Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
2017-08-07NathanBirenbaumMatthewMacEwanWilsonRay
Nathan K. Birenbaum, Matthew R. MacEwan,, Wilson Z. Ray,,
1 Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, USA
2 Department of Neurosurgery, Washington University School of Medicine, St. Louis, MO, USA
Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
Nathan K. Birenbaum1, Matthew R. MacEwan1,2, Wilson Z. Ray1,2,*
1 Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, USA
2 Department of Neurosurgery, Washington University School of Medicine, St. Louis, MO, USA
Macro-sieve electrodes were implanted in the sciatic nerve of five adult male Lewis rats following spinal cord injury to assess the ability of the macro-sieve electrode to interface regenerated peripheral nerve fibers post-spinal cord injury. Each spinal cord injury was performed via right lateral hemisection of the cord at the T9–10site. Five months post-implantation, the ability of the macro-sieve electrode to interface the regenerated nerve was assessed by stimulating through the macro-sieve electrode and recording both electromyography signals and evoked muscle force from distal musculature. Electromyography measurements were recorded from the tibialis anterior and gastrocnemius muscles, while evoked muscle force measurements were recorded from the tibialis anterior, extensor digitorum longus, and gastrocnemius muscles.e macro-sieve electrode and regenerated sciatic nerve were then explanted for histological evaluation. Successful sciatic nerve regeneration across the macro-sieve electrode interface following spinal cord injury was seen in all five animals. Recorded electromyography signals and muscle force recordings obtained through macro-sieve electrode stimulation confirm the ability of the macro-sieve electrode to successfully recruit distal musculature in this injury model. Taken together, these results demonstrate the macro-sieve electrode as a viable interface for peripheral nerve stimulation in the context of spinal cord injury.
peripheral nerve interface; regenerative electrode; nerve regeneration; spinal cord injury; spinal cord lateral hemisection; electromyography; muscle force
Introduction
Spinal cord injury (SCI) is a debilitating condition that is detrimental to the wellbeing and productivity of affected individuals. In the United States alone, 282,000 individuals are estimated to be living with a SCI, with ~17,000 new cases occurring each year, primarily in young adults (National Spinal Cord Injury Statistical Center, 2016). Injury to the mammalian spinal cord causes neuron death at lesion site with local loss of anterior horn cells.is ultimately results in injury-dependent losses to motor function distal to the site of injury. While axons in the peripheral nervous system (PNS) are capable of robust regeneration post-injury, axons in the central nervous system (CNS) are not and thus loss of function due to SCI is typically permanent (Huebner and Strittmatter, 2009). One promising approach to restoring motor function to SCI patients is the use of peripheral nerve interfaces (PNIs). A PNI is a micro-electrode array used to stimulate or record from a peripheral nerve, in this case one distal to the spinal cord lesion. Many types of PNIs are being developed as potential modalities for neuromuscular control and delivery of functional electrical stimulation. In this paper, we discuss the applicability of the macro-sieve electrode (MSE) as a potential target for restoring motor function following SCI.
Interfacing Peripheral Nerve in the Context of SCI
PNIs are broadly classified into three types—extraneural electrodes, penetrating intraneural electrodes, and regenerative electrodes. Extraneural electrodes, such as the cuff electrode (Veraart et al., 1993) or the flat interface nerve electrode (Tyler and Durand, 2002), are minimally invasive but achieve only limited selective muscular recruitment. Penetrating intraneural electrodes, such as the Utah slant electrode array (Branner et al., 2004) and the transverse intrafascicular multichannel electrode (Boretius et al., 2010), are inserted directly into the target nerve to gain selective intrafascicular control, but suffer from complications such as the breakage of the tines and fibrous encapsulation (Gasson et al., 2004; Christensen et al., 2014). Regenerative electrodes, such as the MSE, interface peripheral nerve that has regenerated across the electrode interface from two surgically opposed nerve stumps (ompson et al., 2016). By interfacing directly with this regenerated nerve, the MSE is able to provide selective control while avoiding issues such as breakage and scarring (MacEwan et al., 2016).
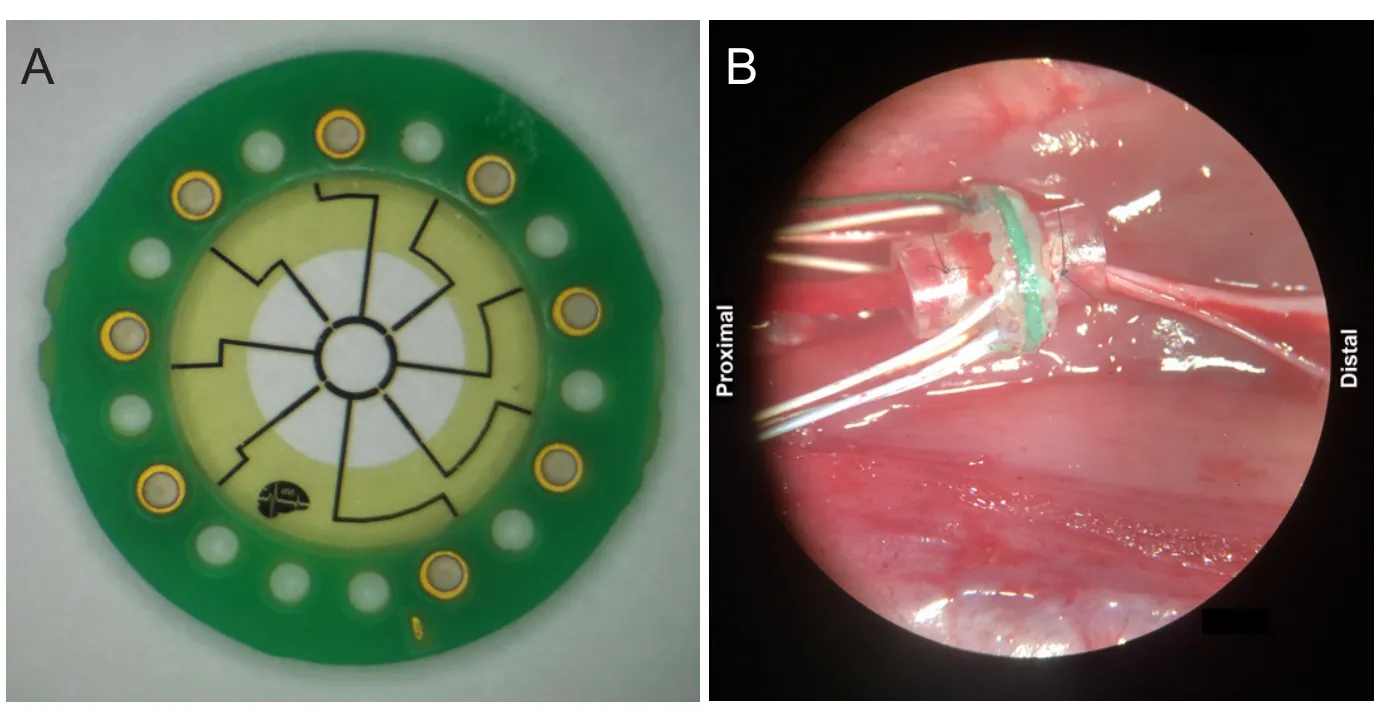
Figure 1 Macrosieve electrode.

Figure 2 Population tibialis anterior (TA) and gastrocnemius (G) EMG data for stimulation through each macro-sieve electrode (MSE) site in each animal.
Direct nerve integration with any regenerative electrode, however, requires transection of the nerve of interest and depends entirely on the regenerative capacity of the nerve —two complicating factors which determine the applicability of regenerative electrodes (Lago et al., 2007). Furthermore, implantation of an MSE subjects the PNS to a second injury following the initial CNS lesion, which intuitively may affect the peripheral nerve’s regenerative capacity, as peripheral nerve axons distal to the SCI lesion have altered structural morphology (Redondo-Castro and Navarro, 2013). Our clinical experience and a growing body of literature demonstrating clinical recovery using peripheral nerve transfers distal to the site of injury suggests that regenerative electrodes such as the MSE may provide an interface that promotes a more widespread use of caudal spinal segments in SCI patients (Ray et al., 2016). We sought to investigate whether a MSE implanted post-SCI could still be used to selectively recruit distal musculature.
Capabilities of the MSE
Details of the MSE design and fabrication have been described previously (MacEwan et al., 2016). Briefly, the MSE is a high-transparency regenerative sieve electrode featuring nine large transit zones, each with an area of approximately 0.285 mm2.ese transit zones are bordered by eight radial spokes and a central ring which are metallized with Pt-Ir to yield four central and four peripheral active electrode sites (Figure 1A). Each MSE assembly also features silicone nerve guidance conduits which project 3 mm from each face and enable the MSE to be secured to the epineurium during implantation.
Several characteristics of the MSE make it especially attractive in the context of SCI compared to other PNIs. The MSE can recruit highly selective groups of regenerated nerve fibers in uninjured animals (MacEwan et al., 2016), meaning that selective motor control can potentially be provided to multiple muscle groups affected by SCI through a single MSE. Specifically, in uninjured animals, the MSE has been able to recruit up to 50% of maximalevoked muscle twitch force using only monopolar stimulation paradigms. Additionally, as the MSE is not implantedviapenetration, it does not experience the mechanical or foreign-body response complications associated with intraneural electrodes (Christensen et al., 2014), furthering its applicability as a long-term PNI for use in SCI. However, as with any regenerative sieve electrode, the ability of the MSE to selectively interface the nerve relies on robust regeneration across its interface, and thus potential hurdles to this regeneration must be carefully evaluated. It is for this reason that we have sought to investigate whether there are any negative downstream effects of SCI on regeneration across the MSE interface.
Evaluation of the MSE in SCI
MSE were implanted in the sciatic nerve of five adult male Lewis rats following SCI to assess the ability of the MSE to interface regenerated peripheral nerve fibers post-SCI. Each SCI was performedviaright lateral hemisection of the cord at the T9–10site. Two weeks post-SCI, an MSE was implanted in the right sciatic nerve. For this procedure, the sciatic nerve was transected 5 mm proximal to the point of trifurcation. An MSE was placed in the transected nerve gap and the proximal and distal nerve stumps were sutured into either end of the silicone nerve guidance conduit by the epineurium (Figure 1B).
Five months post-implantation, the ability of the MSE to interface the regenerated nerve was assessed by stimulating through the MSE and recording both electromyography (EMG) signals and evoked muscle force measurements from distal musculature. An identical set of stimuli was used to stimulate the regenerated nerve for both EMG and muscle force recordings. Individual stimuli consisted of a biphasic, square, symmetrical pulse of current between 100 μA and 500 μA delivered over 1 ms (i.e., 100 μA for 0.5 ms then –100 μA for 0.5 ms). Stimuli were delivered cathodically through the implanted MSE using a MS16 stimulus isolator (Tucker-Davis Technologies, Inc., Alachua, FL, USA) connected to a desktop PCviaoptical cable.
For the EMG measurements, recording needle electrodes were placed in the tibialis anterior (TA) and gastrocnemius (G) muscles. Additional counter and reference needle electrodes were placed subcutaneously in the lower back of the animal. Recorded EMG signals were routed through a RA-16LI-D 16-channel differential recording head stage and amplified using a RA16PA 16-channel medusa preamp (Tucker-Davis Technologies, Inc., Alachua, FL, USA) before being sent to a desktop PCviaoptical cable using custom OpenEx data acquisition soware (Tucker-Davis Technologies, Inc., Alachua, FL, USA).
For muscle force recordings, the anterolateral aspect of the right hind limb was exposed to facilitate access to the tendons of the TA, extensor digitorum longus (EDL), and G muscles. Distal tendons of the target muscles were cut and secured to separate stainless-steel S-hooks using 5-0 nylon suture. The right leg was then immobilized at the femoral condyles by use of a C-clamp. The stainless-steel S-hook was then connected to a 5 N thin-film load cell force sensor (Strain Measurement Devices, Inc., Meriden, CT, USA). Evoked muscle forces for each the TA, EDL, and G muscles were transduced individuallyviathe force sensor and recorded on a desktop PC using the previously described hardware and soware.
All channels in each animal reached an EMG response plateau atstimulation values of 150–200 μA (Figure 2), consistent with data from uninjured animals (MacEwan et al., 2016). A similar plateau was obtained for evoked muscle force recordings, in which recorded muscle force normalized by the maximum muscle force for each muscle also reached a maximum at stimulation values of 150–200 μA. When recording evoked muscle response due to electrical stimulation, a sigmoidal curve typically appears as smaller currents do not cause the motor fibers to reach threshold, while higher currents recruit increasingly more motor fibers until all fibers are recruited and a plateau is reached. However, due to the absence of lower stimulation values and the use of comparatively large current step sizes in this experiment, it is dif ficult to visualize the expected sigmoidal recruitment curve.
Successful sciatic nerve regeneration across the MSE interface following spinal cord injury was visually observed in all five rats. EMG and muscle force recordings obtained following stimulation through the MSE confirm the ability of the MSE to successfully recruit distal musculature in this injury model. Taken together, these results demonstrate that the MSE is a viable interface for providing functional neuromuscular stimulation following SCI.
Conclusion
Acknowledgments:
Author contributions:NKB was the designated guarantor, wrote and revised the manuscript, and performed experimental studies, data acqui-sition, and data analysis. MRM is responsible for study concept and study design. WZR was responsible for study concept, study design, manuscript review, and manuscript editing.
Conflicts of interest:WZR declared provision of consulting services to Globus and Depuy and an ownership interest in Acera Surgical, Inc. All other contributing authors declared no other potential conflicts of interest.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer review reports:
Reviewer 1:Athanasios Chatzisotiriou, Aristotle University ofessaloniki, Greece.
Comments to the author:
Reviewer 2:Christof A. Leicht, Loughborough University, UK.
Comments to the author:.
Additional file:Open peer review report 1, 2.
Boretius T, Badia J, Pascual-Font A, Schuettler M, Navarro X, Yoshida K, Stieglitz T (2010) A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens Bioelectron 26:62-69.
Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann RA (2004) Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng 51:146-157.
Christensen MB, Pearce SM, Ledbetter NM, Warren DJ, Clark GA, Tresco PA (2014)e foreign body response to the Utah Slant Electrode Array in the cat sciatic nerve. Acta Biomater 10:4650-4660.
Huebner EA, Strittmatter SM (2009) Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ 48:339-351.
Lago N, Udina E, Ramachandran A; Navarro X (2007) Neurobiological assessment of regenerative electrodes for bidirectional interfacing injured peripheral nerves. IEEE Trans Biomed Eng 54:1129-1137.
MacEwan MR, Zellmer ER, Wheeler JJ, Burton H, Moran DW (2016) Regenerated sciatic nerve axons stimulated through a chronically implanted macro-sieve electrode. Front Neurosci 10:1-12.
National Spinal Cord Injury Statistical Center (2016) Facts and figures at a glance. 1-2 (https://www.nscisc.uab.edu/Public/Facts%202016. pdf) [Accessed 3/1/2017]
Ray WZ, Chang J, Hawasli A, Wilson TJ, Yangl(2016) Motor nerve transfers: A comprehensive review. Neurosurgery 78:1-26.
Redondo-Castro E, Navarro X (2013) Peripheral nerve alterations aer spinal cord injury in the adult rat. Spinal Cord 51: 630-633.
Tyler DJ, Durand DM (2002) Functionally selective peripheral nerve stimulationwith a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng 10:294-303.
Veraart C, Grill WM, Mortimer JT (1993) Selective control of muscle activation with a multipolar nerve cuffelectrode. IEEE Trans Biomed Eng 40:640-653.
How to cite this article: Birenbaum NK, MacEwan MR, Ray WZ (2017) Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury. Neural Regen Res 12(6):906-909.
*Correspondence to:
Wilson Z. Ray, M.D., rayz@wustl.edu.
orcid:
0000-0002-3006-8562
(Wilson Z. Ray)
10.4103/1673-5374.208565
Accepted: 2017-06-12
杂志排行
中国神经再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease
- Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair