Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease
2017-08-07WillianO.Castillo,AndresFelipeAristizabal-Pachon
Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease
Galantamine commercialized under the name of Razadyne®is the most recently approved AChEi in many countries for AD patients at mild, moderate, and advanced moderate stages. Lately, its ef ficacy has also been observed in patients with AD at severe stage.e ability of this drug to cross the blood-brain barrier makes it suitable for AD patients, and unlike other AChEis, galantamine has a weak AChEi effect. Nevertheless, it has a dual mode of action, since it inhibits AChE and modulates allosterically both the nicotinic and muscarinic acetylcholine receptors (AChRs) to potentiate the sensitivity to acetylcholine (ACh); additionally, galantamine and some of its derivatives exert antioxidant activity which has been associated with the presence of enol group and quaternary nitrogen.e antioxidant activity of the molecule disappears after transformation of the enol group (galantamine) into carbonyl group (galantaminone, narvedine).e same effect is observed aer transformation of the enol group of galantamine hydrobromide; the presence of quaternary nitrogen is not involved in the radical-scavenging action, but is responsible for the increasing of the strength of the scavenging effect (Tsvetkova et al., 2013). Furthermore, Galantamine modulates non-amyloidogenic processing of amyloid precursor protein and inhibits the aggregation and toxicity of Aβ. In summary, accumulating evidences demonstrate that galantamine exerts neuroprotection against Aβ1–42-induced cell loss and neurotoxicity; nevertheless, antigenotoxicity studies were still missing to define the contribution of the drug to neuroprotection mechanism through regulation of DNA damage.
Different types of DNA damage including DNA double-strand breaks (DSBs), DNA single-strand breaks (SSBs), bulky adducts, abasic sites, crosslinking (interstrand and intrastrand), oxidation of specific bases (8-hydroxydeoxyguanosine), insertions and deletions are associated to neurodegenerative diseases (Figure 1). Consequently, cells deploy a diverse repertoire of mechanisms to maintain genetic integrity; however, with advanced age there is a decreasing at both antioxidant system and capacity of the cell to counteracting genotoxic stimulus. Unless repaired in an error-free process, DNA instability can result in mutations and altered cellular behavior (Pearl et al., 2015).e understanding of AD is continually changing; for instance, classical hallmarks of AD, earlier thought to be responsible for the disease development, now rather seem to reflect the damage suffered by neurons over a long time as cellular adaptive strategy to oxidative stress, and although the mechanisms are diverse, neuronal death is the inevitable event in AD (Anand et al., 2014).
The DNA damage responses are essential cellular mechanisms for maintaining the genomic integrity, and its disruption is one of the principal hallmarks of chronic and age-related diseases. Increased production of reactive oxygen species, such as H2O2and NO• are generated by Aβ which may can impact different molecules such as proteins, lipids, RNA and DNA. Under this condition, the brain of AD patients is submitted to increased oxidative stress, coinciding with depletion of antioxidant defense system. Each cell in the human body receives tens of thousands of DNA lesions per day by a variety of sources; therefore, cells have evolved a multifaceted response to counteract the potentially deleterious effect of DNA damage.e cellular response to DNA damage involves execution of DNA repair and activation of a repertoire of DNA damage signaling (Narciso et al., 2016).
We recently assess the effects of galantamine on the cell toxicity and DNA strand breaks induced by Aβ1–42using a set of biomarkers such as clonogenic assay (a cell biology technique for studying the effectiveness of specific agents on the survival and proliferation of cells), cytokinesis block micronucleus cytome (a comprehensive system for measuring DNA damage, cytostasis and cytotoxicity) and comet assay (a sensitive technique for the DNA damage detection at the level of the individual eukaryotic cell) in SH-SY5Y human neuroblastoma cell line as in vitro model in neurotoxicity research. Consistent with previous studies, we reported that Aβ1–42(10 μM) for 24 hours decreased cell proliferation by inducing cell death by necrosis rather than apoptosis; additionally, Aβ1–42treatment had a stronger impact on genomic stability events compared to untreated control. In contrast, Galantamine post-treatments (0.1, 1.0 and 10 μM) significantly improved the rate of cell survival and exerted a high antigenotoxic activity by reducing Aβ1–42-induced DNA damage. Interestingly, the effects of galantamine here reported are at a range of concentrations including blood concentration found in human aer oral administration. Overall, our study provided the first experimental evidence indicating that Galantamine also contributes to the neuroprotection mechanisms through regulation of DNA damage in addition to AChEi activity (Castillo et al., 2016). However, there are many questions about DNA damage and cholinergic impairment in the AD brain that should be answered, including which specific mechanisms are involved in cellular changes associated with AD pathogenesis and progression; and which specific enzymes are involved in cellular changes associated with DNA damage.
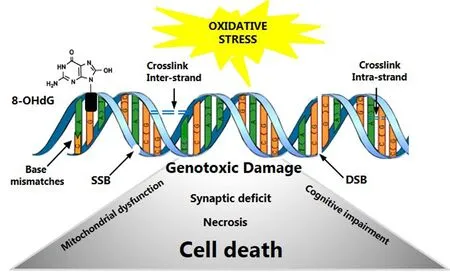
Figure 1 Types of DNA damage due to oxidative stress.
A distinct feature of neurons is that replication associated with DNA repair cannot occur in differentiated, postmitotic cells, and the maintenance of genomic stability becomes a challenge for neuronal survival. Different studies in mice and cell lines have provided insight into the role of repair mechanisms in neurodegeneration and AD. Recently it has been shown that heterozygous mice for the DNA polymerase β, a critical DNA base excision repair enzyme accelerate synaptic and cognitive deficit, including neuronal dysfunction and cell death (Sykora et al., 2015). In addition, studies addressing the involvement of base excision repair (BER) pathways have shown that differentiated human SH-SY5Y cells are more sensitive to oxidative damage than their undifferentiated counterpart (Sykora et al., 2013); according to authors, the attenuated BER in postmitotic neurons seems to be correlated with the decreasing protein levels of long-patch BER components. Still, the literature is scarce and more efforts are needed to explore the relationship between oxidative stress, genotoxicity and repair mechanisms in the brain and its relationship with neurodegenerative diseases such as Alzheimer’s disease.
那时,周启明的合同快要到期了,他担心公司可能会因为自己的这个情况不再续签。承受着巨大的心理压力,于是一点点小事,都能让他怀疑自己是个废人。
With regard to antigenotoxic effect exerted by Galantamine, we speculate that it might be associated with its antioxidant properties which is supported by the fact that an antioxidant is able to prevent the DNA damage by stimulating certain repair enzymes and induce fidelity DNA repair and replication through an effect denominated bio-antimutagenic or acting as a desmutagenic agent that acts directly on mutagens, or on its precursor, in order to inactivate it. Taken together, our results demonstrated an important contribution regarding galantamine effects against Aβ1–42-induced genomic instability.ese observations are fully consistent with antioxidant properties of galantamine. It has been reported that drug reduced the release of reactive oxygen species and prevented the loss of mitochondrial activity.e capability of the drug to scavenge the reactive oxygen species has been associated with the enol group and the presence of the quaternary nitrogen in the molecule; in addition, the conversion of galantamine to galantamine hydrobromide is accompanied by a signif icant increase in the radical-scavenging effect (Tsvetkova et al., 2013).
Our study has reveled an important role of galantamine against Aβ1–42-induced genetic instability. In this context, DNA repair mechanisms and modulation of genetic damages might be studied to gain knowledge about the antigenotoxic mechanisms exerted by Galantamine by analyzing pathways and main proteins that are being regulated by the drug.
Nowadays, there is still no effective treatment for AD and no new therapeutic drugs have been approved since 2003. However, several putative drugs have been thoroughly investigated in preclinical studies, but many of them have failed to produce promising results in the clinical scenario. We speculate that lack of effectiveness might be associated with the fact that these drugs do not take into account the genetic instability as potential mechanism in AD pathogenesis. Galantamine is a natural alkaloid, isolated from bulbs and aerial parts of plant fromAmaryllidaceaefamily. Numerous studies have shown that bioactive compound belongs to a variety of phytochemical groups such as phenolics, pigments, allysulfides, glucosinolates, tannins, flavonoids and phytosterols are effective phytoantigenotoxics in addition to their antioxidant, antimutagenic, anticarcinogenic, antoestrogenic, and antiinflamatory properties which might be beneficial in preventing diseases by improving genomic stability. In previous studies,Amaryllidaceaealkaloids such as galantamine, Lycorine, Homolycorine and Hemantamine have been shown by exerting a high antioxidant activity and AChEi (López et al., 2002, Castillo et al., 2017). Based on these findings and coinciding with antigenotoxic capacity of Galantamine, it is possible that the modulation of DNA damage exerted byAmaryllidaceaealkaloids may represent an important biological property in addition to AChEi activity. We anticipate results obtained in our laboratory, which showed AChEi and antigenotoxic activities of crude extract from a plant belonging toAmaryllidaceaefamily against Aβ1–42-induced cytotoxicity and genotoxicity in SH-SY5Y cells when evaluated trough a set of genotoxic and cytotoxic biomarkers.e results showed that Aβ1–42significantly inhibited cell viability through necrosis rather than apoptosis, increased the DNA damage and exerted mitochondrial morphological alterations; however, treatments with the extract led to a significant recovery of cell survival, decreased necrotic cell death and also exerted antigenotoxic effects; additionally, the extract showed an inhibitory activity of AChE (submitted paper). Overall, the modulation of neurodegeneration by agents with antigenotoxic properties might emerge as a new avenue for the development of interventions that may improve the neural defense response against neurodegeneration.
This research was supported by the Brazilian funding agencies: CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)-No: 5614112 and Student Agreement Program for post graduation –PEC-PG.
Willian O. Castillo*, Andres Felipe Aristizabal-Pachon
Department of Biology, University of Cauca, Popayan, Colombia (Castillo WO)
Department of Genetics, Ribeirão Preto Medical School, University of São Paulo — USP, Brazil (Aristizabal-Pachon AF)
*Correspondence to:Willian O. Castillo, Ph.D., wocastillo@unicauca.edu.co.
Accepted:2017-06-05
orcid:0000-0001-9138-1248 (Willian O. Castillo)
How to cite this article:Castillo WO, Aristizabal-Pachon AF (2017) Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease. Neural Regen Res 12(6):916-917.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Anand R, Gill KD, Mahdi AA (2014)erapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology 76:27-50.
Castillo WO, Aristizabal-Pachon AF, de Lima Montaldi AP, Sakamoto-Hojo ET, Takahashi CS (2016) Galantamine decreases genotoxicity and cell death induced by β-amyloid peptide in SH-SY5Y cell line. Neurotoxicology 57: 291-297.
Castillo WO, Tamarozzi ER, da Silva GM, Aristizabal-Pachon AF, Sakamoto-Hojo ET, Takahashi CS, Giuliatti S (2017) Exploration of the acetylcholinesterase inhibitory activity of some alkaloids from amaryllidaceae family by molecular docking in silico. Neurochem Res doi:10.1007/s11064-017-2295-8.
López S, Bastida J, Viladomat F, Codina C (2002) Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci 71:2521-2529.
Narciso L, Parlanti E, Racaniello M, Simonelli V, Cardinale A, Merlo D, Dogliotti E (2016)e response to oxidative DNA damage in neurons: mechanisms and disease. Neural Plast 2016:3619274.
Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FM (2015)erapeutic opportunities within the DNA damage response. Nat Rev Cancer 15:166-180.
Sanjib Bhattacharya S (2011) Natural antimutagens: a review. Res J Med Plant 5:116-126.
Sykora P, Yang JL, Ferrarelli LK, Tian J, Tadokoro T, Kulkarni A, Weissman L, Keijzers G, Wilson DM, Mattson MP (2013) Modulation of DNA base excision repair during neuronal differentiation. Neurobiol Aging 34:1717-1727.
Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D, Tian J, Baptiste BA, Cong WN, Brenerman BM (2015) DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res 43:943-959.
Tsvetkova D, Obreshkova D, Zheleva-Dimitrova D, Sasol(2013) Antioxidant activity of galantamine and some of its derivatives. Curr Med Chem 20:4595-4608.
10.4103/1673-5374.208572
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- Polyethylene glycol as a promising synthetic material for repair of spinal cord injury
- Novel aspects of extracellular adenosine dynamics revealed by adenosine sensor cells
- Electroacupuncture regulates the stress-injury-repair chain of events after cerebral ischemia/reperfusion injury
- Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke
- High-frequency and brief-pulse stimulation pulses terminate cortical electrical stimulation-induced afterdischarges