肠三叶因子对非酒精性脂肪性肝炎大鼠回肠黏液屏障的影响
2017-08-30孔维宗王迎春
梁 凯, 孔维宗, 陈 娟, 王迎春
(1 大连大学,辽宁 大连 116001;2 大连大学附属中山医院 消化二科, 辽宁 大连 116001)
肠三叶因子对非酒精性脂肪性肝炎大鼠回肠黏液屏障的影响
梁 凯1, 孔维宗2, 陈 娟2, 王迎春2
(1 大连大学,辽宁 大连 116001;2 大连大学附属中山医院 消化二科, 辽宁 大连 116001)
目的 研究非酒精性脂肪性肝炎(NASH)大鼠肠道黏液屏障改变,探讨肠三叶因子(TFF3)对NASH大鼠肠道黏液屏障的影响,及其对NASH的治疗作用。方法 将清洁级雄性SD 大鼠60只随机分为正常组、模型组和治疗组,每组20只。正常组给予普通饲料,模型组和治疗组给予高脂饲料12周,诱导NASH模型后,治疗组给予rhTFF3 1 ml·kg-1·d-1(浓度0.1 mg/ml)腹腔内注射,正常组与模型组给予1 ml·kg-1·d-1生理盐水,连续3周。第15周末,FITC标记的右旋糖酐灌胃检测大鼠回肠通透性后,处死大鼠,检测血清AST、ALT、TC、TG、内毒素(ET)水平及二胺氧化酶(DAO)活性。HE染色观察肝脏和回肠末端组织病理变化,PAS染色观察回肠末端杯状细胞并计数,免疫组化方法检测回肠末端紧密连接蛋白Occludin和TFF3 蛋白表达水平,实时荧光定量PCR检测TFF3 mRNA转录水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。结果 大鼠血清 AST、ALT、TG、TC、DAO、ET水平在模型组升高,而治疗组较模型组明显下降(P值均<0.01);模型组NAS评分较正常组显著增高(P<0.01),而治疗组肝组织炎症改善,NAS评分较模型组明显降低(P<0.01);光镜下模型组回肠末端细胞坏死,绒毛损伤,杯状细胞明显减少,治疗组绒毛损伤修复,杯状细胞增多;模型组大鼠回肠通透性与正常组比显著增加,治疗组较模型组明显降低(P值均<0.01);Occludin和TFF3在模型组表达减少,而治疗组较模型组增多(P值均<0.01);回肠末端TFF3 mRNA转录水平模型组较正常组下调,治疗组表达较模型组上调(P值均<0.01)。结论 NASH大鼠存在肠道杯状细胞损伤,黏液屏障功能障碍,TFF3可以修复回肠末端损伤,促进杯状细胞再生与黏液分泌,修复肠屏障功能,降低肠通透性,对NASH起到治疗作用。
脂肪肝; 肠三叶因子; 杯状细胞; 大鼠, Sprague-Dawley
非酒精性脂肪性肝病(NAFLD)是一种与遗传易感和胰岛素抵抗密切相关的代谢性疾病[1],非酒精性脂肪性肝炎(NASH)在NAFLD的病程中占有重要地位。近来研究[2]发现 NASH与肠道屏障功能异常有关。肠三叶因子(trefoil factor 3, TFF3)是一个重要的黏膜保护因子,主要由肠道黏膜杯状细胞合成并分泌,形成肠黏膜缓冲带,保护肠道上皮细胞。其还有促进黏膜细胞迁移、分化,加速损伤修复,调节炎症与免疫反应等作用[3-6]。关于TFF3对NASH作用的研究鲜有报道,本实验通过给予NASH模型大鼠人重组TFF3(rhTFF3)处理,探讨 TFF3对NASH大鼠回肠末端黏液屏障的影响及对NASH的治疗作用。
1 材料与方法
1.1 材料 清洁级雄性SD大鼠,4周龄,体质量(200±20)g,购自大连医科大学动物研究中心。高脂饲料(88%普通饲料+10%猪油+2%胆固醇),定制于北京华阜康生物科技股份有限公司。鲎试剂盒购自上海伊华生物技术有限公司,二胺氧化酶(DAO)试剂盒由南京建成生物工程研究所提供,rhTFF3购自PrimeGene公司,兔抗TFF3多克隆抗体购自美国Abnova公司,兔抗Occludin单克隆抗体购自香港Abcam有限公司,RNA提取试剂盒、逆转录酶试剂盒、实时荧光定量PCR试剂盒购自大连宝生物公司。
1.2 实验方法
1.2.1 实验动物分组及处理 清洁级SD 大鼠60只,饲养1周后,随机分为正常组、模型组和治疗组,每组20只。采用徐正婕等[7]造模方法,正常组给予普通饲料,模型组及治疗组给予高脂饲料。饲养12周后,每组处死2只大鼠验证NASH模型,治疗组给予rhTFF3 1 ml·kg-1·d-1(浓度为0.1 mg/ml) 腹腔注射[8-9],正常组与模型组给予1 ml·kg-1·d-1生理盐水,连续3周。第15周末,用10%水合氯醛按2 ml/kg腹腔内注射麻醉大鼠。门静脉取血保存后,处死大鼠取出肝脏,吸干后称重,计算肝指数(肝指数=肝质量/体质量×100%)。取部分肝组织及末端回肠组织以中性甲醛固定,部分末端回肠组织用液氮处理,置于-80 ℃保存。
1.2.2 回肠通透性检测 15周末,采用Kwak等[10]的方法,大鼠麻醉前先禁食水4 h后,FITC标记的右旋糖酐4 kD 60 mg/100 g 体质量(浓度为100 mg/ml)给大鼠灌胃,4 h后心脏取血0.8~1.2 ml,4 ℃保存。3000 r/min低温离心15 min后,用Victor3分光光度计(激发光485、放射光525)检测血清样本荧光强度。
1.2.3 血生化指标检测 采用美国强生Vitros 5.1 FS自动生化分析仪测定门静脉血TG、TC、ALT、AST水平。按说明书采用显色基质鲎试剂盒终点显色法对门静脉血中的内毒素(ET)进行检测,分光光度法检测血浆中 DAO活性。
1.2.4 肝组织病理观察 将甲醛固定、石蜡包埋的肝脏及末端回肠组织样本,行常规HE染色,并在光学显微镜下观察。根据《非酒精性脂肪性肝病诊疗指南(2010年修订版)》[11]NAS积分标准,对肝脏进行NAS评分,NAS<3分可排除NASH,NAS>4分则可诊断NASH,介于两者之间为NASH可能。部分末端回肠样本行PAS染色,并采用文献[12]中杯状细胞计数方法,每个样本切片选取5个视野,统计每100个回肠末端上皮细胞中PAS阳性细胞数。
1.2.5 回肠末端组织免疫组化染色 将甲醛固定好的回肠组织脱水、石蜡包埋,在切片机上切成4 μm薄片。用兔抗大鼠TFF3(1∶100)、Occludin(1∶100)进行免疫组化染色,光学显微镜下观察,并用Image-Pro Plus 6.0图像软件分析。
1.2.6 实时荧光定量PCR 设计TFF3引物序列,上游引物:5′-TTCTGGATAACCCTGCTGCT-3′,下游引物:5′-TAGCCACAGTCCACCCTGA-3′。GAPDH上游引物:5′-CCCCCAATGTATCCGTTGTG-3′,下游引物:5′-TAGCCCAGGATGCCCTTTAGT-3′。用Trizol法提取回肠组织总RNA,测定纯度,按逆转录试剂盒说明合成cDNA,然后进行实时荧光定量PCR,以GAPDH为内参引物标准化TFF3含量。循环条件为:95 ℃预变性2 min,95 ℃变性40 s,45 ℃复性40 s,60 ℃延伸1 min,35个循环后60 ℃再延伸7 min。
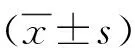
2 结果
2.1 回肠通透性变化 3组大鼠回肠通透性比较,差异有统计学意义(F=614.84,P<0.001)。模型组大鼠血浆中右旋糖酐水平较正常组明显增高[(120.87±10.71) μg/ml vs (36.04±5.70) μg/ml,P<0.01],治疗组较模型组降低[(47.39±6.25) μg/ml vs (120.87±10.71) μg/ml,P<0.01]。结果表明模型组大鼠肠通透性增高,而rhTFF3能改善大鼠肠通透性。
2.2 血清生化指标检测结果 大鼠血清TG、TC、ALT、AST、ET、DAO水平在模型组均较正常组显著升高(P值均<0.01);治疗组较模型组明显下降(P值均<0.01)。模型组大鼠比正常组肝指数明显增高(P<0.01),而rhTFF3处理后,大鼠肝指数较模型组降低(P<0.01)(表1)。
2.3 肝脏病理学改变及NAS评分 光镜下见正常组肝小叶结构正常,细胞呈条索状排列,无明显脂肪变性,未见炎性细胞浸润;模型组肝细胞明显肿大,可见大小不等的脂滴充满细胞,发生脂肪变性,肝小叶内局灶性炎症、细胞坏死;治疗组可见肝细胞脂肪变,小叶内炎性细胞浸润,改变较模型组减轻(图1)。3组大鼠NAS积分比较差异有统计学意义(F=172.31,P<0.001),且模型组较正常组显著增高[(6.97±1.01)分 vs (1.06±0.63)分,P<0.01],而治疗组相比模型组降低[(3.18±1.18)分 vs (6.97±1.01)分,P<0.01]。


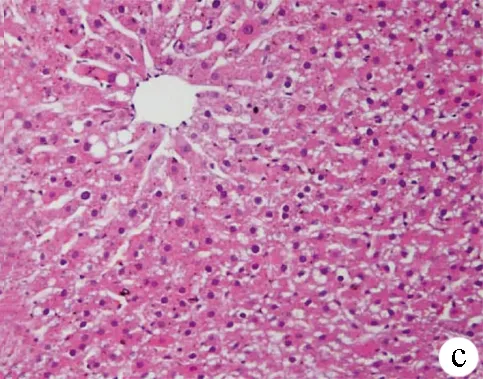
图1 3组大鼠肝组织HE染色结果(×200) a:正常组;b:模型组;c:治疗组
2.4 回肠末端病理改变及杯状细胞计数 正常组回肠末端表面绒毛丰富,排列整齐,黏膜完整连续,未见炎性细胞浸润,杯状细胞位于肠腺上皮与黏膜上皮之间,如酒杯状,数量丰富。模型组回肠绒毛疏松紊乱、萎缩、变钝,黏膜可见水肿、炎性细胞浸润,杯状细胞稀疏,萎缩甚至消失。与模型组比较,治疗组黏膜绒毛较丰富,黏膜水肿、炎性细胞浸润等改变较模型组轻,杯状细胞排列较整齐,数量较多(图2)。PAS染色结果示,阳性杯状细胞显色为深紫色,分布于黏膜上皮及肠腺上皮之间(图3),3组大鼠回肠黏膜杯状细胞计数差异有统计学意义(F=109.31,P<0.001),模型组杯状细胞数量较正常组明显减少[(22.53±2.67)个vs (35.10±3.21)个,P<0.01],而治疗组与模型组相比明显增多[(27.80±1.50)个 vs (22.53±2.67)个,P<0.01]。

表1 3组大鼠各项血清学指标和肝指数检测结果比较
注:与正常组比较,1)P<0.01;与模型组比较,2)P<0.01



图2 3组大鼠回肠末端组织HE染色(×100) a:正常组;b:模型组;c:治疗组

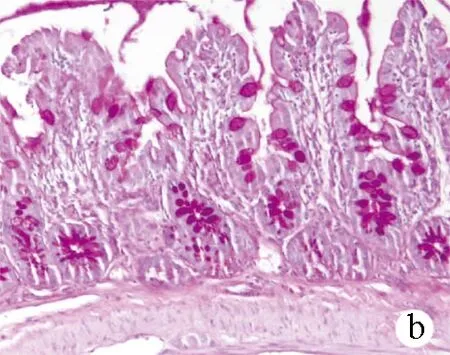
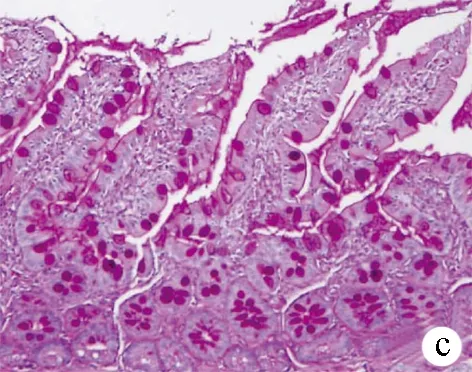
图3 3组大鼠回肠末端组织PAS染色 (×100) a:正常组;b:模型组;c:治疗组
2.5 免疫组化染色观察大鼠回肠末端Occludin及TFF3表达 Occludin分布于回肠上皮细胞连接处,3组大鼠紧密连接蛋白Occludin表达差异有统计学意义(F=255.86,P<0.001)。正常组呈强表达,模型组较正常组表达减弱(12.47±2.16 vs 35.64±4.21,P<0.01);而治疗组较模型组表达增强(16.95±3.08 vs 12.47±2.16,P<0.01)(图4)。从肠隐窝基底部到肠腔表面都可见TFF3阳性染色,3组大鼠TFF3表达差异有统计学意义(F=353.66,P<0.001); 正常组回肠中TFF3主要见于黏膜杯状细胞胞浆和黏膜表面黏液层,表达较强;模型组黏液层稀薄,TFF3表达较正常组明显减弱(23.72±4.01 vs 52.60±2.48,P<0.01);与模型组相比,治疗组信号增多,表达增强(30.22±3.58 vs 23.72±4.01,P<0.01)(图5)。
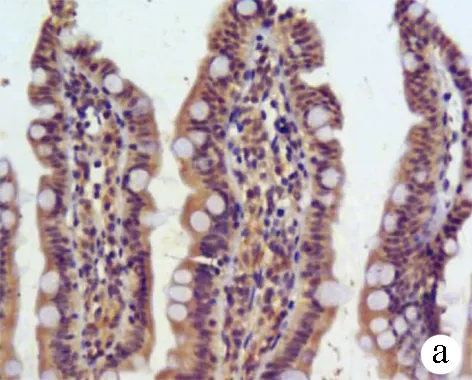


图4 3组大鼠回肠黏膜紧密连接蛋白Occludin的表达(×200) a:正常组;b:模型组;c:治疗组
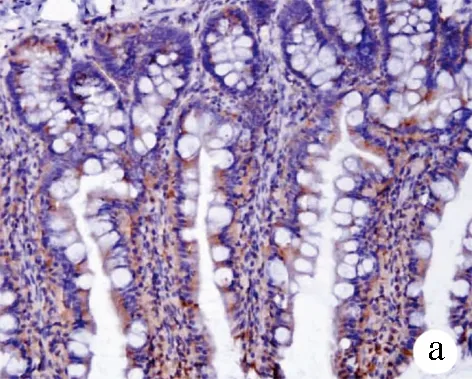

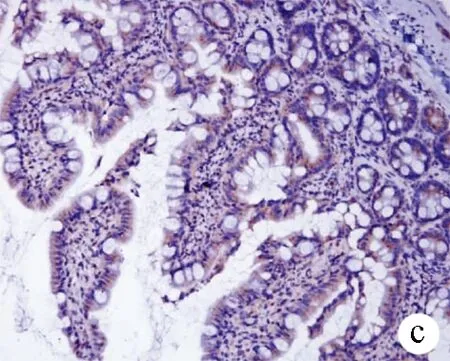
图5 3组大鼠回肠黏膜TFF3的表达(×200) a:正常组;b:模型组;c:治疗组
2.6 大鼠回肠TFF3 mRNA表达变化 3组大鼠TFF3 mRNA表达差异有统计学意义(F=142.50,P<0.001)。与正常组比较,模型组表达明显下调(0.43±0.15 vs 1.03±0.21,P<0.01),而治疗组表达较模型组上调(0.75±0.10 vs 0.43±0.15,P<0.01)。
3 讨论
肠道内含有万亿计的数千种微生物及其产物,还有来自上消化道的各种消化酶、胆汁、酸,以及食物中难消化的纤维素、化合物等,这些构成了肠内复杂的环境[13]。因此,肠道成为细菌及各种毒素入侵机体的重要门户,肠道屏障在抵御肠腔内各种损伤,保护机体方面发挥重要作用。而近来研究[14-15]发现NAFLD的发生发展与肠道屏障功能损伤密切相关 。
本研究结果显示,模型组血清 AST、ALT、TG、TC、DAO、ET水平较正常组明显升高。其中 DAO常用来反映肠道机械屏障完整性及黏膜绒毛损伤程度,DAO水平升高提示存在肠上皮损伤。另外,肠通透性常用于评估肠道屏障功能,模型组大鼠肠通透性较正常组明显增加,表明 NASH大鼠存在肠道黏膜损伤,导致肠道屏障功能障碍,肠通透性增加。并且Occludin作为重要的回肠上皮细胞间紧密连接蛋白之一,在模型组表达明显下调,也提示肠道屏障功能损伤,这都将使血清ET水平升高。而ET随血进入肝脏,刺激炎症因子及生物活性物质释放,进而导致肝脏炎症与坏死[16]。Miele等[17]研究也发现NAFLD患者肠通透性较健康者明显增加,存在明显的小肠菌群过度生长。Friedman[18]认为NAFLD后的肠黏膜屏障损伤,肠通透性增加,导致肠内细菌及毒素入侵机体,诱导炎性反应,促进炎性因子和炎性细胞在肝脏蓄积,这成为NASH发生的重要因素。因此,肠屏障功能异常及通透性增加与NASH密切相关[19]。
近来研究发现TFF3是一个重要的黏膜保护因子。许多疾病状态下,肠黏膜损伤,TFF3表达异常。Hensel等[20]研究儿科炎症性肠病患者,发现伴有炎症的患儿其肠黏膜组织TFF3和MUC2表达显著降低,而无炎症的患儿其表达较健康者水平高。Renes等[21]也发现,在葡聚糖硫酸钠诱导的肠损伤模型上,肠道急性炎性反应和缓解再生阶段,杯状细胞表达 TFF3 mRNA和蛋白明显增多。本研究中NASH大鼠存在明显回肠损伤及炎症,可能是由于TFF3表达异常。TFF3主要由肠道上皮杯状细胞合成并分泌[2],而PAS染色结果显示模型组回肠杯状细胞稀疏,萎缩甚至消失,数量较正常组明显减少。NASH大鼠回肠杯状细胞受损,数量减少,进而合成分泌的TFF3减少。免疫组化染色结果也显示NASH大鼠TFF3表达减少,回肠黏液层稀薄。
TFF3保护肠道有多种机制。其与MUC2共同组成肠上皮黏液层,形成一层保护膜,将肠上皮与肠内环境隔离,成为肠内第一道保护屏障[22],有效的阻止了消化酶、机械应力、酸、肠微生物等对组织损伤。黏液层可分为内外两层:内层比较致密,含细菌少,主要起到保护作用。有研究[23]提出,黏液层只能透过2 μm物质,因而较大物质被阻挡在肠腔内,较小的营养分子则可透过黏液层被上皮细胞吸收。另外在内层黏液中,混有潘氏细胞分泌的抗菌肽和抗菌素,能抑制细菌在黏液内层定植与生长[24]。外层黏液较稀薄,界限不清,是肠道菌群生长的理想场所,因此定居着大量的正常菌群[25]。它们在抑制肠内有害细菌的定植与生长和调节肠内生物屏障方面发挥重要作用。研究[26]发现,在烧伤刺激作用下,杯状细胞结构受损,数量减少,黏液分泌明显减少。另外,肠道上皮的慢性炎症反应也出现杯状细胞减少,MUC2和TFF3分泌减少,有害细菌易穿透黏液层定植在肠上皮而导致黏膜细胞损伤[27]。而在TFF3基因缺陷的小鼠中,发现肠道黏液层稀薄、通透性增高,对肠道损伤敏感性增强,其死亡率也明显升高[28-29]。通过补充TFF3,能明显改善TFF3缺陷导致的肠道损伤,而作为肠道屏障功能密切相关的紧密连接蛋白claudin-1和ZO-1,有研究[30]发现TFF3能促进其表达,调节肠屏障功能,进而改善血小板活化因子诱导的肠黏膜通透性改变。
本实验给予NASH大鼠rhTFF3处理后,回肠末端病理改变明显减轻,紧密连接蛋白Occludin表达明显增多,肠通透性改善,血清ET水平降低。另外黏膜杯状细胞增多,回肠末端组织TFF3 mRNA表达较模型组明显上调。表明TFF3能促进回肠黏膜损伤修复及杯状细胞增生,杯状细胞合成分泌功能增强,进而改善肠道屏障功能,降低肠通透性,减少ET入血。而治疗组大鼠血清 AST、ALT、TG、TC水平较模型组明显下降,肝组织脂肪变性及炎症减轻,NAS评分明显降低,提示TFF3可以改善NASH大鼠肝脏损伤,对NASH起到了治疗作用。但是,TFF3具体作用机制还不清楚,其对肝脏的直接影响也有待进一步研究。
[1] LOOMBA R, SANYAL AJ. The global NAFLD epidemic[J]. Nat Rev Gastroenterol Hepatol, 2013, 10(11): 686-690.
[2] HOFFMANN W, JAGLA W, WIEDE A. Molecular medicine of TFF-peptides: from gut to brain[J]. Histol Histopathol, 2001, 16(1): 319-334.
[3] GE H, GARDNER J, WU X, et al. Trefoil factor 3 (TFF3) is regulated by food intake, improves glucose tolerance and induces mucinous metaplasia[J]. PLoS One, 2015, 10(6): e0126924.
[4] GAO F, PAN S, LIU B, et al. TFF3 knockout in human pituitary adenoma cell HP75 facilitates cell apoptosis via mitochondrial pathway[J]. Int J Clin Exp Pathol, 2015, 8(11): 14568-14573.
[5] ARNOLD P, RICKERT U, HELMERS AK, et al. Trefoil factor 3 shows anti-inflammatory effects on activated microglia[J]. Cell Tissue Res, 2016, 365(1): 3-11.
[6] LAU WH, PANDEY V, KONG X, et al. Trefoil factor-3 (TFF3) stimulates de novo angiogenesis in mammary carcinoma both directly and indirectly via IL-8/CXCR2[J]. PLoS One, 2015, 10(11): e0141947.
[7] XU ZJ, FAN JG, WANG GL, et al. Low dose of endotoxin induced acute liver failure in obese rats with nonalcoholic steatohepfitis[J].Chin J Hepatol, 2004, 12(4): 57-58.(in Chinese) 徐正婕, 范建高, 王国良, 等. 小剂量内毒素导致非酒精性脂肪性肝炎大鼠急性肝功能衰竭[J]. 中华肝脏病杂志, 2004, 12(4): 57-58.
[8] LI J, LUO Y, ZHANG R, et al. Neuropeptide trefoil factor 3 reverses depressive-like behaviors by activation of BDNF-ERK-CREB signaling in olfactory bulbectomized rats[J]. Int J Mol Sci, 2015, 16(12): 28386-28400.
[9] SHI L, ZHOU PH, XI JL, et al. Recombinant human trefoil factor 3 ameliorates bowel injury: its anti-inflammatory effect on experimental necrotizing enterocolitis[J]. Int J Pept, 2014, 2014: 634135.
[10] KWAK DS, LEE OY, LEE KN, et al. The effect of DA-6034 on intestinal permeability in an indomethacin-induced small intestinal injury model[J]. Gut Liver, 2016, 10(3): 406-411.
[11] The Chinese National Workshop on Fatty Liver and Alcoholic Liver disease for the Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition[J]. J Clin Hepatol, 2010, 26(2): 120-124.(in Chinese) 中华医学会肝病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南(2010年修订版)[J]. 临床肝胆病杂志, 2010, 26(2): 120-124.
[12] BERGSTROM KS, GUTTMAN JA, RUMI M, et al. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen[J]. Infect Immun, 2008, 76(2): 796-811.
[13] PAOLELLA G, MANDATO C, PIERRI L, et al. Gut-liver axis and probiotics: their role in non-alcoholic fatty liver disease[J]. World J Gastroenterol, 2014, 20(42): 15518-15531.
[14] PAGLIALUNGA S, DEHN CA. Clinical assessment of hepatic de novo lipogenesis in non-alcoholic fatty liver disease[J]. Lipids Health Dis, 2016, 15(1): 159.
[15] WANG B, SONG HY, YANG JR. The progress on the researches of intestinal barrier function changing in patients with liver diseases[J]. J Clin Hepatol, 2011, 27(8): 893-896. (in Chinese) 王波, 宋怀宇, 杨建荣. 肝病患者肠道屏障功能变化的研究进展[J]. 临床肝胆病杂志, 2011, 27(8): 893-896.
[16] LI CM, ZHUANG LW. Research progress in role of myosin light chain kinase in pathogenesis of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2015, 31(7): 1156-1159. (in Chinese) 李春苗, 庄丽维. 肌球蛋白轻链激酶在非酒精性脂肪性肝病发病机制中的作用[J]. 临床肝胆病杂志, 2015, 31(7): 1156-1159.
[17] MIELE L, VALENZA V, LA TORRE G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease[J]. Hepatology, 2009, 49(6): 1877-1887.
[18] FRIEDMAN SL. Liver fibrosis in 2012: convergent pathways that cause hepatic fibrosis in NASH[J]. Nat Rev Gastroenterol Hepatol, 2013, 10(2): 71-72.
[19] ROH YS, SEKI E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis[J]. J Gastroenterol Hepatol, 2013, 28(Suppl 1): 38-42.
[20] HENSEL KO, BOLAND V, POSTBERG J, et al. Differential expression of mucosal trefoil factors and mucins in pediatric inflammatory bowel diseases[J]. Sci Rep, 2014, 4: 7343.
[21] RENES IB, VERBURG M, van NISPEN DJ, et al. Distinct epithelial responses in experimental colitis: implications for ion uptake and mucosal protection[J]. Am J Physiol Gastrointest Liver Physiol, 2002, 283(1): g169-g179.
[22] JOHANSSON ME, GUSTAFSSON JK, HOLMEN-LARSSON J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis[J]. Gut, 2014, 63(2): 281-291.
[23] ERMUND A, SCHUTTE A, JOHANSSON ME, et al. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer′s patches[J]. Am J Physiol Gastrointest Liver Physiol, 2013, 305(5): g341-g347.
[24] VAISHNAVA S, YAMAMOTO M, SEVERSON KM, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine[J]. Science, 2011, 334(6053): 255-258.
[25] JOHANSSON ME, LARSSON JM, HANSSON GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions[J]. Proc Natl Acad Sci U S A, 2011, 108(Suppl 1): 4659-4665.
[26] PENG X, WANG SL, WANG FJ, et al. Effects of different nutrition support route on changes in intestinal mucus components after burn injury[J]. Parenter Enteral Nutr, 2001, 8(3): 157-160.(in Chinese) 彭曦, 汪仕良, 王凤君, 等. 不同营养支持途径对严重烧伤后大鼠肠黏液成分的影响[J]. 肠外与肠内营养, 2001, 8(3): 157-160.
[27] LIDELL ME, MONCADA DM, CHADEE K, et al. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel[J]. Proc Natl Acad Sci U S A, 2006, 103(24): 9298-9303.
[28] PODOLSKY DK, GERKEN G, EYKING A, et al. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency[J]. Gastroenterology, 2009, 137(1): 209-220.
[29] BECK PL, WONG JF, LI Y, et al. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor[J]. Gastroenterology, 2004, 126(3): 796-808.
[30] XU LF, TENG X, GUO J, et al. Protective effect of intestinal trefoil factor on injury of intestinal epithelial tight junction induced by platelet activating factor[J]. Inflammation, 2012, 35(1): 308-315.
引证本文:LIANG K, KONG WZ, CHEN J, et al. Effect of trefoil factor 3 on intestinal mucous barrier in rats with nonalcoholic steatohepatitis[J]. J Clin Hepatol, 2017, 33(8): 1552-1557. (in Chinese) 梁凯, 孔维宗, 陈娟, 等. 肠三叶因子对非酒精性脂肪性肝炎大鼠回肠黏液屏障的影响[J]. 临床肝胆病杂志, 2017, 33(8): 1552-1557.
(本文编辑:葛 俊)
Effect of trefoil factor 3 on intestinal mucous barrier in rats with nonalcoholic steatohepatitis
LIANGKai,KONGWeizong,CHENJuan,etal.
(DalianUniversity,Dalian,Liaoning116001,China)
Objective To investigate the change in intestinal mucous barrier in rats with nonalcoholic steatohepatitis (NASH), the effect of trefoil factor 3 (TFF3) on intestinal mucous barrier in NASH rats, and the therapeutic effect of TFF3 on NASH. Methods A total of 60 clean male Sprague-Dawley rats were randomly divided into normal group, model group, and treatment group, with 20 rats in each group. The rats in the normal group were given normal diet, and those in the model group and the treatment group were given high-fat diet to induce NASH. The rats in the treatment group were given intraperitoneal injection of rhTFF3 at a dose of 1 ml·kg-1·d-1(a concentration of 0.1 mg/ml), and those in the normal group and the model group were given normal saline at a dose of 1 ml·kg-1·d-1; the course of treatment was 3 weeks for all groups. At the end of week 15, fluorescein isothiocyanate-labeled dextran was given by gavage to evaluate intestinal permeability, and after the rats were sacrificed, serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), and endotoxin (ET) and diamine oxidase (DAO) activity were measured. HE staining was performed to observe the histopathological changes of the liver and the terminal ileum, PAS staining was performed to observe and count the goblet cells of the terminal ileum, immunohistochemistry was used to measure the expression of the tight junction protein Occludin and TFF3 in the terminal ileum, and quantitative real-time PCR was used to measure the mRNA transcription level of TFF3. A one-way analysis of variance was used for comparison between multiple groups, and the least significant differencet-test was used for further comparison between any two groups. Results The model group had significant increases in serum levels of AST, ALT, TC, TG, and ET and DAO activity, and the treatment group had significant reductions compared with the model group (allP<0.01). The model group had a significant increase in NAFLD activity score compared with the normal group (P<0.01), and the treatment group had significant improvement in liver inflammation and a significant reduction in NAFLD activity score compared with the model group (P<0.01). The model group had cell necrosis, damage of the intestinal villi, and a significant reduction in goblet cells in the terminal ileum under a light microscope; in the treatment group, damage of the intestinal villi was repaired and there was an increase in goblet cells. The model group had a significant increase in intestinal permeability compared with the normal group, and the treatment group had a significant reduction compared with the model group (bothP<0.01). The model group had a significant reduction in the expression of Occludin and TFF3, and the treatment group had a significant increase compared with the model group (allP<0.01). The model group had a downregulated TFF3 mRNA transcription level in the terminal ileum compared with the normal group, and the treatment group had an upregulated level compared with the model group (bothP<0.01). Conclusion NASH rats have damaged goblet cells and mucous barrier dysfunction. TFF3 can repair the damaged terminal ileum, promote the regeneration of goblet cells and mucus secretion, restore intestinal barrier function, reduce intestinal permeability, and thus exert its therapeutic effect on NASH.
fatty liver; trefoil factor 3; goblet cells; rats, Sprague-Dawley
10.3969/j.issn.1001-5256.2017.08.029
2017-02-22;
2017-03-26。
梁凯(1990-),男,主要从事非酒精性脂肪性肝病研究。
王迎春,电子信箱:wych_1648@126.com。
R575.5
A
1001-5256(2017)08-1552-06
