Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
2017-08-07ZhanqiongZhongYangXiangXiHuYoucuiWangXiZengXiaomengWangQingjieXiaTinghuaWangXiaoZhang
Zhan-qiong Zhong, Yang Xiang Xi Hu You-cui Wang, Xi Zeng Xiao-meng Wang Qing-jie Xia, Ting-hua Wang, Xiao Zhang
1 Experiment Technology Center of Preclinical Medicine of Chengdu Medical College, Chengdu, Sichuan Province, China
2 School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan Province, China
3 Institute of Neurological Diseases, Center for Translational Neuroscience, Western China Hospital, Sichuan University, Chengdu, Sichuan
Province, China
Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
Zhan-qiong Zhong1,2, Yang Xiang1, Xi Hu1, You-cui Wang3, Xi Zeng1, Xiao-meng Wang1, Qing-jie Xia3, Ting-hua Wang3, Xiao Zhang1,*
1 Experiment Technology Center of Preclinical Medicine of Chengdu Medical College, Chengdu, Sichuan Province, China
2 School of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan Province, China
3 Institute of Neurological Diseases, Center for Translational Neuroscience, Western China Hospital, Sichuan University, Chengdu, Sichuan
Province, China
Graphical Abstract
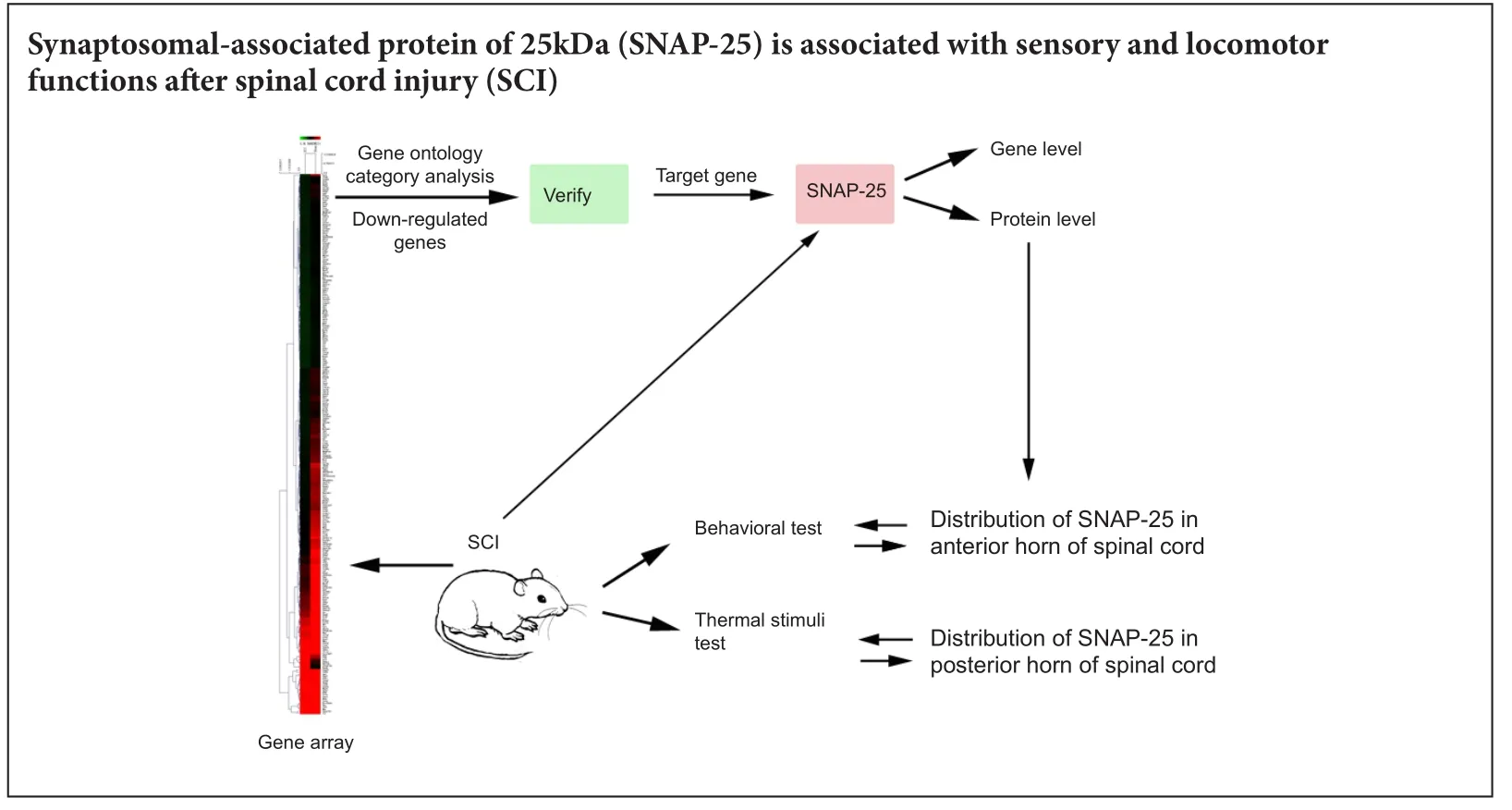
Synaptosomal-associated protein 25 kDa (SNAP-25) is localized on the synapse and participates in exocytosis and neurotransmitter release. Decreased expression of SNAP-25 is associated with Alzheimer’s disease and attention deficit/hyperactivity disorder. However, the expression of SNAP-25 in spinal cord contusion injury is still unclear. We hypothesized that SNAP-25 is associated with sensory and locomotor functions aer spinal cord injury. We established rat models of spinal cord contusion injury to detect gene changes with a gene array. A decreased level of SNAP-25 was detected by quantitative real time-polymerase chain reaction and western blot assay at 1, 3, 7, 14 and 28 days post injury. SNAP-25 was localized in the cytoplasm of neurons of the anterior and posterior horns, which are involved in locomotor and sensory functions. Our data suggest that reduced levels of SNAP-25 are associated with sensory and locomotor functions in rats with spinal cord contusion injury.
nerve regeneration; synaptosomal-associated protein 25 kDa; sensory function; locomotor function; spinal cord injury; gene array; neurons; neural regeneration
Introduction
Spinal cord injury (SCI) is a traumatic injury that leads to devastating motor and sensory dysfunction. The primary and complex secondary injuries, including cell apoptosis and axonal degeneration, both contribute to the dysfunction after SCI (Ahuja and Fehlings, 2016). Identification of key molecules in the mechanism of SCI-induced dysfunction could lead to effective ways to ameliorate the loss of motor and sensory functions (Gensel and Zhang, 2015).
