Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
2017-08-07JianHaoBoLiHuiquanDuanChenxiZhaoYanZhangChaoSunBinPanChangLiuXiaohongKongXueYaoShiqingFeng
Jian Hao, Bo Li, Hui-quan Duan Chen-xi Zhao Yan Zhang Chao Sun Bin Pan Chang Liu, Xiao-hong Kong, Xue Yao, Shi-qing Feng
1 Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin, China
2 School of Medicine, Nankai University, Tianjin, China
3 Tianjin Neurological Institute, Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in Central Nervous System, Ministry of Education, Tianjin, China
Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
Jian Hao1,#, Bo Li1,#, Hui-quan Duan1, Chen-xi Zhao1, Yan Zhang1, Chao Sun1, Bin Pan1, Chang Liu2, Xiao-hong Kong2, Xue Yao1,3,*, Shi-qing Feng1,3,*
1 Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin, China
2 School of Medicine, Nankai University, Tianjin, China
3 Tianjin Neurological Institute, Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in Central Nervous System, Ministry of Education, Tianjin, China
Graphical Abstract
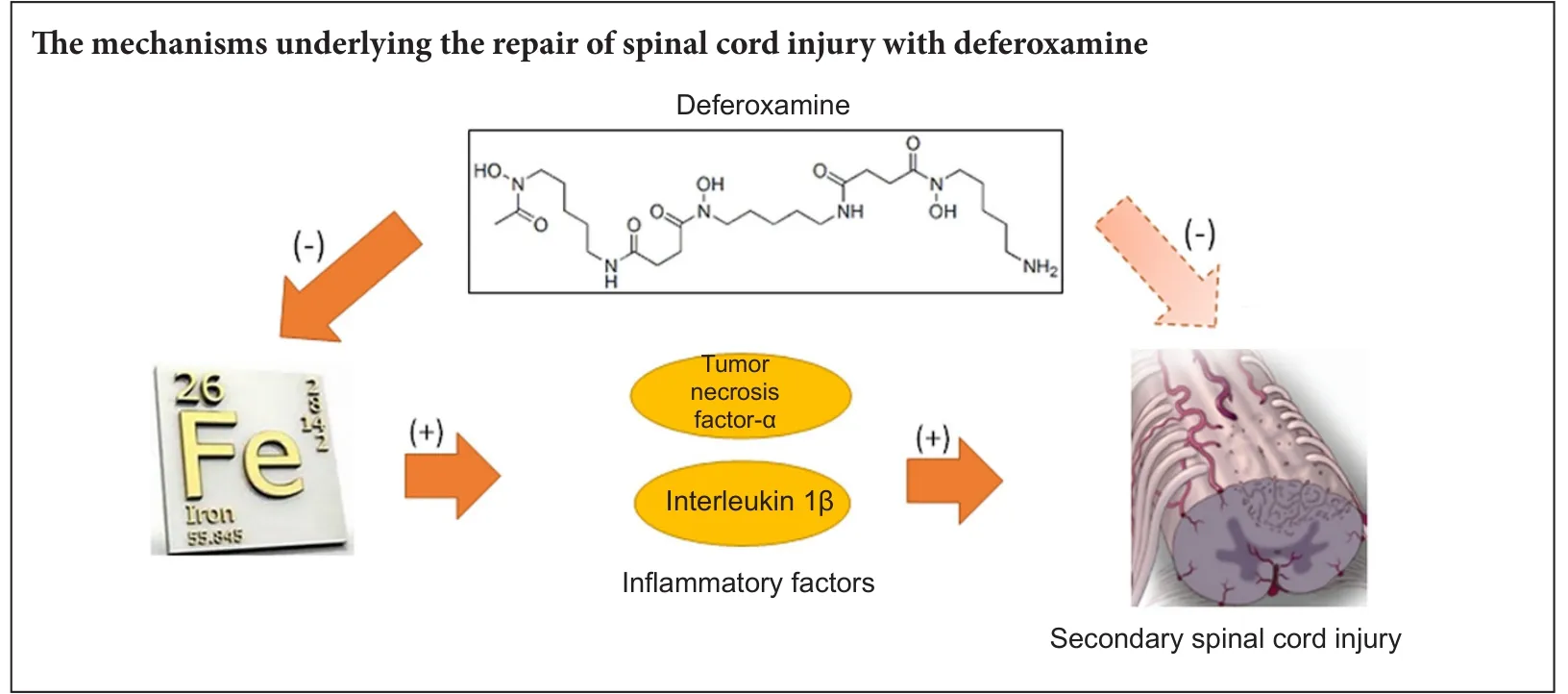
Deferoxamine, a clinically safe drug used for treating iron overload, also repairs spinal cord injury although the mechanism for this action remains unknown. Here, we determined whether deferoxamine was therapeutic in a rat model of spinal cord injury and explored potential mechanisms for this effect. Spinal cord injury was induced by impacting the spinal cord at the thoracic T10vertebra level. One group of injured rats received deferoxamine, a second injured group received saline, and a third group was sham operated. Both 2 days and 2 weeks aer spinal cord injury, total iron ion levels and protein expression levels of the proinflammatory cytokines tumor necrosis factor-α and interleukin-1β and the pro-apoptotic protein caspase-3 in the spinal cords of the injured deferoxamine-treated rats were significantly lower than those in the injured saline-treated group.e percentage of the area positive for glial fibrillary acidic protein immunoreactivity and the number of terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells were also significantly decreased both 2 days and 2 weeks post injury, while the number of NeuN-positive cells and the percentage of the area positive for the oligodendrocyte marker CNPase were increased in the injured deferoxamine-treated rats. At 14—56 days post injury, hind limb motor function in the deferoxamine-treated rats was superior to that in the saline-treated rats.ese results suggest that deferoxamine decreases total iron ion, tumor necrosis factor-α, interleukin-1β, and caspase-3 expression levels aer spinal cord injury and inhibits apoptosis and glial scar formation to promote motor function recovery.
