Perioperative fluid management in major hepatic resection: an integrative review
2017-10-09OsamuYoshinoMarcosViniciusPeriniChristopherChristophiandLaurenceWeinberg
Osamu Yoshino, Marcos Vinicius Perini, Christopher Christophi and Laurence Weinberg
Melbourne, Australia
Perioperative fluid management in major hepatic resection: an integrative review
Osamu Yoshino, Marcos Vinicius Perini, Christopher Christophi and Laurence Weinberg
Melbourne, Australia
BACKGROUND: Fluid intervention and vasoactive pharmacological support during hepatic resection depend on the preference of the attending clinician, institutional resources,and practice culture. Evidence-based recommendations to guide perioperative fluid management are currently limited.erefore, we provide a contemporary clinical integrative overview of the fundamental principles underpinning fluid intervention and hemodynamic optimization for adult patients undergoing major hepatic resection.
DATA SOURCES: A literature review was performed of MEDLINE, EMBASE and the Cochrane Central Registry of Controlled Trials using the terms “surgery”, “anesthesia”, “starch”,“hydroxyethyl starch derivatives”, “albumin”, “gelatin”, “liver resection”, “hepatic resection”, “fluids”, “fluid therapy”, “crystalloid”,“colloid”, “saline”, “plasma-Lyte”, “plasmalyte”, “hartmann's”, “acetate”, and “lactate”. Search results for MEDLINE and EMBASE were additionally limited to studies on human populations that included adult age groups and publications in English.
RESULTS: A total of 113 articles were included aer appropriate inclusion criteria screening. Perioperative fluid management as it relates to various anesthetic and surgical techniques is discussed.
CONCLUSIONS: Clinicians should have a fundamental understanding of the surgical phases of the resection, hemodynamic goals, and anesthesia challenges in attempts to individualize therapy to the patient's underlying pathophysiological condition.erefore, an ideal approach for perioperative fluid therapy is always individualized. Planning and designing large-scale clinical trials are imperative to define the optimal type and amount offluid for patients undergoing major hepatic resection. Further clinical trials evaluating different in-traoperative goal-directed strategies are also eagerly awaited.
(Hepatobiliary Pancreat Dis Int 2017;16:458-469)
hepatic resection;
liver resection;
fluid therapy;
anesthesia;
crystalloid;
colloid;
goal-directed therapy
Introduction
Hepatic resection is a well-established treatment for various liver pathologies with a morbidity rate of up to 25% and reported mortality of up to 5%;[1-3]however, major blood loss and subsequent blood transfusion are common.[4-7]Perioperative fluid intervention and vasoactive pharmacological support during major hepatic resection depend on the preference of the attending clinician, institutional resources, and practice culture.e scientific literature provides little evidence-based guidance regarding the amount (quantitative fluid intervention) or type (qualitative fluid intervention) offluid to optimize outcomes during major hepatic resection. Judicious fluid management is an important strategy to minimize blood loss during hepatic resection;[4]however, there are no guidelines for optimal care.erefore, perioperative fluid management and hemodynamic optimization need to be individualized to account for patient factors and the complexity of surgery.We provide a contemporary integrative overview of the fundamental principles underpinning fluid intervention and hemodynamic optimization for adult patients undergoing major hepatic resection.
Search strategies and results
A literature review was performed of MEDLINE, EMBASE, ande Cochrane Central Registry of ControlledTrials from January 1965 to December 2016 with the following keywords: “surgery”, “anesthesia”, “starch”, “hydroxyethyl starch derivatives”, “albumin”, “gelatin”, “liver resection”, “hepatic resection”, “fluids”, “fluid therapy”,“crystalloid”, “colloid”, “saline”, “plasma-Lyte”, “plasmalyte”, “hartmann's”, “acetate”, and “lactate”. Search results for MEDLINE and EMBASE were additionally limited to studies on human populations that included adult age groups and publications in English. Only published titles and abstracts evaluating the fluid intervention with relevance to major hepatic resection were included.ree authors conducted the search and data extraction(YO, PMV and WL). Two authors analyzed the results(YO and WL). A two-stage process was used for study selection. First, two review authors (YO and WL) independently screened the titles and, if available, the abstracts of the search results to determine if a study met the inclusion criteria. We defined major hepatic resection as the removal of three or more segments and only included papers covering minor hepatic resection if they had specific implications for fluid intervention.Each article was classified as follows: duplicate of another citation, unclear, included, or excluded. References given in the publications were manually assessed for further inclusion in this study. Disagreements were assessed by a third author (PMV) and resolved by consensus. Authors had access to full-text original papers.
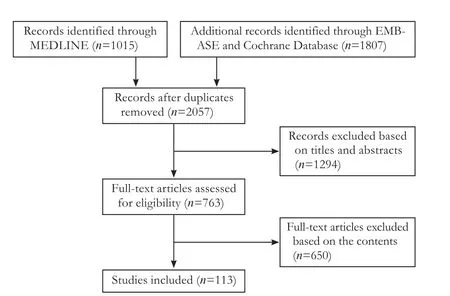
Fig. 1. Study selection offlow diagram.
