氯沙坦通过激活AMPK抑制LPS诱导的小鼠海马GFAP表达*
2017-09-22郭宝璐梁月琴范彦英
廖 雁, 乔 圆, 南 方, 郭宝璐, 梁月琴, 范彦英
(山西医科大学基础医学院药理教研室,山西 太原 030001)
氯沙坦通过激活AMPK抑制LPS诱导的小鼠海马GFAP表达*
廖 雁, 乔 圆, 南 方, 郭宝璐, 梁月琴, 范彦英△
(山西医科大学基础医学院药理教研室,山西 太原 030001)
目的: 探讨氯沙坦对脂多糖(LPS)诱导的星形胶质细胞活化标志物胶质纤维酸性蛋白(GFAP)表达的影响,以及其机制是否与激活腺苷酸活化蛋白激酶(AMPK)有关。方法: 将成年雄性昆明小鼠分为正常对照组、LPS模型组、氯沙坦给药组及氯沙坦与compound C合用组。侧脑室注射相同剂量LPS (24 μg/d,每天1次,共2次),以建立中枢神经炎症损伤模型;氯沙坦(0.5、1 或5 mg·kg-1·d-1)于LPS注射前14 d开始连续每日腹腔注射给药;AMPK抑制剂Compound C (10 mg·kg-1·d-1)于LPS注射前2 d开始连续每日腹腔注射给药。在LPS末次注射后的第3天取各组小鼠的海马脑区组织,利用Western blot法检测GFAP、AMPK、p-AMPK、哺乳动物雷帕霉素靶蛋白(mTOR)和p-mTOR的蛋白水平。结果: 2次LPS注射后可显著诱导GFAP在海马脑区的表达(P<0.01),而氯沙坦可浓度依赖地抑制LPS诱导的GFAP表达,当氯沙坦剂量为5 mg·kg-1·d-1时可显著抑制GFAP表达(P<0.05),同时,在该剂量下,氯沙坦显著提高了AMPK的磷酸化水平(P<0.01),但对mTOR的磷酸化水平无明显调节作用;而AMPK抑制剂Compound C可显著逆转氯沙坦对GFAP表达及AMPK磷酸化的调节作用(P<0.05)。结论: 氯沙坦可抑制LPS诱导的海马GFAP表达,该作用可能与其激活AMPK有关,但并不依赖于mTOR信号通路。
氯沙坦; 脂多糖; 胶质纤维酸性蛋白; 腺苷酸活化蛋白激酶
星形胶质细胞作为中枢神经系统的重要组成部分,可诱导神经元突触的形成,并参与对突触功能、神经元能量供应及神经递质释放的调控过程[1-3],在保持血脑屏障完整和调节脑血流量中发挥重要作用[4]。在感染、创伤、神经退行性疾病等病理条件下,星形胶质细胞被激活,表现为星形胶质细胞胞体肥大,突起增粗、延长,细胞数目增多,胶质纤维酸性蛋白(glial fibrillary acidic protein,GFAP)表达增加[5-7]。尽管活化的星形胶质细胞可通过提高谷氨酸摄取、增加糖原储存、清除自由基、释放营养因子等途径发挥神经保护作用[8],但是过度激活的星形胶质细胞往往会产生大量的炎症因子而损伤神经元及抑制轴突的再生和重建[9]。因此,抑制星形胶质细胞过度激活对防治中枢神经系统的疾病有重要意义。
血管紧张素II受体阻滞剂(angiotensin II receptor blockers,ARBs),也称为AT1受体拮抗剂,是一类常用的抗高血压药物。近年来,越来越多的研究发现,ARBs对许多中枢神经系统疾病,如脑中风、脑外伤、阿尔兹海默病和帕金森病等都具有一定的保护作用[10-12]。最近,Ongali等[13]发现在APP转基因阿尔茨海默病老年小鼠模型上,连续3个月每日给予ARBs类药物氯沙坦(10 mg·kg-1·d-1)可明显抑制星形胶质细胞活化标志物GFAP的表达。但是,ARBs类药物短期给药对星形胶质细胞活化的调节作用及机制尚不清楚。
腺苷酸活化蛋白激酶[adenosine 5’-monophosphate (AMP)-activated protein kinase,AMPK]是一种丝/苏氨酸蛋白激酶。它是生物能量代谢调节的关键分子,也被称为“细胞能量调节器”,可表达于神经元和星形胶质细胞中[14]。研究发现,AMPK信号通路在神经元增殖、迁移和突触传递等方面具有重要的作用[15-16]。Maixner等[17]发现,利用siRNA干扰AMPK表达或条件性敲除星形胶质细胞AMPK基因,可见脊髓损伤区域GFAP表达增加,提示AMPK对GFAP的表达具有调节作用。另有研究发现,ARBs类药物能诱导下丘脑及大脑皮层AMPK的活化[18-19]。然而,ARBs是否通过激活AMPK及其下游哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)来抑制星形胶质细胞的活化尚不清楚。
