Neurological consequences of systemic inflammation in the premature neonate
2017-08-07AparnaPatraHongHuangJohnBauerPeterGiannone
Aparna Patra, Hong Huang, John A. Bauer, Peter J. Giannone
OMNI Academic Service Line and Division of Neonatology, Department of Pediatrics, Kentucky Children’s Hospital, College of Medicine, University of Kentucky, Lexington, KY, USA
Neurological consequences of systemic inflammation in the premature neonate
Aparna Patra*, Hong Huang, John A. Bauer, Peter J. Giannone
OMNI Academic Service Line and Division of Neonatology, Department of Pediatrics, Kentucky Children’s Hospital, College of Medicine, University of Kentucky, Lexington, KY, USA
Despite substantial progress in neonatal care over the past two decades leading to improved survival of extremely premature infants, extreme prematurity continues to be associated with long term neurodevelopmental impairments. Cerebral white matter injury is the predominant form of insult in preterm brain leading to adverse neurological consequences. Such brain injury pattern and unfavorable neurologic sequelae is commonly encountered in premature infants exposed to systemic inflammatory states such as clinical or culture proven sepsis with or without evidence of meningitis, prolonged mechanical ventilation, bronchopulmonary dysplasia, necrotizing enterocolitis and chorioamnionitis. Underlying mechanisms may include cytokine mediated processes without direct entry of pathogens into the brain, developmental differences in immune response and complex neurovascular barrier system that play a critical role in regulating the cerebral response to various systemic inflammatory insults in premature infants. Understanding of these pathologic mechanisms and clinical correlates of such injury based on serum biomarkers or brain imaging findings on magnetic resonance imaging will pave way for future research and translational therapeutic opportunities for the developing brain.
extremely premature infants; systemic inflammation; white matter injury; neurodevelopmental impairment; cytokines
Introduction
Premature birth occurs in 10% of pregnancies in the United States (Centers for Disease Control and Prevention (CDC), 2015) and 5–18% of pregnancies across 184 countries worldwide (Blencowe et al., 2012). Neurodevelopmental impairment such as cerebral palsy (prevalence 5–10%) and cognitive and behavioral deficits (prevalence 40–50%) are a major cause of morbidity in survivors of prematurity (Gargus et al., 2009; Stoll et al., 2010). Despite major progress in neonatal care over the last two decades and improved survival rates for extremely premature infants (born at gestational age ≤ 28 weeks), premature infants continue to contribute inordinately to the burden of early childhood morbidity and long term neurodevelopmental disability (Saigal and Doyle, 2008).e goals of this article are to provide a brief review of current literature with respect to the contribution of inflammatory pathways in preterm infant brain injury risks and outcomes.
Chorioamnionitis diagnosed clinically or histologically is an important cause of premature birth. Presence of histologic evidence of chorioamnionitis has been shown in 65% of placentae in deliveries done at 23–24 weeks of gestational age and can be detected in 30% placentae delivered at 29 weeks of gestation (Lahra and Jeffery, 2004). Presence of intrauterine infection does not always translate to early onset sepsis in preterm neonates. However, it could lead to activation of a fetal inflammatory response which may be associated with cerebral white matter injury and later neurodevelopmental impairment (Wu and Colford, 2000; Yoon et al., 2003). Recent reports have demonstrated that any form of systemic inflammation such as clinical sepsis or culture proven sepsis, meningitis with or without bacteremia, bronchopulmonary dysplasia (BPD) and necrotizing enterocolitis (NEC) were all associated with impaired growth and increased risk of adverse neurologic sequelae in premature infants (Stoll et al., 2004; Schlapbach et al., 2011; Mitha et al., 2013). Interestingly, it has also been reported that late onset sepsis in preterm infants and recurrent neonatal blood stream infections even without evidence of meningitis are associated with increased risk of progressive cerebral white matter injury and acute changes in cerebral function indicated by burst suppression pattern on electrographic studies (Glass et al., 2008; Helderman et al., 2010), pointing tosystemic inflammation from the blood stream infection likely contributing to collateral brain damage.