nerve regeneration; spinal cord injury; deferoxamine; tumor necrosis factor-α; interleukin-1β; apoptosis; iron; anti-inflammatory; glial scar; proinflammatory; rats; motor function; lipid peroxidation; neural regeneration
Introduction
Spinal cord injury (SCI) can be divided into two major phases: the primary injury phase and the secondary injury phase. Investigation into the pathophysiology of secondary injury has revealed a number of proposed underlying mechanisms, including ischemia, edema, ion homeostasis changes, biochemical alterations, and metabolic disorders, that result in cell death and tissue damage (Ahn et al., 2006; Genovese et al., 2007; Oyinbo, 2011; Wang et al., 2011). However, the cascade of the inflammatory responses elicited by tumor necrosis factor-α (TNF-α) is considered to be central to secondary injury (Si et al., 2000; Leal-Filho, 2011; Oyinbo, 2011).
The influx of erythrocytes caused by hemorrhage during SCI provides a rich source of iron (Koszyca et al., 2002). Iron plays an important role in glutamate excitotoxicity in spinal cord motor neurons. Free iron can also lead to formation of reactive oxygen species through the Fenton reaction, producing free radicals that contribute to secondary injury (Emerit et al., 2001; Liu et al., 2003; Routhe and Moos, 2015).
Deferoxamine (DFO), an iron chelator, has a high af finity for trivalent iron ions and is used therapeutically for treatment of chronic hyperferremia and acute iron poisoning. Previous studies have demonstrated that treatment with DFO can also promote recovery aer SCI in rats (Genovese et al., 2007; Liu et al., 2011). Treatment with DFO reduces lesion volumein vivoand increases regenerationin vitro. Chelation of iron appears to block secondary damage, and experiments aimed at elucidating the underlying mechanism revealed that DFO inhibits lipid peroxidation (Chojnacki and Weiss, 2008).
Recently, modulation of inflammatory responses in SCI has come under investigation (Li et al., 2016). Vigorous immune responses induced by SCI have been shown to contribute to secondary cell death (Zoppi et al., 2011). Production of TNF-α, primarily by macrophages and microglial cells, enhances the inflammatory response, upregulates intercellular adhesion molecules, and promotes adhesion and recruitment of leukocytes to the site of injury.ese leukocytes release free radicals, proteases, and toxic oxidative metabolites, triggering a damage cascade that worsens inflammation.
Here, we explored the effect of DFO on levels of the major proinflammatory cytokines, TNF-α and interleukin-1β (IL-1β), to further investigate the understanding of the mechanisms underlying DFO-induced neural repair in SCI. TNF-α and IL-1β have been shown to induce neuronal apoptosis after SCI (Wang et al., 2005; de Rivero Vaccari et al., 2008). We detected whether DFO treatment reduced apoptosis in this rat model and whether DFO prevented glial scar formation.
Materials and Methods
Animals
Female Wistar rats (n= 54) weighing 230—250 g were provided by the Institute of Radiation Medicine, Chinese Academy of Medical Sciences, Tianjin, China. Rats were housed and maintained on a 12-hour light/dark cycle, and allowed free access to food and water.e rats were kept in standard cages, with five animals per cage.
The 54 rats were randomly allocated to three different groups containing 18 animals per group: sham group (sham-operated), injury group (SCI + saline-treated), and injury + DFO group (SCI + DFO-treated). Within each group, 3 rats were used for iron detection at 2 days and 3 more rats were used at 2 weeks post-SCI; 3 rats were used for hematoxylin and eosin staining and glial fibrillary acidic protein (GFAP), NeuN, 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase), and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) detection assays at 2 days post-SCI, and 3 more rats for the same assays at 8 weeks post-SCI; and 3 rats were used for western blot assays at 2 days and 3 more rats at 2 weeks post-SCI.
SCI and intervention
Rats were anesthetized by intraperitoneal injection with 10% chloral hydrate (0.3 mL/100 g body weight). SCI models were established using a modification of Allen’s method (Koozekanani et al., 1976). Briefly, a laminectomy was performed at the T9—10thoracic vertebrae level to expose the spinal cord at T10. SCI was induced by New York University Impactor device (NYU, New York, NY, USA) using a 10 g-rod and a 25-mm drop height.e hind limbs of the rats exhibited involuntary spasms and the tails wriggled, indicating that the injury was consistent with the criteria of SCI in this model. Aer surgery, each rat was placed in a constant-temperature cage until fully recovered from the anesthesia. During the postoperative period, the bladder of each rat was manually voided twice daily until the bladder control reflex was restored.
The sham group was subjected only to laminectomy. The injury + DFO group was subjected to SCI and then treated with intraperitoneal injections from day 0 to day 7 with DFO (100 mg/kg per day; Novartis, Basel, Switzerland; 500 mg dissolved in 5 mL of 0.9% normal saline). Another control group (injury group) was also subjected to SCI, but was treated with intraperitoneal injections of saline (1 mL/kg).