本研究拟在脑室注射脂多糖(lipopolysaccharide,LPS)建立的中枢神经炎症损伤模型上,观察ARBs类药物氯沙坦对星形胶质细胞活化标志物GFAP表达的影响,并探讨其机制是否与激活AMPK及其下游通路有关。
材料和方法
1动物
健康昆明雄性小鼠,8~10周龄,体重(30±2)g,购自山西医科大学动物实验中心。所有的动物实验均遵照国家实验动物饲养和使用指南,动物饲养在温度控制的环境(22±1)oC下,12 h明暗循环,自由饮食和饮水。
2实验方法
2.1小鼠LPS中枢神经炎症模型的制备 将小鼠放于玻璃盒内后通予2.5%异氟烷进行全身诱导麻醉,麻醉后将小鼠固定于手术台上并持续通予1.5%异氟烷来维持小鼠的麻醉状态,侧脑室注射24 μg LPS (6 μL)1次或相同剂量注射2次(每天1次)以建立中枢神经炎症损伤模型,对照组则注射相同体积的生理盐水。
2.2分组和给药方法将动物分为正常对照(control)组、LPS模型组、氯沙坦给药组及氯沙坦与Compound C合用给药组。连续2 d侧脑室注射LPS (24 μg/d)以建立中枢神经炎症损伤模型;氯沙坦(0.5、1 或5 mg·kg-1·d-1)于LPS注射前14 d开始连续每日腹腔注射给药,在LPS刺激当日,氯沙坦于LPS注射前1 h给予;AMPK抑制剂Compound C (10 mg·kg-1·d-1)于LPS注射前2 d开始连续每日腹腔注射给药,并于氯沙坦给药前30 min给予。实验中氯沙坦和Compound C均给药至取材当日。在末次LPS注射后第3天,对各组小鼠取海马脑区组织,液氮速冻,存于-80 ℃备用。
2.3蛋白的提取和Westernblot检测在海马组织中加入RIPA裂解液和蛋白酶抑制剂,提取总蛋白,并采用BCA 法进行蛋白定量。用12% SDS-PAGE 分离蛋白并转印至硝酸纤维素膜用5% BSA 室温封闭90 min,加入抗GFAP (1∶250;博士德)、p-AMPK (1∶1 000;CST)、AMPK (1∶1 000;CST)、p-mTOR (1∶1 000;Abcam)、mTOR (1∶1 000;Abcam)和GAPDH (1∶10 000;Bioworld)抗体4 ℃孵育过夜。次日,反复洗膜后加入IgG-HRP 抗体(1∶3 000;博士德)室温轻摇孵育1 h,并用ECL 化学发光法检测,结果用AlphaView SA 软件分析并以GAPDH为内参照统计GFAP蛋白表达水平,以p-AMPK/AMPK和p-mTOR/mTOR比值反映AMPK和mTOR的磷酸化水平。
3统计学处理
用SPSS 19.0软件进行统计分析。数据用均数±标准误(mean±SEM)表示。单因素方差分析结合Bonferroni post-hoc test进行3组以上数据间差异的分析。以P<0.05为差异有统计学意义。
结 果
1LPS诱导小鼠海马GFAP表达
与对照组相比,LPS (24 μg/d)注射2 次能显著诱导(LPS末次注射后第3天)海马组织表达GFAP(P<0.01),而LPS注射 1次诱导GFAP表达不明显,见图1。因此后续实验均采用2次注射LPS的方法制备中枢神经炎症损伤模型。
2氯沙坦对小鼠海马GFAP表达的调节作用
氯沙坦于LPS注射前14 d开始连续每日给药可浓度依赖性地下调LPS诱导的GFAP表达,其中在0.5 mg·kg-1·d-1和1 mg·kg-1·d-1剂量下,氯沙坦有下调LPS诱导的GFAP表达的趋势,但差异无统计学意义,而5 mg·kg-1·d-1的氯沙坦处理可显著抑制LPS诱导的GFAP表达(P<0.05),见图2A。
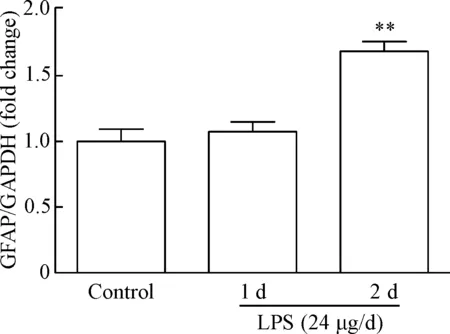
Figure 1. LPS induced GFAP protein expression in the mouse hippocampus. Mean±SEM.n=6.**P<0.01vscontrol group.
图1LPS诱导小鼠海马GFAP表达
3AMPK在氯沙坦调节LPS诱导的GFAP表达中的作用
LPS末次注射后第3天,海马AMPK磷酸化水平与对照组相比并没有明显改变,然而,氯沙坦于LPS注射前14 d开始连续每日给药可浓度依赖性地上调p-AMPK的蛋白水平,而氯沙坦给药剂量为5 mg·kg-1·d-1时,p-AMPK的蛋白水平显著增加(P<0.01),见图2B。
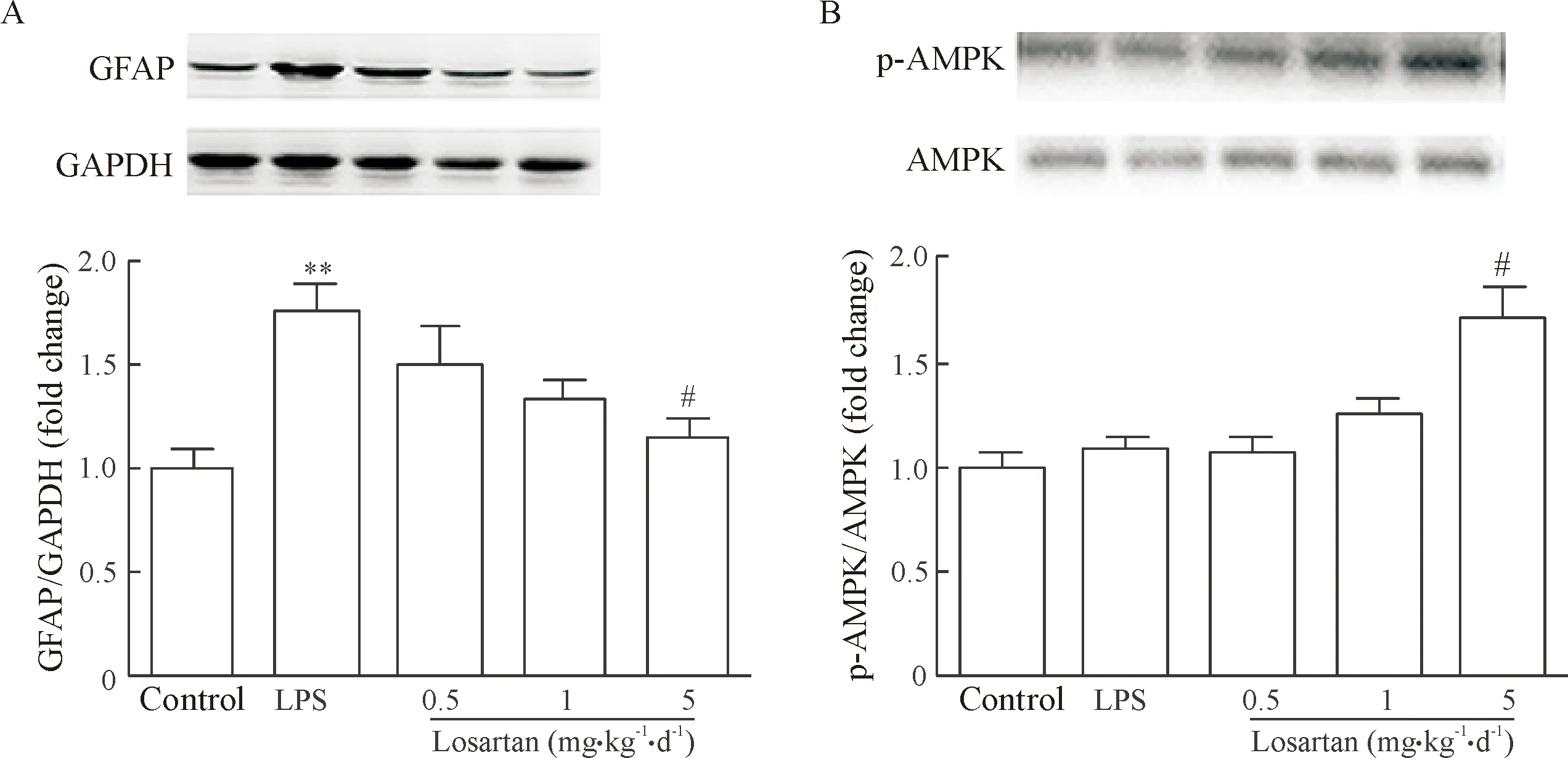
Figure 2. The effects of losartan on GFAP protein expression (A) and AMPK phosphorylation (B) in the mouse hippocampus. Mean±SEM.n=6.**P<0.01vscontrol group;#P<0.05vsLPS group.