BPD, which is characterized by arrested alveolar growth and lung inflammation defined as supplemental oxygen administration at 36 weeks of postmenstrual age for premature infants < 32 weeks of gestation, is known to have a high incidence (55%) in infants born at or < 26 weeks in a recent report of morbidities in prematurity (Stoll et al., 2015) that continues to increase as more extremely low birth weight infants weighing less than 1,000 g (ELBW) are surviving. BPD is a notable precedent of impaired neurodevelopmental and adverse motor outcomes that is independently associated with cognitive impairment in ELBW infants (Natarajan et al., 2012). In another recent cohort, prolonged dependence on mechanical ventilation at 36 weeks of postmenstrual age, oen seen in infants with severe BPD, substantially increased the risk of quadriparesis and diparesis by 6 fold and 4 fold respectively (Van Marter et al., 2011).
Significant neurologic, cognitive and behavioral problems are common sequelae of serious central nervous system (CNS) lesions of prematurity such as severe grade intraventricular hemorrhage with post hemorrhagic ventricular dilatation, hemorrhagic parenchymal infarction or cystic periventricular leukomalacia (PVL). However, the majority of premature infants exhibiting neurocognitive deficits do not manifest such patterns of injury, suggesting that there may be other alterations, insults and remodeling in the developing premature brain resulting in more diffuse injury. Serial brain magnetic resonance imaging (MRI) data from a cohort of 119 premature babies born less than 30 weeks of gestation showed that at least 50% of babies manifest some degree of cerebral white matter injury which accounts for the majority of neurological deficits seen in survivors of prematurity (Dyet et al., 2006). Such cerebral white matter injury characterized by loss of myelination of oligodendrocytes is the predominant form of brain injury seen in premature brain and is associated with high incidence of neurodevelopmental impairment (Khwaja and Volpe, 2008). The exclusive developmental anatomy of the premature brain and its exposure to the abnormal ambience of extrauterine preterm life makes it particularly vulnerable to damage. Systemic inflammatory insults, such as those described in the previous paragraphs, are common phenomena in premature babies. Systemic inflammation activates a cascade of pro-inflammatory cytokines in circulation which leads to activation of microglia locally in the brain along with free radical attack by reactive oxygen and nitrogen species resulting in maturation dependent cell death and apoptosis of neural cells. Further definition of mechanisms that cause such collateral brain injury will hopefully lead to prevention strategies in these settings of systemic inflammatory states.
Cytokine driven tissue injury
Cytokines may gain access to the CNS by blood borne routes or by endogenous production from immune cells, brain endothelial cells, microglia, astrocytes and neurons (Sebire et al., 1993; Banks, 2005). Elevated levels of circulating cytokines in premature neonates were evidenced in cord blood, amniotic fluid, cerebrospinal fluid and cerebral parenchyma of infants diagnosed with inflammation related white matter lesions speaking to potentially direct damage by the systemic cytokines (Yoon et al., 1996, 1997; Martinez et al., 1998). While there is no clear correlation between cytokine profile in plasma and cerebrospinal fluid (CSF) of preterm neonates in response to systemic inflammation or infection, systemic inflammation certainly appears to be associated with premature brain injury (Ellison et al., 2005).
Cytokines may also activate local immune cells in the premature brain. Astrocytes and microglia are the two primary CNS cell types which are involved in intracerebral immune interactions by secretion of cytokines and chemokines. Astrocytes, microglia and oligodendrocyte progenitor cells also express and are activated by cytokine specific receptors such as IL-1β and IFN-γ receptors (Vela et al., 2002). Various cytokines particularly TNF-α and IFN-γ have been isolated directly in neonatal PVL and white matter lesions expressed specifically in microglia/macrophages and astrocytes respectively (Kadhim et al., 2001; Folkerth et al., 2004) with degree of expression correlating with severity of oxidative damage on histopathological studies (Folkerth et al., 2004). Immunohistochemistry staining of preterm infant autopsy brain specimens have demonstrated oxidative and nitrative damage in primarily progenitor premyelinating oligodendrocytes in white matter lesions of the immature brain (Haynes et al., 2003; Back et al., 2005). CSF data followed in a longitudinal study of premature very low birth weight (VLBW) infants also support the role of oxidative damage and elevated levels of oxidative products in CSF of preterm infants with brain MRI evidence of white matter injury at term gestation compared to premature infants without evidence of white matter lesions on MRI (Inder et al., 2002).e extent of the involvement of local microglia in such inflammation associated preterm brain injury may be related to its unique de-velopmental or maturation dependent location. Recent brain autopsy studies have ascertained presence of microglia in the human forebrain starting from 16-22 weeks of gestation with heavy localization in the cerebral white matter (Rezaie et al., 2005; Monier et al., 2006). The density of microglia reaches its peak in the third trimester which is the period for intense susceptibility for the preterm brain to develop white matter injury with significant decline in density at term gestation (Billiards et al., 2006).
Alteration of blood-brain barrier (BBB) function
The BBB and blood cerebrospinal fluid barrier (BCSFB) are two major interfaces which play a critical role in separating the brain parenchyma from systemic circulation, thereby preserving and maintaining a normal physiologic environment paramount for optimal brain growth and function. The BBB is a highly selective diffusion barrier composed of specialized endothelial cells interconnected by an elaborate network of complex tight junction proteins in a basal lamina containing pericytes and perivascular antigen presenting cells enveloped by perivascular astrocytes (Abbott et al., 2010). Contrary to old reports of BBB being under developed in fetuses and neonates, the selective function of the BBB is effective from very early stages of brain development. It is demonstrated by exclusion of passage of proteins and small lipid insoluble molecules between systemic circulation and brain extracellular fluid with a characteristic maturation dependent decline in permeability as the brain develops (Saunders et al., 1999, 2000). Animal studies in fetal and newborn rats and opossums showed increased permeability of the BBB (not BCSFB) to plasma proteins specifically acute phase reactants, IL-1β and TNF-α in response to LPS induced systemic inflammation during a restricted period of postnatal brain development in both species.is was determined immunocytochemically by the presence of related proteins in surrounding cerebral blood vessels and brain parenchyma involving particularly the cerebral white matter (Stolp et al., 2005) again highlighting the location specific nature of perinatal brain injury in association with systemic inflammation in prematurity.
Impaired cerebrovascular autoregulation
It is unclear what role hypotension, cardiovascular instability, and/or impairment of cerebrovascular autoregulation in response to systemic cytokine surge and brain ischemia play in sepsis and fetal inflammatory states. Many premature infants suffer from hypotension and need inotropic support during systemic inflammatory conditions such as sepsis and necrotizing enterocolitis (NEC). Interestingly, decreased systemic blood pressure may not correlate with decreased cerebral blood flow (Volpe, 2008) and pressure passive cerebral circulation is common in sick premature babies (Soul et al., 2007). However, cytokinemia associated with systemic inflammatory states and infection in premature infants may blunt cerebral vascular auto regulatory mechanisms in the developing brain and augment an otherwise weak hypoxic-ischemic insult to a potent damaging one (Ikeda et al., 2004; Larouche et al., 2005; Wang et al., 2007).
Diagnostic Imaging and Biomarkers of Perinatal White Matter Injury in Premature Infants
Biomarkers with accurate diagnostic and prognostic potential are needed for differentiating between infants who have significant neuroinflammation and white matter injury associated with chances of permanent sequelae from the ones who might benefit from therapeutic interventions.e Extremely Low Gestational Age Newborn (ELGAN) study for e newborns at extremely low gestational age demonstrated that serial measurements of plasma levels of vascular endothelial growth factor receptor 1, serum amyloid A, macrophage inflammatory protein 1β on day of life 1 and IL-8 on day of life 7 in extremely premature infants were associated with increased risk ventriculomegaly and diffuse white matter changes on brain MRI (Leviton et al., 2011). Even though plasma cytokine levels for prediction of white matter injury seems to have diagnostic value, earlier studies have failed to establish a correlation between plasma and CSF cytokine levels further implying that certain cytokines such as IL-8 may achieve higher levels in CSF than in plasma which may be more predictive of MRI-defined white matter injury related to systemic infection or inflammation in premature newborns (Ellison et al., 2005). Hence, overt reliance on plasma cytokine levels may delay or prevent early recognition of neurological consequences of systemic inflammatory states. Currently, cytokines are not routinely measured in a neonatal intensive care unit (NICU) setting in preterm inflammatory conditions and will necessitate cost effective standardized validated assay meth-ods prior to integration into standard NICU practice.
Some authors have reported long term follow up studies on cohorts of premature VLBW infants to determine if moderate to severe white matter abnormalities and DEHSI noted on brain MRI are anatomic antecedents of neurological impairments in children born prematurely. In a study of 167 very premature infants (born < 30 weeks of gestation), the presence of any white matter abnormality or moderate to severe white matter abnormalities on MRI were noted to be more sensitive although less specific than ultrasound findings in identifying children who had severe neurodevelopmental impairments in long term follow up at 2 years (corrected for prematurity) age (Woodward et al., 2006).e presence of moderate to severe white matter abnormalities in this study was predictive of severe psychomotor delay (10%), cerebral palsy (10%), cognitive delay (25%) on Bayley scales of Infant Development-II and neurosensory impairment (11%) independently of abnormalities on cranial ultrasound imaging. Another smaller study which looked at diffusion weighted imaging on 38 VLBW infants at term equivalent age found that the presence of DEHSI was strongly associated with lower developmental quotient at 2 years of age (Krishnan et al., 2007). In another cohort of 68 preterm infants who had confirmed sepsis and or NEC, significant white matter abnormalities were noted on brain MRI at term and those infants had poorer psychomotor development at 2 years of age compared to age and gestation matched controls without sepsis and or NEC (Shah et al., 2008). Evidence of thalamic atrophy, loss of myelination especially in posterior limb of internal capsule, and overall loss in cortical white matter on MRI imaging are strong predictors of development of cerebral palsy and inability of independent walking in preterm infants (Inder et al., 1999; Cowan and de Vries, 2005; Ricci et al., 2006).
It is noteworthy that some preterm born children with white matter abnormalities on MRI were free of severe impairments and had normal motor development at 2 years of age in both studies (Woodward et al., 2006; Krishnan et al., 2007) which accentuates that obtaining brain MRI at term equivalent age on prematurely born babies may serve as an important screening tool for closer monitoring of babies at highest risk of neurodevelopmental impairments but also that not all ominous brain MRI findings will translate to severe neurodevelopmental problems in early childhood.is highlights the influence of genetics and home environment in overcoming the aftermath of prematurity and systemic inflammation related comorbidities and determining long term neurodevelopmental outcomes.
Potentialerapeutic Interventions: Directions of Future Research
All novel interventions and therapies in neonatology must go through rigorous evaluation for unexpected long term adverse effects prior to their application in routine practice as the vulnerable population of premature babies is in critical phase of organ development and maturation. Even though experimental animal data is available for several strategies targeting amelioration of systemic inflammation induced cerebral injury, their application in routine patient management has so far been limited. Corticosteroids are potent anti-inflammatory agents and dexamethasone use in treatment and prevention of chronic lung disease of prematurity is a well prevalent practice (Doyle et al., 2010). Corticosteroids have been shown to modulate chorioamnionitis induced fetal inflammatory state in fetal ovine model (Wolfe et al., 2013), however the concern of significantly increased risk of cerebral palsy and adverse neurodevelopmental outcomes associated with prolonged postnatal use of corticosteroids particularly dexamethasone in preterm neonates (Wilson-Costello et al., 2009) has prohibited its frequent clinical use.
N-acetylecysteine has shown promise as a free radical scavenger and antioxidant that has successfully prevented LPS and fetal inflammation-induced degeneration of oligodendrocyte progenitor cells and myelination defects by attenuating intracerebral inflammatory reaction and TNF, IL-1 and inducible nitric oxide synthase expression in mouse models (Paintlia et al., 2004; Chang et al., 2011; Beloosesky et al., 2013). Minocycline has anti-apoptotic, antioxidant and anti-inflammatory properties in animal models by diminishing microglial activation and maintaining BBB integrity in systemic inflammation associated cerebral insult (Stolp et al., 2007). Pentoxifylline, a phosphodiesterase inhibitor that increases intracellular cyclic adenosine monophosphate levels has protective effects in LPS-induced white matter injury in a rat model of PVL (Dilek et al., 2013).e drug has a favorable clinical profile in premature neonates and is currently being studied as an adjunct therapy to decrease mortality and morbidities from sepsis and NEC in premature neonates (Pammi and Haque, 2015).
Studies targeting BBB dysfunction are also being explored. Recently, elevated expression of tight junction proteins such as claudin-3, 5 and occludins were noted after treatment with simvastatin and low molecular weight heparin in animal models of sepsis resulting in improved selective function and decreased permeability of the BBB (Li et al., 2015; Yang et al., 2015). Matrix metalloproteinase-2, 9 inhibitors have been reported to reverse increases in BBB permeability and decrease IL-6 expression in animal model of sepsis related BBB dysfunction (Dal-Pizzol et al., 2013). Various immunological interventions are additionally the subject of ongoing research efforts. Systemic administration of a synthetic im-mune regulator protein 1018 (innate defense regulator peptide) led to protection against white matter injury in a LPS and hypoxia-ischemia induced perinatal brain injury model in mice (Bolouri et al., 2014).
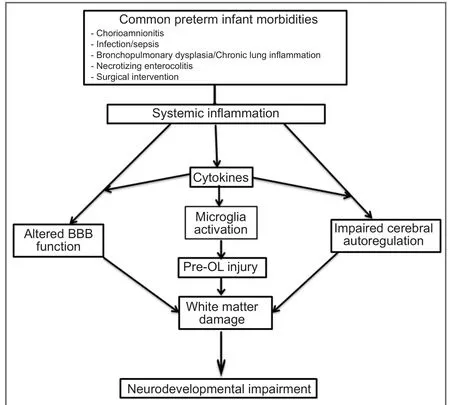
Figure 1 Various mechanisms causing injury to the developing brain in systemic inflammatory state.
Separate from small molecule or protein therapeutics to affect preterm infant brain injury are several recent preclinical studies, and some ongoing clinical trials, exploring the potential of cell-based treatments (Titomanlio et al., 2011; Verina et al., 2013; Chicha et al., 2014; Mitsialis and Kourembanas, 2016). Most of these studies involve the administration of live cells with progenitor/stem-like properties, with the guise that these cells can multiply to grow new tissues replacing the cells damaged from perinatal insults, or (more likely) secrete beneficial factors in specific brain regions and enhance regional growth in a developing organ (Lee et al., 2010; Donega et al., 2015). In many cases the preclinical studies support the potential benefits of this approach, with the most compelling effects being related to direct injections of cell preparations into the lesion (van Velthoven et al., 2010a; Mitsialis and Kourembanas, 2016). Intranasal delivery has also shown some moderate promise (van Velthoven et al., 2010b; Donega et al., 2015). Most data strongly support the ‘paracrine-benefit’ mechanism, wherein the implanted cells release beneficial factors and enhance tissue repair (van Velthoven et al., 2010a; van Velthoven et al., 2013; Park et al., 2015). Although majority of this investigation has been done in hypoxic ischemic perinatal brain injury setting the final pathogenesis of brain damage in both hypoxic ischemic brain injury and systemic inflammatory perinatal insult induced brain injury include excitotoxicity and oxidative damage in which stem-cell based therapies may represent the much awaited answer in the developing premature neonate. Studies in this setting have not as yet investigated the interactions of these cells with inflammatory pathways that can contribute to tissue remodeling.is field is in its very early stages and although there is great potential for benefit and somewhat promising results thus far, some important hurdles include reliable tissue delivery and regulatory challenges in demonstrating long term clinical benefits and avoiding long term disease risks (especially cancer,etc.).
Collectively it is clear that there are several therapeutic strategies that may have real value in reducing the neurodevelopmental morbidities and major costs associated with preterm infant brain injury. For many of these strategies there is ample experimental data to support a conceivable effort toward clinical investigations and make a substantial impact. Given the high prevalence of preterm infant brain injury, and its often-devastating consequences, the diligent pursuit of newer therapies to enhance outcomes is certainly warranted.
Conclusion
Brain injury in preterm infants is a major medical problem and health care cost worldwide; inflammatory mechanisms are very likely contributors to nearly all settings and etiologies. Pertinent knowledge gaps still exist in our detailed understanding of underlying cytokine mediated mechanisms, developmental differences in immune response and complex neurovascular barrier system that play a critical role in in regulating the cerebral response to systemic inflammatory conditions in premature human neonates. A better understanding of the dual role of cytokines and balance between pro- and anti-inflammatory mediators and its role in brain development and maturation is needed to completely appreciate the long term neurodevelopmental consequences in this susceptible population. A valid diagnostic cytokine profile needs to be designed for biomarkers of perinatal brain injury for evaluation in a clinical setting of neonatal sepsis, NEC or other systemic inflammatory state. From currently available studies white matter abnormality on MRI seems to be the anatomic substrate or antecedent for future progression to adverse neurodevelopment in a background of prematurity and systemic inflammation. Hence, advanced brain MR imaging should be considered as part of routine care for every premature baby exposed to systemic inflammatory conditions in their neonatal course and not just as an adjuvant evaluation reserved for babies with severe abnormalities on cranial ultrasound imaging at term equivalent age.ere is now increasing clinical and experimental evidence that systemic inflammatory pathways can damage the preterm brain even in absence of direct entry of infectious agent to the CNS. Given the high rate of preterm birth and huge prevalence of prematurity related inflammatory states there is a dire need for development of novel translational approaches to alleviate long term neurodevelopmental consequences in this highly vulnerable population.
Author contributions:AP, HH, JAB and PJG draed, reviewed and finalized the manuscript.
Conflicts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer review report:
Reviewer:Min Cheol Chang, University of Ulsan College of Medicine, South Korea
Comments to author:The authors provided a brief review of current literatures with respect to the contribution of inflammatory pathways in preterm infant brain injury risks and outcomes.is article is well-written and has good information. In my opinion, it would be enough to be accepted in this state. See details in the additional file.
Additional file:Open peer review report 1.
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41-53.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13-25.
Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD (2009)e association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics 124:268-276.
Back SA, Luo NL, Mallinson RA, O’Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK, Roberts CT, Jr., Murdoch GH, Montine TJ (2005) Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol 58:108-120.
Banks WA (2005) Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 11:973-984.
Bauer B, Hartz AM, Miller DS (2007) Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71:667-675.
Beloosesky R, Ginsberg Y, Khatib N, Maravi N, Ross MG, Itskovitz-Eldor J, Weiner Z (2013) Prophylactic maternal N-acetylcysteine in rats prevents maternal inflammation-induced offspring cerebral injury shown on magnetic resonance imaging. Am J Obstet Gynecol 208:213.e1-6.
Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, Kinney HC (2006) Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol 497:199-208.
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE (2012) National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379:2162-2172.
Bolouri H, Savman K, Wang W, Thomas A, Maurer N, Dullaghan E, Fjell CD, Ek CJ, Hagberg H, Hancock RE, Brown KL, Mallard C (2014) Innate defense regulator peptide 1018 protects against perinatal brain injury. Ann Neurol 75:395-410.
Chang EY, Zhang J, Sullivan S, Newman R, Singhi(2011) N-acetylcysteine attenuates the maternal and fetal proinflammatory response to intrauterine LPS injection in an animal model for preterm birth and brain injury. J Matern Fetal Neonatal Med 24:732-740.
Chicha L, Smith T, Guzman R (2014) Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv Syst 30:37-46.
Cowan FM, de Vries LS (2005)e internal capsule in neonatal imaging. Semin Fetal Neonatal Med 10:461-474.
Dal-Pizzol F, Rojas HA, dos Santos EM, Vuolo F, Constantino L, Feier G, Pasquali M, Comim CM, Petronilho F, Gelain DP, Quevedo J, Moreira JC, Ritter C (2013) Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol 48:62-70.
Dilek M, Kumral A, Okyay E, Ozbal S, Tugyan K, Tuzun F, Sever AH, Yilmaz O, Duman N, Ozkan H (2013) Protective effects of pentoxifylline on lipopolysaccharide-induced white matter injury in a rat model of periventricular leukomalasia. J Matern Fetal Neonatal Med 26:1865-1871.
Donega V, Nijboer CH, van Velthoven CT, Youssef SA, de Bruin A, van Bel F, Kavelaars A, Heijnen CJ (2015) Assessment of long-term safety and ef ficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res 78:520-526.
Doyle LW, Ehrenkranz RA, Halliday HL (2010) Dexamethasone treatment aer the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 98:289-296.
Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, Harrison M, Allsop JM, Hajnal J, Herlihy AH, Edwards B, Laroche S, Cowan FM, Rutherford MA, Edwards AD (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536-548.
Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE (2005)e relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res 57:282-286.
Folkerth RD, Keefe RJ, Haynes RL, Trachtenberg FL, Volpe JJ, Kinney HC (2004) Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol 14:265-274.
Gargus RA, Vohr BR, Tyson JE, High P, Higgins RD, Wrage LA, Poole K (2009) Unimpaired outcomes for extremely low birth weight infants at 18 to 22 months. Pediatrics 124:112-121.
Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, Ferriero DM, Miller SP (2008) Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics 122:299-305.
Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC (2003) Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol 62:441-450.
Helderman JB, Welch CD, Leng X, O’Shea TM (2010) Sepsis-associated electroencephalographic changes in extremely low gestational age neonates. Early Hum Dev 86:509-513.
Ikeda T, Mishima K, Aoo N, Egashira N, Iwasaki K, Fujiwara M, Ikenoue T (2004) Combination treatment of neonatal rats with hypoxia-ischemia and endotoxin induces long-lasting memory and learning impairment that is associated with extended cerebral damage. Am J Obstet Gynecol 191:2132-2141.
Inder T, Mocatta T, Darlow B, Spencer C, Volpe JJ, Winterbourn C (2002) Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr Res 52:213-218.
Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ (1999) Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 46:755-760.
Janeway CA, Jr., Medzhitov R (2002) Innate immune recognition. Ann Rev Immunol 20:197-216.
Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G (2001) Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology 56:1278-1284.
Khwaja O, Volpe JJ (2008) Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 93:F153-161.
Krishnan ML, Dyet LE, Boardman JP, Kapellou O, Allsop JM, Cowan F, Edwards AD, Rutherford MA, Counsell SJ (2007) Relationship between white matter apparent diffusion coef ficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics 120:e604-609.
Lahra MM, Jeffery HE (2004) A fetal response to chorioamnionitis is associated with early survival aer preterm birth. Am J Obstet Gynecol 190:147-151.
Larouche A, Roy M, Kadhim H, Tsanaclis AM, Fortin D, Sebire G (2005) Neuronal injuries induced by perinatal hypoxic-ischemic insults are potentiated by prenatal exposure to lipopolysaccharide: animal model for perinatally acquired encephalopathy. Dev Neurosci 27:134-142.
Lee JA, Kim BI, Jo CH, Choi CW, Kim EK, Kim HS, Yoon KS, Choi JH (2010) Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr Res 67:42-46.
Leviton A, Kuban K, O’Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O (2011)e relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr 158:897-903 e891-895.
Li R, Tong J, Tan Y, Zhu S, Yang J, Ji M (2015) Low molecular weight heparin prevents lipopolysaccharide induced-hippocampus-dependent cognitive impairments in mice. Int J Clin Exp Pathol 8:8881-8891.
Martinez E, Figueroa R, Garry D, Visintainer P, Patel K, Verma U, Sehgal PB, Tejani N (1998) Elevated amniotic fluid interleukin-6 as a predictor of neonatal periventricular leukomalacia and intraventricular hemorrhage. J Matern Fetal Investig 8:101-107.
Mitha A, Foix-L’Helias L, Arnaud C, Marret S, Vieux R, Aujard Y, Thiriez G, Larroque B, Cambonie G, Burguet A, Boileau P, Roze JC, Kaminski M, Truffert P, Ancel PY, Group ES (2013) Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics 132:e372-380.
Mitsialis SA, Kourembanas S (2016) Stem cell-based therapies for the newborn lung and brain: Possibilities and challenges. Semin Perinatol 40:138-151.
Monier A, Evrard P, Gressens P, Verney C (2006) Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol 499:565-582.
Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, Laptook AR, Bell EF, Stoll BJ, Newman N, Hale EC, Bara R, Walsh MC (2012) Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev 88:509-515.
Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK (2004) N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res 78:347-361.
Pammi M, Haque KN (2015) Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev:CD004205.
Park WS, Sung SI, Ahn SY, Yoo HS, Sung DK, Im GH, Choi SJ, Chang YS (2015) Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One 10:e0120893.
Rezaie P, Dean A, Male D, Ulfig N (2005) Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex 15:938-949.
Ricci D, Anker S, Cowan F, Pane M, Gallini F, Luciano R, Donvito V, Baranello G, Cesarini L, Bianco F, Rutherford M, Romagnoli C, Atkinson J, Braddick O, Guzzetta F, Mercuri E (2006)alamic atrophy in infants with PVL and cerebral visual impairment. Early Hum Dev 82:591-595.
Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, Anjari M, Counsell S, Ramenghi LA (2010) Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 52:505-521.
Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E (2007) Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis 195:296-302.
Saigal S, Doyle LW (2008) An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371:261-269.
Saunders NR, Habgood MD, Dziegielewska KM (1999) Barrier mechanisms in the brain, II. Immature brain. Clin Exp Pharmacol Physiol 26:85-91.
Saunders NR, Knott GW, Dziegielewska KM (2000) Barriers in the immature brain. Cell Mol Neurobiol 20:29-40.
Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, Nelle M, Bucher HU, Latal B, Swiss Neonatal N, Follow-Up G (2011) Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 128:e348-357.
Sebire G, Emilie D, Wallon C, Hery C, Devergne O, Delfraissy JF, Galanaud P, Tardieu M (1993) In vitro production of IL-6, IL-1 beta, and tumor necrosis factor-alpha by human embryonic microglial and neural cells. J Immunol 150:1517-1523.
Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE (2008) Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 153:170-175, 175 e171.
Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ (2007) Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res 61:467-473.
Stoll BJ, Hansen NI, Adams-Chapman I, FanaroffAA, Hintz SR, Vohr B, Higgins RD, National Institute of Child H, Human Development Neonatal Research N (2004) Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 292:2357-2365.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP, WyckoffM, Das A, Hale EC, Ball MB, Newman NS, Schibler K, Poindexter BB, Kennedy KA, Cotten CM, Watterberg KL, D’Angio CT, et al. (2015) Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314:1039-1051.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, et al. (2010) Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126:443-456.
Stolp HB, Dziegielewska KM, Ek CJ, Habgood MD, Lane MA, Potter AM, Saunders NR (2005) Breakdown of the blood-brain barrier to proteins in white matter of the developing brain following systemic inflammation. Cell Tissue Res 320:369-378.
Stolp HB, Ek CJ, Johansson PA, Dziegielewska KM, Potter AM, Habgood MD, Saunders NR (2007) Effect of minocycline on inflammation-induced damage to the blood-brain barrier and white matter during development. Eur J Neurosci 26:3465-3474.
Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, Heijnen C, Baud O, Gressens P (2011) Stem cell therapy for neonatal brain injury: perspectives and challenges. Ann Neurol 70:698-712.
Van Marter LJ, Kuban KC, Allred E, Bose C, Dammann O, O’Shea M, Laughon M, Ehrenkranz RA, Schreiber MD, Karna P, Leviton A, Investigators ES (2011) Does bronchopulmonary dysplasia contribute to the occurrence of cerebral palsy among infants born before 28 weeks of gestation? Arch Dis Child Fetal Neonatal Ed 96:F20-29.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ (2010a) Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun 24:387-393.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ (2010b) Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res 68:419-422.
van Velthoven CT, Sheldon RA, Kavelaars A, Derugin N, Vexler ZS, Willemen HL, Maas M, Heijnen CJ, Ferriero DM (2013) Mesenchymal stem cell transplantation attenuates brain injury aer neonatal stroke. Stroke 44:1426-1432.
Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C (2002) Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci 20:489-502.
Verina T, Fatemi A, Johnston MV, Comi AM (2013) Pluripotent possibilities: human umbilical cord blood cell treatment aer neonatal brain injury. Pediatr Neurol 48:346-354.
Volpe JJ (2008) Neurology of the Newborn, 5thEdition. Philadelphia, PA: Saunders Elsevier.
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110-124.
Wang X, Hagberg H, Nie C, Zhu C, Ikeda T, Mallard C (2007) Dual role of intrauterine immune challenge on neonatal and adult brain vulnerability to hypoxia-ischemia. J Neuropathol Exp Neurol 66:552-561.
Weighardt H, Holzmann B (2007) Role of Toll-like receptor responses for sepsis pathogenesis. Immunobiology 212:715-722.
Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, Shankaran S, Finer NN, Van Meurs KP, Engle WA, Das A, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (2009) Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 123:e430-437.
Wolfe KB, Snyder CC, Gisslen T, Kemp MW, Newnham JP, Kramer BW, Jobe AH, Kallapur S (2013) Modulation of lipopolysaccharide-induced chorioamnionitis in fetal sheep by maternal betamethasone. Reprod Sci 20:1447-1454.
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE (2006) Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355:685-694.
Wu YW, Colford JM, Jr. (2000) Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA 284:1417-1424.
Wynn JL, Levy O (2010) Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin Perinatol 37:307-337.
Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL (2008) Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112:1750-1758.
Xaio H, Banks WA, NiehoffML, Morley JE (2001) Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res 896:36-42.
Yang CH, Kao MC, Shih PC, Li KY, Tsai PS, Huang CJ (2015) Simvastatin attenuates sepsis-induced blood-brain barrier integrity loss. J Surg Res 194:591-598.
Yoon BH, Park CW, Chaiworapongsa T (2003) Intrauterine infection and the development of cerebral palsy. BJOG 110:124-127.
Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC (1996) Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 174:1433-1440.
Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG (1997) High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 177:406-411.
How to cite this article: Patra A, Huang H, Bauer JA, Giannone PJ (2017) Neurological consequences of systemic inflammation in the premature neonate. Neural Regen Res 12(6):890-896.
*Correspondence to:
Aparna Patra, M.D.,
aparna.patra@uky.edu.
orcid:
0000-0003-2433-9335
(Aparna Patra)
10.4103/1673-5374.208547 Accepted: 2017-05-23
杂志排行
中国神经再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease