两个基于多金属氧酸盐和冠醚基超分子阳离子的有机无机杂化材料的合成及晶体结构
2017-09-12熊俊吕少仿彭俊军李明李伟杨水彬
熊俊吕少仿彭俊军李明李伟杨水彬
两个基于多金属氧酸盐和冠醚基超分子阳离子的有机无机杂化材料的合成及晶体结构
熊俊*,1吕少仿1彭俊军1李明1李伟1杨水彬*,2
(1武汉纺织大学化学与化工学院,武汉430200)
(2黄冈师范学院化工学院,黄冈438000)
以二苯并18-冠-6,3-氟-4-甲基苯铵盐以及多金属氧酸盐[Mo6O19]2-为原料,使用溶剂挥发法合成了有机无机杂化材料[(3-F-4-MeAnis)(DB[18]crown-6)]2[Mo6O19]·2CH3CN(1)(3-F-4-MeAnis=3-氟-4-甲基苯铵盐;DB[18]crown-6=二苯并18-冠-6);以18-冠-6,2-氟-4-甲基苯铵盐以及多金属氧酸盐[SMo12O40]2-为原料,运用H管扩散法制备了晶体材料[(2-F-4-MeAnis)([18]crown-6)]2[SMo12O40]·2CH3CN(2)(2-F-4-MeAnis=2-氟-4-甲基苯铵盐;[18]crown-6=18-冠-6)。通过红外光谱、元素分析、热重分析和X射线单晶结构分析对化合物进行了表征。结构分析表明,晶体1和2通过非共价键自组装作用构建而成,冠醚基超分子阳离子是通过N-H…O氢键作用形成。在晶体1和2中,超分子阳离子和多酸阴离子交错堆积形成包合物结构,沿着a轴方向观察,发现每个多酸阴离子被6个超分子阳离子包围着形成六边形堆积结构。通过晶体结构研究和热重分析表明,二苯并18-冠-6中的苯环在维持晶体1的稳定性方面具有重要的作用。
有机无机杂化物;超分子自组装;多金属氧酸盐;冠醚;超分子阳离子
Inorganic-organic hybrid materials constructed throughself-assembledinteractionhaveattracted much attention in the past decades due to their fantastic architecture and interesting physicochemical properties[1-4].Supramolecular crystalline structures formed through noncovalent bonding interaction are one of the indispensable part in the crystalline engineering field[5-6].Because of its high directional characteristic and relatively high bonding energy, hydrogen bonding interaction can be used as the cement to link the structural constructing units and modify crystalline structure[7-8].On the other side,the electrostatic interaction between cationic and anionic moieties also effectively affects the crystalline packing pattern[9].Polyoxometallates(POMs)are discrete early transitionmetal-oxideclusteranions,whichis composed of several assembled[MOn]units.POMs can be used as an excellent candidate to construct self-assembled crystalbecauseoftheirstructural characteristic[10-13].First,POMs contains different kind of oxygen atoms,which have many of potential hydrogen bonding sites.Take Keggin POMs[SMo12O40]2-for example,it has three different types of oxygen atoms,which are central oxygen Oc,bridging oxygen Oband terminal oxygen Ot.The outside oxygen atoms are twenty four bridging oxygen Obatoms and twelve terminal oxygen Otatoms act as the potential hydrogen bonding sites[9].Moreover,POMs have different charge and the charge can be modified by electrochemically methods,whichcanchangetheelectrostatic interaction with organic cation causing unexpected and intriguing molecular assembled structure[14].Many of self-assembled inorganic-organic hybrid crystals based on POMs anion and different organic cation such as tetrathiafulvalene and ferrocenyl derivatives have been focused on due to their versatile topological structures and potential applications in magnetic, optical and conductive materials[15-16].Recently,crown ethers have been widely used in constructing supramolecular crystalline structures with POMs due to their structural characteristic[17].First,Crown ethers have large cave,which can capture the cationic moiety including alkali,alkaline earth metal,ammonium cations and inonic/polar organic compounds through hydrogen bonding interaction to construct supramolecular organic cation[18].Second,Crown ethers are the ring structure constructed by the C-C and C-O bond, therefore,the carbon and oxygen atoms are the potential hydrogen bonding sites to construct supramolecular structure with POMs[9].In addition,crown ether has the flexible structural characteristic,which can construct diversity supramolecular cations and versatile inorganic-organic hybrid structure[19-20].
Many of supramolecular cation based on[18] crown-6 and anilinium derivatives have been designed through the N-H…O hydrogen bonding interaction, which were successfully introduced into POMs due to the below advantages[17].The first is that supramolecular cation has many hydrogen bonding sites that can effectively link with POMs.Moreover,giant POMs anion can construct large cave,which can embedded large size of supramolecular cations.Some different structural supramolecular cations have been designed and successfully introduced into Keggin[PMo12O40]n-. Nakamura′s group have designed two kinds of supramolecular cation with different sizes and introduced them into the same Keggin[SMo12O40]2-.It is found that six monovalent supramolecular cations with small size surroundone[SMo12O40]2-,andformhexagonal assembled structures.For the larger size of divalent supramolecularcation,foursupramolecularcation include one[SMo12O40]2-form rectangular assembled structures viewed along certain axis[12].In this paper, we designed two kinds of supramolecular cations and introduced them into[Mo6O19]2-and[SMo12O40]2-,and obtained two inorganic-organic hybrid materials[(3-F-4-MeAnis)(DB[18]crown-6)]2[Mo6O19]·2CH3CN(1)and [(2-F-4-MeAnis)([18]crown-6)]2[SMo12O40]·2CH3CN(2) (3-F-4-MeAnis=3-fluoro-4-methylanilinium,DB[18] crown-6=dibenzo[18]crown-6,2-F-4-MeAnis=2-fluoro-4-methylanilinium).
1 Experimental
1.1 Materials and measurements
[18]crown-6,DB[18]crown-6,3-F-4-MeAni and 2-F-4-MeAni were purchased from Shanghai AladdinBio-Chem Technology Co.,LTD,which were used without further purification.3-F-4-MeAnis,2-F-4-MeAnis,[TBA]2[Mo6O19]and[TBA]2[SMo12O40]were prepared according to similar procedures reported previously[14,21].IR spectra were recorded using a Thermo Scientific Nicolet 6700 FT-IR spectrometer(400~7 800 cm-1).Elemental analysis(C,H,N)was carried out on a CARLO ERBA 1106 analyzer.Thermogravimentic differential thermal analysis(TG-DTA)were carried out using a Rigaku Thermoplus TG8120 thermal analysis station employing an Al2O3reference,in the temperature range from 303 to 773 K with a heating rate of 10 K·min-1under flowing nitrogen gas.
1.2 Syntheses of[(3-F-4-MeAnis)(DB[18]crown-6)]2[Mo6O19]·2CH3CN(1)and[(2-F-4-MeAnis)([18]crown-6)]2[SMo12O40]·2CH3CN (2)
Compound 1 was synthesized using the evaporation method.[TBA]2[Mo6O19](0.14 g,0.1 mmol)was dissolved in 10 mL acetonitrile.To this solution(3-F-4-MeAnis)(BF4)(40 mg,0.20 mmol)and DB[18]crown-6(72 mg,0.20 mmol)dissolved in 10 mL acetonitrile was added slowly with stirring.After one week,yellow block crystals of 1 were obtained.Anal.Calcd.for C58H72F2Mo6N4O31(%):C 35.97,H 3.72,N 2.89;Found (%):C 36.02,H 3.67,N 2.86.IR(KBr pellet,cm-1): 1 493(m),1 240(m),1 120(m),1 060(w),960(s),780(s), 690(w).
Compound 2 was synthesized using the standard diffusion method in an H-shape cell.Saturated CH3CN solution(15 mL)of[TBA]2[SMo12O40],and the acetonitrile solution(15 mL)containing(2-F-4-MeAnis)(BF4) (40 mg,0.20 mmol)and[18]crown-6(52 mg,0.20 mmol) were introduced into opposite sides of the H-shape cell.Then the cell was filled with acetonitrile slowly. After two week,black block crystals of 2 were obtained.Anal.Calcd.for C42H72F2Mo12N4O52S(%):C 18.76,H 2.86,N 2.08;Found(%):C 18.73,H 2.82,N 2.03.IR(KBr pellet,cm-1):1 170(m),1 120(s),1 060 (m),980(s),870(m),790(s).
1.3 Crystal structure determination
Crystallographic data of the single crystals were collected using R-AXIS RAPID diffractometer with Mo Kα radiation(λ=0.071 073 nm)from a graphite monochromator at 173 K.The structures were solved by the direct method(SIR 2004)and expanded using Fourier transfer and refined on F2by the full-matrix least-squares method(SHELXL-97)[21-22].The parameters were refined using anisotropic temperature factors except for the hydrogen atoms,which were refined using the riding model with a fixed C-H bond distance of 0.095 nm.The crystallographic data of compounds 1 and 2 at 173 K are summarized in Table 1.
CCDC:1540103,1;1540105,2.

Table1 Crystallographic parameters for 1 and 2
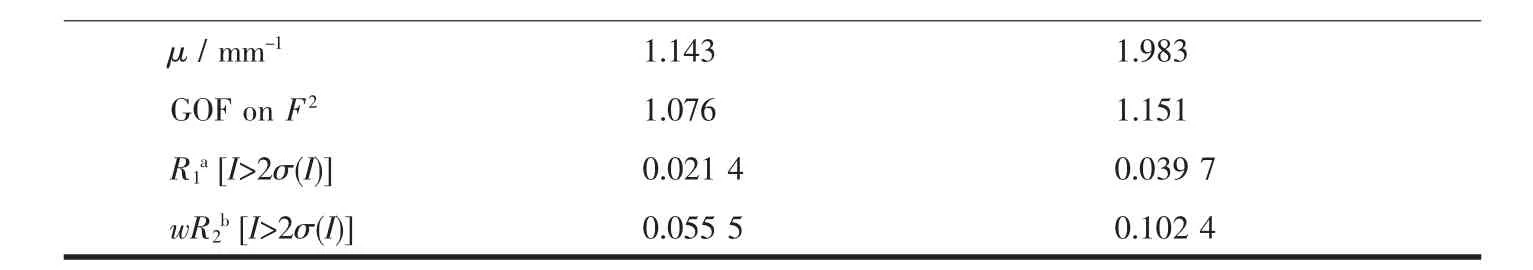
Continued Table 1
2 Results and discussion
2.1 Structure description of 1
Single crystal X-ray diffraction analysis reveals that compound 1 crystallizes in the triclinic space group P21/c(Table 1).As shown in the Fig.1,compound 1 contains one DB[18]crown-6 molecule,one 3-F-4-MeAnis cation and a half[Mo6O19]2-polyoxoanion in the asymmetric unit.Supramolecular cation is construvcted by one DB[18]crown-6 and one 3-F-4-MeAnis through the N-H…O hydrogen bonding interaction. The distances between the H-bonding donor atom(N1) andtheacceptor atoms(O11 to O16)in the macrocyclic assemblies are short(N1-O11,0.294 11 nm;N1-O12, 0.286 92 nm;N1-O13,0.284 26 nm;N1-O14,0.292 04 nm;N1-O15,0.290 06 nm;N1-O16,0.283 99 nm), indicating strong hydrogen bonding interaction.The nitrogen atom(N1)locate at the center of DB[18] crown-6 molecule,and the N1-C2 bond is almost perpendicular to the mean plane constructed by the six oxygen atoms(O11 to O16)of the DB[18]crown-6 molecule.The N1 atom is lying 0.081 96 nm higher from the mean oxygen atoms plane of the crown ring. In the supramolecular cation,DB[18]crown-6 molecule forms a V-shape conformation,and 3-F-4-MeAnis cation inserts into it.The dihedral angle of the two phenyl rings in DB[18]crown-6 is 121.23°,which is smaller than that in compound[(m-FAnis)(DB[18] crown-6)]2[SMo12O40]·4CH3CN[23].This can be attributed to the large size of[SMo12O40],which enhance the steric repulsion of the adjacent supramolecular cations.Polyoxoanion[Mo6O19]2-has an approximate Ohsymmetry and consists of six sharing MoO6octahedrons.[Mo6O19]2-has three kinds of single and doublealternative Mo-O bonds.The average distances of Mo-Oc,Mo-Oband Mo-Otare 0.231 82,0.192 99 and 0.168 56 nm,respectively,which are similar to those reported compounds[24].Each[Mo6O19]2-is surrounded by twelve adjacent[(3-F-4-MeAnis)(DB[18]crown-6)] supramolecular cations and four CH3CN molecules. The shortest O-O distances of adjacent polyoxoanions [Mo6O19]2-are 0.302 62 nm(O8-O8A),0.820 80 nm (O10-O10A)and 0.739 67 nm(O9-O9A)along the a,b and c axis,respectively,which indicates that[Mo6O19]2-polyoxoanions coulddirectlyinteractthroughthe interatomic O…O interaction along the a axis.The divalent[Mo6O19]2-polyoxoanions and monovalent[(3-F-4-MeAnis)(DB[18]crown-6)]assemblies with the molar ratio of 1∶2 are alternately arranged together to build the supramolecular self-assembly structure.In the bc plane,each[Mo6O19]2-polyoxoanion is enclosed bysix[(3-F-4-MeAnis)(DB[18]crown-6)]supramolecular cations,and construct a hexagonal arrangement as shown in Fig.2.This packing structure is similar with the reported compounds[12].
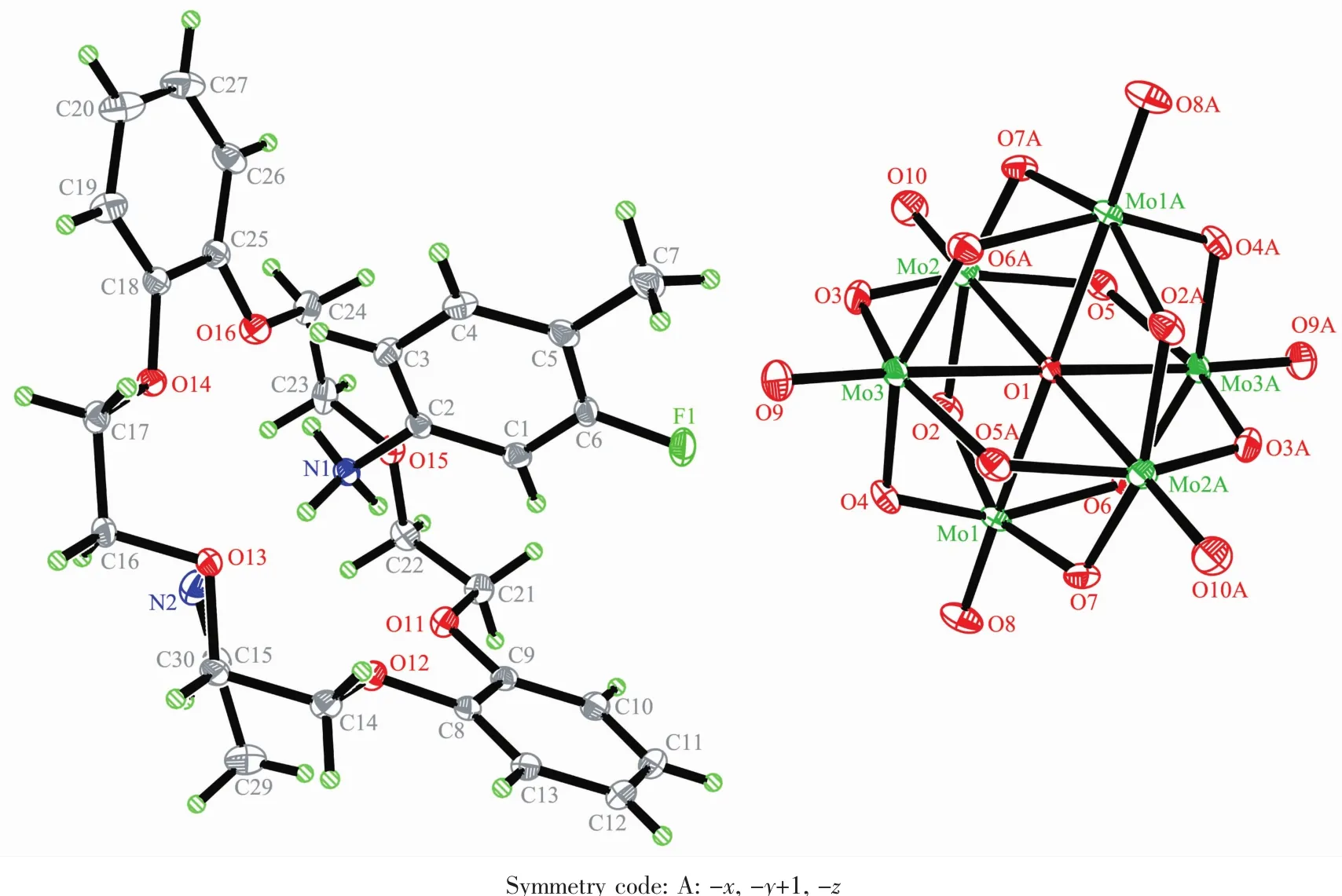
Fig.1 ORTEP diagram of the asymmetric unit of compound 1 with the atomic numbering scheme and 30%thermal ellipsoids

Fig.2 Packing diagram of[Mo6O19]2-polyoxoanion and self-assembly supramolecular cation viewed along the a axis in compound 1
2.2 Structure description of compound 2

Fig.3 ORTEP diagram of the asymmetric unit of compound 2 with the atomic numbering scheme and 30%thermal ellipsoids
Compound 2 crystallizes in the triclinic space group P1.In the asymmetric unit of compound 2, there are half of a[SMo12O40]2-polyoxoanion,one[18] crown-6 molecule,one 2-F-4-MeAnis cation and one CH3CN molecule as shown in Fig.3.Like compound 1, supramolecular cation in compound 2 is constructed through the N-H…O hydrogen bonding interaction between the nitrogen atom of 2-F-4-MeAnis and six oxygen atoms of[18]crown-6 molecule.The hydrogen bonding interaction is strong because the distances between the H-bonding donor atom(N1)and theacceptor atoms(O23 to O28)in the macrocyclic assemblies are short(N1-O23,0.285 55 nm;N1-O24, 0.300 26 nm;N1-O25,0.295 99 nm;N1-O26,0.302 68 nm;N1-O27,0.281 69 nm;N1-O28,0.302 96 nm). The nitrogen atom N1 almost locate at the center of [18]crown-6 molecule,and is 0.012 7 nm higher from the mean[18]crown-6 molecular plane.The dihedral angle of the 2-F-4-MeAnis cation and[18]crown-6 molecule is 87.11°.[SMo12O40]2-polyoxoanion has an approximate Ohsymmetry and consists of twelve sharing MoO6octahedrons.Center S atom and O1-O4(O1AO4A)atoms construct a square-prismatic structure. The population of O1-O4(O1A-O4A)atoms are 0.5.The shortest O-O distances between adjacent[SMo12O40]2-polyoxoanion are 0.308 48 nm(O21-O10),0.350 85 nm(O14-O22)and 0.804 40 nm(O19-O10)along the a,b and c axis,respectively,indi-cating[SMo12O40]2-polyoxoanionshaveweakintera-tomicO…O interaction along the a and b axis.In compound 2, [SMo12O40]2-polyoxoanion is surrounded by eight[(2-F-4-MeAnis)([18]crown-6)]supramolecular cations and four CH3CN molecules.Like compound 1,the divalent [SMo12O40]2-polyoxoanions and monovalent[(2-F-4-MeAnis)([18]crown-6)]assemblieswiththemolar ratio of 1∶2 are alternately arranged together along the c axis,and build the supramolecular self-assembly structure.Each[SMo12O40]2-polyoxoanion in the compound 2 is surrounded by six supramolecular[(2-F-4-MeAnis)([18]crown-6)]cations,which forms a hexagonal arrangement in the bc plane as shown in Fig.4.

Fig.4 Packing diagram of[SMo12O40]2-polyoxoanion and self-assembly supramolecular cation viewed along the a axis in compound 2
2.3 Thermogravimetric analysis
The thermal behaviors of compounds 1 and 2 have been discussed in detail through the TG-DTA measurement as shown in Fig.5.The weight loss of the compound 1 is mainly dominated by losing CH3CN and DB[18]crown-6 molecule during heating process.The weight loss in the compound 1 is observed when the temperature is up to 437 K,whose magnitude is consistent with the loss of two CH3CN molecules(4.28%,Calcd.4.34%).Then,compound 1 lost two DB[18]crown-6 molecules(17.29%,Calcd. 17.37%)when temperature is up to 557 K.However, compound 2 is easy to decompose at room temperature.The reason can be attributed to the using of different crown ethers in the preparation.In compound 1,the phenyl rings in DB[18]crown-6 molecule form C-H…π hydrogen bonding interaction with adjacent molecule.In addition,for the similar compound[(3-F-4-MeAnis)(DB[18]crown-6)]2[SMo12O40]· 2CH3CN previously reported[12],the decomposing temperature is 430 K,which further prove that phenyl rings in DB[18]crown-6 molecule play an important role in maintaining the stability of compound 1.

Fig.5 TG-DTA curve of compound 1
Acknowledgements:The authors thank the foundation of Wuhan Textile University(Grant No.165002)and Hubei Key Laboratory of Biomass Fibers and Eco-dyeing&Finishing and HubeiKeyLaboratoryforProcessingandApplicationof Catalytic Materials for supporting this work.
[1]Kitagawa S,Kitaura R,Noro S.Angew.Chem.Int.Ed.,2004,43:2334-2375
[2]Perry IV J,Perman J,Zaworotko M.Chem.Soc.Rev.,2009, 38:1400-1417
[3]Zhao H,Li D,Ren X,et al.J.Am.Chem.Soc.,2010,132(1): 18-19
[4]GU Wen-Jun(顾文君),GU Jin-Zhong(顾金忠).Chinese J. Inorg.Chem.(无机化学学报),2017,33(2):227-236
[5]Akutagawa T,Takeda S,Nakamura T,et al.J.Am.Chem. Soc.,2004,126(1):18-19
[6]Zhang Q,Wu F,Long L,et al.Angew.Chem.Int.Ed.,2013, 52:12602-12605
[7]Desiraju R.Acc.Chem.Res.,2002,35:565-573
[8]Hua Y,Flood H.Chem.Soc.Rev.,2010,39:1262-1271
[9]Akutagawa T,Cronin L,Nakamura T,et al.Inorg Chem., 2011,50:6711-6718
[10]Du D,Su Z,Wang E,et al.Coord.Chem.Rev.,2013,257: 702-717
[11]Nie S,Hu H,Xue G,et al.J.Solid State Chem.,2010,183: 2957-2962
[12]Xiong J,Akutagawa T,Nakamura T.Cryst.Growth Des., 2016,16:800-807
[13]WANG Lei(王蕾),GU Xiao-Min(顾晓敏),NI Liang(倪良), et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(4): 691-698
[13]Vu T,Bond M,Wedd G,et al.Inorg.Chem.,2001,40:65-72
[14]Coronado E,Gómez-García C.Chem.Rev.,1998,98:273-296
[15]Xu H,Li Z,Xue G,et al.Cryst.Growth Des.,2010,10:1096 -1103
[16]Akutagawa T,Endo D,Nakamura T,et al.Coord.Chem. Rev.,2007,251:2547-2561
[17]Akutagawa T,Noro S,Nakamura T,et al.Inorg.Chem.,2008, 47:5951-5962
[18]Horie M,Suzaki Y,Osakada K.J.Am.Chem.Soc.,2004, 126(12):3684-3685
[19]Langford S,Lau V.Aust.J.Chem.,2004,57:29-32
[20]Nishihara S,Akutagawa T,Nakamura T,et al.Chem.Asian J.,2007,2:1083-1090
[21]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[22]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[23]Xiong J,Akutagawa T,Nakamura T,et al.CrystEngComm, 2015,17:856-861
[24]Niu Y,Ren X,Xue G,et al.J.Organomet.Chem.,2010,695: 1863-1868
Two Inorganic-Organic Hybrid Crystals Based on Polyoxometallates and Supramolecular Cation:Syntheses and Crystal Structures
XIONG Jun*,1LÜ Shao-Fang1PENG Jun-Jun1LI Ming1LI Wei1YANG Shui-Bin*,2
(1College of Chemistry and Chemical Engineering,Wuhan Textile University,Wuhan 430200,China)
(2College of Chemical Engineering,Huanggang Normal University,Huanggang,Hubei 438000,China)
Two new inorganic-organic hybrids[(3-F-4-MeAnis)(DB[18]crown-6)]2[Mo6O19]·2CH3CN(1)and[(2-F-4-MeAnis)([18]crown-6)]2[SMo12O40]·2CH3CN(2)(3-F-4-MeAnis=3-fluoro-4-methylanilinium,DB[18]crown-6=dibenzo [18]crown-6,2-F-4-MeAnis=2-fluoro-4-methylanilinium)have been synthesized and characterized by infrared spectrum(IR),elemental analysis(EA),thermosgravimetric analysis(TGA)and X-ray diffraction.Compounds 1 and 2 are constructed through noncovalent bonding interaction and the polyoxometalates(POMs)and supramolecular cation arrange alternately.Supramolecular cation forms through the N-H…O hydrogen bonding interaction between the nitrogen atom of anilinium and oxygen atoms of crown ether derivatives.In the crystals of 1 and 2, each polyoxoanion is surrounded by six supramolecular cations and forms hexagonal arrangement viewed along the a axis.Crystalline structure and TGA indicated that phenyl rings in DB[18]crown-6 plays an important role in maintaining the stability of compound 1.CCDC:1540103,1;1540105,2.
inorganic-organic hybrids;supramolecular self-assembled;polyoxometallates;crown ether;supramolecular cation
O614.61+2
A
1001-4861(2017)09-1649-07
10.11862/CJIC.2017.171
2017-03-31。收修改稿日期:2017-05-17。
武汉纺织大学未来之星助推计划(No.165002)资助项目。
*通信联系人。E-mail:jxiong@wtu.edu.cn,2416975012@qq.com