图2氯沙坦对小鼠海马GFAP表达及AMPK磷酸化水平的调节作用
给予AMPK的选择性抑制剂Compound C可显著逆转5 mg·kg-1·d-1的氯沙坦对LPS诱导的 GFAP表达的下调作用(P<0.05),见图3A。与此同时,Compound C也明显抑制了5 mg·kg-1·d-1氯沙坦诱导的AMPK磷酸化(P<0.01),见图3B。
4氯沙坦预处理对小鼠海马mTOR磷酸化水平的调节作用
mTOR是AMPK的下游靶点之一,因此我们进一步检测了氯沙坦于LPS注射前14 d开始连续每日给药对mTOR磷酸化水平的影响。结果发现,与对照组相比,LPS和氯沙坦处理(5 mg/kg)对mTOR磷酸化无明显调节作用,见图4。
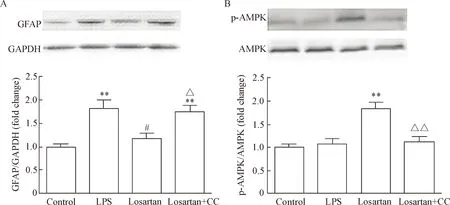
Figure 3. The effects of losartan treatment and Compound C (CC) on GFAP expression (A) and AMPK phosphorylation (B) in the mouse hippocampus. Mean±SEM.n=6~7.**P<0.01vscontrol group;#P<0.05vsLPS group;△P<0.05,△△P<0.01vslosartan group.
图3氯沙坦处理和CompoundC对小鼠海马GFAP蛋白表达及AMPK磷酸化的调节作用
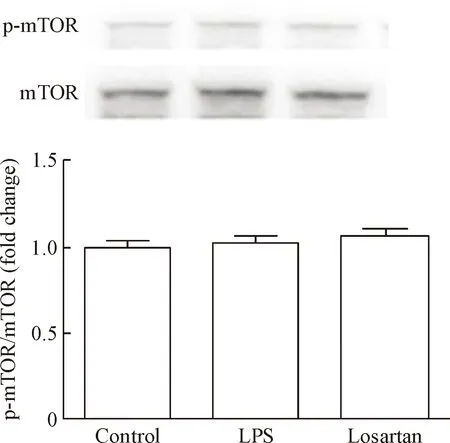
Figure 4. The effects of losartan treatment on mTOR phosphory-lation in the mouse hippocampus. Mean±SEM.n=6.
图4氯沙坦处理对小鼠海马mTOR磷酸化水平的调节作用
讨 论
LPS是一种经典的神经炎症诱导剂,可以诱导脑内星形胶质细胞的活化[20]。在本实验中,为了排除外周炎症的影响,将炎症反应局限在脑内,我们采用了脑室注射LPS的方法。结果发现LPS (24 μg/d)连续注射2 次能显著增加海马脑区 GFAP的表达。在此基础上,我们的研究发现连续数日的氯沙坦处理能显著抑制LPS诱导的GFAP表达。
已有研究发现,在APP转基因阿尔茨海默病模型小鼠上,连续3个月给予氯沙坦(10 mg·kg-1·d-1)可显著抑制星形胶质细胞的反应性增生,改善APP小鼠的空间参考记忆[13]。而本研究发现,在LPS诱导的神经炎症模型上,氯沙坦于LPS注射前短期预防性给药14 d并在LPS刺激后再连续每日给药5 d,可浓度依赖地抑制海马组织GFAP的表达,提示连续多日的氯沙坦给药可抑制LPS诱导的星形胶质细胞的激活。
进一步,本研究发现,氯沙坦于LPS注射前短期预防性给药14 d并在LPS刺激后再连续每日给药5 d可浓度依赖性地提高 LPS注射后第3天时海马AMPK磷酸化水平,而AMPK的选择性抑制剂Compound C可逆转氯沙坦对GFAP表达的下调作用。相似地,最近的研究发现,siRNA干扰AMPK表达或条件性敲除星形胶质细胞AMPK基因可显著增加脊髓GFAP的表达[17]。因此,促进AMPK的激活可能是氯沙坦抑制星形胶质细胞活化的重要机制之一。但是,氯沙坦是否通过直接激活星形胶质细胞内的AMPK来抑制GFAP的表达仍有待研究。
mTOR是AMPK所调节的下游信号靶点之一,其活性可被活化的AMPK所抑制[21]。最近Li等[22]在离体培养的星形胶质细胞缺糖缺氧模型中发现,抑制mTOR通路可减少星形胶质细胞的活化。然而,令人意外的是,尽管氯沙坦可激活AMPK,但对AMPK下游的mTOR活化并没有显著的调节作用。因此,氯沙坦抑制GFAP表达的机制并不依赖于mTOR信号通路。
综上所述,氯沙坦可抑制LPS诱导的小鼠海马GFAP表达,而这一作用与激活AMPK有关,但并不依赖于AMPK下游的mTOR信号通路。另外,已有研究证实氯沙坦等ARBs类药物可以增强星形胶质细胞对神经元的保护功能,如促进谷氨酸摄取等[23],加之该类药物对神经元尚有直接保护作用,因此氯沙坦作为临床上常用的抗高血压药物,长期应用对中枢神经系统疾病的预防和治疗可能具有重要意义。然而,氯沙坦通过激活APMK抑制星形胶质细胞活化的下游分子机制有待进一步研究。
[1] Eroglu C, Barres BA. Regulation of synaptic connectivity by glia[J]. Nature, 2011, 468(7321):223-231.
[2] Pellerin L, Bouziersore AK, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update[J]. Glia, 2007, 55(12):1251-1262.
[3] Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission[J]. Science, 2008, 322(5907):1551-1555.
[4] Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood-brain barrier development[J]. Int J Dev Biol, 2011, 55(4-5):467-476.
[5] Dong Y, Benveniste EN. Immune function of astrocytes[J]. Glia, 2001, 36(2):180-190.
[6] Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury[J]. Neurosignals, 2008, 16(2-3):154-164.
[7] Choudhury GR, Ryou MG, Poteet E, et al. Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke[J]. Brain Res, 2014, 1551(11):45-58.
[8] Abeysinghe HCS, Phillips EL, Chin-Cheng H, et al. Modulating astrocyte transition after stroke to promote brain rescue and functional recovery: emerging targets include Rho kinase[J]. Int J Mol Sci, 2016, 17(3):288.
[9] Silver J, Miller JH. Regeneration beyond the glial scar[J]. Nat Rev Neurosci, 2004, 5(2):146-156.
[10] Zhang TL, Fu JL, Geng Z, et al. The neuroprotective effect of losartan through inhibiting AT1/ASK1/MKK4/JNK3 pathway following cerebral I/R in rat hippocampal CA1 region[J]. CNS Neurosci Ther, 2012, 18(12):981-987.
[11] Pratap R, Pillai KK, Khanam R, et al. Protective effect of irbesartan, an angiotensin II receptor antagonist, alone and in combination with aspirin on middle cerebral artery occlusion model of focal cerebral ischemia in rats[J]. Hum Exp Toxicol, 2011, 30(5):354-362.
[12] Benicky J, Sánchez-Lemus E, Honda M, et al. Angiotensin II AT1receptor blockade ameliorates brain inflammation[J]. Neuropsychopharmacology, 2011, 36(4):857-870.
[13] Ongali B, Nicolakakis N, Tong XK, et al. Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model[J]. Neurobiol Dis, 2014, 68:126-136.
[14] Mccullough LD, Zeng Z, Li H, et al. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke[J]. J Biol Chem, 2005, 280(21):20493-20502.
[15] Dhar SS, Wong-Riley MT. Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochromecoxidase and NMDA glutamate receptor subunit genes[J]. J Neurosci, 2009, 29(2):483-492.
[16] Potter WB, O’Riordan KJ, Barnett D, et al. Metabolic regulation of neuronal plasticity by the energy sensor AMPK[J]. PLoS One, 2010, 5(2):e8996.
[17] Maixner DW, Yan X, Gao M, et al. Adenosine monophosphate-activated protein kinase regulates interleukin-1β expression and glial glutamate transporter function in rodents with neuropathic pain[J]. Anesthesiology, 2015, 122(6):1401-1413.
[18] Yoshida T, Semprunprieto L, Wainford RD, et al. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus[J]. Endocrinology, 2012, 153(153):1411-1420.
[19] Xu Y, Xu Y, Wang Y, et al. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation[J]. Brain Behav Immun, 2015, 50:298-313.
[20] Minghetti L, Walsh DT, Levi G, et al.Invivoexpression of cyclooxygenase-2 in rat brain following intraparenchymal injection of bacterial endotoxin and inflammatory cytokines[J]. J Neuropathol Exp Neurol, 1999, 58(11):1184-1191.
[21] Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival[J]. Cell, 2003, 115(5):577-590.
[22] Li CY, Li X, Liu SF, et al. Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation[J]. Neurochem Int, 2015, 83-84:9-18.
[23] Wu X, Kihara T, Hongo H, et al. Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen-glucose depletion[J]. Br J Pharmacol, 2010, 161(1):33-50.
(责任编辑: 陈妙玲, 罗 森)
Losartan inhibits LPS-induced GFAP expression via AMPK activation in mouse hippocampus
LIAO Yan, QIAO Yuan, NAN Fang, GUO Bao-lu, LIANG Yue-qin, FAN Yan-ying
(Department of Pharmacology, School of Basic Medical Sciences, Shanxi Medical University, Taiyuan 030001, China. E-mail: fyanying6@hotmail.com)
AIM: To investigate the effects of losartan on lipopolysaccharide (LPS)-induced glial fibrillary acidic protein (GFAP) expression, and to determine whether adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) activation is involved in the mechanism.METHODS: Adult male KM mice were divided into control group, LPS model group, losartan treatment group, and losartan and Compound C co-treatment group. To establish a model of central nervous system inflammation, the mice
daily intracerebroventricular injection of LPS (24 μg/d) for 2 d. Daily losartan administration (0.5, 1 or 5 mg·kg-1·d-1, ip) initiated at 14 d prior to LPS injection. Compound C (10 mg/kg, ip), a selective AMPK inhibitor, started to be injected daily at 2 d prior to LPS injection. The hippocampal tissues in each group were isolated at 3 d after the last LPS injection, and then the protein levels of GFAP, AMPK, p-AMPK, mammalian target of rapamycin (mTOR) and p-mTOR were determined by Western blot.RESULTS: Twice LPS injections significantly increased the expression of GFAP in the hippocampus (P<0.01). Losartan inhibited LPS-induced GFAP expression in a concentration-dependent way, and losartan at 5 mg·kg-1·d-1significantly inhibited GFAP expression and AMPK activation (P<0.05), but it had no obvious effect on mTOR activation. Furthermore, Compound C significantly reversed the effect of losartan treatment on LPS-induced GFAP expression and AMPK phosphorylation (P<0.05).CONCLUSION: Losartan inhibits LPS-induced GFAP expression in the mouse hippocampus, and AMPK activation but not mTOR, is involved in the mechanism.
Losartan; Lipopolysaccharide; Glial fibrillary acidic protein; Adenosine 5′-monophosphate-activated protein kinase
1000- 4718(2017)09- 1593- 05
2016- 12- 19 [
] 2017- 04- 13
国家自然科学基金资助项目(No. 81202520);山西省青年科技研究基金资助项目(No.2014021038-1);山西省重点学科建设经费资助项目。
R363.2+1; R741
A
10.3969/j.issn.1000- 4718.2017.09.009
△通讯作者Tel: 0351-4135079; E-mail: fyanying6@hotmail.com