Histological analysis
Rats were sacrificed at 2 days or 56 days post-SCI. Two rats randomly selected from each group were intracardially perfused with 4% paraformaldehyde under anesthesia.e spinal cord, including the entire T9—10segment, was dissected and fixed in 4% paraformaldehyde solution.e specimens were embedded in paraf fin and cut into 5-μm cross sections using a cryostat.e sections were stained with hematoxylinand eosin.e histopathological analysis was performed by a pathologist who was blinded to the experimental design.e number of nuclei was calculated to show the pathological differences among the groups.
Immunohistochemistry and immunofluorescence
Rats were sacrificed 2 days or 8 weeks post-SCI. For immunohistochemical staining, paraffin sections (5 μm) from each specimen were deparaffinized with xylene (two times for 30 minutes each), rehydrated in graded concentrations of ethanol (twice exposures to each of the following concentrations: 100%, 95%, 85%, and 75%), and washed in phosphate-buffered saline (PBS) for 5 minutes. The sections were then incubated with a rabbit anti-rat NeuN monoclonal antibody (1:500; Abcam, Branford, CT, USA) to detect neurons or a rabbit anti-rat CNPase monoclonal antibody (1:100; Proteintech Rosemont, IL, USA) to detect oligodendrocytes overnight at 4°C. After being washed in PBS for 5 minutes, the sections were incubated with goat anti-rabbit IgG (1:200; Beyotime, Haimen, Jiangsu Province, China) for 30 minutes at 37°C. Subsequently, the sections were incubated with avidin-biotin-peroxidase (Beyotime) for 45 minutes at 37°C and then transferred to a solution containing 30% hydrogen peroxide in 0.05 M PBS containing 1 drop of 3,3′-diaminobenzidine (0.0125 g/25 mL) for 10 minutes at 37°C. After being washed in PBS, the sections were dehydrated in series of increasing concentrations of ethanol (25%, 50%, 80%, 100%; twice at each concentration), washed twice with xylene, and then mounted onto glass slides for microscopic analysis. Image J software (version 2.1.4.7; National Institutes of Health, Bethesda, MD, USA) was used to assess the percentages of the areas in sections that were immunopositive.
For immunofluorescence, the sections were rehydrated as described above and incubated with a primary antibody (polyclonal goat anti-rat GFAP, 1:400; Abcam) overnight at 4°C. The next day, the sections were washed twice for 5 minutes each in PBS and incubated with a Cy3-labeled donkey anti-goat IgG secondary antibody (1:200; Beyotime) for 60 minutes at room temperature. PBS was used to wash the sections, and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, 1 g/mL; Biosharp, Hefei, Anhui Province, China) for 5 minutes.e sections were washed, and one drop of anti-fade mounting medium (Solarbio, Beijing, China) was placed on each slide, and a coverslip was placed on top of each sample. All sections were photographed using an inverted fluorescence microscope (IX71, Olympus, Tokyo, Japan).
Western blot assay
Rats were sacrificed 2 days or 2 weeks post-SCI. Aer being anesthetized, the rats were intracardially perfused with 9% saline (300 mL per rat; 4°C). Control and injured spinal cord tissue (0.5 cm above and below the site of injury) was dissected, frozen in liquid nitrogen, and stored at -80°C. Aer being homogenized, tissue samples were centrifuged at 12,000 ×gfor 15 minutes at 4°C.e supernatants were collected, and total protein was extracted from tissues and quantified using a Bicinchoninic Acid Protein Assay kit according to the manufacturer’s instructions (Wanleibio; Shenyang, Liaoning Province, China). Proteins were resolved on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene fluoride membranes.e membranes were blocked in 5% fat-free milk in PBS for 60 minutes and then incubated overnight at 4°C with the following primary antibodies: anti-IL-1β (1:500; Wanleibio), anti-TNF-α (1:500; Wanleibio), anti-caspase-3 (1:500; Wanleibio), and anti-β-actin (1:10,000; Wanleibio). The membranes were then washed with PBS and subsequently incubated at 37°C for 45 minutes with goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibodies (1:10,000; Wanleibio). Bands were visualized using chemiluminescence on X-ray film (Kodak, Rochester, NY, USA). Band grayscale values were quantified using Gel-Pro Analyzer software (Media Cybernetics, Rockville, MD, USA), and all grayscale values were normalized to that of β-actin. All assays were performed in triplicate.
Total iron detection
Spinal cord samples were collected as described above and homogenized under ice-cold conditions. Iron levels of spinal cords at 2 days and 2 weeks post-SCI were detected by following the tissue iron assay kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China). All assays were performed in triplicate.
TUNEL assay
Rats were sacrificed 2 days or 2 weeks post-SCI. The TUNEL assay was performed using an In Situ Cell Death Detection kit (Roche, USA) according to the manufacturer’s recommended instructions. Briefly, spinal cord sections were rehydrated and incubated for 15—30 minutes at 37°C with the Proteinase K working solution. Subsequently, the slides were rinsed twice with PBS. The Converter-POD solution (50 μL) was added to the sections, which were then incubated in a humidified chamber for 30 minutes at 37°C and washed three times with PBS.e 3,3′-diaminobenzidine substrate (50—100 μL) was added to each sample, which was then incubated for 10 minutes at 25°C and washed three times.e samples were mounted under glass coverslips using glycerol.
Behavioral evaluation
Recovery of locomotor function post-SCI was evaluated using the Basso, Beattie, Bresnahan (BBB) locomotor rating scale (Barros Filho and Molina, 2008). This scale ranges from 0 to 21, with complete paralysis scored 0 and normal locomotor function scored 21. Each rat was evaluated weekly post-SCI by two independent observers blinded to the experimental design.e rats were allowed to move freely in an open field apparatus (1 m × 1 m), and their movements were recorded for 5 minutes. The BBB scores were determined 0, 7, 14, 21, 28, 35, 42, 49, and 56 days post-SCI to assess locomotor recovery.
Statistical analysis
All data are presented as the mean ± SEM. All statistical analyses were conducted using SPSS 19.0 software (IBM Corporation, NY, USA). Significant differences among groups were assessed by one-way analysis of variance followed by Tukey’spost hoctest.P-values less than 0.05 were considered statistically significant.
Results
DFO treatment blocked the SCI-induced increase in iron levels

Figure 1 Deferoxamine (DFO) effects on total iron levels in rats with spinal cord injury.
Iron overload in injured spinal cord tissue plays a detrimental role in secondary injury. To analyze changes in iron concentrations post-SCI, iron levels were analyzed in rats from the sham, injury, and injury + DFO groups at 2 days and 2 weeks after injury (Figure 1). Compared with those in the sham group, iron levels significantly increased in the injury group 2 and 14 days post-SCI (P< 0.01,P< 0.001). By contrast, iron levels in the injured rats treated with DFO were not significantly different from those in the sham group, but were significantly lower than those in saline-treated SCI rats 2 days and 2 weeks post-SCI (P< 0.01 andP< 0.001, respectively).us, DFO exhibited a strong ef ficacy to maintain the iron level of the injured spinal cord at a level similar to that of uninjured tissue.
DFO treatment blocked the SCI-induced increase in IL-1β and TNF-α protein expression
TNF-α and IL-1β, as key proinflammatory cytokines, play a dominant role in inflammatory reactions during the secondary injury phase. To assess the anti-inflammatory effects of DFO, we analyzed protein expression of TNF-α and IL-1β 2 days and 2 weeks post-SCI (Figure 2). We found that TNF-α and IL-1β protein levels were significantly increased 2 days and 2 weeks post-SCI (P< 0.001). By contrast, injured rats treated with DFO showed TNF-α and IL-1β protein levels similar to those in sham-operated rats and significantly lower than those in saline-treated injured rats. These results suggested a prolonged inhibitory effect of DFO on the expression of TNF-α and IL-1β during both the acute and chronic phases post-SCI (P< 0.001) and provided evidence for the effective anti-inflammatory activity of DFO aer SCI.
DFO ameliorated SCI-induced pathological morphology

Figure 2 DFO effects on TNF-α, IL-1β, and caspase-3 protein expression levels in the injured spinal cord of rats.
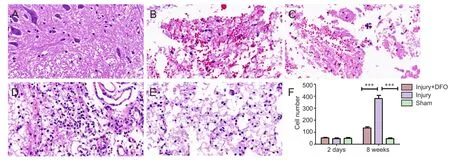
Figure 3 DFO effects on pathological morphology in injured spinal cord of rats.
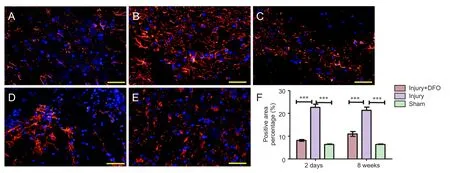
Figure 4 DFO effects on glial scars in the injured spinal cord of rats.
To determine the effects of DFO on the histological changes post-SCI, hematoxylin and eosin staining was conducted 2 days and 8 weeks post injury on tissue obtained from each group (Figure 3). Compared with spinal cord tissue from rats in the sham group, tissue from both DFO- and saline-treated rats with SCI showed obvious morphological changes, leaked erythrocytes, and severe spinal cord structural disorder 2 days post injury. However, 8 weeks post-SCI, we observed a substantial and significant increase in cell number (P< 0.001 compared with sham-operated rats) as well as increased neovascularization in the injury group, whereas neovascularization in injured rats treated with DFO was decreased.
DFO treatment reduced glial scar formation post-SCI
To analyze the effects of DFO on scar formation post-SCI, we examined the expression of GFAP, a major component of the scar matrix (Figure 4). Compared with that in the injury group, the percentage of the GFAP-positive area in the injured rats treated with DFO was significantly lower, and it was only slightly higher than that observed in thesham group 2 days post-SCI (P< 0.001). At 8 weeks post-SCI, glial scar formation was observed surrounding the crushed area in the injured saline-treated rats. The percentage of the area with glial scars was significantly lower in the DFO-treated rats than in the saline-treated rats (P< 0.001).ese results indicated that DFO treatment decreased scar formation aer SCI.
