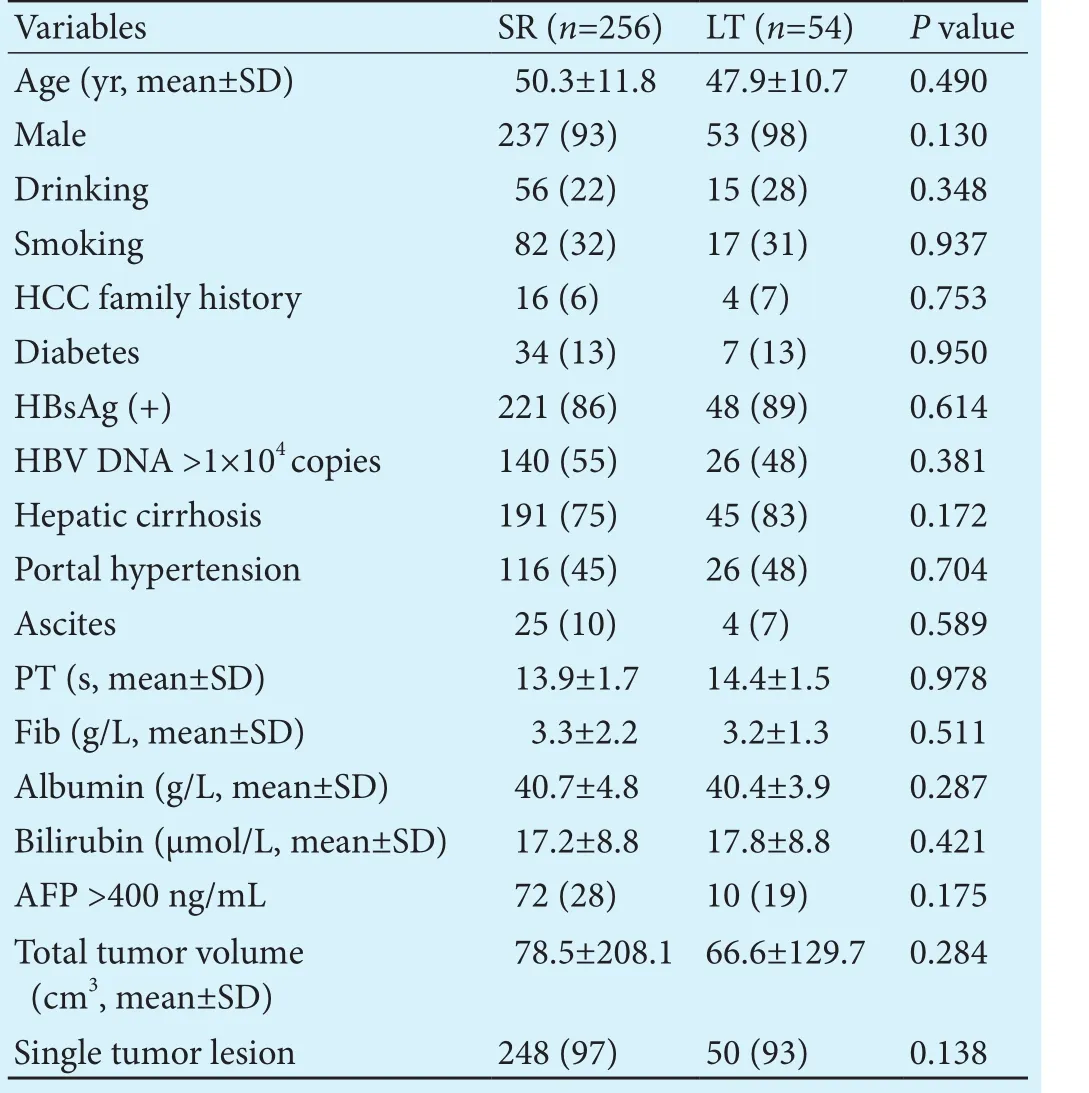
Table 1.Baseline demographics for the HCC patients within the Hangzhou criteria undergoing SR or LT (n, %)

Fig. 1.A: Recurrence-free survival (RFS) for the hepatocellular carcinoma patients undergoing surgical resection (SR) and liver transplantation (LT);B: Overall survival (OS) for the hepatocellular carcinoma patients undergoing SR and LT.
Risk group classification and survival analysis
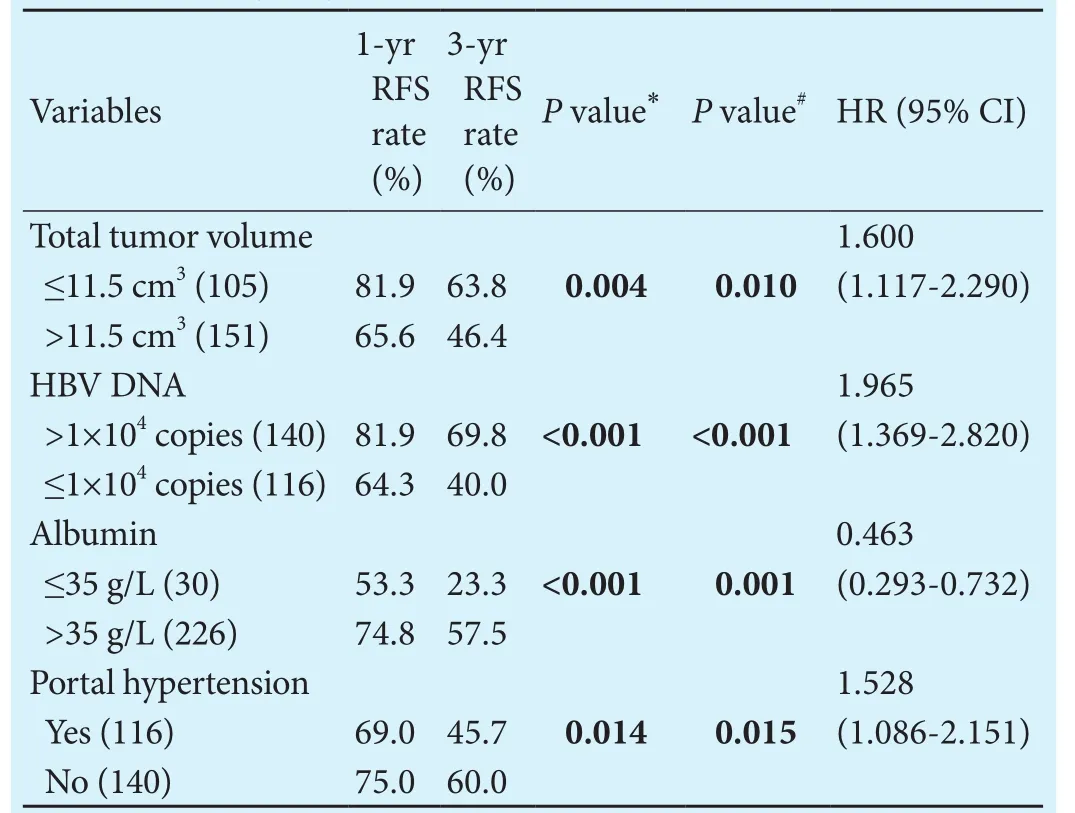
Table 2.Multivariate regression results for recurrence in HCC patients undergoing SR (n=256)

Fig. 2.A: Nomogram predicting recurrence-free survival (RFS)rate for the hepatocellular carcinoma patients within the Hangzhou criteria undergoing surgcal resection;B:e calibration plot of the nomogram;C:e ROC curve of the nomogram in assessing the 3-year RFS rate.
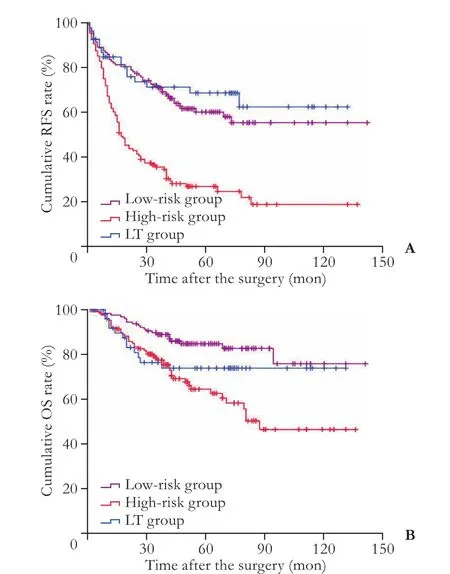
Fig. 3.A: Recurrence-free survival (RFS) for the hepatocellular carcinoma patients undergoing SR in the low-risk, high-risk groups and patients undergoing LT;B: Overall survival (OS) for the hepatocellular carcinoma patients undergoing SR in the lowrisk, high-risk groups and patients undergoing LT.
Discussion
SR and LT are both recommended first-line therapies for patients with early-stage HCC.[8]With the expansion of the surgical criteria for both SR and LT, selection of the appropriate first-line treatment for HCC is controversial due to the lack of adequately powered randomized controlled trials.[23-25]We found that LT within the Hangzhou criteria was associated with a better RFS and similar OS compared with patients undergoing SR which was in concordance with previously published data.[26]It has been shown that patients without HCC recurrence had significantly better survival compared with those with HCC recurrence.[27]It is reasonably postulated that tumor recurrence is crucial to determine the prognosis.erefore, we aimed to develop a clinically relevant method to predict tumor recurrence in patients fulfilling the Hangzhou criteria and undergoing SR.
With a large patient cohort within the Hangzhou criteria undergoing SR for HCC, we constructed a predictive nomogram that was capable of generating a personalized risk evaluation for tumor recurrence aer SR.e easy-to-use graphical tool consisted of ordinary clinical variables, included TTV, serum albumin level, HBV DNA copy number and portal hypertension.ese variables included tumor factors and surrogates for underlying liver conditions. HBV DNA copy number as a predictor could be managed by nucleotide analogue antiviral treatment.e management of HBV DNA copy number might bring hope for HBV-related HCC patients for improved prognosis before surgery. More importantly, the nomogram did not incorporate pathological features and was derived solely from pretreatment clinical variables.ese features enable the nomogram to be an integral part of pretreatment counseling and decision-making.
We further divided these patients by their nomogramscores into two distinct risk groups: low-risk and highrisk. We compared their survival with the patients undergoing LT. Aer dividing the SR patients into two distinct risk groups, LT was still consistently not superior to either group in terms of OS.e option of LT was limited by the stricter requirement for tumor burden, and more importantly, by a lack of donors.[28]As such, SR remains a more viable choice from a cost-effectiveness perspective.[29]In addition to OS, LT was associated with a similar RFS as the low-risk SR patients. Our results indicate that the acceptable long-term survival, lower financial costs, and salvage treatment of LT render SR a favorable treatment option for patients with low-risk recurrence profiles.
However, LT patients had better RFS compared with SR patients in high-risk group. LT is traditionally considered the preferred treatment option for HCC patients with poor liver function.[9]LT offers the potential beneif ts of complete tumor eradication by removing both detectable and undetectable tumor lesions, particularly in patients with an underlying liver disease.[4]For patients with a higher risk of HCC recurrence, it may be of prime importance for patients to receive aggressive therapy to achieve better tumor control and therefore improve longterm survival. Our results imply that the nomogram could serve as a prognostic marker in treatment planning and allocation.
When constructing a nomogram for tumor recurrence, both numbers of recurrences and non-recurrences should be greater than 10 times the number of predictors to reduce the expected error. In this study, the number of non-recurrence was 114, which was 28 times greater than the number of predictors used in the nomogram.We also applied an internal validation with a resampling method by bootstrapping the entire cohort to calibrate the nomogram.ese approaches further increase the validity and data integrity of our results.
We have constructed a clinically relevant and easyto-use nomogram that consistently predicts tumor recurrence aer SR for HCC patients within the Hangzhou criteria. With this nomogram, investigators are able to provide specific information with an individual prognosis and to classify patients undergoing SR into low- and high-risk groups. We further demonstrate that LT offers a better RFS but similar OS compared with high-risk patients undergoing SR. However, LT is associated with a similar RFS and OS, compared with low-risk patients undergoing SR. Due to the heterogeneity of HCC patients within the same clinical stage, this nomogram could serve as a useful instrument to improve treatment selection for HCC patients fulfilling the Hangzhou criteria.Although these results require external validation and further justification from adequately designed trials, our findings support the use of a nomogram for risk stratif ication and the treatment of high-risk patients with LT to achieve less recurrence, which could translate into better quality of life.
Contributors:JN proposed the study. LY, RDY and JN performed the research and wrote the first dra. LY and RDY collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further dras. LY and RDY contributed equally to this article. JN is the guarantor.
Funding:is study was supported by grants from the National Natural Science Foundation of China (81572368), Guangdong Natural Science Foundation (2016A030313278), Science and Technology Planning Project of Guangdong Province, China (2014A020212084)and National Natural Science Youth Foundation of China (81600505).
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D.Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2 Mazzaferro V, Regalia E, Doci R,s Andreola S, Pulvirenti A,Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-699.
3 Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005;40:225-235.
4 Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY,Marsh JW, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol 2008;15:1001-1007.
5 Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-1732.
6 Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma:expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-1403.
7 Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut 2016;65:1035-1041.
8 European Association Fore Study Ofe Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-943.
9 Gomaa AI, Waked I. Recent advances in multidisciplinarymanagement of hepatocellular carcinoma. World J Hepatol 2015;7:673-687.
10 McCormack L, Petrowsky H, Clavien PA. Surgical therapy of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2005;17:497-503.
11 Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G,Hashizume K, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg 2003;238:508-519.
12 Schlachterman A, CraWW Jr, Hilgenfeldt E, Mitra A, Cabrera R. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol 2015;21:8478-8491.
13 Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-1370.
14 Liu H, Shen Z, Wang X, Zhang H, Qin J, Qin X, et al. Increased expression of C-C motif ligand 2 associates with poor prognosis in patients with gastric cancer aer gastrectomy. Tumour Biol 2016;37:3285-3293.
15 Ma B, Li M, Zhang L, Huang M, Lei JB, Fu GH, et al. Upregulation of long non-coding RNA TUG1 correlates with poor prognosis and disease status in osteosarcoma. Tumour Biol 2016;37:4445-4455.
16 Mazouni C, Fina F, Romain S, Bonnier P, Ouafik L, Martin PM.Post-operative nomogram for predicting freedom from recurrence aer surgery in localised breast cancer receiving adjuvant hormone therapy. J Cancer Res Clin Oncol 2015;141:1083-1088.
17 Pan QZ, Wang QJ, Dan JQ, Pan K, Li YQ, Zhang YJ, et al. A nomogram for predicting the benefit of adjuvant cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Sci Rep 2015;5:9202.
18 Li J, Liu Y, Yan Z, Wan X, Xia Y, Wang K, et al. A nomogram predicting pulmonary metastasis of hepatocellular carcinoma following partial hepatectomy. Br J Cancer 2014;110:1110-1117.
19 Shim JH, Jun MJ, Han S, Lee YJ, Lee SG, Kim KM, et al. Prognostic nomograms for prediction of recurrence and survival aer curative liver resection for hepatocellular carcinoma. Ann Surg 2015;261:939-946.
20 Liu PH, Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, et al.When to perform surgical resection or radiofrequency ablation for early hepatocellular carcinoma?: a nomogram-guided treatment strategy. Medicine (Baltimore) 2015;94:e1808.
21 Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, et al.Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2008;14:1107-1115.
22 Jr HF. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001.
23 TaefiA, Abrishami A, Nasseri-Moghaddam S, Eghtesad B,Sherman M. Surgical resection versus liver transplant for patients with hepatocellular carcinoma. Cochrane Database Syst Rev 2013;(6):CD006935.
24 Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K,Durand F, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg 2003;238:885-893.
25 Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434-1440.
26 Li Y, Ruan DY, Yi HM, Wang GY, Yang Y, Jiang N. A three-factor preoperative scoring model predicts risk of recurrence aer liver resection or transplantation in hepatocellular carcinoma patients with preserved liver function. Hepatobiliary Pancreat Dis Int 2015;14:477-484.
27 Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer aer resection: patterns,treatments, and prognosis. Ann Surg 2015;261:947-955.
28 Ribero D, Curley SA, Imamura H, MadoffDC, Nagorney DM,Ng KK, et al. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol 2008;15:986-992.
29 Sarasin FP, Giostra E, Mentha G, Hadengue A. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology 1998;28:436-442.
September 20, 2016
Accepted after revision February 3, 2017
Author Affiliations: Department of Hepatic Surgery and Liver Transplantation Center of theird Affiliated Hospital, Organ Transplantation Institute of Sun Yat-Sen University, Organ Transplantation Research Center of Guangdong Province, Guangzhou 510630, China (Li Y, Zhao H,Wang GY, Yang Y and Jiang N); Department of Medical Oncology (Ruan DY) and Department of Biotherapy (Jia CC), theird Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, China
Nan Jiang, MD, PhD, Department of Hepatic Surgery and Liver Transplantation Center of theird Affiliated Hospital, Organ Transplantation Institute of Sun Yat-Sen University, Organ Transplantation Research Center of Guangdong Province, Guangzhou 510630, China(Tel: +86-20-85252177; Fax: +86-20-85252276; Email: njiang163@163.com)© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60052-3
Published online August 8, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy
- Prospective comparison of prophylactic antibiotic use between intravenous moxifloxacin and ceftriaxone for high-risk patients with post-ERCP cholangitis
- Helicobacter pyloriand 17β-estradiol induce human intrahepatic biliary epithelial cell abnormal proliferation and oxidative DNA damage
- Tailored pancreatic reconstruction after pancreaticoduodenectomy: a single-center experience of 892 cases
- Comparative study of the effects of terlipressin versus splenectomy on liver regeneration after partial hepatectomy in rats
- The “Colonial Wig” pancreaticojejunostomy:zero leaks with a novel technique for reconstruction after pancreaticoduodenectomy