Synaptosomal-associated protein 25 kDa (SNAP-25) is a member of a family of evolutionarily conserved proteins and is essential for neurotransmitter release. SNAP-25, syntaxin-1 and synaptobrevin form the stable soluble N-ethylmaleimide sensitive factor attachment protein receptor complex, and their interaction is involved in the release of synaptic vesicles (Jahn et al., 2003; Jahn and Scheller, 2006).SNAP-25 is located on the synapse and is involved in rapid and slow endocytosis of secretory vesicles (Xu et al., 2013). In neuroendocrine cells, SNAP-25 is localized to the plasma membrane where it functions in regulated secretory vesicle exocytosis, but it is also found on intracellular membranes (Aikawa et al., 2006). In neonatal deep cerebellar nuclei, SNAP-25 is detectable in both vesicular glutamate transporter 1- and 2-immunoreactive synaptic contacts, with a higher prevalence in vesicular glutamate transporter 1-positive terminals (Manca et al., 2014).e distribution and the role of SNAP-25 in spinal cord are still unclear.

Figure 1 Results of gene array in the spinal cord of rats with spinal cord contusion injury.
Other studies have recently reported the relationship of SNAP-25 with Alzheimer’s disease (Brinkmalm et al., 2014), attention deficit/hyperactivity disorder (Galvez et al., 2014), and autism (Braida et al., 2015). Increased levels of SNAP-25 were found in Alzheimer’s disease patients, in whom cerebrospinal fluid levels of SNAP-25 could serve as a marker for the diagnosis of Alzheimer’s disease (Brinkmalm et al., 2014; Guerini et al., 2014). Barakauskas et al. (2016) have shown that lower levels of SNAP-25 in schizophrenia may be associated with greater illness severity, and polymorphisms of SNAP-25 have been associated with attention deficit/hyperactivity disorder (Galvez et al., 2014).us, we hypothesized that the expression of SNAP-25 is associated with sensory function or locomotor function in rats with SCI. In the present study, we aimed to investigate whether SNAP-25 was associated with SCI and determine its role in SCI.
Materials and Methods
Animals

Figure 3 Significant genes in the spinal cord of rats with spinal cord contusion injury.

Figure 2 Analysis of downregulated genes in the spinal cord of rats with spinal cord contusion injury.
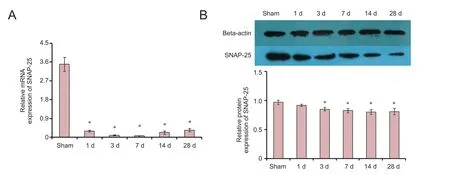
Figure 4 SNAP-25 expression in rats with spinal cord injury.
A total of 240 adult female Sprague-Dawley rats aged 12 weeks and weighing 200—220 g were purchased from the Dashuo of Laboratory Animal Co., Ltd., Chengdu, China (SYXK (Chuan) 2014-189). Rats were randomly divided into a sham group (n= 120) and a SCI group (n= 120), and the survival periods for subgroups were 1, 3, 7, 14 and 28 days post injury, with 24 rats in each group.
Rats were maintained in strict conditions at 22—25°C, 30—40% humidity and 12/12-hour light/dark cycles. All animal care and experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1986). This research was approved by the Institutional Animal Care and Use Committee, Chengdu Medical College, China (approval number: 201401120411).

Table 1 Gene sequences
SCI induction
Surgical procedures were performed in a standard method as previously described (Zhang et al., 2015). In brief, after anesthesia with 3.6% chloral hydrate (1 mL/100 g; Kelong, Chengdu, Sichuan Province, China), the position of tenth thoracic vertebra was identified and the skin was sterilized. A laminectomy was performed, exposing the T11spinal cord. A weight-drop device (Chengdu Taimeng, Chengdu, Sichuan Province, China) was used to injure the spinal cord, with a drop of 30 mm. The sham group received only a laminectomy without injury. Rats received benzylpenicillin (80,000 U/kg/d, intramuscularly) and normal saline (5 mL, intramuscularly) after surgery. Urinary bladders were manually expressed until autonomous micturition recovery.
Gene array and gene ontology (GO) category analysis
Rats in the sham group and 3-day group (n= 3) were anesthetized with 3.6% chloral hydrate, and tissue from the SCI site was taken and used for gene array. Purified RNA was performed using Affymetrix GeneChip Gene 2.0 Array (Genminix Informatics Ltd., Co., Shanghai, China) according to the manufacturer’s protocol.
GO category analysis was used to classify hierarchical categories and predict the most effective differential genes based on the biological process. GO category analysis was used to analyze the data in the gene array. At-test was used to detect the difference between the two groups. False discovery rate was used to determine whether the difference was significant by random chance, withP< 0.001 and false discovery rate < 0.05 (Chen et al., 2010).
Quantitative real time-polymerase chain reaction (qRT-PCR)
qRT-PCR (Applied Biosystems, Waltham, MA, USA) was used to verify the differential expression of genes that were detected by the Affymetrix GeneChip.e procedures were conducted as described previously (Chen et al., 2005).e gene sequences are shown inTable 1.e expression levels of genes were shown as an increase in cycle threshold relative to the expression of β-actin.e relative gene expression in the sample was calculated as 2–ΔCT(Meng et al., 2011).
To detect the change of SNAP-25 levels in the spinal cord, the center of the contused spinal cord was obtained from the sham and SCI groups (1, 3, 7, 14 and 28 days,n= 6 per group) aer rats were anesthetized with 3.6% chloral hydrate and perfused with normal saline (Kelun, Chengdu, China). RNA was extracted from spinal cord with Trizol reagent (Invitrogen, Waltham, MA, USA), cDNA was obtained with RevertAid First Strand cDNA Synthesis Kit (ermo Fisher Scientific, Waltham, MA, USA), and the amplified primers of the gene were list inTable 1. All of the procedures were conducted according to the manufacturer’s protocol.
Western blot assay
Spinal cord tissues were harvested as described previously. Tissues were homogenized in protein extraction reagent (Beyotime Biotechnology, Nantong, China). In detail, proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA) in transfer buffer. Membranes were blocked by 5% skim milk and incubated with monoclonal rabbit anti-SNAP-25 (1:200; Boster, Wuhan, Hebei Province, China) or mouse anti-beta-actin (1:4,000; ProteinTech, Chicago, IL, USA) overnight at 4°C. Horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG (1:10,000; ProteinTech) antibodies were applied for 2 hours at 37°C aer membranes were washed by Tris-buffered saline containing 0.5% Tween-20. Membranes were detected by Enhanced Chemiluminescence (Beyotime Biotechnology) and exposure to film in the dark.e optical density intensity of each band was measured using Quantity One software (BioRad, Waltham, MA, USA). Results were shown as the optical density ratio to beta-actin.
Test of pain thresholds and locomotor function
To evaluate the change of sensory function in SCI rats, the latency of the hind limbs’ withdrawal response to thermal stimuli (Chengdu Taimeng) was assessed. The rats in the sham group and 28-day group (n= 15) were subjected to thermal stimuli before surgery, and 1, 7, 14, 21 and 28 days aer injury. Each rat was measured three times with a 3-minute interval between tests.
To detect the change of motor function aer SCI, rats were tested with the Basso, Beattie, Bresnahan Locomotor Rating Scale (BBB) (Basso et al., 1995).e rats in the sham group and 28-day group (n= 15) were measured at 1, 7, 14, 21 and 28 days aer surgery. Rats were allowed to move in an open field for 5 minutes. A lower BBB score indicates worse locomotor function.
Thermal stimuli tests and BBB assessments were performed by three experts and independent observers. All of them were blinded to the treatments of each group.
Immunohistochemistry
Immunohistochemistry was used to detect changes in morphology and SNAP-25 expression in the posterior and anterior horns of spinal cord aer SCI.e primary antibody was mouse anti-SNAP-25 (1:100, monoclonal, Boster). Rats in the SCI and sham groups (n= 3 per group) were anesthetized with 3.6% chloral hydrate and perfused with 4% paraformaldehyde. Briefly, spinal cord tissues were fixed in paraf fin. Paraf fin sections were cut transversely at 4 μm. After dewaxing and hydration, high-pressure antigen retrieval was performed. The sections were rinsed in 0.05 M phosphate-buffered saline and endogenous enzymes were inactivated by 3% H2O2. Before adding the primary antibody, 5% goat serum with 0.3% Triton X-100 was used. Sections were incubated overnight at 4°C with the primary antibody followed by horseradish peroxidase-conjugated goat anti-mouse IgG (1:200; ZSGB-BIO, Beijing, China). Images of the epicenter of the injured spinal cord were photographed using an Olympus BX-DSU microscope (Olympus, Tokyo, Japan). The positive staining was quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All of the data were analyzed by SPSS 17.0 (SPSS, Chicago, IL, USA). Values are presented as the mean ± standard error of the mean. Data were analyzed using one-way analysis of variance with the least significant difference or Student’st-test. Values ofP< 0.05 were considered statistically significant.
Results
Gene expression changes in the spinal cord of rat models of SCI
A gene array was used to detect changes of gene expression in the sham and SCI groups. Based on a 1.5—2.5-fold change in the gene array data, displayed as a heat-map, 13 genes were upregulated and 249 genes were downregulated in the SCI group compared with the sham group (Figure 1). Because a majority of the significant genes were downregulated on the cluster heat map, we chose the downregulated genes for further examination. The top 10 implicated biological processes were identified based on the number of different genes in each biological process (Figure 2A). In addition, the top 15 significantly downregulated genes are shown inFigure 2B, and the negative of the log function ofP-values of the significantly downregulated genes in the biological processes are shown inFigure 2C.
To further clarify the affected processes in the spinal cord, we analyzed the pathways of significantly downregulated genes.e top 15 identified pathways are involved in: GABAergic synapse, glutamatergic synapse, retrograde endocannabinoid signaling, morphine addiction, nicotine addiction, circadian entrainment, insulin secretion, gastric acid secretion, synaptic vesicle cycle, amphetamine addiction, cocaine addiction, steroid biosynthesis, citrate cycle, proximal tubule bicarbonate reclamation and terpenoid backbone biosynthesis (Figure 2D).
According to the analyses of biological processes, the downregulated genes were involved in synaptic transmission, neurotransmitter secretion, sensory perception of pain, axonogenesis, locomotor behavior and adult walking behavior.
SNAP-25 chosen for further analysis
From the results of the differential genes combined with the biological process analysis, we pursued SNAP-25, phosphatidylethanolamine binding protein 1 (PEBP1), beta-synuclein (SNCB) and aquaporin 4 (AQP4) genes.e fold change of SNCB was highest among them (Figure 3A). SNAP-25 exhibited the highest expression in the SCI and sham groups based on gene array (Figure 3B). Finally, qRT-PCR was used to verify the accuracy of the gene array analysis.e levels of SNAP-25, PEBP1, SNCB and AQP4 were significantly decreased in the SCI group when compared with the sham group (Figure 3C), and SNAP-25 was most highly expressed in the two groups. In summary, we selected SNAP-25 for further research in this study.
SNAP-25 expression in rats with SCI
To detect the change of SNAP-25 expression in rats with SCI, we performed qRT-PCR and western blot assay in spinal cord tissue. SNAP-25 mRNA expression was significantly decreased at 1, 3, 7, 14 and 28 days (Figure 4A). Furthermore, SNAP-25 protein expression was significantly reduced at 3, 7, 14 and 28 days (Figure 4B).ese results showed that SNAP-25 expression might be associated with SCI.
SNAP-25 associated with sensory function in rats with SCI
We hypothesized that SNAP-25 was involved in the sensory function of SCI rats, and therefore measured pain sensitivity.e latency of the hind limb response to the mechanical stimulus was reduced at 7, 14, 21 and 28 days when compared with the sham group (Figure 5). To investigate the localization of SNAP-25 in the spinal cord, we performed immunohistochemistry. Dense immunoreactivity was found on axons of neurons and glial cells in the dorsal horn of the spinal cord in the sham and SCI groups (Figure 6A—G).e density of SNAP-25 immunoreactivity at 1, 3, 7, 14 and 28 days was reduced in the SCI group compared with the sham group (Figure 6H).e results suggest that reduced levels of SNAP-25 are associated with sensory function in rats with SCI.
SNAP-25 associated with locomotor function in rats with SCI
We examined the locomotor behavior of rats in both the SCI and sham groups. Locomotor function decreased immediately aer SCI, but gradually increased over time.e BBB scores were significantly lower in the SCI group than in the sham group at each time point (Figure 7). Similarly, the BBB scores of SCI rats at 7 and 14 days were significantly higher than at 1 and 7 days, respectively.

Figure 5 Changes of pain sensitivity in rats with SCI.
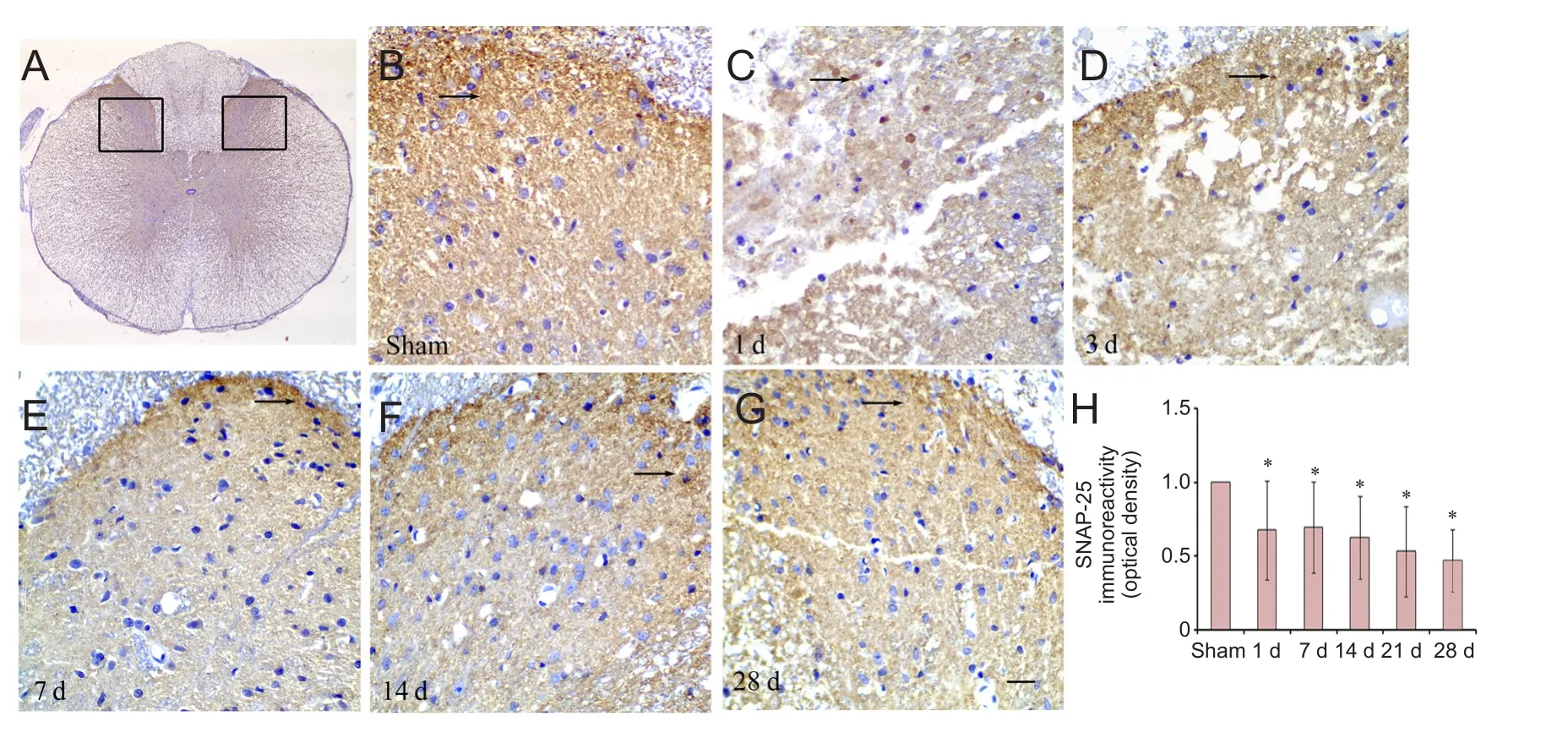
Figure 6 Distribution of SNAP-25 in the posterior horn of spinal cord.

Figure 7 Change of locomotor function in rats with SCI.
Interestingly, the immunoreactivity of SNAP-25 was located in the anterior horn of the spinal cord, in which the majority of immunoreactivity was localized in the axon or cytoplasm of the neurons (Figure 8A—G). The number of SNAP-25-immunoreactive cells significantly decreased at 1, 3, 14 and 28 days (Figure 8H).us, we presumed that the decrease of SNAP-25 expression was associated with the locomotor function aer SCI.
Discussion
In this study, we speculated that SNAP-25 might be associated with sensory function and locomotor function in an SCI rat model. Gene array and GO analysis were used to identify significantly different genes in SCI rats. Results of qRT-PCR and western blot assay showed that SNAP-25 expression decreased aer SCI. Furthermore, immunohistochemistry and behavioral tests demonstrated that SNAP-25 was located in the posterior and anterior horns of the spinal cord, and was associated with sensory function and locomotor function in SCI rats.erefore, SNAP-25 may be beneficial to neural regeneration following SCI, and might be a potential target gene for the treatment of SCI.

Figure 8 Distribution and role of SNAP-25 in the anterior horn of spinal cord.
An association of SNAP-25 levels with clinical symptoms suggest that SNAP-25 plays a role in disease. We hypothesized that SNAP-25 was associated with SCI. In this study, SNAP-25 gene and protein expression levels were significantly lower in the SCI group than in the sham group, which indicates that SNAP-25 has a direct or indirect relationship with the SCI contusion. Our results are consistent with previous studies, which show that SNAP-25 expression is decreased in children affected by autism spectrum disorders and in patients with diverticular disease (Barrenschee et al., 2015; Braida et al., 2015). However, SNAP-25 protein expression was significantly higher in cerebrospinal fluid when measured using af finity purification and mass spectrometry in Alzheimer’s disease (Brinkmalm et al., 2014). Fibromyalgia syndrome patients have an increased frequency of a SNAP-25 gene polymorphism compared with matched healthy women, which is associated with the behavioral, personality and psychological disorders in fibromyalgia syndrome patients (Balkarli et al., 2014). Our results differ with the findings from these two reports, which occurred because SNAP-25 might play different roles in different diseases or in different regions of the peripheral and central nervous systems. For example, SNAP-25 was downregulated in the hippocampus of Alzheimer’s disease (Kamat et al., 2016).
Neuropathic pain is a characteristic feature of SCI (Tan and Waxman, 2015). Recently, a study has shown that SNAP-25 is associated with the function of protein kinase C gamma that regulates neuropathic pain in rat models of chronic constriction injury-induced neuropathic pain (Zou et al., 2012). Additionally, Liu et al. (2013) has found that SNAP-25 is correlated with the relief of nociceptive responses in chronic constriction injury rats through botulinum toxin serotype A treatment. In this study, SNAP-25 immunoreactivity was found in the posterior horn of the spinal cord, and the density of SNAP-25 immunoreactivity was significantly reduced after SCI. Thus, we presume that SNAP-25 is associated with neuropathic pain. Interestingly, we detected the latency of hind limb withdrawal response to temperature stimulation and found that the latency was reduced aer SCI, which is similar to previous reports (Houeland et al., 2007; Wang et al., 2014) suggesting that SNAP-25 has an important role in sensory function.
We suggest that SNAP-25 may influence the recovery of locomotor function aer SCIviaaxon plasticity and neural regeneration. Together, our findings support that a decreased level of SNAP-25 plays an important role in SCI rats, which might be associated with sensory function and locomotor function. SNAP-25 provides a new direction for the role of SNAP-25 in SCI.
Acknowledgments:We owe special thanks to Professor Mary Galea from the University of Melbourne (Clinical Science, Royal Melbourne Hospital), who was the foundation chair of clinical physiotherapy at the Univer-sity of Melbourne and Austin Health, and one of the founding members of Spinal and Research Institute, for the review of grammatical errors.e same gratitude goes to Mr. Joseph Z. Muyeye, a current student at the University of Melbourne, Australia.
Author contributions:ZQZ, XZ (Xiao Zhang) and THW designed the experiments. ZQZ wrote the main manuscript text. YCW and XZ (Xi Zeng) prepared all figures. ZQZ and YX conducted the western blot experiments. ZQZ, YX and XZ (Xi Zeng) established animal models. XMW, YCW and XH conducted the behavioral tests. QJX conducted qRT-PCR. ZQZ and XZ (Xi Zeng) conducted the immunohistochemistry. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:The study protocol was approved by the Institutional Animal Care and Use Committee, Chengdu Medical College, China (Approval number: 201401120411). The experiment followed the National Institutes of Health Guide for the Care and Use of Laboratory animals (NIH Publications No. 8023, revised 1978), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE). The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Adler M, Sheridan RE, Deshpande SS, Oyler GA (2001) Neuromuscular transmission and muscle contractility in SNAP-25-deficient coloboma mice. Neurotoxicology 22:775-786.
Ahuja CS, Fehlings M (2016) Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies inspinal cord injury. Stem Cells Transl Med 5:914-924.
Aikawa Y, Xia X, Martin TF (2006) SNAP25, but not syntaxin 1A, recycles via an ARF6-regulated pathway in neuroendocrine cells. Mol Biol Cell 17:711-722.
Balkarli A, Sengul C, Tepeli E, Balkarli H, Cobankara V (2014) Synaptosomal-associated protein 25 (Snap-25) gene polymorphism frequency in fibromyalgia syndrome and relationship with clinical symptoms. BMC Musculoskelet Disord 15:191.
Barakauskas VE, Moradian A, Barr AM, Beasley CL, Rosoklija G, Mann JJ, Ilievski B, Stankov A, Dwork AJ, Falkai P, Morin GB, Honer WG (2016) Quantitative mass spectrometry reveals changes in SNAP-25 isoforms in schizophrenia. Schizophr Res 177:44-51.
Barrenschee M, Bottner M, Harde J, Lange C, Cossais F, Ebsen M, Vogel I, Wedel T (2015) SNAP-25 is abundantly expressed in enteric neuronal networks and upregulated by the neurotrophic factor GDNF. Histochem Cell Biol 143:611-623.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1-21.
Braida D, Guerini FR, Ponzoni L, Corradini I, De Astis S, Pattini L, Bolognesi E, Benfante R, Fornasari D, Chiappedi M, Ghezzo A, Clerici M, Matteoli M, Sala M (2015) Association between SNAP-25 gene polymorphisms and cognition in autism: functional consequences and potential therapeutic strategies. Transl Psychiatry 5:e500.
Brinkmalm A, Brinkmalm G, Honer WG, Frolich L, Hausner L, Minthon L, Hansson O, Wallin A, Zetterberg H, Blennow K, Öhrfelt A (2014) SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener 9:53.
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ (2005) Real-time quantification of microRNAs by stemloop RT-PCR. Nucleic Acids Res 33:e179.
Chen F, Zhu HH, Zhou LF, Li J, Zhao LY, Wu SS, Wang J, Liu W, Chen Z (2010) Genes related to the very early stage of ConA-induced fulminant hepatitis: a gene-chip-based study in a mouse model. BMC Genomics 11:240.
Fossati G, Morini R, Corradini I, Antonucci F, Trepte P, Edry E, Sharma V, Papale A, Pozzi D, Defilippi P, Meier JC, Brambilla R, Turco E, Rosenblum K, Wanker EE, Ziv NE, Menna E, Matteoli M (2015) Reduced SNAP-25 increases PSD-95 mobility and impairs spine morphogenesis. Cell Death Differ 22:1425-1436.
Galvez JM, Forero DA, Fonseca DJ, Mateus HE, Talero-Gutierrez C, Velez-van-Meerbeke A (2014) Evidence of association between SNAP25 gene and attention deficit hyperactivity disorder in a Latin American sample. Atten Defic Hyperact Disord 6:19-23.
Gensel JC, Zhang B (2015) Macrophage activation and its role in repair and pathology aer spinal cord injury. Brain Res 1619:1-11.
Guerini FR, Agliardi C, Sironi M, Arosio B, Calabrese E, Zanzottera M, Bolognesi E, Ricci C, Costa AS, Galimberti D, Griffanti L, Bianchi A, Savazzi F, Mari D, Scarpini E, Baglio F, Nemni R, Clerici M (2014) Possible association between SNAP-25 single nucleotide polymorphisms and alterations of categorical fluency and functional MRI parameters in Alzheimer’s disease. J Alzheimers Dis 42:1015-1028.
Houeland G, Nakhost A, Sossin WS, Castellucci VF (2007) PKC modulation of transmitter release by SNAP-25 at sensory-to-motor synapses in aplysia. J Neurophysiol 97:134-143.
Islamov RR, Samigullin DV, Rizvanov AA, Bondarenko NI, Nikolskiy EE (2015) Synaptosome-associated protein 25 (SNAP25) synthesis in terminal buttons of mouse motor neuron. Dokl Biochem Biophys 464:272-274.
Jahn R, Scheller RH (2006) SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol 7:631-643.
Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112:519-533.
Kamat PK, Kyles P, Kalani A, Tyagi N (2016) Hydrogen sulfide ameliorates homocysteine-induced Alzheimer’s disease-like pathology, blood-brain barrier disruption, and synaptic disorder. Mol Neurobiol 53:2451-2467.
Lawrence GW, Wang J, Brin MF, Aoki KR, Wheeler L, Dolly JO (2014) Fusion of Golgi-derived vesicles mediated by SNAP-25 is essential for sympathetic neuron outgrowth but relatively insensitive to botulinum neurotoxins in vitro. FEBS J 281:3243-3260.
Liu C, Guo QL, Huang CS, Zou WY, Song ZB (2013) Suppressing SNAP-25 and reversing glial glutamate transporters relieves neuropathic pain in rats by ameliorating imbalanced neurotransmission. Chin Med J (Engl) 126:4100-4104.
Manca P, Mameli O, Caria MA, Torrejon-Escribano B, Blasi J (2014) Distribution of SNAP25, VAMP1 and VAMP2 in mature and developing deep cerebellar nuclei aer estrogen administration. Neuroscience 266:102-115.
Meng W, Xia Q, Wu L, Chen S, He X, Zhang L, Gao Q, Zhou H (2011) Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer 11:88.
Tan AM, Waxman SG (2015) Dendritic spine dysgenesis in neuropathic pain. Neurosci Lett 601:54-60.
Wang W, Wang F, Liu J, Zhao W, Zhao Q, He M, Qian BJ, Xu Y, Liu R, Liu SJ, Liu W, Liu J, Zhou XF, Wang TH (2014) SNAP25 ameliorates sensory deficit in rats with spinal cord transection. Mol Neurobiol 50:290-304.
Xu J, Luo F, Zhang Z, Xue L, Wu XS, Chiang HC, Shin W, Wu LG (2013) SNARE proteins synaptobrevin, SNAP-25, and syntaxin are involved in rapid and slow endocytosis at synapses. Cell Rep 3:1414-1421.
Yang H, Zha X, Cai Y, Wang F, Wu Z, Wu B, Jia X, Zhu D (2015) Impacts of thyroxine combined with donepezil on hippocampal ultrastructures and expressions of synaptotagmin-1 and SNAP-25 in adult rats with hypothyroidism. Int J Clin Exp Med 8:17922-17931.
Zhang X, Shi LL, Gao X, Jiang D, Zhong ZQ, Zeng X, Rao Y, Hu X, Li TZ, Li XJ, Li L, Chen JM, Xia Q, Wang TH (2015) Lentivirus-mediated inhibition of tumour necrosis factor-alpha improves motor function associated with PRDX6 in spinal cord contusion rats. Sci Rep 5:8486.
Zou W, Zhan X, Li M, Song Z, Liu C, Peng F, Guo Q (2012) Identification of differentially expressed proteins in the spinal cord of neuropathic pain models with PKCgamma silence by proteomic analysis. Brain Res 1440:34-46.
Copyedited by McCollum L, Robens J, Yu J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Zhong ZQ, Xiang Y, Hu X, Wang YC, Zeng X, Wang XM, Xia QJ, Wang TH, Zhang X (2017) Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions aer spinal cord contusion. Neural Regen Res 12(6):969-976.
*Correspondence to:
Xiao Zhang, Ph.D.,
zhangxiao1985007@126.com.
orcid:
0000-0001-6597-8825
(Xiao Zhang)
10.4103/1673-5374.208592
Accepted: 2017-05-05
杂志排行
中国神经再生研究(英文版)的其它文章
- Polyethylene glycol as a promising synthetic material for repair of spinal cord injury
- Novel aspects of extracellular adenosine dynamics revealed by adenosine sensor cells
- Electroacupuncture regulates the stress-injury-repair chain of events after cerebral ischemia/reperfusion injury
- Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke
- High-frequency and brief-pulse stimulation pulses terminate cortical electrical stimulation-induced afterdischarges
- Mild closed head traumatic brain injury-induced changes in monoamine neurotransmitters in the trigeminal subnuclei of a rat model: mechanisms underlying orofacial allodynias and headache