Minimally invasive and open gallbladder cancer resections: 30- vs 90-day mortality
2017-08-16NaeemGoussousMotaharHosseiniAnneSillandStevenCunningham
Naeem Goussous, Motahar Hosseini, Anne M Sill and Steven C Cunningham
Baltimore, USA
Minimally invasive and open gallbladder cancer resections: 30- vs 90-day mortality
Naeem Goussous, Motahar Hosseini, Anne M Sill and Steven C Cunningham
Baltimore, USA
BACKGROUND:Minimally invasive surgery is increasingly used for gallbladder cancer resection. Postoperative mortality at 30 days is low, but 90-day mortality is underreported.
METHODS:Using National Cancer Database (1998-2012), all resection patients were included. Thirty- and 90-day mortality rates were compared.
RESULTS:A total of 36 067 patients were identif i ed, 19 139 (53%) of whom underwent resection. Median age was 71 years and 70.7% were female. Ninety-day mortality following surgical resection was 2.3-fold higher than 30-mortality (17.1% vs 7.4%). There was a statistically signif i cant increase in 30- and 90-day mortality with poorly differentiated tumors, presence of lymphovascular invasion, tumor stage, incomplete surgical resection and low-volume centers (P<0.001 for all). Even for the 1885 patients who underwent minimally invasive resection between 2010 and 2012, the 90-day mortality was 2.8-fold higher than the 30-day mortality (12.0% vs 4.3%).
CONCLUSIONS:Ninety-day mortality following gallbladder cancer resection is signif i cantly higher than 30-day mortality. Postoperative mortality is associated with tumor grade, lymphovascular invasion, tumor stage, type and completeness of surgical resection as well as type and volume of facility.
(Hepatobiliary Pancreat Dis Int 2017;16:405-411)
gallbladder cancer; laparoscopic; minimally invasive; survival; radical cholecystectomy; complications
Introduction
Gallbladder cancer (GBC) is the most frequent malignancy of the biliary tree.[1]The annual incidence of GBC is around 1.13 cases per 100 000 making it the 5th most common cancer of the gastrointestinal tract,[2]especially in high-risk ethnic groups.[3]The overall 5-year survival, even after resection, is reported to be 5%-21%,[4-9]likely due to the aggressive nature of the cancer, delayed presentation, and lack of effective adjuvant therapy.
Complete resection of GBC with negative margins is the only potentially curative treatment for this malignancy. Curative-intent resection recommended for T1b and more invasive tumors[10,11]includes cholecystectomy withen-blocliver resection and portal lymphadenectomy. If the cystic-duct margin is involved, then excision of the extrahepatic biliary tree is necessary. Postoperative 30-day mortality following resection of GBC has been reported to be between 1.7% and 4.2%.[5,11]Minimally invasive surgery has long been shown to decrease length of hospital stay, recovery time, and complications following simple cholecystectomy. Laparoscopic and robotic radical cholecystectomy for GBC has been suggested to be safe, with equivalent outcomes to open resection, but generally 90-day morbidity and mortality is not reported.[12-15]
Indeed, 90-day postoperative mortality may be a more accurate marker of true postoperative mortality due to complications, since complication-related deaths are widely recognized to occur after 30 days (very few cancer-related deaths are expected within 90 days). Indeed, 90-day mortality has recently been shown to be almost double that of the 30-day mortality followingpancreatic resection for malignancy.[16]However, data on 90-day mortality following surgical resection of GBC are limited. We hypothesized that 90-day mortality would be signif i cantly higher than 30-day mortality following resection of GBC, but that this difference may be mitigated by minimally invasive surgery.
Methods
A retrospective review of the National Cancer Database (NCDB) was performed of all patients who were diagnosed with GBC between 1998 and 2012. NCDB is a clinical oncology database jointly sponsored by the American College of Surgeons (ACS) and the American Cancer Society. The database is a compilation of cancer registries of more than 1500 facilities accredited by the ACS Commission on Cancer and captures around 70% of the newly diagnosed cancers across the United States, including their treatments and outcomes.
Data for all patients who underwent surgical resection for a pathologically conf i rmed malignancy originating from the gallbladder were included. Patient comorbidities were evaluated using the Charlson/Deyo score.[17]The scores were truncated to 0, 1 and 2 where a score of 0 indicated no comorbidities, a score of 1 indicated one comorbid condition present and a score of 2 indicated more than one comorbid condition. Def i nitions for surgical resections were def i ned in NCDB and obtained from the Facility Oncology Registry Data Standards (FORDS).[18]Only patients with FORDS codes 30-60 were included, which thereby included only patients who underwent surgical resection of the gallbladder. Patients with pathologically proven malignancy originating from the gallbladder were selected as def i ned by the International Classif i cation of Diseases of Oncology (ICD-O-3) histology codes.[19]Patients with codes: 8160, 8161, 8162, 8170, 8180, 8430, 8453, 8500, 8503, 8504, 8507 were excluded as these malignant diseases were not believed to have originated from the gallbladder. Pathological stage of the tumor (pTNM) was def i ned using the 5th, 6th, or 7th edition of the American Joint Commission on Cancer based on the year when the tumor was diagnosed. Patients with a pathological tumor stage of pT0 and pTx were also excluded.
The stage of the tumor was def i ned using the NCDB analytic stage group which represents the pathological stage group if reported, or the clinical stage group if the pathological stage group is not recorded. Data on the surgical approach were available only for patients diagnosed between 2010 and 2012. Surgical approaches included open, minimally invasive (laparoscopic or robotic), and minimally invasive converted to open. Hospital volume was calculated as the number of cases of GBC performed at the facility during the entire study period. Annual hospital volume was calculated by dividing hospital volume by duration of the study. Hospital volume groups and annual hospital volume groups were divided based on the quartiles. Status of margins was def i ned as:R0 for complete resection, R1 for incomplete resection with microscopic residual disease, and R2 for incomplete resection with macroscopic residual disease.
Patient demographic data including: age, gender, race, insurance type, income, education and distance from hospital in miles were collected. Type and location of the facility where the surgery was performed were analyzed. Histological grade of the tumor, presence of lymphovascular invasion, pathological TNM stage, and the NCDB stage were obtained. Type of surgical procedures, surgical approach, margins and the performance of lymphadenectomy were reviewed. Thirty- and 90-day mortality was compared by demographic, facility-related, pathologic and surgical variables using Fisher’s exact and Chi-square tests.
Continuous variables are presented as median with interquartile range (IQR) and compared using the Wilcoxon two-sample test. Categorical variables are presented as number (percentage) and analyzed using the Fisher’s exact test. Statistical signif i cance was accepted atP<0.05. When comparing 30- and 90-day mortality, statistical signif i cance was def i ned as nonoverlapping 95% conf i dence intervals (CI).
Results
We identif i ed 36 067 patients in the NCDB with the diagnosis of GBC, 19 139 (53%) of whom underwent resection and were included in our study. The median age at diagnosis was 71 years (IQR: 62-79), and the majority were female (70.7%) and white (81.8%). The majority of the patients had insurance, either Medicare or private (59.6% and 29.0%, respectively) (Table 1).
Proportionally, GBC prevalence was greater in the South and Mid Atlantic regions and in the East North Central region (20.0%, 17.5% and 18.7%, respectively). The majority (53.5%) of operations were performed in comprehensive community cancer programs followed by academic and research programs (33.6%). The median number of resections performed by each facility during the study period was 21 cases (IQR: 13-34). Median annual hospital volume was 1.4 cases/hospital per year (IQR:0.87-2.26) (Table 1). There was a steady increase in the number of newly diagnosed cases of GBC each year between 1998 and 2012 (Fig.).
Tumor characteristics are shown in Table 2. The vastmajority (94.2%) of the tumors were invasive cancers, as opposed to 5.8% being carcinomain situ. Lymphovascular invasion was present in 40.6% of cases. Lymph node involvement was present in 25.5% of patients, absent in 47.0%, and unknown (Nx) in 27.5%. NCDB stage 4 disease was present in 22.8% of patients at the time of the diagnosis.

Fig. Trend of gallbladder cancer cases per year.
Of all the operations performed 70.6% were total cholecystectomies, 19.1% were partial cholecystectomies, 9.1% were radical cholecystectomies and 1.1% were debulking. Complete surgical excision def i ned as R0 was completed in 69.9% of the cases. Lymphadenectomy was performed in 45.6% of the patients. Chemotherapy was used in 29.6% and radiotherapy was used in 18.2% of the patients (Table 3).
Table 4 compares patient demographic and facility characteristics of patients who died within 30 vs 90 days following resection. While 30-day mortality for the entire cohort was already substantial at 7.4% (95% CI: 7.0%-7.8%), the 90-day mortality was signif i cantly even greater (17.1%; 95% CI: 16.5%-17.7%). Thirty-and 90-day mortality increased as age group increased. Patients with Medicare insurance had the highest 30-and 90-day mortality at 9.4% and 20.4%, respectively. Patients living in the Mid Atlantic and Pacif i c regions had lower mortality rates as compared to other regions. There was a decrease in the 30- and 90-day mortality with increasing annual hospital volumes, and for patients who received their care in a comprehensive community cancer center or academic center, compared to community cancer programs. As the Charlson/Deyo score increased, rates of 30- and 90-day mortality increased accordingly; with each increasing level of comorbidity death rates at 90 days were approximately twice that as at 30 days (Table 4).
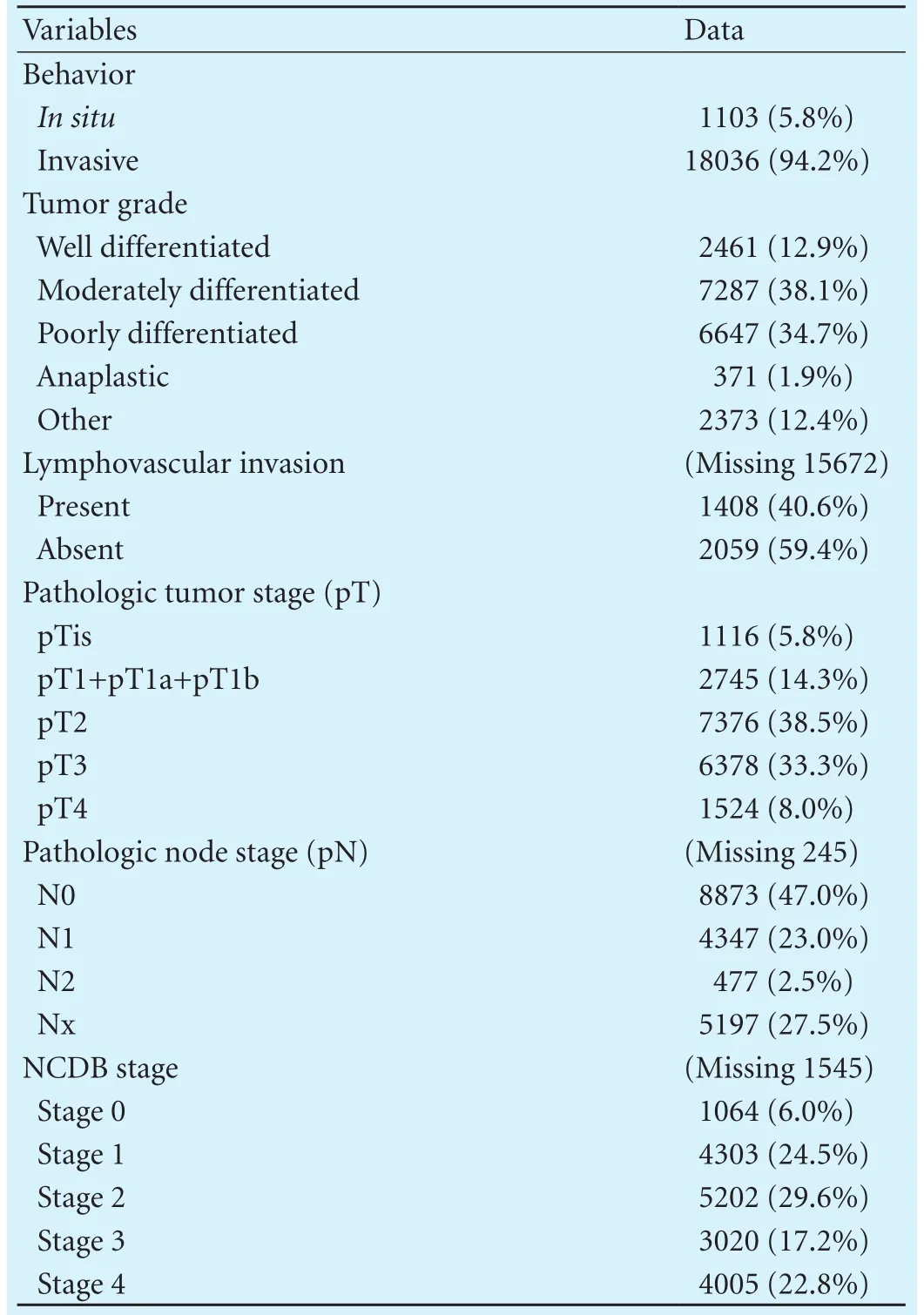
Table 2. Tumor characteristics
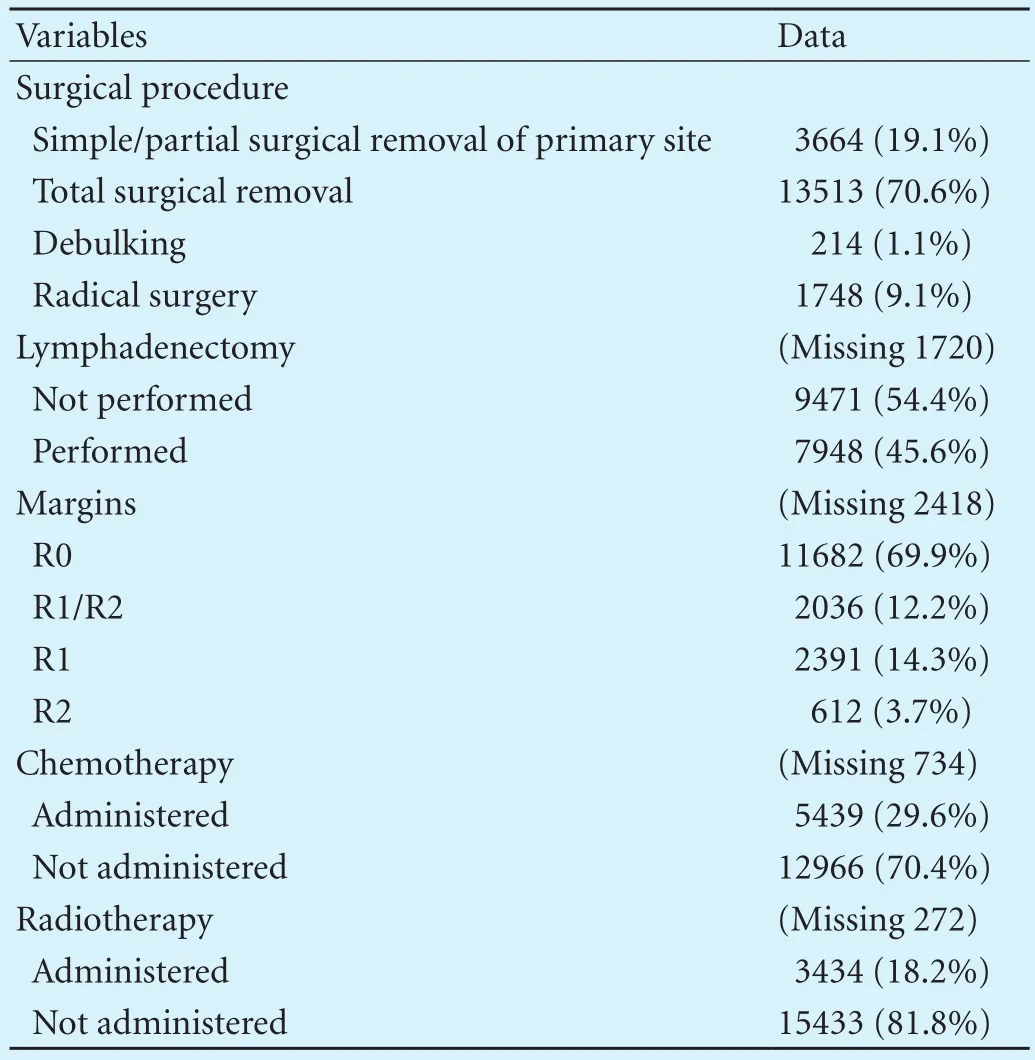
Table 3. Surgical therapy characteristics and utilization of chemotherapy and radiotherapy
Regarding tumor pathology, both 30- and 90-day mortality were higher with increasing pT stage, NCDB stage, presence of lymphovascular invasion, invasive tumor and worsening tumor differentiation (Table 5). Patients who underwent R0 resection had a better survival as compared to patients with R1 or R2 resection. As for the type of resection, patients who underwent radical cholecystectomy had the lowest mortality compared to other resections (Table 5).
Operative approach was available for 4589 patients diagnosed between 2010 and 2012. Median age at presentation was 70 years (IQR: 61-79) and 70% were female. An open approach was used in 34.4%, and a minimally invasive (either laparoscopic or robotic) approach in 41.1%, while 10.6% had a conversion from minimallyinvasive to open. Mortality was lower for minimally invasive patients compared to open patients (Table 6) but even for this minimally invasive group, the 90-day mortality was signif i cantly (2.8-fold) greater than the 30-day mortality.
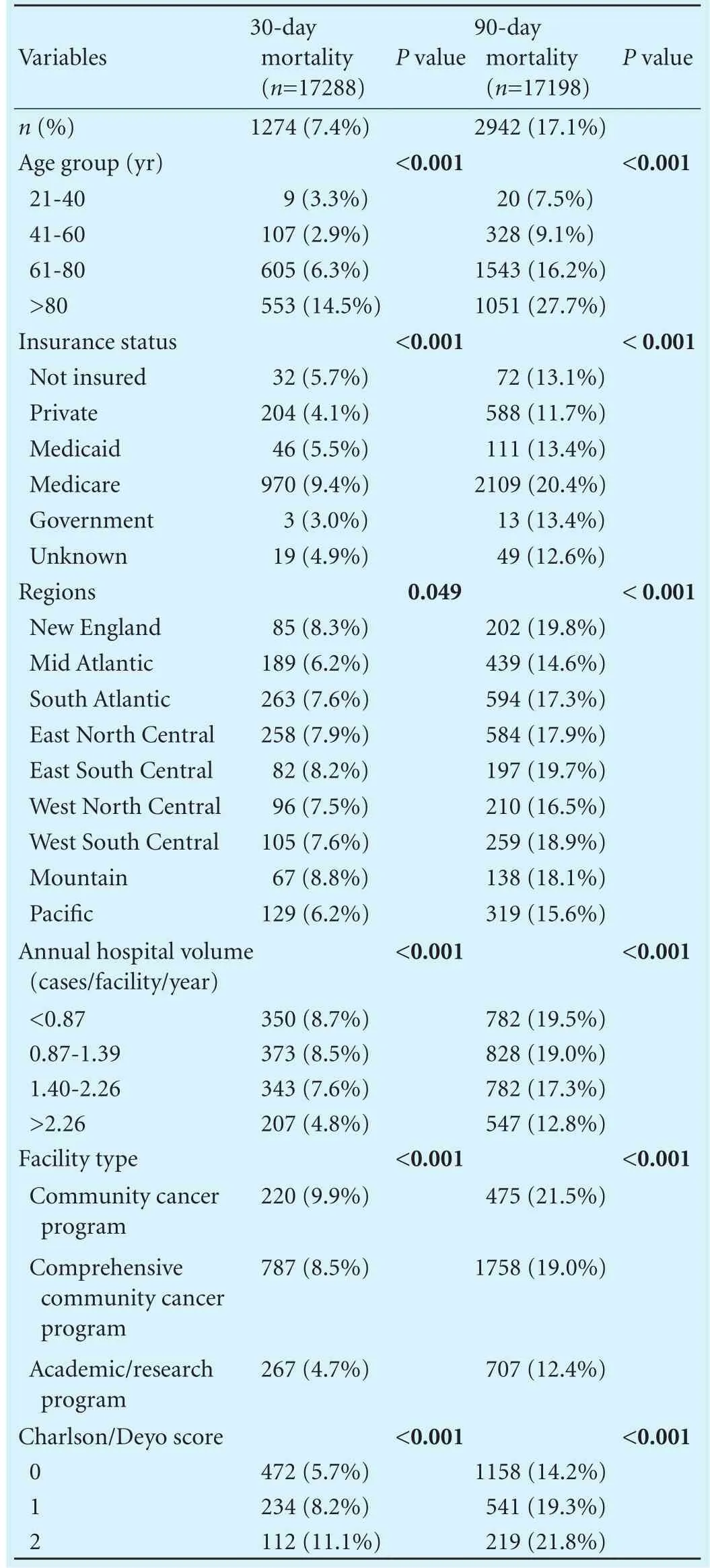
Table 4. Comparison between 30- and 90-day mortality based on demographic and facility-related data

Table 5. Comparison between 30- and 90-day mortality based on tumor characteristics and surgical procedure
Discussion
Postoperative outcomes are traditionally assessed by 30-day mortality. Recent studies[19,20]have suggested that 90-day mortality may be a better measure of postoperative outcomes. Swanson et al, for example, reported a doubling in 90-day mortality following pancreatic resection for malignancy as compared to 30-day mortality.[16]Compared with that study, the rate of increase from 30 to 90 days is similar in our study (2.3 times higher at 90vs 30 days), but the absolute rate of mortality following GBC resections in our study is much higher than even the postpancreatectomy mortality rate in Swanson et al, perhaps due to less well-def i ned criteria for unresectability in GBC. Following resection of esophagogastric cancers, Fischer et al reported a 90-day mortality of 4.4% which was almost double the 30-day mortality of 2.3%.[20]In our study, postoperative mortality at 90 days following GBC resection was 2.3 times greater than the mortality at 30 days, and nearly 3 times greater when only minimally invasive cases were included.
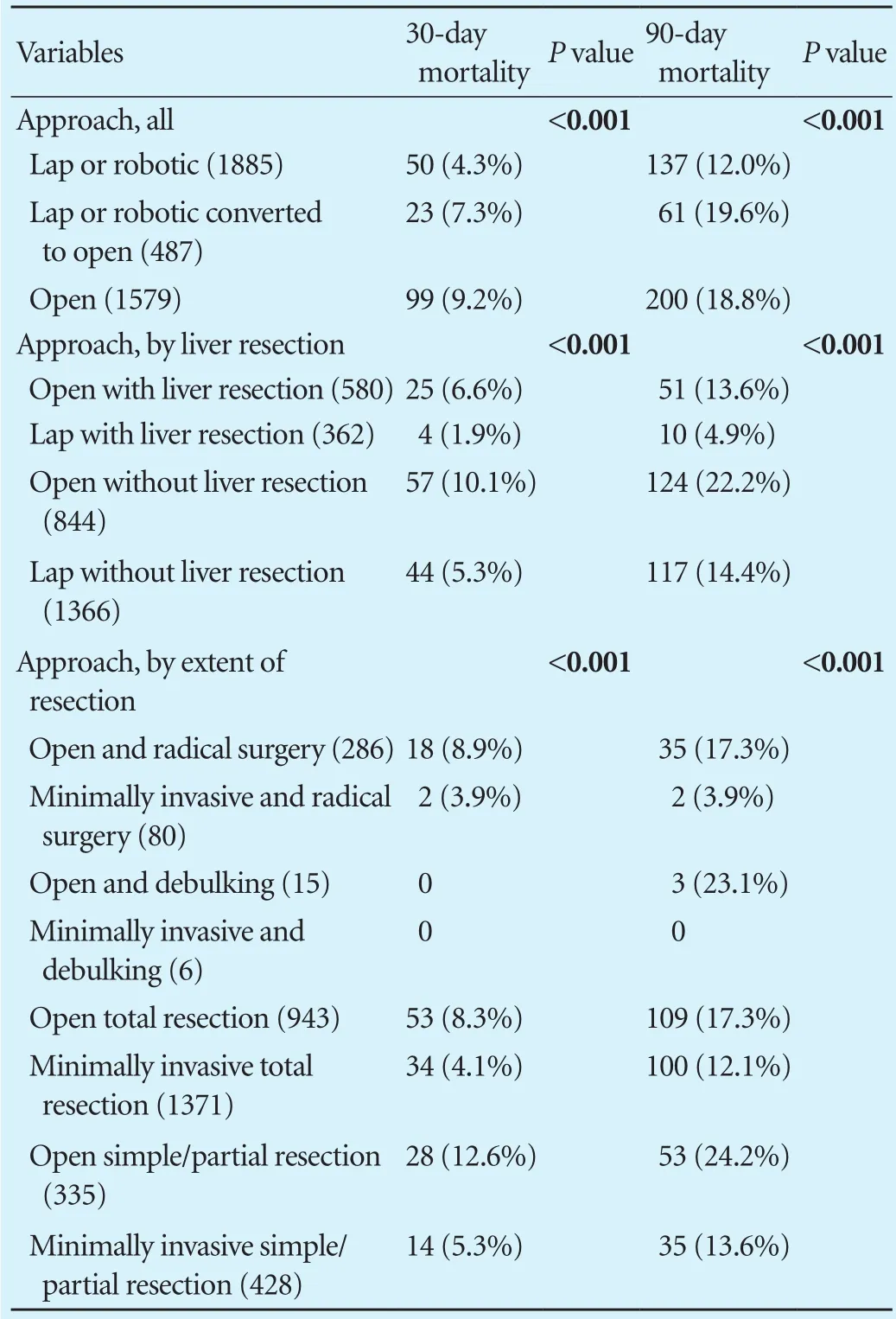
Table 6. Comparison between 30- and 90-day mortality based on surgical approach for patients who surgery between 2010 and 2012
There are many known determinants of postoperative mortality. In multiple large-scale studies, larger hospital volume and hospital teaching status have been shown to correlate with lower postoperative mortality for complex surgical procedures including pancreatectomies, liver resections and esophagectomies.[21-23]We found that this observation holds true for GBC as well, as highervolume centers and academic facilities had lower 30- and90-day mortality. This was not surprising, given that complete oncologic resection of GBC usually entails at least a liver resection and portal lymphadenectomy, if not an excision of the extrahepatic biliary tree, except for the early-stage T1a where a simple cholecystectomy is suff i cient. The complexity of these operations attests to the importance of hepato-pancreato-biliary specialty training and high-volume hospitals.
What was surprising, however, especially given a recent report claiming that “pure laparoscopic radical cholecystectomy with lymph node dissection is safe”,[24]was that even for minimally invasive cases in the NCDB, the 90-day mortality was not just double, but was nearly triple the 30-day mortality. While excellent results like those of Shirobe et al[24]are to be applauded, the lack of 90-day morality reporting in the literature, and the signif i cantly worse mortality we observed in this study, do raise concern that mortality may be underestimated in the literature.
The importance of achieving complete radical resection for GBC with negative macroscopic and microscopic margins (R0) is reemphasized in our study. Patients who underwent R0 resection had signif i cantly lower mortality as compared to patients who underwent incomplete resection with microscopic or macroscopic positive margins. Similarly, our data also support previous reports showing worse prognosis and decreased survival in patients with advanced disease at presentation. Presence of lymphovascular invasion and worsening tumor differentiation negatively affected both 30- and 90-day mortality.
Our study has several limitations. First, this is a retrospective study, making it impossible to determine causal relationships. Second, the NCDB lacks details about certain clinicopathologic variables such as the presence of bile spillage intraoperatively, which is important since bile spillage has been shown to adversely affect the prognosis of GBC.[25-28]Third, most GBC cases are diagnosed incidentally during cholecystectomy for presumed benign disease,[29,30]and this database does not reveal which patients received a subsequent formal oncologic resection; in other words, identif i cation of incidental GBC was not possible in this dataset. Fourth, biases inherent in the selection of patients for different operations and different operative approaches are well known to exist in the NCDB dataset.
In conclusion, 90-day mortality is signif i cantly worse than, and may be a more reliable metric of postoperative outcomes than, 30-day mortality for patients with GBC. Surprisingly, this difference is even more pronounced for minimally invasive operations. Even though selection bias may hamper comparisons between different operations and operative approaches, it is reasonable to conclude that minimally invasive approaches for earlystage GBC are likely still appropriate, especially in skilled hands at high-volume centers, and especially that more data on 90-day mortality are required from these centers to better understand the reality of postoperative mortality.
Contributors: CSC conceived of the study. GN and HM drafted the article. All authors contributed to the design and interpretation of the study and to the analysis and interpretation of data. CSC is the guarantor.
Funding: None.
Ethical approval: Not needed.
Competing interest: No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Lai CH, Lau WY. Gallbladder cancer--a comprehensive review. Surgeon 2008;6:101-110.
2 Henley SJ, Weir HK, Jim MA, Watson M, Richardson LC. Gallbladder cancer incidence and mortality, United States 1999-2011. Cancer Epidemiol Biomarkers Prev 2015;24:1319-1326.
3 Cunningham SC, Alexander HR. Porcelain gallbladder and cancer: ethnicity explains a discrepant literature? Am J Med 2007;120:e17-18.
4 Wilkinson DS. Carcinoma of the gall-bladder: an experience and review of the literature. Aust N Z J Surg 1995;65:724-727.
5 Mayo SC, Shore AD, Nathan H, Edil B, Wolfgang CL, Hirose K, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years:a population-based analysis. J Gastrointest Surg 2010;14:1578-1591.
6 Kiran RP, Pokala N, Dudrick SJ. Incidence pattern and survival for gallbladder cancer over three decades--an analysis of 10301 patients. Ann Surg Oncol 2007;14:827-832.
7 Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg 1994;219:275-280.
8 Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2008. Available from: https://seer.cancer.gov/archive/csr/1975_2008.
9 Taner CB, Nagorney DM, Donohue JH. Surgical treatment of gallbladder cancer. J Gastrointest Surg 2004;8:83-89.
10 Chan SY, Poon RT, Lo CM, Ng KK, Fan ST. Management of carcinoma of the gallbladder: a single-institution experience in 16 years. J Surg Oncol 2008;97:156-164.
11 Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol 2008;34:306-312.
12 Gumbs AA, Jarufe N, Gayet B. Minimally invasive approaches to extrapancreatic cholangiocarcinoma. Surg Endosc 2013;27:406-414.
13 Cho JY, Han HS, Yoon YS, Ahn KS, Kim YH, Lee KH. Laparoscopic approach for suspected early-stage gallbladder carcinoma. Arch Surg 2010;145:128-133.
14 Agarwal AK, Javed A, Kalayarasan R, Sakhuja P. Minimally invasive versus the conventional open surgical approach of aradical cholecystectomy for gallbladder cancer: a retrospective comparative study. HPB (Oxford) 2015;17:536-541.
15 Qadan M, Kingham TP. Technical aspects of gallbladder cancer surgery. Surg Clin North Am 2016;96:229-245.
16 Swanson RS, Pezzi CM, Mallin K, Loomis AM, Winchester DP. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20 000 resections from the national cancer data base. Ann Surg Oncol 2014;21:4059-4067.
17 Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-619.
18 FORDS. FORDS (Facility Oncology Registry Data Standards Revised) for 2013, appendix B, p395. Available from: http:// www.facs.org/cancer/coc/fords/fords-manual-2013.pdf.
19 National Cancer Institute, Surveillance Research Program, Cancer Statistics Branch. Surveillance, Epidemiology, and End Results (SEER) Program; SEER*Stat Database: Incidence -SEER 17 Regs Public-Use, Nov 2005 Sub (1973-2003 varying), released April 2006. Available from: www.seer.cancer.gov.
20 Fischer C, Lingsma H, Hardwick R, Cromwell DA, Steyerberg E, Groene O. Risk adjustment models for short-term outcomes after surgical resection for oesophagogastric cancer. Br J Surg 2016;103:105-116.
21 Kotwall CA, Maxwell JG, Brinker CC, Koch GG, Covington DL. National estimates of mortality rates for radical pancreaticoduodenectomy in 25 000 patients. Ann Surg Oncol 2002;9:847-854.
22 Dimick JB, Cowan JA Jr, Colletti LM, Upchurch GR Jr. Hospital teaching status and outcomes of complex surgical procedures in the United States. Arch Surg 2004;139:137-141.
23 Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-1751.
24 Shirobe T, Maruyama S. Laparoscopic radical cholecystectomy with lymph node dissection for gallbladder carcinoma. Surg Endosc 2015;29:2244-2250.
25 Sarli L, Contini S, Sansebastiano G, Gobbi S, Costi R, Roncoroni L. Does laparoscopic cholecystectomy worsen the prognosis of unsuspected gallbladder cancer? Arch Surg 2000;135:1340-1344.
26 Weiland ST, Mahvi DM, Niederhuber JE, Heisey DM, Chicks DS, Rikkers LF. Should suspected early gallbladder cancer be treated laparoscopically? J Gastrointest Surg 2002;6:50-57.
27 Ouchi K, Mikuni J, Kakugawa Y; Organizing Committee, The 30th Annual Congress of the Japanese Society of Biliary Surgery. Laparoscopic cholecystectomy for gallbladder carcinoma:results of a Japanese survey of 498 patients. J Hepatobiliary Pancreat Surg 2002;9:256-260.
28 Wullstein C, Woeste G, Barkhausen S, Gross E, Hopt UT. Do complications related to laparoscopic cholecystectomy inf l uence the prognosis of gallbladder cancer? Surg Endosc 2002;16:828-832.
29 Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of fi nding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 2007;11:1478-1487.
30 Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-489.
November 20, 2016
Accepted after revision March 17, 2017
Author Aff i liations: Department of Surgery, Saint Agnes Hospital, Baltimore, MD, USA (Goussous N, Hosseini M, Sill AM and Cunningham SC)
Steven C Cunningham, MD, FACS, Director of Pancreatic and Hepatobiliary Surgery, Director of Research, Saint Agnes Hospital, 900 Caton Avenue, MB 207, Baltimore, MD 21229, USA (Tel:+410-368-8815; Fax: +410-719-0094; Email: Steven.Cunningham@ stagnes.org)
This study was presented at the 57th annual (2016) meeting of the Society for Surgery of the Alimentary Tract, during the Digestive Disease Week (DDW), San Diego, CA, USA
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60025-0
Published online May 26, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Indocyanine green fl uoroscopy and liver transplantation: a new technique for the intraoperative assessment of bile duct vascularization
- Effects of multimodal fast-track surgery on liver transplantation outcomes
- Characteristics of recipients with complete immunosuppressant withdrawal after adult liver transplantation
- Predictive value of C-reactive protein/albumin ratio in acute pancreatitis
- Bilioenteric anastomotic stricture in patients with benign and malignant tumors: prevalence, risk factors and treatment
- Interaction between insulin-like growth factor binding protein-related protein 1 and transforming growth factor beta 1 in primary hepatic stellate cells