Major blood loss during hepatic resection: a changing landscape
A study by Foster from nearly 40 years ago evaluated patients undergoing major hepatic resection and reported a mortality rate of greater than 20%.[3]e leading cause of death was hemorrhage directly related to hepatic parenchymal transection resulting in massive intraoperative and postoperative exsanguination. Over the last two decades, blood loss associated with hepatic resection has significantly decreased, with intraoperative losses of less than 500 mL now commonly reported in high-volume centers[8-10]and mortality rates as low as 0.[9]ere is a strong association between intraoperative blood loss and major morbidity and in-hospital mortality; however, intraoperative blood loss during hepatocellular carcinoma resection is also an independent prognostic factor for tumor recurrence and long-term disease survival.[5]As the numbers of surgical techniques and innovative strategies to minimize blood loss improve, the leading causes of death in major hepatic resection continue to shiaway from massive perioperative exsanguination and toward hepatic failure and infectious complications.[11-15]Notably,post-surgical pulmonary complications are also common and include pleural effusion, atelectasis, pneumonia, and pulmonary congestion.[9,13,16]e development of these complications may be directly caused or adversely impacted by improper fluid management.[17]
Surgical techniques that impact blood loss and fluid intervention
Hepatic resection can be broadly divided into three surgical phases.e first phase involves mobilization and control of inflow and outflow. During this stage, major hemorrhage is usually uncommon; however, massive blood loss from unexpected vascular injury can be profound, and clinicians should always be prepared for rapid and aggressive fluid resuscitation. Hypotension can occur if the liver pushes against the vena cava, which impairs venous return to the heart and is reversible upon returning the liver to its normal anatomical position.e second phase involves parenchymal resection beginning with the transection of the liver parenchyma and concluding when the resection is completed. If a vascular occlusion strategy is applied, sudden changes in circulation volume may significantly influence fluid and anesthetic management. Furthermore, pharmacological support, including inotrope with or without fluid intervention, may require maintaining organ perfusion pressure. Intraoperative hemorrhage primarily occurs in this phase. Strategies involved in inflow and outflow control include the Pringle maneuver, liver exclusion, total or partial clamping of the interior vena cava and theuse of low central venous pressure (CVP) anesthesia.e reverse Trendelenburg position and the use of intraoperative cell salvage may be utilized.[18]Finally, the third phase involves surgical hemostasis and closure. Blood loss minimization and blood transfusion avoidance are vital during each stage to prevent perioperative morbidity and mortality.[19-21]e amount of blood loss further dictates the type and amount offluid therapy required.A detailed discussion between the anesthesiologist and surgeon is paramount prior to surgery.is discussion should outline the nature of the resection, the quality of the underlying liver (cirrhosis, steatosis, cholestasis, etc.),the surgical plan, the need for clamping, and techniques to be employed to minimize bleeding.
Various vaso-occlusive surgical techniques have been performed in major hepatic surgery to reduce perioperative bleeding. In 1908, Pringle performed a hepatic inflow occlusion technique (Pringle maneuver) to minimize hepatic hemorrhage.[22]Pringle described that clamping the hepatoduodenal ligament leads to interrupted blood flow to the liver through the hepatic artery and portal vein.Currently, this technique is commonly used for major hepatic resection as well as liver trauma where significant hemorrhage occurs.e maneuver can be performed continuously or intermittently to reduce blood loss.e Pringle maneuver can be oen performed for 15-20 minutes continuously with a clamp-free time of 5-10 minutes(even with impaired liver function) without increasing hepatic complications such as ischemic hepatic failure.[23-25]Generally, the total length of the maneuver in the majority of major liver centers is reported to require less than 45 minutes.[26,27]Clearly, the length of the Pringle maneuver should be adjusted by surgeons according to the quality and quantity of the liver.
Common techniques for vascular inflow control in addition to the Pringle maneuver include i) selective inflow occlusion (with or without hilar dissection), ii) segmental liver occlusion, and iii) total vascular exclusion,which can be o ff ered in patients with hepatic venous bleeding aer inflow control. For right or lehepatic resection, hilar control with or without hilar dissection can be performed. Inflow control can be performed more selectively aer the preparation of the hilum. In addition,an extraglissonian approach has been demonstrated to be safe and useful in major hepatic resections, in particular laparoscopic hepatic resection.[28-30]A selective clamping approach avoids ischemia to the other hemi-liver and is associated with stable hemodynamics. An infrahepatic vena cava clamp represents another potentially useful technique. A reduction in the inflow to the vena cava potentially prevents hemorrhage from the hepatic veins;however, this technique has not been shown to reduce intraoperative blood loss.[31]
Laparoscopic hepatic resection was first reported more than two decades ago.[32]New devices (staplers/retractors) and energy sources (LigaSure®, Harmonic®,underbeat®) have contributed to reductions in blood loss during parenchymal transection. During laparoscopic surgery, pneumoperitoneum can reduce venous blood pressure, leading to less intraoperative blood loss.Laparoscopic surgery is associated with less blood loss and pain, shorter ICU stay and hospital stay, and fewer complications;[33-35]however, superiority in major hepatic resection is unproven. Laparoscopic hepatic resection will likely represent the mainstay for treating non-vascular encasing liver tumors (tumors invading the interior vena cava, hepatic veins and portal veins requiring graing) in the future. Nevertheless, a limited number of experienced liver surgeons are currently performing laparoscopic major hepatic resections, and there appears to be few differences compared with short-term outcomes in high-volume centers.[36]e principles of surgery and anesthesia for laparoscopic surgery are identical to those of open hepatic resection and are discussed elsewhere in this review.
Non-anatomical resection can be performed for various purposes, oen for colorectal metastasis, as a streamlining component of a parenchyma sparing strategy, in addition to radiofrequency ablation or trans-arterial radioembolization (selective internal radiotherapy), or in repeated hepatic resection.[37]Whether non-anatomical resection or anatomical resection is superior in terms of long-term outcome remains controversial. However,recent studies[37-41]reported that non-anatomical resection is associated with reduced blood transfusion, more future remanent liver, and shorter hospital stay; notably,non-anatomical resection was associated with comparable disease-free and overall survival.
Anesthesia techniques to reduce perioperative blood loss
Anesthesia considerations to minimize bleeding and reduce portal pressures during hepatic resection include reverse Trendelenburg positioning, restrictive fluid intervention, low CVP or high stroke volume variation anesthesia, venodilatation using glyceryl trinitrate, autologous venesection with normovolemic hemodilution,the use of diuretics to decrease blood volume, the use of vasoactive medications to support mean arterial pressure, and the use of intraoperative cell salvage.[18]Patients should be managed on an individualized basis. No single approach will be effective in all cases; thus, understand-ing the principles offluid intervention and maintaining hemodynamic stability are important.
Fluid intervention for major hepatic resection
More recently, a larger clinical trial assessed 78 critically ill trauma patients resuscitated with sodium acetate as an alternative to 0.9% saline or lactated ringers.e patients receiving acetate had stable biochemical and hematological profiles without evidence of hemodynamic instability. Normalization of hyperchloremia and metabolic acidosis occurred faster in the patients who received acetate.[51]Further, acetate metabolism is not dependent on age.[52]Acetate may also prevent malnutrition by acting as a fat substitute or oxidative fuel without causing hyperglycemia or affecting glucose homeostasis.[53,54]Finally, the metabolism of acetate does not affect glucose or insulin homeostasis,[55]whereas the administration of Hartmann's solution can result in hyperglycemia as a result of lactate conversion to glucose via gluconeogenesis.[56]In diabetic patients, intraoperative glucose levels have been reported to double following the administration of exogenous lactate solutions.[55]
Avoidance of hyperchloremia in general surgical and ICU patients: implications for major hepatic resection
4.2.2 目标不在视野范围内 解决办法:(1)血细胞计数盘的划线区域一定要对准物镜头;(2)较少的标本,涂片区域较小,观察时要注意不要超出涂片范围。
Fluid intervention studies for patients undergoing hepatic resection
Fluid administration can also impact surgical outcomes in patients undergoing hepatic resection.[42,43]Two recent studies evaluating fluid intervention in major hepatic resection supported the notion that lactate in Hartmann's solution can independently increase lac-tatemia.[64,65]A randomized controlled trial involving 104 donors undergoing right hepatic resection compared acid base status, lactate concentrations, and liver function tests in patients who received an acetate- or lactate-buffered solution.[65]e acetate-buffered solution resulted in lower lactate and bilirubin levels, lower and prothrombin times, and higher albumin levels compared to patients receiving Hartmann's solution.is single-center study reported no significant differences in complications or hospital stay duration between groups. However, a recent multicenter randomized study in patients receiving major hepatic resection[64]found that an acetate-buffered solution had improved biochemical and hematological profiles (acid base homeostasis, electrolyte balance, and coagulation status) as well as fewer complications and reduced hospital stay duration. Finally, McFarlane and Lee compared the preoperative and postoperative acid base status of patients who received either normal saline or plasma-Lyte 148 while undergoing major hepatobiliary or pancreatic surgery.[66]Consistent with the above studies,the patients who received normal saline intraoperatively were more hyperchloremic and acidemic compared to those who received plasma-Lyte 148.e most favorable crystalloid solution for patients undergoing major hepatic surgery remains unknown.[67]
Physiochemical considerations for the choice offluids for major hepatic resection
Hepatic resection can impose a major pathophysiological insult on the patient; thus, the volume and type offluid intervention depends on the presence and severity of biochemical derangements and coagulopathy. Severe biochemical derangements are common and include metabolic acidosis and hyperlactatemia. Massive blood transfusion results in large volumes of administered citrated blood and the development of metabolic acidosis.Liver function to metabolize citrate is limited or compromised following major hepatic resection; therefore, citrate intoxication can occur with resultant hypocalcemia.Ionized calcium levels must be frequently monitored,and calcium chloride should be administered when appropriate.
Fluid intervention for the management of coagulopathy is beyond the scope of this review. However, there are multiple causes of coagulopathy during hepatic resection.ese include pre-existing coagulopathy due to chronic hepatic insufficiency and reduced synthesis of clotting factors, as well as coagulopathy from hemodilution and massive blood transfusion. Hypothermia slows enzymatic reactions, prolongs factor reaction time, and reduces platelet aggregation.e liver plays a vital function in acid/base regulation, and severe metabolic derangements during major resections are common and frequently profound.e choice offluid should take into account the important role that the liver plays in the maintenance of acid base homeostasis. Clinicians should have a fundamental understanding of the physiochemical properties of the available intravenous solutions to individualizetherapy to the patient's underlying pathophysiological condition.e physiochemical compositions of the commonly available crystalloid solutions used in hepatic resection are summarized in Table.

Table.Physiochemical properties of the common crystalloid solutions
Colloids and hepatic resection
Albumin is a common colloid solution that is frequently used in patients undergoing major surgery.Advantages specific to hepatic resection include the maintenance of colloid osmotic pressure, the preservation of renal function, pleiotropic physiological benefits on endothelial integrity, improved endothelial integrity by substantially protecting the glycocalyx of endothelial cells,[86,87]the facilitation of negative fluid balance in the hypoproteinemia states that are common in transplant patients, and the maintenance of glomerular filtration via hemodynamic and oncotic mechanisms.[88-90]Biological plausibility, freedom from nephrotoxicity (safety), and reductions in renal morbidity in liver cirrhosis (effectiveness) support the use of albumin in hepatic resection surgery.[91,92]In contrast to other colloids, fluid resuscitation with human albumin is not considered nephrotoxic.Albumin has been extensively reappraised as a resuscitation fluid by Finfer.[93]Available as iso-oncotic andiso-osmolar 4%-5% solutions and hyperoncotic 20%-25% solutions, albumin is suspended in saline containing octanoate as an anion stabilizer. Albumin remains an attractive colloid for hepatic resection with 4%-5% albumin expanding plasma by an amount equal to that of the volume infused and concentrated albumin expanding plasma by 4-5-fold the volume infused.[93]e SAFE study randomized 7000 patients requiring fluid resuscitation in the ICU to albumin or 0.9% saline and reported no difference in mortality between the groups.[74]However, a subgroup analysis reported that albumin maybe harmful in the setting of traumatic brain injury.[94]erefore, large volumes of albumin should be used cautiously in fulminant liver transplant recipients with raised intracranial pressure.e pooled analysis of mortality data from large studies of volume therapy with human albumin in sepsis, namely SAFE and ALBIOS, confirm that albumin administration could reduce mortality in severe sepsis or patients with septic shock.[74,95]In the ALBIOS study,patients in the albumin group not only achieved more frequent hemodynamic stabilization but also had prognostically favorable negative fluid balance.[95]In a meta-analysis by Patel et al, albumin was reported to be safe in the setting of critical illness.[96]
Goal-directed therapy and hepatic resection
In 2009, Kim et al evaluated the relationship between bleeding and CVP during major hepatic resection and identified factors associated with blood loss during hepatic resection.[98]No strong association was observed,and CVP was not shown to be a useful hemodynamic modifiable parameter in preventing or predicting surgical bleeding. In contrast, a recent review and meta-analysis of eight randomized trials evaluated the effects of CVP on clinical outcomes and reported that low CVP during hepatic resection was associated with reduced intraoperative bleeding.[99]Moggia et al[100]performed the largest network meta-analysis to date evaluating methods to decrease blood loss during hepatic resection.ey stated that there was low-quality evidence to suggest that blood transfusion is lower with acute normovolemic hemodilution combined with low CVP compared to low CVP alone.e application of acute normovolemic hemodilution combined with low CVP in hepatic resection has also been shown to reduce intraoperative blood loss and blood transfusions in recent small clinical trials.[101]e dogma that the CVP reflects the volume of circulation is misleading because there is no proven association between CVP and right-ventricular end-diastolic pressure and fluid responsiveness.[102,103]Nevertheless, CVP remains one of the most traditional physiological parameters to reduce intraoperative bleeding during major hepatic resection.[4]e maintenance of low CVP (less than 5 cmH2O in the authors' institution) during hepatic parenchymal resection has been associated with decreased hemorrhage and subsequent blood loss leading to a reduced blood transfusion amount. Some centers advocate specific anesthesia techniques to reduce CVP; however,none of these assertions are evidence-based.ese techniques include the reverse Trendelenburg position to decrease portal venous congestion and a restrictive fluid therapy with low CVP.
Pharmacological therapies, e.g., glyceryl trinitrate(venodilatation) or diuresis with furosemide or mannitol(decreased intravascular blood volume), autologous venesection (decreased intravascular blood volume) with return of blood post-hepatic resection, and autologous venesection with normovolemic hemodilution,[104]have also been employed to reduce bleeding. Generally, a volume reduction of this magnitude can be tolerated if a change in the mean arterial pressure is within 20% of the patient's baseline blood pressure and the cardiac index is greater than 2.0 L/min/m2.e use of vasoconstrictors,e.g., metaraminol, phenylephrine, or noradrenaline to support mean arterial pressure is oen required. Autologous venesection with controlled hypovolemia prior to hepatic resection appears feasible and safe; however,comparative studies determining its effectiveness are needed.[105]
In addition to low CVP anesthesia, the goal-directed therapy algorithm used in the authors' institution is presented in Fig. 2.is hemodynamic optimization protocol takes into consideration the patient's volume responsiveness (using stroke volume variation), their mean arterial pressure, and whether the cardiac index is low,normal, or high. Stroke volume variation is an indicator offluid responsiveness measured with a standardized algorithm using pulse contour analysis in mechanically ventilated patients. Stroke volume variation may be used as a continuous preload variable allowing for optimal fluid management during hepatic resection.
Other high-volume centers have recently published algorithms utilizing stroke volume variation to guide fluid management during major hepatic resection.[4]Emerging research explores the role of dynamic metrics of volume responsiveness (such as stroke volume variation)as simple and sensitive indicators for evaluating fluid responsiveness and predicting intraoperative blood loss.Similar to our institution's experience, other larger hepatobiliary units also advocate that stroke volume variationcan be maintained at 10%-20% with fluid restriction and the optional administration of diuretics during major hepatic resection.[4]
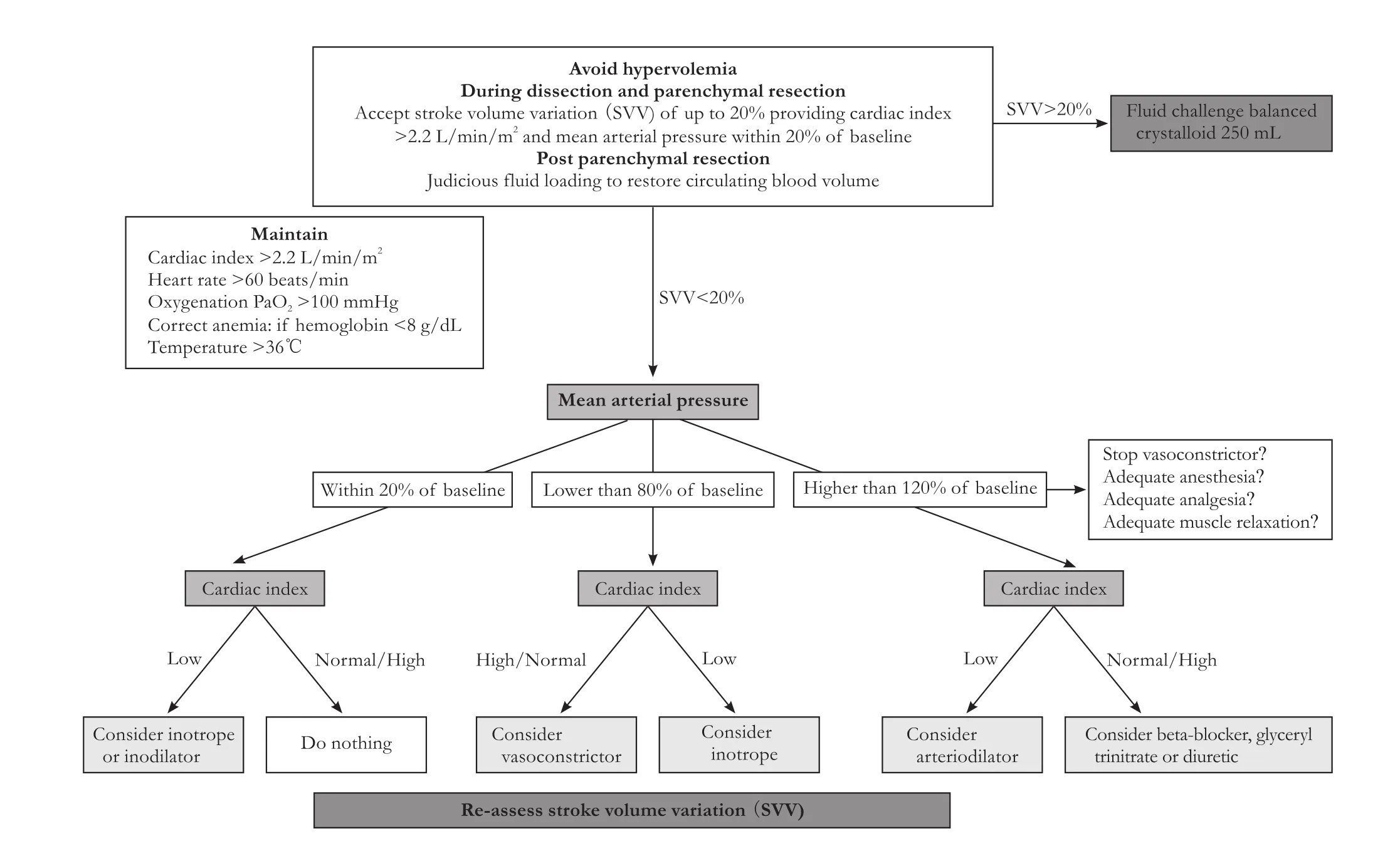
Fig. 2.Goal-directed therapy protocol used at the authors' institution for patients undergoing major hepatic resection.
Conclusions
Fluid prescription and intervention for major hepatic resection are complex.ere are physiologically rational and biologically plausible data to suggest that certain fluids may confer metabolic, organ, and outcome benefits.However, there is currently no substantial evidence to support or refute the use of any fluid in this setting. Similarly, there is no perfect goal-directed therapy protocol or algorithm delineating the optimum amount offluid intervention needed or the type of vasoactive theories that support fluid intervention during major hepatic resection. Clinicians should have a fundamental understanding of the surgical phases of the resection and should understand the hemodynamic goals and anesthesia challenges to individualize therapy to the patient's underlying pathophysiological condition because perioperative management is substantially dependent on anesthetic and surgical strategies.erefore, the ideal approach for perioperative fluid therapy is always individualized and involves a qualitative and quantitative approach: i) the right amount offluid ii) with a favorable physiochemical composition iii) administered at the right time iv) at the right rate, and most importantly, fluid that is v) individualized to both the patient's physiological state and guided by a surgery-specific hemodynamic algorithm. Further large-scale clinical trials to define the optimal type, dose,and amount offluid for patients undergoing major hepatic resection are imperative. Further clinical trials evaluating different intraoperative goal-directed strategies are also eagerly awaited.
Acknowledgement:We thank Dr. Sarah Anthony for her proof-reading.
Contributors:CC proposed the study. YO, PMV and WL conducted the search and data extraction. YO and WL analyzed the results and wrote the first dra. All authors reviewed and contributed to further dras and revision. YO is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, et al.Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford)2009;11:510-515.
2 Blumgart LH, Belghiti J. Surgery of the liver, biliary tract, and pancreas, 4th ed. Philadelphia, PA: Saunders Elsevier;2007.
3 Foster JH. Survival aer liver resection for secondary tumors.Am J Surg 1978;135:389-394.
4 Choi SS, Kim SH, Kim YK. Fluid management in living donor hepatectomy: Recent issues and perspectives. World J Gastroenterol 2015;21:12757-12766.
5 Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, et al.Operative blood loss independently predicts recurrence and survival aer resection of hepatocellular carcinoma. Ann Surg 2009;249:617-623.
6 Kusano T, Sasaki A, Kai S, Endo Y, Iwaki K, Shibata K, et al.Predictors and prognostic significance of operative complications in patients with hepatocellular carcinoma who underwent hepatic resection. Eur J Surg Oncol 2009;35:1179-1185.
7 Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH,et al. Intraoperative blood loss is a risk factor for complications in donors aer living donor hepatectomy. Liver Transpl 2006;12:950-957.
8 Aramaki O, Takayama T, Higaki T, Nakayama H, Ohkubo T,Midorikawa Y, et al. Decreased blood loss reduces postoperative complications in resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2014;21:585-591.
9 Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y,Sano K, et al. One thousand fiy-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198-1206.
10 Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M,Cavallari A, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg 2001;234:71-78.
11 Palavecino M, Kishi Y, Chun YS, Brown DL, Gottumukkala VN, Lichtiger B, et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1557 consecutive liver resections. Surgery 2010;147:40-48.
12 Huang ZQ, Xu LN, Yang T, Zhang WZ, Huang XQ, Cai SW, et al. Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl)2009;122:2268-2277.
13 Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G,Ravaioli M, et al. Trends in perioperative outcome aer hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg 2009;249:995-1002.
14 Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival aer hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-135.
15 Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 2006;244:897-908.
16 Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al.Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698-710.
17 Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L,Little S, et al. Improvement in perioperative outcome aer hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg 2002;236:397-407.
18 Miller RD. Miller's anesthesia, 6th ed. New York: Elsevier/Churchill Livingstone; 2005.
19 Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al.Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-330.
20 Jamieson GG, Corbel L, Campion JP, Launois B. Major liver resection without a blood transfusion: is it a realistic objective?Surgery 1992;112:32-36.
21 Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR,Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860-870.
22 Pringle JH. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908;48:541-549.
23 Ishizaki Y, Yoshimoto J, Miwa K, Sugo H, Kawasaki S. Safety of prolonged intermittent pringle maneuver during hepatic resection. Arch Surg 2006;141:649-654.
24 Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg 2006;244:921-930.
25 Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg 1999;229:369-375.
26 Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704-713.
27 Scatton O, Zalinski S, Jegou D, Compagnon P, Lesurtel M,Belghiti J, et al. Randomized clinical trial of ischaemic preconditioning in major liver resection with intermittent Pringle manoeuvre. Br J Surg 2011;98:1236-1243.
28 Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H,Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-629.
29 Launois B, Jamieson GG.e posterior intrahepatic approach for hepatectomy or removal of segments of the liver. Surg Gynecol Obstet 1992;174:155-158.
30 Herman P, Perini MV, Coelho F, Saad W, D'Albuquerque LA.Half-Pringle maneuver: a useful tool in laparoscopic liver resection. J Laparoendosc Adv Surg Tech A 2010;20:35-37.
31 Kato M, Kubota K, Kita J, Shimoda M, Rokkaku K, Sawada T.Effect of infra-hepatic inferior vena cava clamping on bleeding during hepatic dissection: a prospective, randomized, controlled study. World J Surg 2008;32:1082-1087.
32 Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-958.
33 Belli G, Fantini C, D'Agostino A, CioffiL, Langella S, Russolillo N, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc 2007;21:2004-2011.
34 Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 2003;138:763-769.
35 Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348-356.
36 Medbery RL, Chadid TS, Sweeney JF, Knechtle SJ, Kooby DA,Maithel SK, et al. Laparoscopic vs open right hepatectomy: a value-based analysis. J Am Coll Surg 2014;218:929-939.
37 Matsuki R, Mise Y, Saiura A, Inoue Y, Ishizawa T, Takahashi Y.Parenchymal-sparing hepatectomy for deep-placed colorectal liver metastases. Surgery 2016;160:1256-1263.
38 Lalmahomed ZS, Ayez N, van der Pool AE, Verheij J, IJzermans JN, Verhoef C. Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg 2011;35:656-661.
39 Memeo R, de Blasi V, Adam R, Goere D, Azoulay D, Ayav A, et al. Parenchymal-sparing hepatectomies (PSH) for bilobar colorectal liver metastases are associated with a lower morbidity and similar oncological results: a propensity score matching analysis. HPB (Oxford) 2016;18:781-790.
40 Stewart GD, O'Suilleabhain CB, Madhavan KK, Wigmore SJ,Parks RW, Garden OJ.e extent of resection influences outcome following hepatectomy for colorectal liver metastases.Eur J Surg Oncol 2004;30:370-376.
41 Imamura H, Seyama Y, Kokudo N, Aoki T, Sano K, Minagawa M, et al. Single and multiple resections of multiple hepatic metastases of colorectal origin. Surgery 2004;135:508-517.
42 Kim Y, Ejaz A, Gani F, Wasey JO, Xu L, Frank SM, et al. Crystalloid administration among patients undergoing liver surgery: Defining patient- and provider-level variation. Surgery 2016;159:389-398.
43 Lilot M, Ehrenfeld JM, Lee C, Harrington B, Cannesson M,Rinehart J. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. Br J Anaesth 2015;114:767-776.
44 Zander R. Fluid management. Second edition ed. Germany:Die Deutsche Bibliothek;2015:71.
45 Watanabe I, Mayumi T, Arishima T, Takahashi H, Shikano T,Nakao A, et al. Hyperlactemia can predict the prognosis of liver resection. Shock 2007;28:35-38.
46 Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med 2009;37:2827-2839.
47 Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg 1996;171:221-226.
48 Kveim M, Nesbakken R. Utilization of exogenous acetate during canine haemorrhagic shock. Scand J Clin Lab Invest 1979;39:653-658.
49 Mudge GH, Manning JA, Gilman A. Sodium acetate as a source offixed base. Proc Soc Exp Biol Med 1949;71:136-138.
50 Hamada T, Yamamoto M, Nakamaru K, Iwaki K, Ito Y, Koizumi T.e pharmacokinetics of D-lactate, L-lactate and acetate in humans. Masui 1997;46:229-236.
51 McCague A, Dermendjieva M, Hutchinson R, Wong DT, Dao N. Sodium acetate infusion in critically ill trauma patients for hyperchloremic acidosis. Scand J Trauma Resusc Emerg Med 2011;19:24.
52 Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA.Plasma acetate turnover and oxidation. J Clin Invest 1979;64:708-713.
53 Akanji AO, Bruce MA, Frayn KN. Effect of acetate infusion on energy expenditure and substrate oxidation rates in non-diabetic and diabetic subjects. Eur J Clin Nutr 1989;43:107-115.
54 Akanji AO, Hockaday TD. Acetate tolerance and the kinetics of acetate utilization in diabetic and nondiabetic subjects. Am J Clin Nutr 1990;51:112-118.
56 Arai K, Kawamoto M, Yuge O, Shiraki H, Mukaida K, Horibe M, et al. A comparative study of lactated Ringer and acetated Ringer solution as intraoperative fluids in patients with liver dysfunction. Masui 1986;35:793-799.
57 McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L,Tait G, Beattie WS. Hyperchloremia aer noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg 2013;117:412-421.
58 Yunos NM, Bellomo R, Bailey M. Chloride-restrictive fluid administration and incidence of acute kidney injury--reply.JAMA 2013;309:543-544.
59 Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg 2015;102:24-36.
60 Young P, Bailey M, Beasley R, Henderson S, Mackle D,McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA 2015;314:1701-1710.
61 Reddy SK, Young PJ, Beasley RW, Mackle DM, McGuinness SP, McArthur CJ, et al. Overview of the study protocols and statistical analysis plan for the Saline versus Plasma-Lyte 148 for Intravenous Fluiderapy (SPLIT) research program. Crit Care Resusc 2015;17:29-36.
62 Ince C, Groeneveld AB.e case for 0.9% NaCl: is the undefendable, defensible? Kidney Int 2014;86:1087-1095.
63 Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay offluid resuscitation to prevent ‘pre-renal' acute kidney injury?: con. Kidney Int 2014;86:1096-1105.
64 Weinberg L, Pearce B, Sullivan R, Siu L, Scurrah N, Tan C, et al.e effects of plasmalyte-148 vs. Hartmann's solution during major liver resection: a multicentre, double-blind, randomized controlled trial. Minerva Anestesiol 2015;81:1288-1297.
65 Shin WJ, Kim YK, Bang JY, Cho SK, Han SM, Hwang GS. Lactate and liver function tests aer living donor right hepatectomy: a comparison of solutions with and without lactate. Acta Anaesthesiol Scand 2011;55:558-564.
66 McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia 1994;49:779-781.
67 Weinberg L, Pearce B, Bellomo R. Plasma-Lyte or Hartmann's for major liver resection: which is the best solution? Minerva Anestesiol 2016;82:123-124.
68 Mukhtar A, Aboulfetouh F, Obayah G, Salah M, Emam M,Khater Y, et al.e safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesth Analg 2009;109:924-930.
69 Zhou ZB, Shao XX, Yang XY, Zhang T, Xian DF, Huang CY, et al. Influence of hydroxyethyl starch on renal function aer orthotopic liver transplantation. Transplant Proc 2015;47:1616-1619.
70 Hand WR, Whiteley JR, Epperson TI, Tam L, Crego H, Wolf B, et al. Hydroxyethyl starch and acute kidney injury in orthotopic liver transplantation: a single-center retrospective review.Anesth Analg 2015;120:619-626.
71 Bagshaw SM, Chawla LS. Hydroxyethyl starch for fluid resuscitation in critically ill patients. Can J Anaesth 2013;60:709-713.
72 Haase N, Perner A, Hennings LI, Siegemund M, Lauridsen B, Wetterslev M, et al. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ 2013;346:f839.
73 Haase N, Perner A. Hydroxyethyl starch for resuscitation. Curr Opin Crit Care 2013;19:321-325.
74 Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367:1901-1911.
75 Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124-134.
76 Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL,McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013;309:678-688.
77 Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 2013;2:CD000567.
78 Dart AB, Mutter TC, Ruth CA, Taback SP. Hydroxyethyl starch(HES) versus other fluid therapies: effects on kidney function.Cochrane Database Syst Rev 2010;1:CD007594.
79 Mutter TC, Ruth CA, Dart AB. Hydroxyethyl starch (HES)versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev 2013;7:CD007594.
80 Niemi TT, Suojaranta-Ylinen RT, Kukkonen SI, Kuitunen AH. Gelatin and hydroxyethyl starch, but not albumin, impair hemostasis aer cardiac surgery. Anesth Analg 2006;102:998-1006.
81 Konrad C, Markl T, Schuepfer G, Gerber H, Tschopp M.e effects of in vitro hemodilution with gelatin, hydroxyethyl starch, and lactated Ringer's solution on markers of coagulation:an analysis using SONOCLOT. Anesth Analg 1999;88:483-488.
82 Hartog CS, Reuter D, Loesche W, Hofmann M, Reinhart K. Influence of hydroxyethyl starch (HES) 130/0.4 on hemostasis as measured by viscoelastic device analysis: a systematic review.Intensive Care Med 2011;37:1725-1737.
83 Casutt M, Kristoffy A, Schuepfer G, Spahn DR, Konrad C.Effects on coagulation of balanced (130/0.42) and non-balanced (130/0.4) hydroxyethyl starch or gelatin compared with balanced Ringer's solution: an in vitro study using two different viscoelastic coagulation tests ROTEMTM and SONOCLOTTM. Br J Anaesth 2010;105:273-281.
84 Demir A, Aydınlı B, Toprak HI, Karadeniz ü, Yılmaz FM,Züngün C, et al. Impact of 6% starch 130/0.4 and 4% gelatin infusion on kidney function in living-donor liver transplantation. Transplant Proc 2015;47:1883-1889.
86 Jacob M, Paul O, Mehringer L, Chappell D, Rehm M, Welsch U,et al. Albumin augmentation improves condition of guinea pig hearts aer 4 hr of cold ischemia. Transplantation 2009;87:956-965.
87 Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg 2011;112:1289-1295.
88 Dawidson IJ, Sandor ZF, Coorpender L, Palmer B, Peters P,Lu C, et al. Intraoperative albumin administration affects the outcome of cadaver renal transplantation. Transplantation 1992;53:774-782.
89 Pockaj BA, Yang JC, Lotze MT, Lange JR, Spencer WF, Steinberg SM, et al. A prospective randomized trial evaluating colloid versus crystalloid resuscitation in the treatment of the vascular leak syndrome associated with interleukin-2 therapy.J Immunother Emphasis Tumor Immunol 1994;15:22-28.
90 Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am J Physiol Lung Cell Mol Physiol 2007;293:L328-335.
91 Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol 2013;11:123-130.
92 Wiedermann CJ, Joannidis M. Nephroprotective potential of human albumin infusion: a narrative review. Gastroenterol Res Pract 2015;2015:912839.
93 Finfer S. Reappraising the role of albumin for resuscitation.Curr Opin Crit Care 2013;19:315-320.
94 SAFE Study Investigators1; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J, Cooper DJ, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 2007;357:874-884.
95 Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A,Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014;370:1412-1421.
96 Patel A, Laffan MA, Waheed U, Brett SJ. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ 2014;349:g4561.
97 Howdieshell TR, Wood M, Swayne M, Duvall R, Mooney S,Stark N. Effects of intraluminal and extracorporeal inferior vena caval bypass on canine hemodynamics. Crit Care Med 1996;24:631-634.
98 Kim YK, Chin JH, Kang SJ, Jun IG, Song JG, Jeong SM, et al.Association between central venous pressure and blood loss during hepatic resection in 984 living donors. Acta Anaesthesiol Scand 2009;53:601-606.
99 Hughes MJ, Ventham NT, Harrison EM, Wigmore SJ. Central venous pressure and liver resection: a systematic review and meta-analysis. HPB (Oxford) 2015;17:863-871.
100 Moggia E, Rouse B, Simillis C, Li T, Vaughan J, Davidson BR, et al. Methods to decrease blood loss during liver resection: a network meta-analysis. Cochrane Database Syst Rev 2016;10:CD010683.
101 Guo JR, Shen HC, Liu Y, Xu F, Zhang YW, Shao Y, et al. Effect of acute normovolemic hemodilution combined with controlled low central venous pressure on blood coagulation function and blood loss in patients undergoing resection of liver cancer operation. Hepatogastroenterology 2015;62:992-996.
102 Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008;134:172-178.
103 Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med 2013;41:1774-1781.
104 Jarnagin WR, Gonen M, Maithel SK, Fong Y, D'Angelica MI,Dematteo RP, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg 2008;248:360-369.
105 Rekman J, Wherrett C, Bennett S, Gostimir M, Saeed S,Lemon K, et al. Safety and feasibility of phlebotomy with controlled hypovolemia to minimize blood loss in liver resections. Surgery 2017;161:650-657.
106 Esper SA, Waters JH. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus 2011;9:139-147.
107 Han S, Kim G, Ko JS, Sinn DH, Yang JD, Joh JW, et al. Safety of the use of blood salvage and autotransfusion during liver transplantation for hepatocellular carcinoma. Ann Surg 2016;264:339-343.
108 Kim JM, Kim GS, Joh JW, Suh KS, Park JB, Ko JS, et al. Longterm results for living donor liver transplant recipients with hepatocellular carcinoma using intraoperative blood salvage with leukocyte depletion filter. Transpl Int 2013;26:84-89.
109 Waters JH, Donnenberg AD. Blood salvage and cancer surgery: should we do it? Transfusion 2009;49:2016-2018.
110 Zulim RA, Rocco M, Goodnight JE Jr, Smith GJ, Krag DN,Schneider PD. Intraoperative autotransfusion in hepatic resection for malignancy. Is it safe? Arch Surg 1993;128:206-211.
111 Davis M, Sofer M, Gomez-Marin O, Bruck D, Soloway MS.e use of cell salvage during radical retropubic prostatectomy: does it influence cancer recurrence? BJU Int 2003;91:474-476.
112 Gray CL, Amling CL, Polston GR, Powell CR, Kane CJ. Intraoperative cell salvage in radical retropubic prostatectomy.Urology 2001;58:740-745.
113 Hart OJ 3rd, Klimberg IW, Wajsman Z, Baker J. Intraoperative autotransfusion in radical cystectomy for carcinoma of the bladder. Surg Gynecol Obstet 1989;168:302-306.
November 5, 2016
Accepted after revision April 10, 2017
Author Affiliations: Department of Surgery, Austin Hospital and University of Melbourne, Melbourne, Victoria, Australia (Yoshino O, Perini MV,Christophi C and Weinberg L); Anaesthesia Perioperative Pain Medicine Unit, University of Melbourne, Melbourne, Victoria, Australia (Weinberg L)
Osamu Yoshino, MD, PhD, Department of Surgery, Austin Hospital, Austin Health, 145 Studley Rd, Heidelberg VIC 3084, Australia (Tel: +61-3-9496-5000; Fax: +61-3-9458-1650: Email:osamu.yoshino@austin.org.au, Osamu1979@mac.com)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60055-9
Published online September 4, 2017.
猜你喜欢
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors and managements of hemorrhage associated with pancreatic fistula after pancreaticoduodenectomy
- Prospective comparison of prophylactic antibiotic use between intravenous moxifloxacin and ceftriaxone for high-risk patients with post-ERCP cholangitis
- Helicobacter pyloriand 17β-estradiol induce human intrahepatic biliary epithelial cell abnormal proliferation and oxidative DNA damage
- Tailored pancreatic reconstruction after pancreaticoduodenectomy: a single-center experience of 892 cases
- Comparative study of the effects of terlipressin versus splenectomy on liver regeneration after partial hepatectomy in rats
- The “Colonial Wig” pancreaticojejunostomy:zero leaks with a novel technique for reconstruction after pancreaticoduodenectomy