DFO treatment protected neurons and increased expression of CNPase
Neurons are critical for the normal functioning of the central nervous system, and neuronal death results in permanent functional loss.e myelin sheath increases the speed of impulse propagation along myelinated neuronal fibers. In the sham group, a large number of NeuN-positive (+) cells were detected and appeared to have normal morphology (Figure 5). Although NeuN(+) cells were also present in the injury group 2 days post-SCI, they were smaller in size and appeared markedly degenerated. In comparison, the number of surviving neurons was higher in the DFO-treated rats 2 days post-SCI (P< 0.05). At 8 weeks post-SCI, few NeuN(+) cells were found in the injured saline-treated rats, and a large glial scar was observed. By contrast, numerous NeuN(+) cells were present at 8 weeks in rats treated with DFO (P< 0.01).
Compared with that from the sham group, spinal cord tissue derived from both DFO- and saline-treated injured rats displayed similar reductions in the percentage of area that was CNPase(+) 2 days post-SCI (P< 0.001). However, DFO treatment was associated with a significant increase in the CNPase(+) area at 8 weeks compared with that for rats in the injury group (P< 0.01;Figure 6).ese results indicated that DFO had a protective effect on neuronal survival and maintenance of the myelin sheath post-SCI.
DFO inhibited apoptosis post-SCI
To determine whether apoptosis was involved in the response to SCI, we analyzed caspase-3 protein expression and conducted a TUNEL assay (Figure 7). At 2 days post-SCI, caspase-3 expression was markedly and significantly increased in the injury group compared with that in the sham group. However, DFO-treated rats displayed slightly higher caspase-3 protein expression levels than the sham-operated rats and significantly lower expression than the injured saline-treated rats. Furthermore, in contrast to the continued high expression level observed in the injured saline-treated rats, caspase-3 levels in DFO-treated rats were comparable to those in the sham-operated rats 2 weeks post-SCI (Figure 7A,B). The TUNEL assay was conducted to confirm the differences in apoptosis levels among the treatment groups. Consistent with the caspase-3 results, a significantly higher number of TUNEL-positive cells was observed in the injury group relative to both the sham group, which had zero TUNEL-positive cells, and injury + DFO group, which showed a few scattered TUNEL-positive cells 2 days post-SCI. These results provided evidence that DFO treatment strongly inhibits apoptosis in injured spinal cord tissue.
DFO treatment promotee locomotor recovery aer SCI
Discussion
Secondary SCI is an important process for early treatment of SCI (Huang et al., 2015; Young et al., 2015; Zhao et al., 2015). SCI-induced inflammatory responses play an important role in the secondary injury phase. In flammatory cytokines and mediators are the basic effector molecules that participate in and control the in flammatory response. TNF-α, IL-1β, and other in flammatory mediators in filtrate the injured spinal cord tissue, thereby causing a strong inflammatory cascade response (Yin et al., 2012).
Expression of these cytokines and the resulting inflammatory responses are the initiating factors that lead to neuronal apoptosis (Paulson et al., 2013), which is the key form of secondary injury after the initial insult. Therefore, control of the initial inflammatory response is very important for treating SCI and preventing further injury (David et al., 2012). Thus, identification of therapeutic means to inhibit inflammatory responses aer SCI could help to reduce secondary SCI and protect the injured spinal cord.
As an important regulator of neuroinflammation, TNF-α is produced by many immune and non-immune cells, including macrophages, T cells, mast cells, granulocytes, natural killer cells, fibroblasts, neurons, and has been shown to play important roles in signal transduction and posttraumatic inflammatory responses (Esposito and Cuzzocrea, 2011; Olmos and Llado, 2014).
Harrington et al. (2005) demonstrated increased expression of TNF-α and its receptors in neurons 6 hours post-SCI. Previous studies have shown that rapid and sustained high expression of TNF-α increases the inflammatory response and promotes secondary injury (Kwon et al., 2012). Thus, preventing the upregulation of TNF-α during the early stage of SCI could reduce inflammatory responses at the injured site. Macrophages are triggered by central nervous system injury and contribute to secondary injury and functional damage (David and Kroner, 2011).
Post-SCI, macrophage phagocytosis of myelin and erythrocytes, released following hemorrhage and tissue damage, results in high iron levels in cells, which could induce overexpression of TNF-α and transformation ofmacrophages from the M2 to the M1 phenotype and then aggravate secondary injury (Kroner et al., 2014). Importantly, M2 macrophages reduce local inflammation and protect the spinal cord from further injury (Kigerl et al., 2009), whereas M1 macrophages aggravate the inflammatory response and promote secondary injury, suggesting a potential underlying mechanism explaining the positive effects of DFO treatment. As an iron chelator, DFO may reduce the iron ion level, and our results showed that DFO treatment significantly reduced the iron concentration at the site of SCI, especially 2 weeks post injury, consistent with previous studies (de Castro et al., 1999).
Our data also confirmed that DFO inhibited the expression of TNF-α, as well as IL-1β. Thus DFO reduced the concentration of iron ions in the injured segment and inhibited TNF-α expression, which may block the transformation of M2 macrophages to the M1 phenotype, thereby reducing inflammation and protecting against further SCI.
Our results demonstrated that DFO reduced the SCI-induced expression of IL-1β, as well as caspase-3, and decreased the neuronal apoptosis observed aer SCI. Interleukin-1β has been shown to inhibit nerve regeneration aer SCI (David and Kroner, 2011), and IL-1 receptor antagonists reduce neuronal apoptosis, alleviating damage after injury and serving as protective agents (Nesic et al., 2001).
Previous studies have demonstrated that DFO can inhibit nuclear factor-κB signaling pathways and reduce infiltration of neutrophils into the spinal cord, thereby diminishing the local inflammatory response (Paterniti et al., 2010). However, the present study did not address the effect of DFO on inflammatory factors, which are the major mediators of the inflammatory response.erefore, in future studies, it will be important to determine DFO effects on these inflammatory factors to better understand the protective mechanisms underlying the benefits of DFO aer SCI. Our results demonstrated that DFO decreased IL-1β expression and reduced the inflammatory response after injury, decreasing neuronal apoptosis and protecting against further SCI. The mechanism by which DFO reduces the inflammatory response may be through inhibition of high mobility group box 1 production or decreased expression of toll-like receptor-4 and the receptor for advanced glycation end-products, interrupting the vicious inflammatory response cycle and protecting against SCI. Further experiments will be required to test these hypotheses.
DFO directly decreased iron levels at the injured site and reduced apoptosis. The underlying relationship of reduced iron levels and apoptosis may be affected by myriad factors. For example, reactive oxygen species, induced by iron, usually leads to cell death upon injury; apoptosis, which is a form of programmed cell death, is reduced by DFO treatment post-SCI (Fankhauser et al., 2000). Additionally, TNF-α and IL-1β have been implicated as signals to induce apoptosis in neurons, astrocytes, and oligodendrocytes after SCI. The reduction of proinflammatory cytokines aer DFO treatment abolishes the apoptotic cascade (Ehrlich et al., 1999). DFO promotes neuronal survival, as we showed in the present study using a TUNEL assay. The neuroprotective effect can also be observed as the protection of oligodendrocytes, which create the neuronal myelin sheaths (Takahashi et al., 2003).erefore, DFO showed a protective effect on nerve function following SCI. Recent studies indicate that other forms of cell death are also found aer SCI, such as necroptosis, ferroptosis and autophagy.e relationship between iron levels and other types of cell death in SCI remains to be elucidated.
In summary, DFO exhibited treatment ef ficacy in an animal model of SCI by ameliorating pathophysiological morphology and improving locomotor function. The underlying mechanism may involve reduction of local iron concentrations resulting from hemorrhage in the injured spinal cord and subsequent decreases in TNF-α and IL-1β levels released by macrophages and microglia as a result of the reduced iron levels. These changes in cytokine expression may facilitate macrophage shis from the M1 to the M2 phenotype, thereby inhibiting inflammatory reactions during secondary SCI. Consequent reductions in levels of apoptosis post-SCI and rescue of remaining neurons and oligodendrocytes likely underlie both the ameliorated pathophysiology and recovery of locomotor function.is study provided a potential new pharmacologic strategy for treatment of SCI, began to elucidate the detailed underlying mechanism for this therapy, and provided evidence indicating that further preclinical and clinical investigation for the therapeutic use of DFO in SCI is warranted.
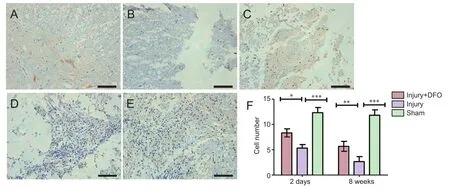
Figure 5 DFO effects on the number of NeuN(+) neurons in injured spinal cord of rats.

Figure 6 DFO effects on the number of oligodendrocytes in injured spinal cord of rats (inverted fluorescence microscope).
Author contributions:JH, BL and XY designed this study. HQD, CS andBP performed this study. YZ, CXZ and CL analyzed data. XY, BL and XHK wrote the paper. BL and XY provided critical revision of the paper. XY and SQF provided the funding and supervision. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:
Open access statement:
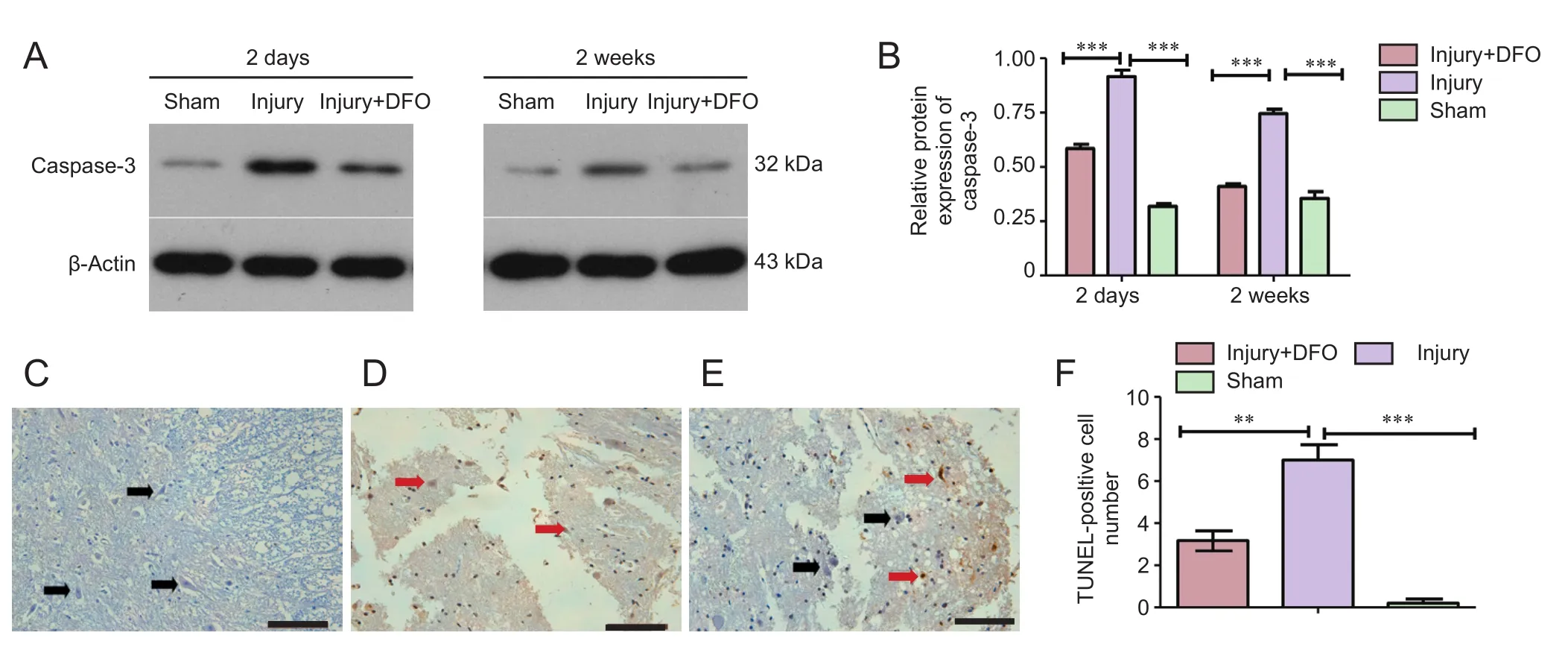
Figure 7 DFO effects on cell apoptosis in the injured spinal cord of rats.
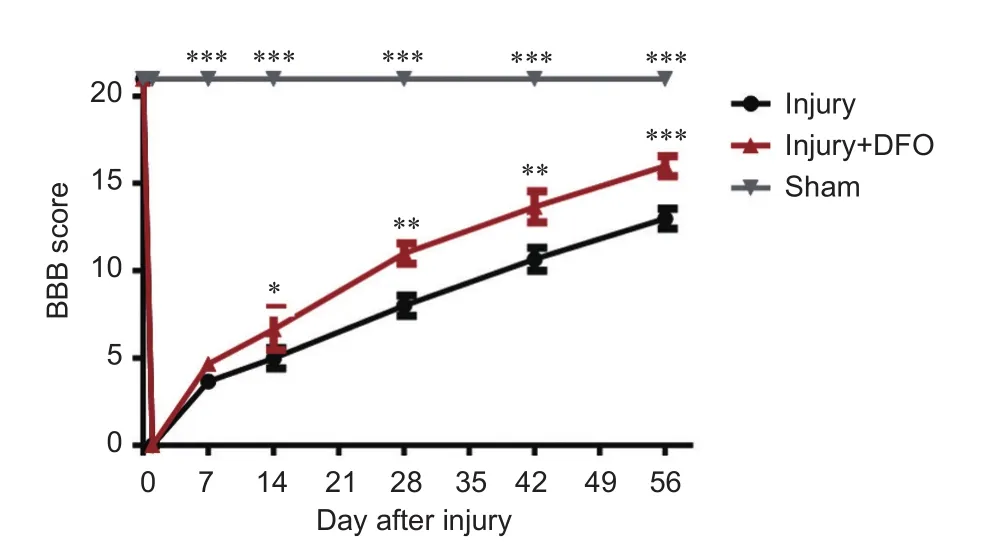
Figure 8 DFO effects on motor function in rats aer spinal cord injury.
Contributor agreement:A statement of “Publishing Agreement” has beensigned by an authorized author on behalf of all authors prior to publication.Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Ahn YH, Lee G, Kang SK (2006) Molecular insights of the injured lesions of rat spinal cords: Inflammation, apoptosis, and cell survival. Biochem Biophys Res Commun 348:560-570.
Barros Filho TE, Molina AE (2008) Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics (Sao Paulo) 63:103-108.
Chojnacki A, Weiss S (2008) Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nat Protoc 3:935-940.
David S, Kroner A (2011) Repertoire of microglial and macrophage responses aer spinal cord injury. Nat Rev Neurosci 12:388-399.
David S, Zarruk JG, Ghasemlou N (2012) Inflammatory pathways in spinal cord injury. Int Rev Neurobiol 106:127-152.
de Castro R, Jr., Alcock NW, McAdoo DJ (1999) Sampling of low molecular weight iron by microdialysis following spinal cord injury. J Neurosci Res 57:735-739.
de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW (2008) A molecular platform in neurons regulates inflammation aer spinal cord injury. J Neurosci 28:3404-3414.
Didangelos A, Puglia M, Iberl M, Sanchez-Bellot C, Roschitzki B, Bradbury EJ (2016) High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation aer spinal cord injury. Sci Rep 6:21607.
Ehrlich LC, Peterson PK, Hu S (1999) Interleukin (IL)-1beta-mediated apoptosis of human astrocytes. Neuroreport 10:1849-1852.
Emerit J, Beaumont C, Trivinf(2001) Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother 55:333-339.
Esposito E, Cuzzocrea S (2011) Anti-TNF therapy in the injured spinal cord. Trends Pharmacol Sci 32:107-115.
Fankhauser C, Friedlander RM, Gagliardini V (2000) Prevention of nuclear localization of activated caspases correlates with inhibition of apoptosis. Apoptosis 5:117-132.
