Comparison of cytological and histological preparations in the diagnosis of pancreatic malignancies using endoscopic ultrasoundguided fi ne needle aspiration
2017-08-16DongKeeJangSangHyubLeeJunKyuLeeWooHyunPaikKwangHyunChungBanSeokLeeJunHyukSonJaeWooLeeJiKonRyuYongTaeKimandKyoungBunLee
Dong Kee Jang, Sang Hyub Lee, Jun Kyu Lee, Woo Hyun Paik, Kwang Hyun Chung, Ban Seok Lee, Jun Hyuk Son, Jae Woo Lee, Ji Kon Ryu, Yong-Tae Kim and Kyoung-Bun Lee
Seoul, Korea
Comparison of cytological and histological preparations in the diagnosis of pancreatic malignancies using endoscopic ultrasoundguided fi ne needle aspiration
Dong Kee Jang, Sang Hyub Lee, Jun Kyu Lee, Woo Hyun Paik, Kwang Hyun Chung, Ban Seok Lee, Jun Hyuk Son, Jae Woo Lee, Ji Kon Ryu, Yong-Tae Kim and Kyoung-Bun Lee
Seoul, Korea
BACKGROUND:Endoscopic ultrasound-guided fi ne needle aspiration (EUS-FNA) has become a crucial diagnostic technique for pancreatic malignancies. The specimen obtained by EUS-FNA can be prepared for either cytological or histological examinations. This study was to compare diagnostic performance of cytological and histological preparations using EUSFNA in the same lesions when pancreatic malignancies were suspected.
METHODS:One hundred and eighteen patients who underwent EUS-FNA for suspected pancreatic malignancies were consecutively enrolled. All procedures were conducted by a single echoendoscopist under the same conditions. Four adequate preparations were obtained by 22-gauge needles with 20 to-and-fro movements for each pass. The 4 preparations included 2 cytological and 2 histological specimens. The pathologic reviews of all specimens were conducted independently by a single experienced cytopathologist. Sensitivity, specif i city, and accuracy of the 2 preparations were compared.
RESULTS:The enrolled patients consisted of 62 males (52.5%), with the mean age of 64.6±10.5 years. Surgery was performed in 23 (19.5%) patients. One hundred and sixteen (98.3%) lesions were classif i ed as malignant, while 2 (1.7%) were benign. Sensitivity of cytology and histology were 87.9% and 81.9%, respectively, with no signif i cant difference (P=0.190). Accuracy was also not signif i cantly different. Cytological preparation was more sensitive when the size of lesion was <3 cm (86.7% vs 68.9%,P=0.033).
CONCLUSIONS:Our results suggested that the diagnostic performances of cytological and histological preparations are not signif i cantly different for the diagnosis of pancreatic malignancies. However, cytological preparation might be more sensitive for pancreatic lesions <3 cm.
(Hepatobiliary Pancreat Dis Int 2017;16:418-423)
endoscopic ultrasound-guided fi ne needle aspiration; pancreatic neoplasms; cytology; pathology; histology
Introduction
Currently, endoscopic ultrasound-guided fi neneedle aspiration (EUS-FNA) is an established standard method for tissue diagnosis in patients with suspected pancreatic neoplasm.[1-3]The recently reported sensitivity, specif i city, and accuracy of EUS-FNA were 77%-94.3%, 85.9%-100% and 80%-95%, respectively.[4-7]Many factors such as the sample size, def i nition of positive results, types of neoplasm, and methods of tissue sampling may affect these values. Accurate, prompt diagnosis of pancreatic neoplasms is essential in directing surgical or medical treatment of patients,because pancreatic cancer has a poor prognosis. Hence, there have been various efforts to improve the diagnostic performance of EUS-FNA, including development of needles or helpful techniques, and accompanying on-site cytopathologists.[8-13]
The two methods of tissue sampling with the same needle in EUS-FNA differ in the way the samples are processed. In cytological preparation, an aspirate of cells is smeared on a slide and fi xed in alcohol; while in histological preparation, aspirated cells fi xed in formalin are sectioned from a paraff i n block. The quantity and quality of the collected materials often affect the preference. Smear cytological examination is a traditional and standard method for cytological diagnosis, which should be interpreted by an experienced cytopathologist.[3,11,12]Histological preparations provide more pathological information, especially when combined with immunohistochemistry (IHC). The IHC is remarkably useful for the discovery of non-morphological markers.[14-17]These two methods are considered complementary, according to previous studies.[18-20]However, little is known about the specif i c contribution of each method in the same target lesion. The aim of this study was to compare diagnostic performance of cytological and histological preparations using EUS-FNA in the same lesions when pancreatic malignancies were suspected. We also attempted to determine the superior method under certain circumstances.
Methods
Patients and study design
The patients who underwent EUS-FNA for suspected pancreatic malignancies at Seoul National University Hospital between September 2013 and February 2015 were consecutively enrolled. Patients who had pure cystic neoplasms or specimens obtained by methods other than our study protocol were excluded. We reviewed patients’entire medical records, EUS-FNA results, and pathology reports. This study was approved by the Institutional Ethics Review Board of Seoul National University Hospital and conducted in accordance with theDeclaration of Helsinki(IRB No. 1505-033-670).
EUS-FNA procedures and cytopathological examinations
All EUS-FNA procedures in the included patients were performed by a single, experienced echoendoscopist (Lee SH) according to the following protocol with the same types of instruments. Linear array echoendoscope (GF-UCT240; Olympus Ltd., Tokyo, Japan) was used for EUS. FNA was performed with a 22-gauge needle (EchoTip® Ultra; Wilson Cook Medical, Winston-Salem, NC, USA) regardless of the lesions’ location. A transduodenal approach was applied for proximal lesions located in the head or uncinate process of the pancreas; whereas, a transgastric approach was used for distal lesions in the pancreatic body or tail. Each session included at least 4 needle passes to make 4 adequate preparations without an exception, and aspirated material by each pass was subjected to cytological (C) or histological (H) preparation in the pre-set order of C-H-H-C or H-C-C-H. The order of preparations was alternately assigned to each patient, making the fi nal proportion the same. In the cytological preparation, an aspirate was thinly smeared on microscope glass slides and immediately fi xed in 95% alcohol solution. In the histological preparation, aspirated material was placed in a container with 10% formaldehyde solution, which was then interpreted after appropriate staining such as hematoxylin and eosin stain. Needles were washed after each pass and fl ushed with air before reinsertion. Fanning technique was applied if possible, as described previously,[8]and 20 to-and-fro movements were performed in each needle pass with negative pressure created by a 10 mL syringe. There was no available on-site cytopathologists at our institution. The collected specimens were sent to the department of pathology for microscopic analysis. Pathologic reviews of all specimens were independently conducted and conf i rmed by a single experienced cytopathologist (Lee KB) after all of the patients were enrolled.
Def i nitions
Malignancy or suspicion of malignancy in the pathologic review was categorized as malignancy, while atypical or benign cells were categorized as benign. Final diagnosis was determined by the result of EUS-FNA itself or another biopsy of metastatic lesions or pathologic analysis of surgically resected specimens. Size was def i ned by the longest diameter measured by EUS. Lesions in the head or uncinate process of the pancreas were classif i ed as proximal lesions, while those in the body or tail were regarded as distal lesions.
Statistical analysis
Continuous data was expressed as mean±standard deviation, while categorical variables were presented as frequencies and percentages. Sensitivity, specif i city, and accuracy of the two preparations were compared by the McNemar Chi-square test.[21]All analyses were calculated with the statistics program IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA). A two-sidedPvalue of <0.05 was considered to indicate statistically signif i cant.
Results
Patient characteristics
This study enrolled 118 patients. Among them, 62 (52.5%) were male, and a mean age was 64.6±10.5 years (range 34-82). Table 1 shows the overall clinical characteristics of patients. The fi nal diagnosis included 116 (98.3%) malignant and 2 (1.7%) benign diagnosis. The preparation order was equally distributed to C-H-H-C and H-C-C-H, 50.0% for each. The clinical characteristics of the patient groups categorized by the preparation order are described in Table 2. There was no signif i cant difference between the two groups. Fig. shows a case of 70-year-old female patient diagnosed with pancreatic ductal adenocarcinoma by EUS-FNA in our study.
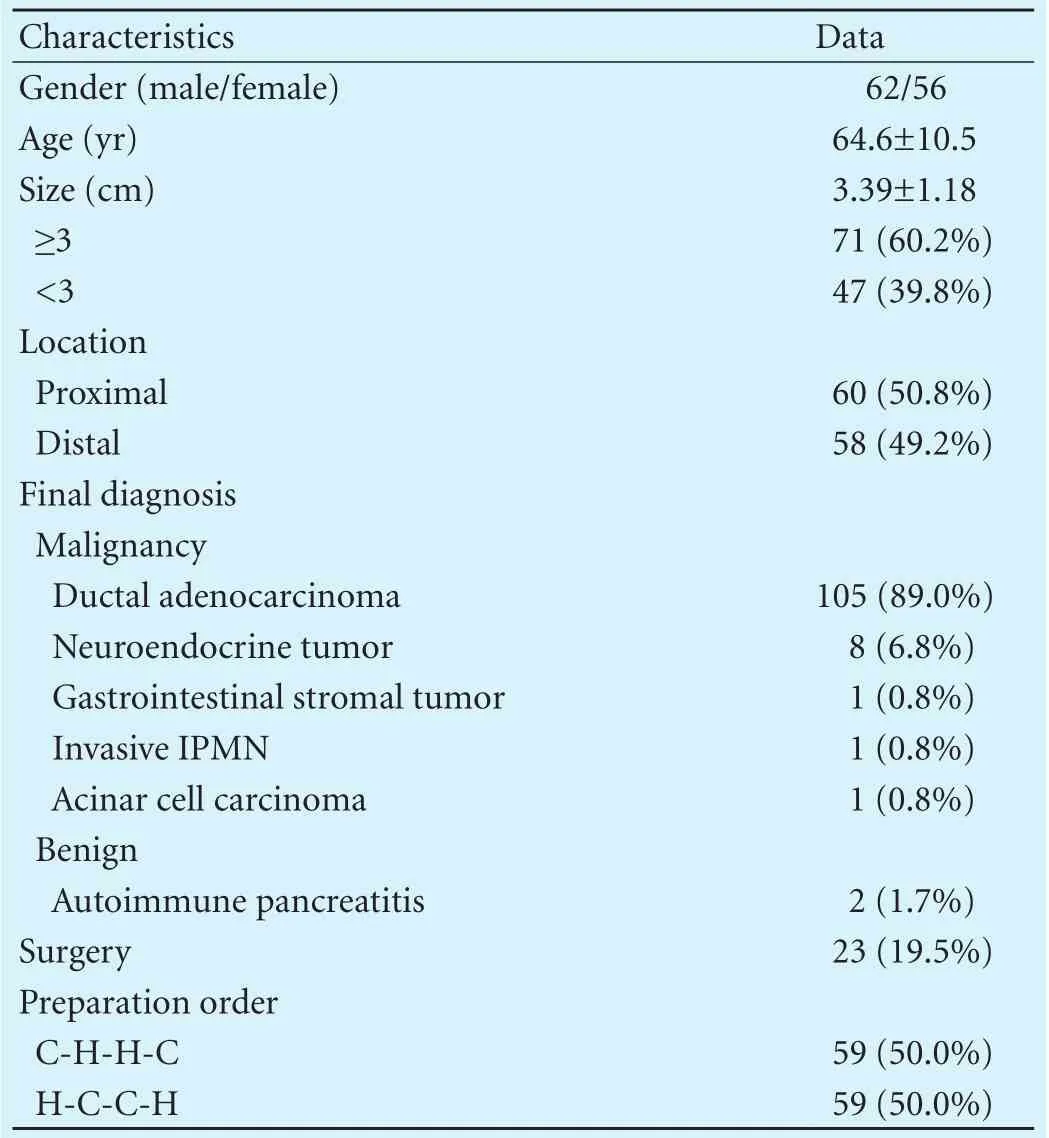
Table 1. Baseline characteristics (n=118)

Table 2. Clinical characteristics of patient groups categorized by the preparation order
Comparison of diagnostic performance according to the method of preparation
There were 2 inadequate samples in each preparation that were excluded in the analysis. Sensitivity, specif i city and accuracy of the two preparations are summarized in Table 3. Sensitivity of cytology and histology were 87.9% and 81.9%, respectively, with no signif i cant difference (P=0.190). Accuracy was also not signif i cantly different. Combining these two preparations, sensitivity and accuracy increased up to 94.0% and 94.1%, respectively. In the subgroup analysis, a signif i cant difference of sensitivity was observed in the lesion of size <3 cm (Table 4). Cytological preparation was more sensitive when the size of lesion was <3 cm. Other parameters such as location,type of malignancy, and preparation order did not affect the difference of sensitivity. IHC was performed in 12 (10.2%) patients. Most cases did not depend on the result of IHC alone for the fi nal diagnosis, except for 2 cases that were conf i rmed by IHC. The 2 cases were neuroendocrine tumor and gastrointestinal stromal tumor.
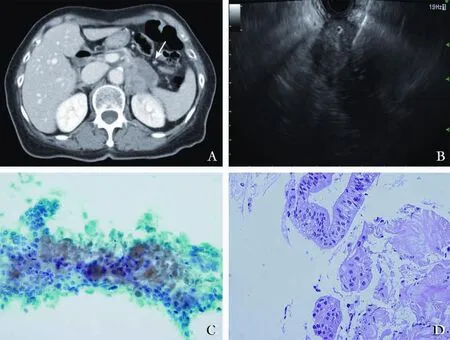
Fig. A case of 70-year-old female patient diagnosed with pancreatic ductal adenocarcinoma by EUS-FNA. A: Abdomen computed tomography scans revealed a 4-cm-sized presumed pancreatic tail cancer (arrow). B: EUS-FNA was performed according to our preset protocol. C: Some sheets of adenocarcinoma cells with nuclear atypia are observed in the EUS-FNA cytological preparation (Papanicolaou and Diff-Quick stain, original magnification ×400). D: Malignant glands with desmoplasia are demonstrated in the EUS-FNA histological preparation (Hematoxylin and eosin stain, original magnif i cation ×400).
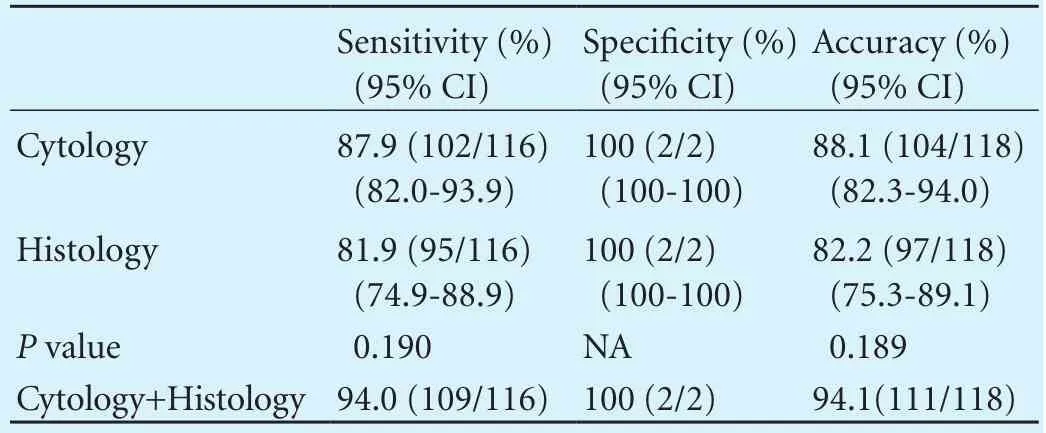
Table 3. Comparison between cytological and histological preparations (per-lesion analysis)
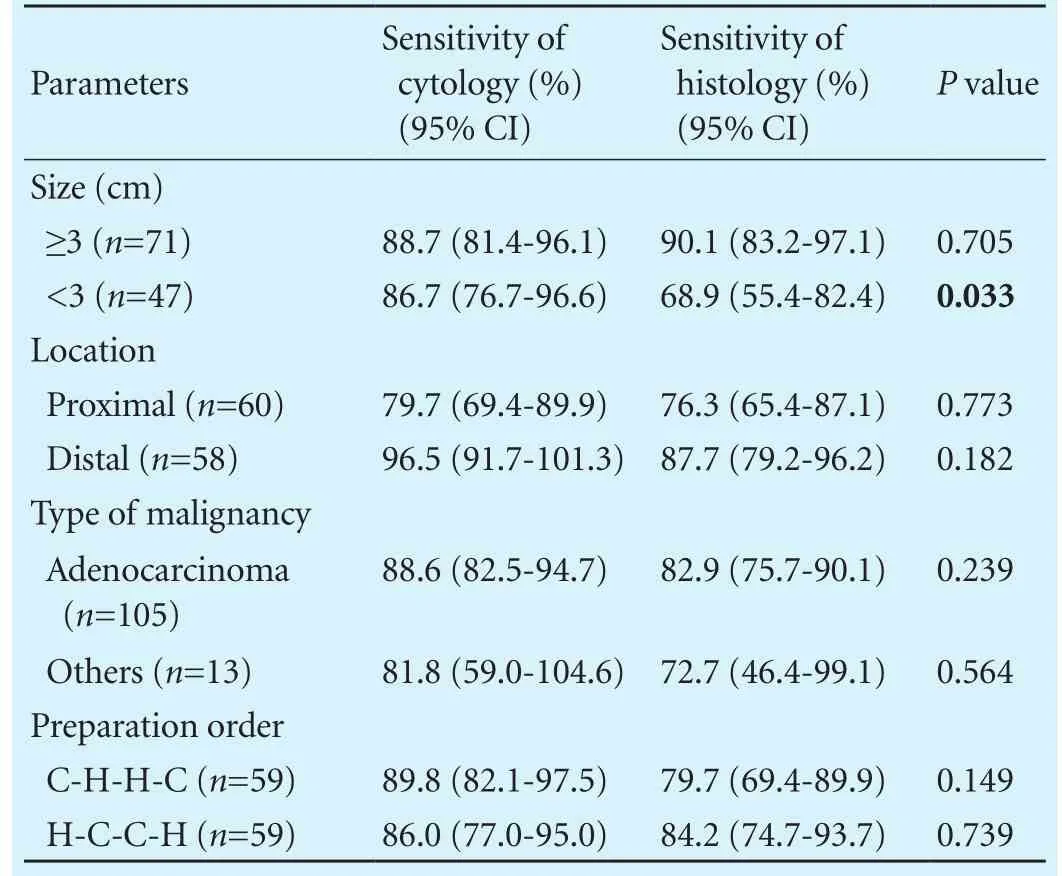
Table 4. A subgroup analysis for the difference of sensitivity between cytological and histological preparations (per-lesion analysis)
Discussion
EUS-FNA is currently an essential procedure for the diagnosis of pancreatic malignancies. However, its diagnostic eff i cacy varies based on several factors. In the present study, we focused on the methods of specimen preparation in EUS-FNA, and evaluated the diagnostic eff i cacy of cytological and histological preparation in consecutive patients with suspected pancreatic malignancies. This study showed that the diagnostic eff i cacy was not signif icantly different between two methods, and the combination resulted in the improvement of accuracy, as well as sensitivity for the diagnosis of pancreatic malignancies.
The number of needle passes was determined by a previous report,[22]while that of to-and-fro movements were based on the operator’s experience because of lack of reliable previous data. Although more needle passes are more likely to yield better accuracy, needles usually wear out after a few passes.[19]Accordingly, 4 needle passes is considered the most appropriate number in clinical situations based on the results of the previous study.[22]The type of needle was also identical regardless of lesions’location. Our results demonstrated that the diagnostic eff i cacy of the cytological and histological preparation was not signif i cantly different in the diagnosis of pancreatic malignancies in terms of sensitivity, specif i city, and accuracy. Particularly, the sensitivity was signif i cantly higher in the cytological preparation when a lesion was<3 cm in size. In addition, the combination of two methods resulted in a noteworthy improvement of sensitivity and accuracy, which suggests that the two methods are complementary for accurate diagnosis. However, this interpretation should be accepted cautiously because we did not have combinations of single preparation such as C-C-C-C or H-H-H-H. Nevertheless, in restricted conditions, cytological preparation might be better when a lesion is <3 cm in size.
Previous studies[19,23,24]showed conf l icting results regarding the diagnostic eff i cacy. Kopelman et al[19]reported that EUS-FNA cytology was more sensitive than cell block histology but less specif i c for the diagnosis of pancreatic lesions. They explained that cytological preparations enable us to interpret cancerous characteristics of single cells sensitively, and become less specif i c when inf l ammatory changes are present. However, they could not directly compare the two methods because not all patients had both preparations. On the other hand, Noda et al[23]reported that histological method showed a signif i cantly higher diagnostic accuracy (93.9% vs 60.6%) and sensitivity (92.0% vs 60.0%) than smear cytology in 33 patients. They performed IHC for all histological specimens, which probably resulted in higher eff i cacy. Recently, Qin et al[24]reported the results of comparative study on the diagnostic eff i cacy of cell block IHC, smear cytology, and liquid-based cytology in EUS-FNA of pancreatic lesions. They suggested that cell block IHC offers a higher diagnostic eff i cacy than smear cytology and liquid-based cytology. These conf l icting results are probably caused by their various different settings. In contrast, our results were obtained under the homogenous conditions so that accurate comparison could be guaranteed.
In our study, although the diagnostic performances of the two methods were not signif i cantly different, cytology showed better sensitivity and accuracy. This result might be affected by pathologists’ expertise or experience.[4]Accompanying on-site cytopathologist or obtaining both cytological and histological preparations, if possible, leads to a satisfactory diagnostic yield.[4,10,11,18-20,23-25]However, we frequently encounter cases wherein both preparations are not possible due to lack of time or tissue amount. There also may be a cost burden for both preparations. Furthermore, an on-site cytological evaluation is usually unavailable in most centers including our institution. Therefore, if we are to select just one method, cytology seems to be better when a lesion is <3 cm in size in viewof the result of our subgroup analysis. Considering small amount of tissue is not feasible for histological evaluation, cytology might be more sensitive for a small lesion in which to-and-fro movement is limited. All aspirated materials are smeared in the cytological preparation, while some sections may not include malignant portion in the histological preparation. This happens more readily in the small lesions. A previous report also indicated that cytology yielded better results for lesions <2 cm.[19]
The present study had several limitations. First, our results might not be accepted generally because the capability of echoendoscopists or pathologists varies with institutions. To minimize variety, the single specialist was involved in the EUS-FNA procedure and pathological examinations in our study. Second, the number of preparation orders was just 2, i.e., C-H-H-C or H-C-C-H. The other 4 combinations were not applied. Therefore, these two orders did not represent random distribution of the preparation methods. Third, the number of benign cases was too small to compare specif i city and positive or negative predictive values between the methods. With advances in the interpretation of radiologic images, fi nal benign cases become rare when pancreatic malignancies are suspected in the initial radiologic imaging. Accordingly, benign cases tend to be rare in the clinical settings. Fourth, “indeterminate” cytology specimens were excluded, which might affect the rate of accuracy. Efforts such as cell block procedures with IHC may help to classify those specimens correctly.[26,27]Despite these limitations, our study is still valuable for its strictly controlled condition; otherwise variable factors including an EUSFNA protocol, operators, or instruments could have infl uenced the results. We tried to maintain the same conditions except the method of tissue preparation in each needle pass. All procedures were performed by the single echoendoscopist, with 4 needle passes and 20 to-and-fro movements in the same lesions.
Contributors: LSH proposed the study. JDK and LSH performed the research and wrote the fi rst draft. LKB performed independent pathological reviews. CKH, LBS, SJH and LJW collected the data. LJK, PWH, RJK and KYT analyzed the data. All authors contributed to the design and interpretation of the study. LSH is the guarantor.
Funding: None.
Ethical approval: This study was approved by the Institutional Ethics Review Board of Seoul National University Hospital and conducted in accordance with theDeclaration of Helsinki(IRB No. 1505-033-670).
Competing interest: No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2011;43:897-912.
2 ASGE Standards of Practice Committee, Eloubeidi MA, Decker GA, Chandrasekhara V, Chathadi KV, Early DS, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc 2016;83:17-28.
将充分发挥党组织领导把关作用与发扬民主结合起来,将平时考核与年度考核结合起来,将干部考核与任职考察结合起来,发挥各种考核机制的叠加效力。坚持全面地看干部,把“识德”和“识才”统一起来,既从细微处辨德才,更在大事上识德才;坚持历史地看干部,把“一时”和“一贯”统一起来;坚持辩证地看干部,既看干部的一贯表现,又观察干部落实重大决策部署、完成重点工作任务、解决突出矛盾的表现。
3 Pitman MB, Layf i eld LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: a review. Cancer Cytopathol 2014;122:399-411.
4 Bergeron JP, Perry KD, Houser PM, Yang J. Endoscopic ultrasound-guided pancreatic fi ne-needle aspiration: potential pitfalls in one institution’s experience of 1212 procedures. Cancer Cytopathol 2015;123:98-107.
5 Fisher L, Segarajasingam DS, Stewart C, Deboer WB, Yusoff IF. Endoscopic ultrasound guided fi ne needle aspiration of solid pancreatic lesions: performance and outcomes. J Gastroenterol Hepatol 2009;24:90-96.
6 Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012;75:319-331.
7 Turner BG, Cizginer S, Agarwal D, Yang J, Pitman MB, Brugge WR. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc 2010;71:91-98.
8 Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fi ne-needle aspiration of solid pancreatic mass lesions. Endoscopy 2013;45:445-450.
9 Costache MI, Iordache S, Karstensen JG, Săftoiu A, Vilmann P. Endoscopic ultrasound-guided fi ne needle aspiration: from the past to the future. Endosc Ultrasound 2013;2:77-85.
10 Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fi ne needle aspiration cytology for pancreatic adenocarcinoma:a meta-analysis. Cytopathology 2013;24:159-171.
11 Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fi ne needle aspiration. Am J Gastroenterol 2003;98:1289-1294.
12 Layf i eld LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fi ne-needle aspiration smears: a cost and compensation analysis. Cancer 2001;93:319-322.
13 Matsubayashi H, Matsui T, Yabuuchi Y, Imai K, Tanaka M, Kakushima N, et al. Endoscopic ultrasonography guided-f i ne needle aspiration for the diagnosis of solid pancreaticobiliary lesions: clinical aspects to improve the diagnosis. World J Gastroenterol 2016;22:628-640.
14 Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its eff i cacy in diagnostic cytology. Am J Clin Pathol 2000;114:599-606.
15 Puleo F, Demetter P, Eisendrath P, Maréchal R, Verset L, Toussaint E, et al. Feasibility of immunohistochemistry on endoscopic ultrasound fi ne-needle aspiration samples for evaluating predictive biomarkers in pancreatic cancer management. Pancreas 2016;45:e50-52.
16 Kasajima A, Yazdani S, Sasano H. Pathology diagnosis of pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci 2015;22:586-593.
17 Lin F, Chen ZE, Wang HL. Utility of immunohistochemistry in the pancreatobiliary tract. Arch Pathol Lab Med 2015;139:24-38.
18 Kim TH, Choi KH, Song HS, Kim JW, Jeon BJ. Histology combined with cytology by endoscopic ultrasound-guided fi ne needle aspiration for the diagnosis of solid pancreatic mass and intra-abdominal lymphadenopathy. Gut Liver 2013;7:605-610.
19 Kopelman Y, Marmor S, Ashkenazi I, Fireman Z. Value of EUSFNA cytological preparations compared with cell block sections in the diagnosis of pancreatic solid tumours. Cytopathology 2011;22:174-178.
20 Möller K, Papanikolaou IS, Toermer T, Delicha EM, Sarbia M, Schenck U, et al. EUS-guided FNA of solid pancreatic masses:high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc 2009;70:60-69.
21 Hawass NE. Comparing the sensitivities and specif i cities of two diagnostic procedures performed on the same group of patients. Br J Radiol 1997;70:360-366.
22 Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Sato A, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fi ne-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc 2012;24:452-456.
23 Noda Y, Fujita N, Kobayashi G, Itoh K, Horaguchi J, Takasawa O, et al. Diagnostic eff i cacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fi ne-needle aspiration. J Gastroenterol 2010;45:868-875.
24 Qin SY, Zhou Y, Li P, Jiang HX. Diagnostic eff i cacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fi ne-needle aspiration of pancreatic lesions: a single-institution experience. PLoS One 2014;9:e108762.
25 Haba S, Yamao K, Bhatia V, Mizuno N, Hara K, Hijioka S, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fi ne needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol 2013;48:973-981.
26 Ieni A, Barresi V, Todaro P, Caruso RA, Tuccari G. Cell-block procedure in endoscopic ultrasound-guided-f i ne-needleaspiration of gastrointestinal solid neoplastic lesions. World J Gastrointest Endosc 2015;7:1014-1022.
27 Ieni A, Todaro P, Crino SF, Barresi V, Tuccari G. Endoscopic ultrasound-guided fi ne-needle aspiration cytology in pancreaticobiliary carcinomas: diagnostic eff i cacy of cell-block immunocytochemistry. Hepatobiliary Pancreat Dis Int 2015;14:305-312.
November 13, 2016
Accepted after revision March 15, 2017
Author Aff i liations: Department of Internal Medicine, Dongguk University Ilsan Medical Center, Goyang-si, Gyeonggi-do, Korea (Jang DK and Lee JK); Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea (Jang DK, Lee SH, Paik WH, Chung KH, Lee BS, Lee JW, Ryu JK and Kim YT); Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang-si, Gyeonggi-do, Korea (Son JH); Department of Pathology, Seoul National University College of Medicine, Seoul, Korea (Lee KB)
Sang Hyub Lee, MD, PhD, Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea (Tel: +82-2-2072-4892; Fax: +82-2-762-9662; Email: gidoctor@snuh.org)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60035-3
Published online June 30, 2017.
猜你喜欢
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Indocyanine green fl uoroscopy and liver transplantation: a new technique for the intraoperative assessment of bile duct vascularization
- Effects of multimodal fast-track surgery on liver transplantation outcomes
- Characteristics of recipients with complete immunosuppressant withdrawal after adult liver transplantation
- Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
- Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment
- Interaction between insulin-like growth factor binding protein-related protein 1 and transforming growth factor beta 1 in primary hepatic stellate cells