Genovese T, Mazzon E, Crisafulli C, Esposito E, Di Paola R, Muia C, Di Bella P, Meli R, Bramanti P, Cuzzocrea S (2007) Combination of dexamethasone and etanercept reduces secondary damage in experimental spinal cord trauma. Neuroscience 150:168-181.
Harrington JF, Messier AA, Levine A, Szmydynger-Chodobska J, Chodobski A (2005) Shedding of tumor necrosis factor type 1 receptor aer experimental spinal cord injury. J Neurotrauma 22:919-928.
Huang H, Mao G, Chen L, AL (2015) Progress and challenges with clinical cell therapy in neurorestoratology. J Neurorestoratol 3:91-95.
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435-13444.
Koozekanani SH, Vise WM, Hashemi RM, McGhee RB (1976) Possible mechanisms for observed pathophysiological variability in experimental spinal cord injury by the method of Allen. J Neurosurg 44:429-434.
Koszyca B, Manavis J, Cornish RJ, Blumbergs PC (2002) Patterns of immunocytochemical staining for ferritin and transferrin in the human spinal cord following traumatic injury. J Clin Neurosci 9:298-301.
Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S (2014) TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83:1098-1116.
Kwon BK, Ghag A, Dvorak MF, TetzlaffW, Illes J (2012) Expectations of benefit and tolerance to risk of individuals with spinal cord injury regarding potential participation in clinical trials. J Neurotrauma 29:2727-2737.
Leal-Filho MB (2011) Spinal cord injury: From inflammation to glial scar. Surg Neurol Int 2:112.
Li XG, Lin XJ, Du JH, Xu SZ, Lou XF, Chen Z (2016) Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function aer spinal cord injury. Neural Regen Res 11:1678-1684.
Liu D, Liu J, Sun D, Alcock NW, Wen J (2003) Spinal cord injury increases iron levels: catalytic production of hydroxyl radicals. Free Radic Biol Med 34:64-71.
Liu J, Tang T, Yang H (2011) Protective effect of deferoxamine on experimental spinal cord injury in rat. Injury 42:742-745.
Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R (2001) IL-1 receptor antagonist prevents apoptosis and caspase-3 activation aer spinal cord injury. J Neurotrauma 18:947-956.
Olmos G, Llado J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014:861231.
Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 71:281-299.
Paterniti I, Mazzon E, Emanuela E, Paola RD, Galuppo M, Bramanti P, Cuzzocrea S (2010) Modulation of inflammatory response aer spinal cord trauma with deferoxamine, an iron chelator. Free Radic Res 44:694-709.
Paulson TA, Goosey-Tolfrey VL, Lenton JP, Leicht CA, Bishop NC (2013) Spinal cord injury level and the circulating cytokine response to strenuous exercise. Med Sci Sports Exerc 45:1649-1655.
Routhe LJ, Moos T (2015) Handling iron in restorative neuroscience. Neural Regen Res 10:1558-1559.
Si Q, Nakamura Y, Kataoka K (2000) A serum factor enhances production of nitric oxide and tumor necrosis factor-alpha from cultured microglia. Exp Neurol 162:89-97.
Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW (2003) Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol 53:588-595.
Wang CY, Chen JK, Wu YT, Tsai MJ, Shyue SK, Yang CS, Tzeng SF (2011) Reduction in antioxidant enzyme expression and sustained inflammation enhance tissue damage in the subacute phase of spinal cord contusive injury. J Biomed Sci 18:13.
Wang XJ, Kong KM, Qi WL, Ye WL, Song PS (2005) Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity aer spinal cord injury. Acta Pharmacol Sin 26:934-942.
Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou WG, Sun SK, Luo ZJ (2012) Tanshinone IIA attenuates the inflammatory response and apoptosis aer traumatic injury of the spinal cord in adult rats. PLoS One 7:e38381.
Young W, AlZoubi ZM, Saberi H, Sharma A, Muresanu D, Feng S, Chen L, HH (2015) Beijing declaration of international association of neurorestoratology. J Neurorestoratol 3:121-122.
Yu Y, Kovacevic Z, Richardson DR (2007) Tuning cell cycle regulation with an iron key. Cell Cycle 6:1982-1994.
Zhao WT, Yuan HB, Li PP, Zhang HF, Liu MQ (2015) Methylprednisolone for acute spinal cord injury: a meta-analysis of therapeutic ef ficacy and adverse reactions. Zhongguo Zuzhi Gongcheng Yanjiu 19:6868 -6874.
Zoppi S, Perez Nievas BG, Madrigal JL, Manzanares J, Leza JC, Garcia-Bueno B (2011) Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology 36:805-818.
Copyedited by Smith T, Pack M, Wang J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Hao J, Li B, Duan HQ, Zhao CX, Zhang Y, Sun C, Pan B, Liu C, Kong XH, Yao X, Feng SQ (2017) Mechanisms underlying the promotion of functional recovery by deferoxamine aer spinal cord injury in rats. Neural Regen Res 12(6):959-968.
*Correspondence to:
Xue Yao, Ph.D. or Shi-qing Feng, M.D., xueyao@tmu.edu.cn or sqfeng@tmu.edu.cn.
orcid:
0000-0001-9437-7674
(Shi-qing Feng)
0000-0003-4904-7697
(Xue Yao)
10.4103/1673-5374.208591
Accepted: 2017-05-22
杂志排行
中国神经再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease
- Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair