The crossed phrenic phenomenon
2017-08-07MichaelGeorgeZakiGhali
Michael George Zaki Ghali
Department of Neurobiology & Anatomy, Drexel University College of Medicine, Philadelphia, PA, USA
INVITED REVIEW
The crossed phrenic phenomenon
Michael George Zaki Ghali*
Department of Neurobiology & Anatomy, Drexel University College of Medicine, Philadelphia, PA, USA
spinal cord injury; SCI; cervical; C1; C2; hemisection; respiratory; recovery; phrenic; diaphragm; hemidiaphragm; paralysis; neuroplasticity
Introduction
Respiratory insuf ficiency is a common complication in patients sustaining spinal cord injury (SCI; Winslow and Rozovsky, 2003).is accounts for a significant proportion of the morbidity and mortality burden in patients with cervical SCI (DiMarco, 2005) and may (Oo et al., 1999) or may not result in ventilator dependency.e higher the level of injury, the more pronounced the degree of ventilatory dysfunction (Winslow and Rozovsky, 2003). Patients rendered ventilator-dependent by cervical SCI may benefit from phrenic nerve stimulation or pacing of respiratory (diaphragm, intercostal) muscles (DiMarco, 2009).e programming of such devices may benefit from determining the time-frequency pattern of individual phrenic motoneurons (PhMNs), which we have investigated in a previous study (Marchenko et al., 2012).
Functional consequences of hemidiaphragmatic paralysis include decreases in ventilation, perfusion, and ventilation/ perfusion matching in the lower lobe of the lung ipsilateral to paresis. Small decreases in PO2and vital capacity in the supine position along with a minimal increase in the alveolar-arterial oxygen difference may also occur (Arobrelius et al., 1975; Ridyard et al., 1976; Clague et al., 1979; Easton et al., 1983). Unilateral diaphragm paralysis is generally minimally symptomatic, but patients may exhibit mild compensatory tachypnea and dyspnea upon exertion (Easton et al., 1983). Patients with bilateral diaphragm paralysis, in contrast, typically are severely tachypneic and orthopneic, exhibiting dyspnea at rest and atelectasis (Gibson et al., 1989) and a respiratory acidosis that is more marked during sleep (Newsom et al., 1976).
The development of animal models of respiratory dysfunction following high cervical SCI has been instructive in elucidating underlying mechanisms contributing to recovery of previously silent neural respiratory outputs. As will be discussed, the C2-hemisected rat has dominated this exciting field and investigations have revealed mechanisms underlying spontaneous recovery following complete loss of ipsilateral medullophrenic drive. C2hemisection (HSx) of the spinal cord silences the ipsilateral phrenic nerve (PhN) and hemidiaphragm (Goshgarian, 1979; O’Hara and Goshgarian, 1991), as well as the ipsilateral intercostal motoneuron pools (Dougherty et al., 2012b; Beth Zimmer et al., 2015).e crossed phrenic phenomenon (CPP), strictly defined, involves re-emergence of PhN or hemidiaphragm activity ipsilateral to a C2HSx in response to respiratory stressors. Crossed phrenic activity, broadly defined, involves any re-emergence of PhN or hemidaphragm activity ipsilateral to injury: this may occur spontaneously (acutely or chronically) or in response to respiratory stressors (CPP).ese observations demonstrate the potential for the phrenic motor neuron pool ipsilateral to hemisection to be driven by pathways in the contralateral hemicord crossing at cervical spinal levels and may be exploited therapeuticallyviaa variety of means.
We will first discuss the historical context for our understanding of crossed phrenic activity, the models used to study the CPP, and changes in respiratory (mechanical and blood gas) parameters following high cervical SCI. We will then critically compare and evaluate the different electrophysio-logic methods used to measure respiratory neural output (phrenic electroneurography and diaphragm electromyography (EMG)). We will then discuss neuroanatomical, neurochemical, and molecular mechanisms underlying crossed phrenic recovery, then conclude by discussing the potential for regenerative therapeutic approaches to be used in respiratory neurorehabilitation following cervical SCI.
In a well-designed set of investigations by Porter (1895), the supraspinal source for primary respiratory drive was confirmed and the CPP was systematically characterized. A major goal of Porter’s studies was to disprove a spinal origin of respiration proposed by Brown-Séquard (1858), Langendorff(1880), and Wertheimer (1886), who reported observing breathing following spinal transection (Ghali and Marchenko, 2016a).ose advocating for the existence of a spinal respiratory central pattern generator proposed that hemisective or transective spinal cord injury transiently interrupts respiration by spinal shock inhibition. Porter challenged these findings: “irregular contractions have been seen in the diaphragm aer its isolation from the cord by the section of the phrenic nerves; and that rhythmical contractions of this muscle have occurred from the stimulation of the phrenic nerve by the current of action of the contracting heart. To these sources of erroriwould add one previously undescribed, namely, the changes of intrathoracic pressure caused by the contractions of the trapezius and sterno-cleido-mastoideus muscles….I shall show….that long inhibition of respiration from section of the upper cervical cord does not exist.”
Brown-Sequard (1869), and previously Vulpian (1866), demonstrated that a HSx performed between C1–C4occasionally does not affect breathing on either side and bilateral diaphragm contractions were later reported following C1/C2HSx by Bert (1970). In two separate studies by Knoll (1885, 1888), respiration continued aer HSx at the calamus scriptorius. The interpretation of these results is of course confounded by the subjectiveness of determining whether diaphragmatic/chestmovementis active or passive. Porter goes on: “”.
In his first experiment investigating crossed phrenic activity, Porter (1895) performed aHSx at the C2level on a morphine-sedated dog. “On cutting the right phrenic nerve just above the first rib, the right side of the diaphragm ceased to contract, while the leside, which had hitherto been passive, contracted strongly.” He repeated the same study on rabbits and confirmed his previous results.us, the CPPproperis defined as such: the appearance of phrenic or diaphragmatic activity ipsilateral to a supra-phreninuclear HSx of the cervical spinal cord following a phrenicotomy contralateral to the same in a spontaneously breathing animal.e latter distinction is significant in that phrenicotomy may induce CPPvia1) increased respiratory drive consequent to decreased tidal volume or 2) the severing of phrenic afferents that may inhibit contralateral PhMN output. The definition of the CPP has been extended to include induction by a variety of respiratory stressors, including hypercapnia, hypoxia, and asphyxia.
In contrast to current understanding of CPP, Porter (1895) did not hypothesize the existence of a bulbophrenic projectioncoursingcontralateral to bulbar origin. Instead, he hypothesized PhMN dendrites crossing the midline receive inputs from descending projections in the contralateral lateral funiculus. He suggested that these contacts are insuf ficient by number and strength under resting conditions but are induced to activity following phrenicotomy on account of‘redirection of phrenic impulses’: “.”
In 1951, Lewis and Brookhart investigated CPP in nembutal-anesthetizedartificially-ventilatedcats, recording diaphragm EMGs. Following a high cervical HSx between C1/C2, minute ventilation (VE, mL/min) was altered before and aer phrenic nerve transection or cold block. No breathing was found at VEof 600 mL. With progressive decreases in VE, tonic, followed by phasic, respiratory activity, appeared on the intact and injured sides, with the injured side’s threshold higher than the intact side. At 240 mL, both hemidiaphragms exhibited phasic activity. Additionally, intravenous injection of potassium cyanide, a potent peripheral chemoreceptor stimulant (Franchini and Krieger, 1993) with VEset at the apneic threshold, induced diaphragmatic activity bilaterally. Lastly, phrenicotomy or phrenic nerve cold block under conditions of artificial ventilation did not induce CPP, however, and it was concluded that increased respiratory drive is the mechanism underlying crossed phrenic activity.
Animal Models of Respiratory Function and Recovery following SCI
Overview
High- and mid-cervical contusion injury models of high cervical SCI
El Bohy et al. (1998) developed a lateralized C2contusioninjury model in the rat by applying an impactor to the lateral aspect of the hemicord. Terminal experiments were performed 5 weeks following injury under chloral hydrate anesthesia. Rats with a C2contusion used approximately 78% and 55% of respiratory reserve at rest, as determined by expressing PhN amplitude during eupnea as a percentage of that during asphyxia, ipsi- and contralateral to HSx, respectively; the latter value was not significantly different from spinal-intact animals.
Baussart et al. (2006) also utilized a C2contusion model. Diaphragm activity recorded from spontaneously-breathing pentobarbital-anesthetized rats at one week (as % of contralateral control) was 27.4% and not significantly different in the subset of animals tested later at 1 month. Regrettably, there is no mention of activity recorded immediately following injury. In terminal experiments on pentobarbital-anesthetized artificially-ventilated rats, PhN activity ipsilateral to HSx was 38.2% of the contralateral; in a qualitative substudy on these animals (n= 2), a contralateral right C1HSx eliminated ipsilateral PhN activity and reduced recovery in the HSx-ipsilateral PhN by approximately 50– 75%, demonstrating the involvement of contralateral projections in recovery of phrenic output.
A modified version of the cervical contusion model, the dual injury, hemi-contusion post-contralateral hemisection, was developed by Awad et al. (2013). In this model, spared phrenic activity ipsilateral to a hemi-contusion is abolished by contralateral hemisection. The authors posit this model may be more clinically relevant for investigating cervical SCI, wherein diaphragm activity is more directly compromised. In order to create models more representative of human cervical SCI, other investigators have used contusions of the mid-cervical cord, which compromise white matter and damaged PhMNs simultaneously (Choi et al., 2005; Golder et al., 2011; Lane et al., 2012). Contusions involving C5(+/–C4) resulted in acute tachypnea with subacute resolution, whereas higher contusions (C3/C4) did not change respiratory pattern. Importantly, although contusion and incomplete HSx injuries exhibit high variability in anatomical extent and physiological deficits even under the most controlled conditions, they are more representative of human cervical SCI and may prove useful in testing therapies that have been shown to improve respiratory recovery in the C2-hemisected animal.
Far lateral-restricted model of high cervical SCI
Vinit and colleagues (2006, 2008) developed a far lateral C2HSx model for cervical SCI with the purpose of sparing the ventromedial funiculus to serve as a potential pathway for recovery. This group has shown variable PhN and diaphragm recovery (see below for comparison of 2006vs. 2008 study) in terminal experiments conducted on anesthetized rats. Further studies are required to more thoroughly characterize recovery patterns in this model under different conditions.
The lateralized contusion injury model (El Bohy et al., 1998; Baussart et al., 2006) is advantageous in that it more closely resembles spinal cord injuries occurring in patients (than precise HSx without impact and cold block without injury). By preserving parenchymal medial continuity, this model allows the testing of more therapeutic approaches for neurorehabilitation following high cervical SCI. (i.e., attempts at regeneration through the ipsilateral hemicord).
Cold block model of high cervical SCI
Castro-Moure and Goshgarian (1996) developed a technique using cold block of the ventrolateral funiculus at C2in the rat. Much of the innovation comes from the surgical approach, especially with regards to avoiding injury to the bilaterally-paired rostrocaudally-oriented large epidural venous complex in this area. Using recirculation of cooled ethyl alcohol through a cooling probe in contact with a thermo-paste placed on the spinal cord (to avoid direct contact), surface temperature of the spinal cord was reduced to 7°C.is resulted in a complete silencing of ipsilateral diaphragm EMG activity and crossed phrenic activity occurred in response to asphyxia. Diaphragm EMG activity was restored following reversal of the cold block. The cold block model is not ideal as aninjurymodelper se, but it is useful in dissecting out the effects of interrupted descending drive from parenchymal damage proper.
1-hemisected unanesthetized decerebrate rat
We have recently shown progressive acute recovery of spontaneous crossed phrenic activity occurring over 2 hours in the C1-hemisected unanesthetized decerebrate adult rat (Figure 1; Ghali and Marchenko, 2015). This new model offers several advantages, by avoiding the neural-suppressant effects of anesthesia (Figure 2) and sparing the C1–2pre-phrenic interneuron group. Our findings suggest that crossed phrenic activity is state-dependent and/or that C1–2pre-phrenic interneurons play a role in respiratory recovery following SCI. Anesthesia potently suppresses neural activity, including the respiratory network, insofar that administration of isoflurane anesthesia essentially eliminated acutely recovered spontaneous crossed phrenic activity in C1-hemisected decerebrate rats.
4-hemisected anesthetized rat
Lee et al. (2014) compared and contrasted the effects of C2versus C4hemisection in an effort to produce an anatomically precise lesion of the mid-cervical cord, which is the most common site of human cervical SCI, and distinguish the effects of white matter from combined white and gray matter injury seen in mid-cervical contusion models (Choi et al., 2005; Golder et al., 2011; Lane et al., 2012).ey found mid-cervical hemisection to be associated with improved tidal volume in comparison to high-cervical hemisectionat restbut not under hypercapnic conditions and a diminished response to asphyxia aer the mid-cervical lesion compared with the high-cervical one.
Sex differences in phrenic recovery following cervical SCI
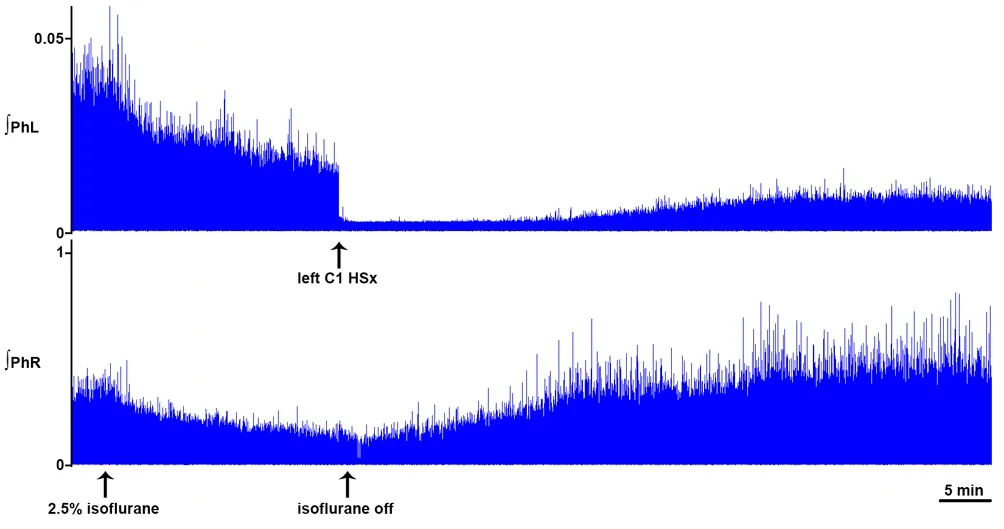
Figure 1 Dynamic changes in phrenic motor output following hemisection.
Possible sex difference in CPP are suggested by the finding that increases in PhN amplitude ipsilateral to C2HSx in response to hypoxia and asphyxia, as well as increases in tidal volume in response to hypercapnia, are greater in female rats than male and ovariectomized female counterparts (Doperalski et al., 2008). Examples of well-characterized hormonal-based differences in respiratory neural networks include the involvement of testosterone aromatization in long-term facilitation of phrenic and hypoglossal discharges in male rats in response to intermittent hypoxia (Zabka et al., 2006).us, sex differences may account for experimental variability and should be taken into account when making crossstudy comparisons.is is clinically significant insofar that female patients exhibit better neurological recovery compared to male patients following SCI (Sipski et al., 2004), though this remains debated (Greenwald et al., 2001; Chan et al., 2013).
Changes in Respiratory Parameters and Arterial Blood Gases following Upper Cervical SCI
Goshgarian et al. (1986) were the first to systematically investigate changes in respiratory parameters and arterial blood gases following high cervical SCI. Twenty-four hours following C2HSx, increases in respiratory rate, PO2, and arterial pH were observed, along with a non-significant decrease in PaCO2and increase in HCO3–in unanesthetized rats (Goshgarian et al., 1986). One to two months following C2HSx, spontaneously-breathing urethane-anesthetized rats exhibit increased respiratory frequency, diminished tidal volume, and respiratory alkalosis relative to control (Golder et al., 2001a, b), similar to breathing in humans following SCI (Estenne and De Troyer, 1987; Loveridge and Dubo, 1990; Loveridge et al., 1992). Recovery of minute ventilation and tidal volume occurs progressively over weeks to months following C2HSx (Fuller et al., 2008; Dougherty et al., 2012a) and phrenicotomy reduces tidal volume when performed 2 or 8 weeks, but not 1–3 days, following injury, providing evidence for phrenic-mediated functional recovery (Dougherty et al., 2012a).

Figure 2 Anesthesia potently suppresses crossed phrenic activity.
Functionally, upper cervical SCI results in an acute reduction in tidal volume compensated for increased respiratory frequency in both awake (e.g., Fuller et al., 2006) and spontaneously breathing anesthetized (e.g., Golder et al., 2001b) rats, resulting in unchanged minute ventilation (e.g., Fuller et al., 2006). It has been suggested that the increases in respiratory frequency compensating for diminished tidal volume capacity results from a vagal-dependent mechanism (Golder et al., 2001b), although chemoreceptor-drive likely plays an equally important role. Vagal-dependent pathways relaying pulmonary stretch receptor information may also tonically inhibit crossed phrenic activity, as the incidence of spontaneously-active CPP at 2 and 8 weeks was increased by intra-investigational bilateral vagotomy in artificially-ventilated urethane-anesthetized rats (Lee et al., 2010). It should be noted that the central respiratory rhythm synchronizes to the ventilator in vagus-intact animals (e.g., Ghali, 2015; Ghali and Marchenko, 2016b); vagotomy results in decoupling of the animal’s central respiratory rhythm from the ventilator and decreased baseline respiratory frequency.
Tachypnea was observed 2 months following C2HSx in anesthetized, vagotomized, artificially-ventilated rats (Golder et al., 2001a). Bilateral vagotomy in controls and upper C2-hemisected animals effected a reduction of respiratory frequency and augmentation of tidal volume that resulted in no statistical differences of these respiratory parameters between both groups (Golder et al., 2001b, 2003).is suggests that information regarding altered pulmonary mechanics is relayed centrallyviavagal afferents to induce tachypneic hypopnea.
Curiously, vagotomy reduced minute ventilation but decreased PaCO2, suggesting a significant increase in alveolar ventilation occurringviareduction in deadspace by improved ventilation/perfusion matching in spinal-injured animals (Golder et al., 2001b). Reduced PaCO2following vagotomy may also theoretically reflect decreased metabolic production as a consequence of gastrointestinal hypomotility(Macleod, 1974). Other changes in peripheral vagal modulation of respiratory function include diminution (Tsai and Lee, 2014) and attenuated recovery (Lee and Chang, 2014) of the pulmonary component of the Bezold-Jarisch reflex in C2-hemisected animals, which may permit recovery of the phrenic motor system as well as contribute to protection of minute ventilation following SCI (Lee, 2016).
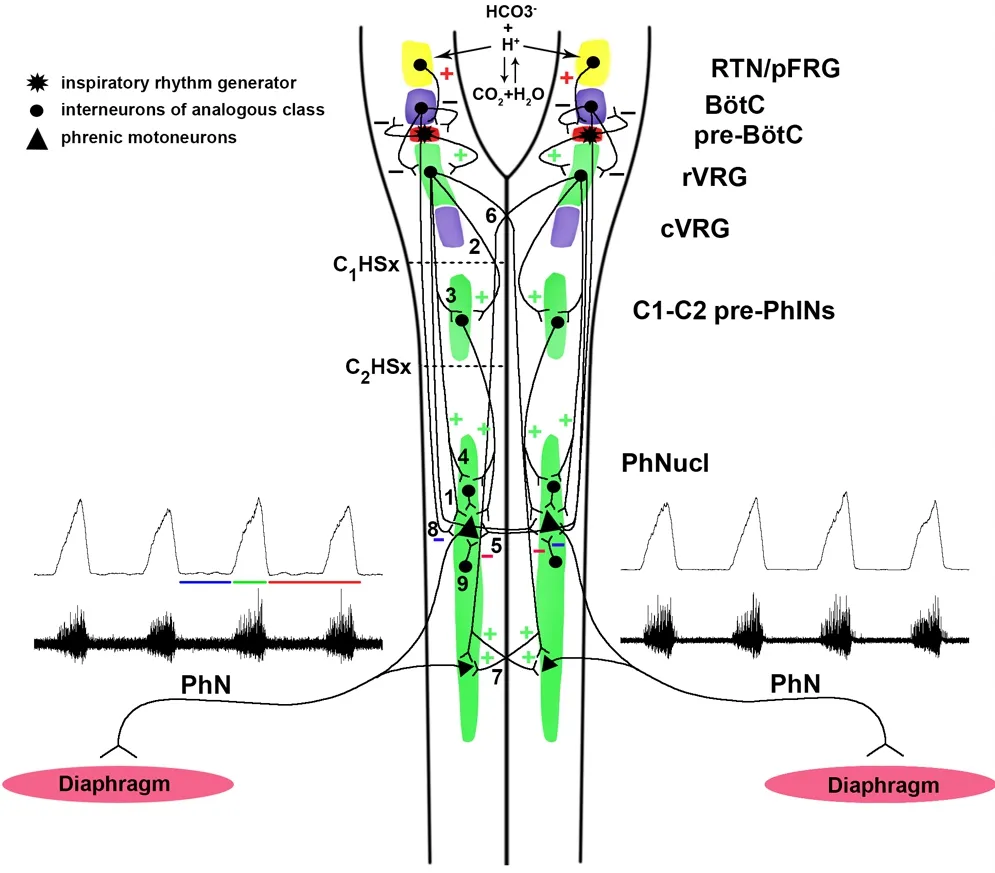
Figure 3 Bulbophrenic network organization.
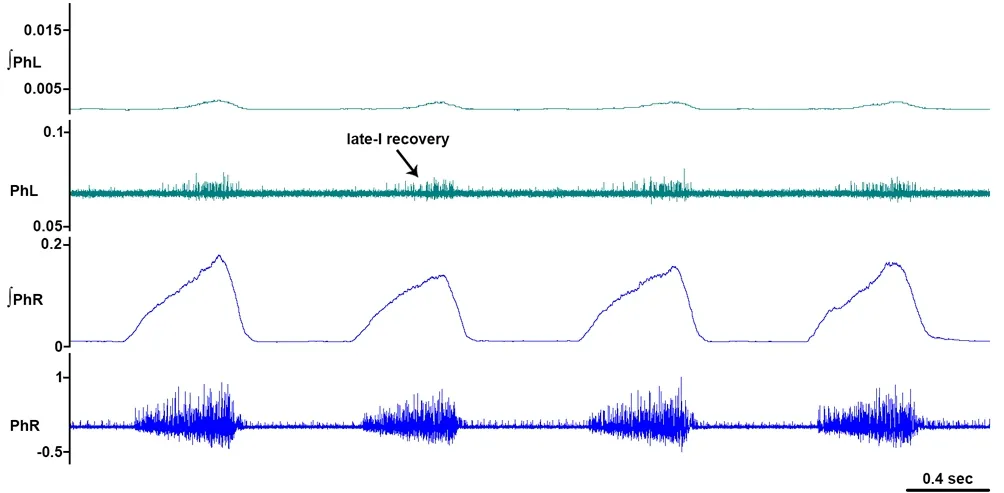
Figure 4 Recovery of late-inspiratory activity ipsilateral to hemisection.
The frequency response to hypercapnia in C2-hemisected unanesthetized rats is not different from spinal-intact animals, but some have shown that tidal volume is blunted and fails to improve with time (Fuller et al., 2006) and crossed phrenic activity remains delayed in onset in response to hypercapnia compared to contralateral PhN (Fuller et al., 2008). Dougherty et al. (2012a) demonstrated a progressive recovery in tidal volume responses to hypercapnia, but, at 2 months post-C2HSx, this failed to approach the magnitude of those by spinal-intact animals. Analogously to responses to hypercapnia, those to hypoxia improve in C2-hemisected animals over the same time frame but do not normalize (Fuller et al., 2008).
Electrophysiologic Determination of Recovery Following High Cervical SCI
Overview
A major short-coming of studies investigating CPP is the high variability of results between and within different preparation types. This was first noted by Lewis and Brookhart (1951): “no group of experiments dealing with this subject has been productive of uniform results; inter- and intraspecies variations in the degree of respiratory hemiparesis produced by the spinal hemisection and in the amount of crossing produced by experimental manipulations are reported in every study.” One possible reason may involve inconsistencies in the electrophysiological recording of neural and diaphragmatic respiratory output.
Diaphragm electromyography assessment of crossed phrenic recovery: inter-study inconsistency
In spontaneously-breathing chloral hydrate-anesthetized rats, spontaneous recovery of CPP in diaphragm EMG occurred as early as 6 weeks following C2Hsx, progressively increasing up to 16 weeks, but no recovery was observed at 4 weeks (Nantwi et al., 1999). The incidence of spontaneous recovery in vagotomized, artificially-ventilated, anesthetized rats was shown to be 25% at 1 month and 73% at 2 months (Golder et al., 2001a). Corroborating these findings, far-lateral C2HSx (HSxL) (but neither medial nor dorsolateral section) silenced both the phrenic electroneurogram and diaphragmatic electromyogram in pentobarbital/ morphine-anesthetized rats (with paracetamol/codeine on board; Vinit et al., 2006) acutely. However, spontaneous recovery occurred at 3 months in PhN but not diaphragm, in which CPP was activated only in response to contralateral phrenicotomy. In one study, HSx-ipsilateral diaphragm EMG remained silent 2 weeks following a C2HSx sparing the dorsal funiculus, even in response to combined hypercapnia and hypoxia (5% CO2, 10% O2), in sponatenously-breathing ketamine/xylazine-anesthetized rats (Mantilla et al., 2007). These results underscore the better sensitivity of phrenic electroneurography compared to diaphragm EMG for detecting recovery of the phrenic motor system, especially in anesthetized animals (Sapru and Krieger, 1979).
In a later study, Vinit et al. (2008) showed spontaneous activity in ipsilateral PhN and/or diaphragm (in contradistinction to their 2006 study) within one week aer injury which was greater at 3 months in the same animal model (pentobarbital/morphine-anesthetized rats with C2HSxL). That spontaneous diaphragm activity was found as early as one week following injury in the 2008 study (Vinit et al., 2008), but not at 3 months in the 2006 study (Vinit et al., 2006) in the exact same animal model implicates inter-experimental variation (e.g., thoroughness of and avoidance of tissue distortion during HSx), inter-animal variability (e.g., hemodynamics, arterial blood gases, temperature stability), and/or spinal shock as important and over-looked confounders in interpreting the HSx literature.
Diaphragm electromyography assessment of crossed phrenic recovery: sources of confounding
It should be noted that since many studies do not quantify EMG activity, variability in threshold used to determine presence or absence of muscle unit firing may lead to inconsistency in the reporting of results leading to different conclusions regarding whether or not diaphragmatic recovery was obtained (Vinit and Kastner, 2009). One should also note the possibility of picking up contralateral diaphragm-synchronous motion artifact and reporting the same as recovery.e use of anesthesia, by state-dependently suppressing phasic and/or tonic descending bulbospinal drive, may also result in overestimation of the time-to-recovery of spontaneous CPP. Failure to observe recovery in diaphragm EMG aer C2HSx in the subacute period of recovery (Vinit et al., 2006; Mantilla et al., 2007) could also represent poor sensitivity as a consequence of the few motor units sampled by such recordings and may be addressed by recording from multiple diaphragm sites.
As a final consideration, — an unlikely scenario but nevertheless interesting and deserving of special mention —the Vulpian-Heidenhain-Sherrington phenomenon, first described in the 19thcentury, involves slow muscular contraction in the absence of neuromuscular innervation in response to activation of cholinergic fibers supplying associated vasculature (Kusurkar, 2004) and may underlie diaphragmatic contractions that have been infrequently seen following bilateral phrenicotomy (Porter, 1895). In theory, this phenomenon could account for false-positive determination of post-HSx recovery as assessed by diaphragm EMG, but would not confound results obtained by phrenic electroneurography.
Phrenic electroneurography assessment of crossed phrenic recovery: sources of confounding
Neuroanatomical Basis for Recovery of Crossed Phrenic Activity
Adult animals
Since ipsilateral PhN activity becomes silent after acute C2HSx in guinea pigs (Guth, 1976), mice (Minor et al., 2006) and rats (Goshgarian, 1979; Ling et al., 1994), it has beenproposed that CPP does not contribute to PhN output at rest, but can generally be elicited in response to respiratory stressors such as contralateral phrenicotomy (Lewis and Brookhart, 1951), asphyxia (e.g., O’Hara and Goshgarian 1991; Yu and Goshgarian, 1993; Gould and Goshgarian, 1999; Golder et al., 2003; Doperalski et al., 2008), and chronic intermittent hypoxia (Fuller et al., 2003). In fact, asphyxia applied several minutes aer C2HSx has been shown to recover PhN activity ipsilateral to injury (Porter, 1895; Rosenbleuth and Ortiz, 1936).
Despite extensive structural and functional characterization of bulbospinal pathways in several animal models, to what extent ipsi- versus contralateral and phasic versus tonic pathways are active under uninjured versus injured and unstressed versus stressed conditions remains to be clarified (Figure 3) (Ghali, 2017). CPP is thought to be mediated by descending bulbospinal pathways coursing in the lateral funiculus contralateral to the injury (Goshgarian, 2003) and crossing within the spinal cord at the level of the phrenic nucleus (Goshgarian et al., 1991; Moreno et al., 1992) in the ventral white commissure to supply PhMNs in the contralateral ventral horn. Alternatively, these axons may not actually cross in the ventral white, but may make synaptic contact on contralateral PhMN dendrites extending past midline (Prakash et al., 2000; Boulenguez et al., 2007), as first proposed by Porter (1895).
Additionally, the ventromedial funiculus may also contribute to CPP by providing excitatory drive, as CPP elicited by a contralateral phrenicotomy in mice was greater if the ventromedial funiculus was spared (Minor et al., 2006). In another study, crossed phrenic activity occurred in all control rats where integrity of the ventral funiculus was preserved, but in none wherein HSx was complete (Li et al., 2003). Moreover, during terminal experiments in pentobarbital/morphine-anesthetized rats (Vinit et al., 2008), a complete C2HSx performed contralateral to the initial HSxL eliminated spontaneous activity ipsilateral to the original side of injury in animals studied 7 days, but not 3 months, aer initial C2HSxL.us, subacute and chronic recovery may involve different pathways; in subacute recovery, contralateral bulbophrenic tracts descending in the lateral and ventral funiculi are utilized whereas, in delayed recovery, the ipsilateral ventral funiculus is involved.
Neonatal animals
Overview
In vitro studies
Prior to our study in C1-hemisected unanesthetized decerebrate rats (Ghali and Marchenko, 2015), spontaneous crossed phrenic activity occurring acutely following injury was only shown in neonatal animals (see below), initially in thein vitrobrainstem-spinal corden blocpreparation (pontomedullary and T8transection; cervical and thoracic dorsal rhizotomy). Recovery ipsilateral to injury was approximately 20% of ipsilateral activity pre-injury (peak amplitude), although the raw recordings show that integrated burst area may be significantly less. Importantly, the respiratory discharge was irregular, exhibiting decrementing firing dynamics suggestive of gasping, consistent with the fact that this preparation is pontomedullary transected (St. John and Paton, 2004).
In vivo studies
Studies conductedin vivoin neonates reveal a rapid entrance into latency of crossed phrenic activity occurring well before the juvenile period. In spontaneously-breathing anesthetized rats, crossed activity is manifest throughout the entire diaphragm in early neonatal (P2–P4) animals, becoming restricted to the ventral diaphragm in late neonatal (P7–P28) animals, and completely abolished in P35 animals (Huang and Goshgarian, 2009a). Retrograde diaphragm labeling reveals midline-crossing PhMN dendrites in P2 animals which become diminished in P7 and P28 rats, and are not observed by P35, suggesting that retraction of midline-crossing PhMN dendrites are responsible for maturational changes in crossed phrenic activity (Prakash et al., 2000; Huang and Goshgarian, 2009c).
Juvenile and young adult animals
Remarkably, more than two decades prior to demonstrated spontaneously-active crossed phrenic activity in neonates, Goshgarian (1979) showed that there is a longer delay to elicit CPP following C2HSx in juvenile rats (5–9 weeks old) compared to older animals (26 weeks old), suggesting a latent pathway contributing to contralateral phrenic activity that becomes functional over maturation. Another previous study by this lab (Yu and Goshgarian, 1993) investigated age differences in inducible CPP, noting a greater amplitude in older than younger animals but a similar overall incidence in the two groups. In our experience, we observe diminution, but persistence of phrenic nerve activity ipsilateral to a C1hemisection with gradual recovery in the artificially-perfusedin situpreparation of the decerebrate juvenile rat (unpublished data).
Mechanisms underlying prominent spontaneous crossed phrenic activity in younger animals
Spontaneous crossed phrenic activity in neonatal animals is likely attributed in large part to midline-crossing PhMN dendrites (Allan and Greer, 1997; Song et al., 2000), which represent a higher proportion of the PhMN pool in younger animals (Prakash et al., 2000), perhaps facilitated by removal of tonic inhibitory influence of the pons on respiration(Hilaire et al., 1989) in theen blocpreparation (St. John and Paton, 2004). Midline-decussating PhMNs may become retracted with age. Additionally, spontaneous crossed phrenic activity may be more prominent in neonatal animals as a consequence of higher input resistance of smaller PhMNs, necessitating smaller amounts of current to reach action potential threshold.
To reconcile the apparent contradiction of spontaneously-active crossed phrenic activity in neonates and more facile induction of crossed phrenic activity in older than younger animals that have passed the neonatal period, one may argue that weak but consistently active crossed activity mediated by PhMN dendrites crossing the midline in the neonate diminishes rapidly with growth and age-related increases in projections result in a stronger but latent inducible crossed phrenic drive.
Cellular and Neurochemical Adaptations Underlying Recovery of Crossed Phrenic Activity
Acute cellular adaptations following SCI
Structural adaptations
It is rather noteworthy that within 2–4 hours following C2hemisection, the neural respiratory network within the spinal cord undergoes significant ultrastructural changes (Goshgarian et al., 1989; Sperry and Goshgarian, 1993; Castro-Moure and Gosharian, 1997; Hadley et al., 1999a).ese include, but are not limited to, glial process retraction along with a significant increase in dendro-dendritic oppositions and double (and multiple) synapses. Under normal conditions, glial processes are interposed between and separate the cell body and primary dendrities; retraction of glial processes may effect the unmasking of dendro-dendritic appositions, which may improve coupling between and amongst PhMNs, amplifying bulbophrenic phasic and tonic synaptic inputs. Analogous ultrastructural adaptations occur in rats sacrificed 4 hours following hemi-cold block at the C2level, indicating acute plasticity is a consequence of loss of descending drive rather than representing a non-specific effect of trauma (Castro-Moure and Gosharian, 1997).
Acute morphologic changes in the phrenic nucleus induced by a C2HSx are attenuated by pre-treatment with an inhibitor of tryptophan hydroxylase, para-chlorophenylalanine (Hadley et al., 1999a), and the same treatment reduces asphyxia-inducibility of CPP acutely following C2HSx (Hadley et al., 1999b).is suggests an important role for serotonergic signaling in respiratory cellular neuroplasticity. Acute recovery of inducible CPP occurring over several hours following HSx may parallel morphological adaptations, as asphyxia-induced crossed phrenic activity progressively increases over 1–6 hours following a C2HSx, but does not improve significantly at 12 and 24 hours post-injury (O’Hara and Goshgarian, 1991). Mechanisms operant during the hyperacute/acute post-injury period may differ fundamentally from those occurring subacutely and chronically and understanding these differences may reveal new treatment targets to “protect” the respiratory network in the spinal cord, in addition to the current protocol of intravenous methylprednisolone aimed at protecting neural parenchyma in general.
Electrophysiological adaptation
A study by El-Bohy and Goshgarian (1999) provides some insight into the single cell firing properties of crossed pathways. They performed individual PhN axon recordings ipsilateral to C2HSx along with intact HSx-contralateral PhN recordings and induced crossed phrenic activity by asphyxia 2–3 hours following HSx. Responses included increased single unit firing frequency as well as recruitment of previously silent PhMNs, with a greater increase in late-onset inspiratory (I) than early-onsetiunits.eophylline-induced crossed phrenic activity was shown in one set of investigations to elicit predominantly late-I activity in PhN discharge (described by the investigators as “asynchrony”), further corroborating the hypothesis that PhMNs/diaphragm motor units underlying CPP are recruited from silence in an orderly manner (Nantwi et al., 1996), presumably in accordance with the size principle of Henneman (1965a, b). Moreover, hypoxia-induced short-term potentiation appears to preferentially affect late-recruited inspiratory PhMNs (Lee et al., 2015).
Recovery of predominantly late-I units was observed by our group (Figure 4; Ghali and Marchenko, 2015) and delayed burst onset in recovering PhMNs ipsilateral to a C2hemisection was similarly observed by Lee and colleagues (Lee et al., 2013; Lee, 2016). Late-onsetiunits tend to fire with lower frequencies while early-onsetiunits are comprised by PhMNs underlying synchronous medium- and high-frequency oscillations in PhN spectra (Marchenko et al., 2012). Uncovering the recruitment order and activation patterns of specific subpopulations of the PhMN pool following HSx in response spontaneously and in response to respiratory stressors would greatly further current understanding of laterality of bulbophrenic inputs controlling PhMNs with different firing patterns and overall respiratory network organization. This knowledge, in turn, could be exploited to optimize the design of phrenic and diaphragm pacing devices (i.e., time-frequency pattern of stimulation).
Role of neurochemical pathways in crossed phrenic activity
Overview
Zimmer and Goshgarian (2007a) have shown that CPP is tonically inhibited by a mechanism utilizing GABAAergic pathways, demonstrating that fast inhibitory synaptic transmission plays a critical role in CPP, as it contributes to phrenic pattern formation in normal uninjured conditions (Marchenko and Rogers, 2009; Marchenko et al., 2015). Additionally, other investigators have demonstrated the involvement of serotonergic signaling in CPP (Ling et al., 1994; Zhou et al., 2001). Thus, a variety of neurochemical pathways appear to interact to regulate phrenic output at rest as well as modulate respiratory plasticity following injury. For example, it has been shown that serotonergic transmissionis involved in long-term potentiation in PhMNs induced by stimulation of pre-motor neurons (Fuller et al., 2002) and administration of a 5-HT1Aagonist increases PhN output (Zimmer and Goshgarian, 2006). Moreover, 5-HT1Aagonists have been shown to hyperpolarize membrane potential of dorsal horn neurons (Hains et al., 2003b), which, in turn, have been shown to inhibit PhMNs by a GABAA-dependent, but glycine-independent, mechanism (Zimmer and Goshgarian, 2007a). Clearly, therefore, it is the interaction of and intersection between neurochemical pathways which underlies spontaneous CPP as well as acute, subacute, and chronic recovery of neural respiratory function.
Role of fast inhibitory transmission
Evidence for the role of fast inhibitory synaptic transmission (i.e., GABAA- and glycinergic signaling) following SCI comes from a study by Zimmer and Goshgarian (2007a). In vagotomized, paralyzed, artificially-ventilated, urethane-anesthetized rats, GABAA- or glycinergic antagonists were topically applied to the dorsal surface of the cervical spinal cord 1 week following C2HSx.e method of application was as follows: laminectomy and durotomy inclusive of C3–7were performed and drugs pipetted across this length during bilateral PhN recordings. Bicuculline and gabazine (GABAAergic receptor antagonists), but not strychnine (glycinergic receptor antagonist), induced CPP, and the effects of the former were reversed by application of muscimol.is suggests that CPP is tonically inhibitedviaGABAA- but not glycinergic mechanisms. Elevation of the animal’s head and unchanged respiratory frequency argue against the proposition that these drugs affected supraspinal respiratory centers, although it is not inconceivable that the activity of upper cervical inspiratory neurons at C1/C2(Lipski et al., 1993; Tian and Duf fin, 1996a, b) was somehow modified.
GABAAergic inhibition of CPP may derive from supraspinal regions, local intraspinal, or both. Irrespective of the origin, gradual down-regulation of GABAAergic inhibition over time may account for the observation that CPP becomes spontaneous (i.e., in the absence of respiratory stress) eventually (Nantwi et al., 1999). Other than GABAAergic mechanisms, serotonergic signaling may be implicated in inhibitory control of phrenic output. For example, it has been shown that activation of 5-HT1Areceptors increases PhN activity on the side of a C2HSx (Zimmer and Goshgarian, 2006) and dorsal rhizotomy can activate a latent CPP (Fuller et al., 2002), suggesting PhN afferents activate dorsal horn neurons (DHNs), which consequently tonically inhibit CPP.
Role of serotonergic mechanisms
Intraventricular administration of the serotonergic-selective neurotoxin 5,7-dihydroxytryptamine has been shown to reduce the incidence of spontaneous CPP recovery 2 months following C2HSx in vagotomized artificially-ventilated anesthetized rats (Golder et al., 2001a) and blockade of 5-HT2Areceptors prevents acute intermittent hypoxia-induced long term facilitation in PhN activity ipsilateral to a C2HSx in the same preparation (Golder and Mitchell, 2005). Conversely, intravenous administration of 5-hydroxytryptophan (Zhou et al., 2000) or the 5-HT2A/2Cagonist DOI (Zhou et al., 2001) one day following HSX recovered ipsilateral PhN activity. Activation of 5-HT2receptors has been shown to enhance theophylline-induced crossed phrenic activity (Basura et al., 2002), providing evidence for interaction among pathways. The usefulness of these findings is challenged by a study showing enhancement of hemidiaphragm EMG ipsilateral to HSx, but not tidal volume, in response to systemic serotonergic stimulation (Hsu and Lee, 2015).e therapeutic utility of manipulating specific serotonergic signaling pathways in respiratory neurorehabilitation following SCI requires further investigations.
Role of adenosinergic signaling
A role for adenosinergic signaling in recovery of crossed phrenic activity has been extensively investigated and holds promise as a therapeutic target (Bascom et al., 2005). Nantwi et al. (1996) demonstrated that intravenous administration of theophylline during recording induced crossed phrenic activity in chloral hydrate-anesthetized rats one day following C2HSx. Crossed phrenic activity could not be induced by theophylline if the animal was pre-treated with an adenosine receptor agonist (L-PIA), nor in response to a methylxanthine lacking adenosine antagonistic properties (enprofylline; Nantwi et al., 1998b; Nantwi and Goshgarian, 2001) nor one that is peripherally selective lacking central activity (8-(p-sulfophenyl)theophylline; Nantwi and Goshgarian, 2001). Later, it was shown blockade of adenosine A1, but not A2, receptors following HSx which is specificially responsible for inducing the crossed phrenic phenomenon. These investigations establish theophylline as promising in the treatment of respiratory dysfunction following SCI and implicate central adenosine A1receptor antagonism as an important mechanism underlying observed crossed phrenic recovery in response to methylxanthines. Ef ficacy of methylxanthines in respiratory recovery has also been shown in animal models of cervical contusion (Hoy and Alilain, 2015). Since blockade of adenosine 2A receptors has been shown to improve long-term facilitation in diaphragm in response to chronic intermittent hypoxia (Navarette-Opazo et al., 2014), adenosinergic pathways involved respiratory neuroplasticity and recovery following SCI are complex and a more thorough understanding of the same will require further investigations.
As adenosine A1receptors are Gi-protein coupled, their blockade results in increased activity of adenylate cyclase, greater cAMP synthesis, and consequent protein kinase A activity and pharmacological manipulation of these pathways should elicit similar effects to those observed with methylxanthines.is, indeed, is the case, as crossed phrenic activity has been induced in rats following C2HSx by treatment with the cAMP analogue 8-Br-cAMP (Kajana and Goshgarian, 2008a), the phosphodiesterase (PDE) inhibitors pentoxyfylline (Kajana and Goshgarian, 2008b) and rolipram (Kajana and Goshgarian, 2008b, 2009), and the adenosine A1receptor antagonist DPCPX (Nantwi and Goshgarian, 2002;Kajana and Goshgarian, 2008b).
Dose-dependent efficacy for use of theophylline during subacute/chronic recovery has been shown in rats treated with theophylline for 3 – 30 days aer a C2HSx. In these animals, crossed phrenic activity was evident at post-treatment recording times but absent in untreated animals (Nantwi et al., 1998b, 2003a) and persisted following discontinuation of treatment (in some cases improving with increasing duration of post-treatment discontinuation, see Nantwi et al., 2003a). However, theophylline treatment for 4 months following HSx suppressed or did not affect crossed phrenic recovery that had occurred spontaneously (Nantwi et al., 2003b), suggesting a critical period wherein this treatment is effective and aer which may be harmful.
Modulation of respiratory recovery following SCI by concomitant pre-phrenicotomy
Animals with combined C2HSx and ipsilateral phrenicotomy studied 2 months later did not exhibit CPP spontaneously although crossed output was inducible by hypoxia (Golder et al., 2003), in contrast to HSx-only animals, 12 of 15 of which had spontaneous phrenic activity ipsilateral to injury.is may be attributable to axotomy induced molecular and cytoarchitectural alterations in PhMN somata intraspinally (Gould and Goshgarian, 1997; Liou and Goshgarian, 1994, 1997) and altered firing properties of phrenic axons (Titmus and Faber, 1990; Liou and Goshgarian 1994). This process can loosely be considered as the transynaptic variant of double crush syndrome (Upton and McComas, 1973), whereby a single lesion activates plasticity mechanisms but a preceding second lesion significantly compromises recovery.
Gould and Goshgarian (1999) suggest that a microglial, but not astrocytic, proliferative response occurs in response to phrenicotomy. They showed that intracisternal treatment prior to HSx with the mitotic inhibitor cytarabine reduces microglial proliferation and diminished phrenicotomy-related attenuation of recovery following SCI. Microglial proliferation may be induced by the axotomy of the phrenic nerve and subsequent activation of these cells may include features such as increased cytokine signaling capacity which may underlie morphological alterations at the phrenic pre-MN-PhMN synapse (Giulian and Baker, 1985; Barron et al., 1990; Giulian et al., 1994).is has important implications for multi-trauma patients, who may sustain cervical SCI in conjunction with peripheral nerve (e.g., phrenic) damage.
Role of afferents in recovery of crossed phrenic activity
Overview
Lewis and Brookhart (1951) demonstrated that CPP is elicited by phrenicotomy as a consequence of increased central respiratory drive as opposed to the transection of fibersproper, which argues somewhat against a role for phrenic afferents in CPP inducibility. However, PhN stimulation was shown to result in inhibition of 40% of the contralateral PhMN pool (as determined by intracellular recording) and this effect was abolished by sectioning dorsal roots of the stimulated PhN (Gill and Kuno, 1963) and unaffected by complete spinalization above the phrenic nucleus. These, among other, findings led investigators studying high cervical hemisection to hypothesize that loss of afferent inhibition may additionally play a role in phrenicotomy-induced CPP (Goshgarian, 1981). An anatomical basis forhas been shown in mammals, where afferent fibers terminate in the contralateral ventral (Escolar, 1948; Sprague, 1958; Kerr, 1961; Sprague and Hongchien, 1964) and dorsal (Culberson et al, 1979) horns.
Several investigators have suggested a role for phrenic afferents in CPP recovery (Hadley et al., 1999a, b), relaying ventrallyviadorsal horn neurons. Notably, and in contrast to other muscle groups, there exist a paucity of muscle spindles in the diaphragm (Corda et al., 1965; Von Euler, 1968), yet PhN stimulation results in increased neurogram amplitude (Butler et al., 2003), suggesting that the few afferents that do exist in the phrenic system are capable of producing marked changes in the motor output. It has been suggested that sensory neurons in the PhN may subsume a greater role after injury than normal conditions and may contribute to recovery following cervical SCI (Corda et al., 1965).
Dorsal rhizotomy in the subacute recovery period following C2HSx elicits CPP (Goshgarian, 1981), suggesting afferents may play a predominantly inhibitory role in phrenic recovery. Additionally, following a far-lateral C2HSx, PhN transection eliminates previously recovered activity; this does not occur in cervical dorsal root-gangliectomized animals, supporting the hypothesis that the effect is due to afferent as opposed to motoneuron axotomy (Vinit et al., 2007). Since axoaxonic synapses occur in the phrenic nucleus (Goshgarian and Rafols, 1984), presynaptic inhibition is another candidate mechanism by which phrenic afferents may inhibit CPP or phrenic output in general. Understanding afferent regulation of crossed phrenic activity may lead to the development of medical therapies targeting specific neurochemical pathways or the use of direct surgical intervention, such as selective C3–6dorsal rhizotomy, to improve respiratory function following SCI.
Experimental studies
Three to twenty-eight days following C2HSx, terminal experiments were performed on chloral hydrate-anesthetized C2-hemisected rats. Dorsal rhizotomy of the cervical spinal cord (C3–6; a few additionally at C7–8) on the intact side (contralateral to HSx) elicited CPP (diaphragm EMG) ipsilateral to HSx and sometimes augmented activity ipsilateral to rhi-zotomy (Goshgarian, 1981). In the animals in which additional C7–8dorsal rhizotomization was performed, CPP was further enhanced. These findings physiologically demonstrate that phrenic nucleus receive contralateral inhibition from afferents at and below phrenic nucleus levels.e role of phrenic primary afferents in inhibiting CPP was later revisited by Zimmer and Goshgarian (2006) who performed C2HSx in vagotomized, paralyzed, artificially-ventilated, anesthetized rats. Following variable postoperative periods (1 day, 2 days, 1 week, 16 weeks), the 5-HT1Aagonist 8-OHDPAT was topically applied to the dorsal spinal cord surface or administered systemically, resulting in activation of CPP. It is instructive to comment on a series of investigations by Fuller and colleagues (2002). One week following cervical dorsal rhizotomy (C3–6), vagotomized, paralyzed, artificially-ventilated, urethane-anesthetized rats were hemisected at C2. Relative to cervical afferent-intact animals, dorsally-rhizotomized rats exhibited greater PhN amplitude in response to contralateral ventrolateral funiculus stimulated rostral to HSx level. Responses in rhizotomized animals were unaffected by intravenous administration of the serotonin antagonist methysergide, arguing against a role for 5-HT mediated inhibition. However, since the animals were phrenicotomized (for PhN recordings), what was actually being investigated was the role ofnon-phreniccervical afferents, which may tonically inhibit PhMN outputviaserotonin-independent mechanisms.
Mechanisms underlying phrenic aerent inhibition of crossed phrenic activity
There are several candidate pathways mediating motor inhibition by phrenic afferents: primary afferent-ascending relay-descending inhibition,viasynapses on cervical respiratory-modulated neurons,viadirect synapse on PhMNs, andvianon-respiratory-modulated DHNs (Iscoe and Duffin, 1996).e latter two are the most viable options, as phrenic afferents in close approximation to PhMNs (Song et al., 1999) have been demonstrated and the activity of a subset of non-respiratory-modulated DHNs nevertheless responds to PhN stimulation (Cleland and Getting, 1993; Iscoe and Duffin, 1996). Descending inhibition of dorsal horn neurons has been well demonstrated in locomotor systems. For example, multireceptive DHNs in the lumbar spinal cord become more excitable following a T13HSx (Hains et al., 2003b) and fire more frequently in response to a rostrally-placed cold block (Wall, 1967; Brown, 1971; Handwerker et al., 1975), in part as a result of the elimination of descending serotonergic inhibition (Yaksh and Wilson, 1979; Hains et al., 2002).
Following SCI, 5-HT levels are signi ficantly reduced in the spinal cord (Hains et al., 2002; Golder and Mitchell, 2005) and DHNs become hyperexcitable (Hains et al., 2003a, b). Since DHNs are known to be inhibited by descending serotonergic (5-HT1A) signaling (or et al., 1993), it is possible that 8-OH-DPAT mediates its effect by silencing of DHNs and facilitation of PhMN activity.us, loss of descending serotonergic inhibition of serotonergic inhibitory DHNs may account for reduced responsiveness (i.e., excessive hyperpolarization) of PhMNs caudal to a HSx to contralateral bulbophrenic inputs. Descending serotonergic inputs, originating from raphe magnus, are bilateral and have intraspinal decussations through lamina X (Skagerberg and Bjorklund, 1985) and bilateral monosynaptic supply to PhMNs from raphe structures has been demonstrated by Dobbins and Feldman (1994), suggesting some sparing following HSx and possible involvement in acute and/or chronic respiratory recovery.
Long-Term Respiratory Recovery following SCI
Functional considerations
Long-term respiratory recovery has been demonstrated in humans, with diaphragmatic recovery occurring in approximately one-third of patients ipsilateral to SCI in one series (Oo et al., 1999) and some individuals achieving ventilator independence (DiMarco, 2005). Although spontaneous CPP may be observed a few days aer C2HSx in animal models, a progressive increase in PhN output is observed over long time scales in rats (Nantwi et al., 1999; Fuller et al., 2006; Vinit et al., 2006, 2008); recovery after 1 week is approximately 25% and aer 3 months is 40% of control (Vinit et al., 2008). Despite progressive recovery of the PhN ipsilateral to C2HSx over the months following injury (Nantwi et al., 1999), this is not correlated with increasedrestingtidal volume (Fuller et al., 2006), but phrenicotomy ipsilateral to the side of injury 2 months post-C2HSx reduces tidal volume suggesting improved PhN output account for functional recovery (Golder et al., 2003). Additionally, increased vital capacity is demonstrated by the finding that chronically-injured animals exhibit larger tidal volumes under respiratory stressors (i.e., hypercapnia, hypoxia, asphyxia; Golder et al., 2003) compared to acutely-injured animals.
As normoxemic normocapnia typically characterizes arterial blood gases following C2HSx (Goshgarian et al., 1986; Golder and Mitchell, 2005; Doperalski and Fuller, 2006; Fuller et al., 2006), theprogressiveincrease in PhN output observed during chronic recovery is not the consequence of increased chemo-drive, although the increased respiratory frequency may be sustained by, in addition to vagal afferent feedback mechanisms, minor alterations in arterial pH from decreased tidal volume which is adequately compensated by the tachypnea. Although animal models of high cervical SCI remain normoxic, chronic (Fuller et al., 2003) and acute (Golder and Mitchell, 2005; Doperalski and Fuller, 2006; Lee et al., 2015), intermittent hypoxia does enhance recovery of PhN activity ipsilateral to C2HSx.is is significant insofar that patients may develop hypoxemia following trauma due to a variety of causes (i.e., pneumonia, atelectasis, pulmonary embolism, respiratory insuf ficiency), which may play a role in long-term respiratory neuroplasticity contributing to functional recovery (Oo et al., 1999).
Neuroanatomical substrate for long-term recovery of crossed phrenic activity
A fundamental question is whether phrenic recovery following SCI reflects activation of pre-existing pathways or a regenerative process, or both. In chronic recovery of afar-lateral C2-sectioned animal, PhN recovery is eliminated by damage to the contralateral medial spinal cord, but unaffected by damage to the contralateral lateral funiculus (Vinit et al., 2008), consistent with a hypothesis whereby long-term recovery involves pathways projecting in the ventromedial funiculus and not requiring the lateral funiculus. Despite the observation that PhN output increases time-dependently, no such correlation is observed for PhN activity elicited from electrical stimulation of contralateral lateral funiculus (location of CPP; Fuller et al., 2006) and in far-lateral C2-sectioned animals, PhN recovery occurred only if the ventromedial funiculus was preserved (Li et al., 2003), providing further evidence for CPP-independence of long-term recovery.e latter finding goes a step further to suggest that the acute/subacute recovery observed with activation of latent CPP not only is not responsible for long-term recovery but in some manner becomes silenced. Another study showed that at 1 month following C2HSx, respiratory frequency was lower and tidal volume higher in ventromedially-spared animals compared to those in which the ventromedial funiculus was lesioned (Fuller et al., 2009). Vinit et al. (2006, 2007) suggest that bulbophrenic projections in the ventromedial funiculus are not used normally and are too weak to be involved in acute/subacute recovery, being recruited later by the induction of long-term respiratory plasticity.
Sprouting of ventral corticospinal tract (CST) axons into ventral horns has been demonstrated following damage to the dorsal CSTviaa dorsal funiculus-limited HSx in rats (Weidener et al., 2002) and mice (Steward et al., 2008), demonstrating that the animal may utilize pathways other than the native projections to achieve functional recovery.is has also been shown for the respiratory system in rats following a C2HSx and transplantation with olfactory ensheathing cells at the lesion site (Li et al., 2003; see below).
There are several proposed mechanisms for increased functionality of the normally dormant ventromedial bulbophrenic synapses onto PhMNs in chronic injury models. Goshgarian (2003) suggests that nerve terminals already synapsing onto PhMNs become more strongly coupled to the latter. Evidence for this is provided by a study by Alilain and Goshgarian (2008) demonstrating an increase in the expression of medullary NR2A and GluR1 aer SCI, suggesting the activation of mechanisms involved in strengthening of glutamatergic synapses. Moreover, both serotonin and 5-HT2A receptors are found in greater abundance following C2HSx (Golder and Mitchell, 2005; Fuller et al., 2006). Additionally, axons that normally supply adjacent non-phrenic motoneurons may form collaterals onto PhMNs or propriospinal interneurons may be recruited into the phrenic network (Vinit and Kastner, 2009).
Molecular mechanisms underlying long-term recovery of crossed phrenic activity
Molecular basis for phrenic motor system neuroplasticity
Increased excitability of PhMNs ipsilateral to hemisection is thought to underlie long-term recovery of crossed phrenic activity.is is believed to be mediated, in part, by changes in glutamate receptor subtype expression. It is well known that recovery of crossed phrenic activity is associated with upregulation of NMDA receptor subunits and a downregulation of AMPA receptor subunits (Alilain and Goshgarian, 2008). Specifically, chronic recovery following C2HSx is characterized by increased expression of NR2A and GluR1 and decreased expression of GluR2 in the phrenic nucleus, consistent with enhanced NMDA and non-NMDA glutamatergic signaling (Alilain and Goshgarian, 2008) within the PhMN pool. Glutamate receptor expression is in turn governed by tropomyosin-related kinase receptor subtype B (TrkB), which has been shown to be critically involved in phrenic recovery following high cervical spinal cord injury during eupnea (Mantilla et al., 2013, 2014; Martínez-Gálvez et al., 2016; Gill et al., 2016; Gransee et al., 2013, 2017; Hernandez-Torres et al., 2017) and other respiratory behaviors (Hernandez-Torres et al., 2017).ese changes act in concert to render PhMNs more excitable and receptive to descending inputs.
Transfection of PhMNs with adenoviral-associated vector-delivered TrkB increases the probability of phrenic recovery following SCI and effects a corresponding large increase in the expression of NMDA receptor subunit 1. Hemisection proper diminishes the expression of the AMPA receptor subunit 1, which is expressed to a greater degree in animals with greater degrees of recovery (Gransee et al., 2013, 2017). Intrathecal administration of BDNF to stimulate TrkB signaling was shown to enhance phrenic recovery following high cervical hemisective cord injury and blockade of the same using TrkB-Fc soluble fusion protein or siRNA targeting TrkB was shown to prevent respiratory recovery (Mantilla et al., 2013). Whether this recovery was a consequence of phrenic versus supraspinal effects remains to be clarified. Importantly, expression of glutamatergic signaling machinery can be targeted using therapeutic administration of brain-derived neurotrophic factor (BDNF) to stimulate the TrkB pathway.
Following axonal transection, increased expression of GAP-43 and c-Jun, which promotes axonal sprouting (Raivich et al., 2004). Increased expression of GAP-43 in the ventral horn caudal to C2HSx, but a decrease in entire ipsilateral C1–6hemicord, suggests that increased capacity for functional plasticity isspecificfor MN pools (Vinit et al., 2009). In contradistinction, upregulation of GAP-43 in the contralateral cervical hemicord is observed, suggesting that recovery of bulbophrenic input involvesde novoaxonogenesisviaprimarily crossed pathways (Vinit et al., 2009). Moreover, it has been shown that regenerative potential is inversely proportional to the distance between the site of injury and corresponding soma (Jenkins et al., 1993). Following SCI at cervical levels, bulbospinal and rubrospinal neuron somata increase the expression of genes associated with regeneration (Jenkins et al., 1993; Houle et al., 1998; Vinit et al., 2005, 2009), while corticospinal tract somata do not (Mason et al., 2003), since they are further from the site of injury. Specifically, c-Jun increases in rubrospinal neurons following HSx at C3(Jenkins et al., 1993; Houle et al., 1998) but not T10(Jenkins et al., 1993) and increased expression of genes implicated in regenerative processes occur in layer V pyramidalneurons of CST following axotomy within the cortex but not at the cervical spinal cord (Mason et al., 2003).
Involvement of nitric oxide in respiratory neuroplasticity was demonstrated by Capková et al. (2011), wherein staining for neuronal nitric oxide synthase in terminals near PhMNs and b1 subunit of guanylyl cyclase in phrenic motoneurons was significantly reduced contralateral to a C2–3hemisection and nearly abolished ipsilateral to the cord injury. Further studies are necessary to elucidate the role of nitric oxidergic signaling in respiratory neuroplasticity following SCI, an enhanced understanding of which could hold significant therapeutic promise, given the wide availability of pharmacological compounds acting on this pathway.
Molecular basis for supraspinal neuroplasticity
In afferent-intact spontaneously-breathing C2-hemisected animals, respiratory rate is increased to compensate for the decrease in tidal volume (Goshgarian et al., 1986; Fuller et al., 2006; Dougherty et al., 2012a); however, invagotomized, anesthetized, paralyzed, and artificially-ventilated rats (Golder et al., 2001a), as well as thein vitrobrainstem-spinal corden blocpreparation of the neonatal rat (Zimmer and Goshgarian, 2007b), respiratory frequency islowerfollowing C2HSx, demonstrating that central drive is reduced. Moreover, in thein vitroneonatal rat preparation, the response to hypercapnia is paradoxically characterized by shorter burst duration and smaller burst area than control.e observed increase in respiratory rate following SCIin vivoin vagus-intact animals and decrease in respiratory ratein vivoin vagotomized animals andin vitroare likely consequences of supraspinal plasticity secondary to alterations in pulmonary stretch receptor, chemoreceptor, and spinobulbar pathway activity.
Adaptations of supraspinal respiratory circuitry following high cervical SCI (Buttry and Goshgarian, 2014; Ghali and Marchenko, 2015) likely underlie changes incentraldrive and increased phrenic/hemidiaphragm activity contralateral to hemisection (Ghali and Marchenko, 2015; Lee and Hsu, 2017). In seeking to uncover molecular mechanisms underlying the observed plasticity, Zimmer and Goshgarian (2007b) quantified changes in neurotransmitter receptor subunit levels two days following a C2HSx in thein vitrobrainstem-spinal corden blocpreparation of P0/P1 neonatal rats. Medullary levels of glutamate decarboxylase (65 kDa isoform; GAD65) and NR2B (NMDA receptor subunits) were increased ipsilateral to HSx and of A1(adenosine receptor subunits) and neurokinin-1 (NK1) contralateral to HSx. Levels of NR1, phospho-NR2B/totalNR2B, GluR2, and NK1 were decreased ipsilateral to HSx and levels of phospho-NR1, phospho-NR1/totalNR1, phospho-NR2, phospho-NR2/totalNR2, and GluR2 were decreased contralateral to HSx.
Role of interneurons in respiratory plasticity following high cervical hemisection
It has been suggested that following injury, spinal circuitry undergoes such significant reorganization to the degree that its microanatomy and neurocytoarchitecture are fundamentally altered (Dimitrijevic et al., 1997). One of the mechanisms utilized in recovery of PhN output ipsilateral to a C2HSx, contusion, or analogous injury may involve the recruitment and incorporation of interneurons (Lane et al., 2009b; Sandhu et al., 2009) as shown in other motor systems (Jankowska, 2001; Bareyre et al., 2004; Courtine et al., 2008, 2009).
In contrast to spinal-intact animals, where pseudorabies virus transynaptic retrograde tracing fails to label the C1–2respiratory-related interneuron pool, following C2HSx, retrograde tracing of the diaphragm contralateral to injury demonstrates extensive bilateral labeling of pre-phrenic interneurons at the C1–2spinal levels (Lane et al., 2009a). Recruitment of these cells subsequent to hemisection and their coaptation by the phrenic motor network may be a consequence of development of polysynaptic projections to the phrenic nucleus. Whether these units are one and the same as the C1–2respiratory-related units previously described and shown to receive supraspinal input (Lipski et al., 1993), as described in the rat (Lipski et al., 1994) and cat (Hoskin and Duf fin, 1987a, b; Mateika and Duf fin, 1989), remains to be determined.
Diaphragmatic changes following cervical SCI
Plasticity of the neural respiratory network following SCI involves not only neural elements subserving respiration but also effector muscles. Thus, therapeutic interventions aimed at improving respiratory functionvianeurorehabilitation should take into consideration concomitant changes occurring in the target muscle over time, which may lead to changes in the recruitment order and firing patterns of motoneurons and motor units (Henneman et al., 1965a, b; Webber and Pleschka, 1976; Marchenko et al., 2012).
Following C2HSx, the diaphragm atrophies (Gill et al., 2014). Neuromuscular junction (NMJ) microanatomy (planar area, nerve terminal and motor end plate length) of myosin heavy chain (MHC)slowand MHC2Afibers does not change significantly, while an increase in planar area occurs at MHC2Xand MHC2BNMJs, perhaps as an adaptation to enhance synaptic efficiency (Prakash et al., 1999). In fact, neuromuscular transmission failure in an isolated phrenic nerve-diaphragm preparation taken from C2-hemisected animals two weeks following injury was less than that observed in spinal-intact animals, possibly reflecting increased synaptic overlap at MHCfast(2A, 2X, 2B) fibers (Prakash et al., 1999).
Respiratory and locomotor recovery: similarities and differences
Differences between the pre-existing pathways subserving respiration and locomotion are reflected in differential mechanisms underlying recovery following injury (Majczyński and Sławińska, 2007; Ghali and Marchenko, 2015; Ghali, 2017). For example, the corticospinal tract relays to lumbosacral MNs polysynapticallyviainterposed pre-motor propriospinal interneurons (Courtine et al., 2008). As a result, locomotor recovery is characterized mainly by the outgrowth of collaterals (Fouad et al., 2001; Weidner et al., 2001) and specific interruption of the dorsal corticospinal tract will result in sprouting of collaterals from the ventromedial corticospinal tract (Weidner et al., 2001; Steward et al., 2008). In contrast, as the bulbospinal respiratory network, especially the bulbophrenic network, is characterized by direct monosynaptic bulbospinal projections (Dobbins and Feldman, 1994; Kastner and Gauthier, 2008; Vinit and Kastner, 2009), a potential substrate for recovery is the recruitment and incorporation of extra-motor propriospinal interneurons into descending pathways for use as relays and/or amplifiers (Vinit and Kastner, 2009). However, the few pre-phrenic interneurons (Lipski et al., 1993, 1994; Tian and Duf fin, 1996a, b; Lu et al., 2004; Lane et al., 2008) that do exist, and which possibly function in a minor polysynaptic pathway, remain unchanged in number 2 weeks aer C2HSx (Lane et al., 2008), arguing against this as a primary mechanism facilitating long-term respiratory recovery.
Regenerative and Non-Regenerativeerapeutic Approaches
Overview
Respiratory muscle training regimens and phrenic nerve pacing are useful therapeutic modalities (Winslow and Rozovsky, 2003), but they are limited in the degree to which they can achieve lasting improvement of intrinsic function. While many studies in animal models of respiratory dysfunction following SCI have emphasized basic physiology and underlying neurochemical mechanisms, a few studies have looked at the applicability and utility of regenerative neurobiology, which may be combined with traditional treatments, to achieve functional recovery of the respiratory system. Recently, techniques in regenerative neurobiology, including implantation of olfactory ensheathing cells across an SCI site, nerve gras, as well as intraspinal microstimulation, have been applied toward enhancing respiratory recovery in spinal cord-injured animals and offer promising potential for patients suffering ventilator-dependency following spinal trauma.
Nerve grafts provide the central nervous system circuits with the microenvironmental milieu of the peripheral nervous system to encourage regrowth of axons and regeneration (Houle, 1991). Gauthier et al. (2002) implanted the proximal end of a nerve graft in the ventrolateral medulla (control graimplanted in dorsolateral medulla) 3 months before performing a HSx at C3followed by implantation of the distal nerve grain C4in phrenic nucleus. PhN activity was elicited by stimulation of grafts implanted in the medulla ventrolaterally, but not those implanted dorsolaterally, suggesting a specific innervation of the phrenic nucleus by inspiratory pre-motor neurons.e use of medullo-phreninuclear bridges to achieve respiratory recovery is a promising approach in patients with extensive SCI significantly compromising descending respiratory drive.
Olfactory ensheathing cells
Olfactory ensheathing cells (OECs) have attracted interest on account of their propensity to encourage regeneration inthe central nervous system (Li et al., 1998). Following an upper cervical HSx (approach through atlanto-occipital membrane without laminectomy), animals were transplanted with OECs and neural respiratory recovery compared to controls (Li et al., 2003). Terminal experiments were performed 2 months later on rats under paralyzed artificially-ventilated, gallamine-paralyzed, and anesthetized (unspecified drug) conditions. Ipsilateral to HSx, spontaneous PhN activity in control animals occurred only if there was sparing of the ventral funiculus and no PhN recovery was observed during asphyxia in rats with a histologically-confirmed complete HSx. In 19 of 24 treated animals, PhN activity recovered under spontaneous breathing conditions. Curiously, this recovery only persisted in 17 animals following paralysis with gallamine and asphyxia, suggesting the unspecified anesthetic type and depth confounded the results.e authors found neural repair of descending tracts in the ventral funiculus correlated with PhN recovery in treated animals; whether this represents regeneration of axotomized fibers (Li et al., 1997, 1998; Ramón-Cueto et al., 1998) orde novoaxonogenesis (allmair et al., 1998).
Polentes et al. (2004) performed a similar experiment to Li and colleagues (1998), wherein OECs were transplanted into the lesion site in C2-hemisected rats, with a recovery period was 3–6 (rather than 2) months. Subsequently, PhN and diaphragm activity ipsilateral to C2HSx were 80.7 and 73% of the side contralateral to injury, respectively, in terminal experiments conducted under spontaneously-breathing pentobarbital-anesthetized conditions. Subsequently, a C1HSx performed contralateral to initial C2HSx eliminated, reduced, or did not change recovery in the phrenic neurogram, suggesting variable contributions of regenerative processes on the ipsilateral side, crossed pathways, and possible effects of spinal shock. Subsequent mid-sagittal section of the cervical spinal cord (C1–6) did not change PhN activity ipsilateral to HSx, arguing against the role of crossed and re-crossed pathways. Moreover, stimulation ipsilateral and rostral to the original C2HSx elicited PhN responses before, and weaker responses aer, contralateral C1HSx in transplanted (6/8), but not in control, animals.ese findings suggest that therapeutic intervention may shiregenerative processes from natural mechanisms of recovery toward pathways that would otherwise be unused.
Evidence for the utility of other neural stem cells in promoting similar recovery derives from the work of Sandhu et al. (2017). Neural progenitor cells derived from the subventricular zone transplanted intraspinally below the C2lesion site in adult rats enhanced phrenic recovery. This recovery correlated with the proportion of surviving transplanted cells. They localized principally to the white matter and a subset of them was found to have differentiated into glia.
Intraspinal microstimulation
Use of intraspinal microstimulation has been extensively investigated in the context of locomotor recovery following spinal cord injury, but no such study had explored the utility of this intervention in respiratory recovery following SCI until recently. Mercier et al. (2017) found that genioglossus-triggered intraspinal microstimulation at the ventral aspect of spinal segment C4in rats effectively enhanced phrenic recovery acutely and subacutely following a rostrally-related C2hemisection of the cord.ese data suggest this approach to be promising in promoting respiratory recovery following SCI.
Conclusion and Perspectives
The present-day conceptual model underlying crossed phrenic activity still remains nebulous.e crossed phrenic phenomenon currently is understood as a latent pathway that can be recruited under conditions of moderate to severe respiratory stress. However, one may propose the more general conclusion that CPP is “state-dependent.” Redefining crossed phrenic activity in this manner, then, suggests that it is not necessarily the loss of ipsilateralphasiccontrol from the rostral ventral respiratory group which fully accounts for silence in PhN activity (and hemidiaphragmatic paresis) ipsilateral to C2HSx, that is unrecoverable by the contralateral projection acutely, but rather, the loss of descendingtonicexcitatory support of the membrane potential.is would be the case if 1) tonic drive is primarily ipsilateral and/or 2) contralateral tonic drive is polysynaptic, whereas ipsilateral tonic drive is monosynaptic, which would make the former more state-dependently sensitive to suppression by anesthesia, hypocapnia, or lung stretch receptors in experimental investigations. In this regard, it becomes critical to note that many studies investigating the C2-hemisected rat model of acute SCI do so under conditions of anesthesia, which may confound results by diminishing the already reduced tonic drive to the ipsilateral phrenic nucleus, leading to inaccurate inferences about the native configuration of the system.
Current management of SCI involves standard resuscitation, intensive care, and/or surgical measures and thus, has remained rudimentary at best, failing to parallel the tremendous leaps made in the fields of regenerative neurobiology and network neuroscience.e study of CPP following high cervical spinal cord hemisection in the rat has significantly furthered our understanding of the respiratory network’s capacity to adapt following injury. It has also served as an impetus for the development of novel therapeutic approaches aimed at recovering respiratory function in patients sustaining SCI, including neuropharmacological agonists and antagonists targeting serotonin and/or adenosine receptors. Additional strategies which appear promising include use of brief periods of mild hypoxia to induce long-term facilitation in respiratory networks and dorsal rhizotomy to disinhibit crossed phrenic activity. Regenerative approaches have also been used successfully in experimental models to enhance respiratory recovery following SCI, including nerve grafts to promote axonal regeneration (into PhN or from the medulla into the phrenic nucleus), implantation of OECs at the injury site to encourage axonal growth, as well as intraspinal microstimulation.ese strategies require extensive further investigation before they can become candidate for human clinical trials, but nevertheless hold tremendous promise as therapeutic options for respiratory neurorehabilitation following SCI.
Author contributions:
Conflicts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Additional file:
Additional Table 1:Crossed phrenic phenomenon in rats as assessed by diaphragm electromyography.
Additional Table 2:Crossed phrenic phenomenon in rats as assessed by phrenic electroneurography.
Alilain WJ, Goshgarian HG (2008) Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol 212:348-357.
Allan DW, Greer JJ (1997) Development of phrenic motoneuron morphology in the fetal rat. J Comp Neurol 382:469- 479.
Arborelius M, Lilja B, Senyk J (1975) Regional and total lung function in patients with hemidiaphragmatic paralysis. Respiration 32:253-264.
Awad BI, Warren PM, Steinmetz MP, Alilain WJ (2013)e role of the crossed phrenic pathway aer cervical contusion injury and a new model to evaluate therapeutic interventions. Exp Neurol 248:398-405.
Bareyre FM (2007) Neuronal repair and replacement in spinal cord injury. J Neurol Sci 265:63-72.
Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME (2004)e injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7:269-277.
Barron KD, Marciano FF, Amundson R, Mankes R (1990) Perineuronal glial responses aer axotomy of central and peripheral axons: A comparison. Brain Res 523:219-229.
Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG (2005) Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest 127:658-661.
Basura GJ, Nantwi KD, Goshgarian HG (2002)eophylline-induced respiratory recovery following cervical spinal cord hemisection is augmented by serotonin 2 receptor stimulation. Brain Res 956:1-13.
Baussart B, Stamegna JC, Polentes J, Tadié M, Gauthier P (2006) A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis 22:562-574.
Bert P (1870) Leçons sur la respiration. Paris: Kessinger Publishing.
Beth Zimmer M, Grant JS, Ayar AE, Goshgarian HG (2015) Ipsilateral inspiratory intercostal muscle activity aer C2 spinal cord hemisection in rats. J Spinal Cord Med 38:224-230.
Bittigau P, Ikonomidou C (1997) Glutamate in neurologic diseases. J Child Neurol 12:471-485.
Boulenguez P, Gauthier P, Kastner A (2007) Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motor neurons aer C2 hemisection. Brain Res 1148:96-104.
Brown AG (1971) Effects of descending impulses on transmission through the spinocervical tract. J Physiol 219:103-125
Brown-Séquard CE (1860) Course of lectures on the physiology and pathology of the central nervous system: delivered at the Royal College of Surgeons of England in May 1858. Philadelphia: University of California Libraries.
Brown-Séquard CE (1869) De l’influence de la section du cordon latéral de la moelle sur la respiration. Comptes rendus de la société de biologie. Paris I:64-65. Also Archives de physiologie, II:299-300.
Butler JE, McKenzie DK, Gandevia SC (2003) Reflex inhibition of human inspiratory muscles in response to contralateral phrenic nerve stimulation. Respir Physiol Neurobiol 138:87-96.
Buttry JL, Goshgarian HG (2014) Injection of WGA-Alexa 488 into the ipsilateral hemidiaphragm of acutely and chronically C2 hemisected rats reveals activity-dependent synaptic plasticity in the respiratory motor pathways. Exp Neurol 261:440-450.
Capková L, Dávidová A, Pavel J, Kucharíková A, Radoňak J, Kuchárová K, Cigánková V, Maršala M, Lukáčová N (2011) Identification of NO/ sGC signalling in the bulbospinal respiratory pathway after C2-C3 hemisection of the spinal cord in rats. Acta Histochem 113:749-755.
Castro-Moure F, Goshgarian HG (1996) Reversible cervical hemispinalization of the rat spinal cord by a cooling device. Exp Neurol 141:102-12.
Castro-Moure F, Goshgarian HG (1997) Morphological plasticity induced in the phrenic nucleus following cervical cold block of descending respiratory drive. Exp Neurol 147:299-310.
Chan WM, Mohammed Y, Lee I, Pearse DD (2013) Effect of gender on recovery aer spinal cord injury. Transl Stroke Res 4:447-461.
Choi H, Liao WL, Newton KM, Onario RC, King AM, Desilets FC, Woodard EJ, Eichler ME, Frontera WR, Sabharwal S, Teng YD (2005) Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci 25:4550-4559.
Clague HW, Hall DR (1979) Effect of posture on lung volume: airway closure and gas exchange in hemidiaphragmatic paralysis. Thorax 34:523-526.
Cleland CL, Getting PA (1993) Respiratory-modulated and phrenic afferent-driven neurons in the cervical spinal cord (C4-C6) of the fluorocarbon-perfused guinea pig. Exp Brain Res 93:307-311.
Corda M, Von Euler C, Lennerstrand G (1965) Proprioceptive innervation of the diaphragm. J Physiol 178:161-177.
Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV (2008) Recovery of supraspinal control of stepping via indirect propriospinal relay connections aer spinal cord injury. Nat Med 14:69-74.
Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR (2009) Transformation of nonfunctional spinal circuits into functional states aer the loss of brain input. Nat Neurosci 12:1333-1342.
Culberson JL, Haines DE, Kimmel DL, Brown PB (1979) Contralateral projection of primary afferent fibers to mammalian spinal cord. Exp Neurol 64:83-97.
DiMarco AF (2005) Restoration of respiratory muscle function following spinal cord injury; Review of electrical and magnetic stimulation techniques. Resp Physiol Neurobiol 147:273-287.
Dimitrijevic MR, McKay WB, Sherwood AM (1997) Motor control physiology below spinal cord injury: residual volitional control of motor units in paretic and paralyzed muscles. Adv Neurol 72:335-345.
Dobbins EG, Feldman JL (1994) Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347:64-86.
Doperalski NJ, Fuller DD (2006) Long-term facilitation of ipsilateral but not contralateral phrenic output aer cervical spinal cord hemisection. Exp Neurol 200:74-81.
Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD (2008) Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir Physiol Neurobiol 162:160-167.
Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD (2012a) Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol 112:96-105.
Dougherty BJ, Lee KZ, Gonzalez-Rothi EJ, Lane MA, Reier PJ, Fuller DD (2012b) Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respir Physiol Neurobiol 183:186-192.
Easton PA, Fleetham JA, de la Rocha A, Anthonisen NR (1983) Respiratory function aer paralysis of the right hemidiaphragm. Am Rev Respir Dis 127:125-128.
El Bohy AA, Goshgarian HG (1999) The use of single phrenic axon recordings to assess diaphragm recovery after cervical spinal cord injury. Exp Neurol 156:172-179.
El Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG (1998) Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol 150:143-152.
Estenne M, De Troyer A (1987) Mechanism of postural dependence of vital capacity in tetraplegic subjects. Am Rev Respir Dis 135:367-371.
Fouad K, Pedersen V, Schwab ME, Brösamle C (2001) Cervical sprouting of corticospinal fibers aer thoracic spinal cord injury accompanies shis in evoked motor responses. Curr Biol 11:1766-1770.
Fournier M, Sieck GC (1988) Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59:1055-1066.
Franchini KG, Krieger EM (1993) Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst 42:63-69.
Fuller DD, Johnson SM, Johnson RA, Mitchell GS (2002) Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett 323:25-28.
Fuller DD, Johnson SM, Olson EB Jr, Mitchell GS (2003) Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23:2993-3000.
Fuller DD, Golder FJ, Olson EB Jr, Mitchell GS (2006) Recovery of phrenic activity and ventilation aer cervical spinal hemisection in rats. J Appl Physiol 100:800-806.
Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ (2008) Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211:97-106.
Gauthier P, Réga P, Lammari-Barreault N, Polentes J (2002) Functional reconnections established by central respiratory neurons regenerating axons into a nerve grabridging the respiratory centers to the cervical spinal cord. J Neurosci Res 70:65-81.
Gauthier P, Baussart B, Stamegna JC, Tadié M, Vinit S (2006) Diaphragm recovery by laryngeal innervation after bilateral phrenicotomy or complete C2 spinal section in rats. Neurobiol Dis 24:53-66.
Geiger PC, Cody MJ, Macken RL, Sieck GC (2000) Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol 89:695-703.
Ghali MG (2015) Vagal modulation of pre-inspiratory activity in hypoglossal discharge in the decerebrate rat. Respir Physiol Neurobiol 215:47-50.
Ghali MG, Marchenko V (2015) Dynamic changes in phrenic motor output following high cervical hemisection in the decerebrate rat. Exp Neurol 271:379-389.
Ghali MG, Marchenko V (2016a) Patterns of phrenic nerve discharge following complete high cervical spinal cord injury in the decerebrate rat. J Neurotrauma 33:1115-1127.
Ghali MG, Marchenko V (2016b) Effects of vagotomy on hypoglossal and phrenic responses to hypercapnia in the decerebrate rat. Resp Physiol Neurobiol 232:13-21.
Gibson GJ (1989) Diaphragmatic paresis: pathophysiology, clinical features, and investigation.orax 44:960-970.
Gill LC, Gransee HM, Sieck GC, Mantilla CB (2016) Functional recovery aer cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons. Respir Physiol Neurobiol 226:128-36.
Gill LC, Ross HH, Lee KZ, Gonzalez-Rothi EJ, Dougherty BJ, Judge AR, Fuller DD (2014) Rapid diaphragm atrophy following cervical spinal cord hemisection. Respir Physiol Neurobiol 192:66-73.
Gill PK, Kuno M (1963) Excitatory and inhibitory actions on phrenic motoneurons J Physiol 168:274-289.
Giulian D, Li J, Li X, George J, Rutecki PA (1994)e impact of microglia-derived cytokines upon gliosis in the CNS. Dev Neurosci 16:128-136.
Giulian D, Baker TJ (1985) Peptides released by ameboid microglia regulate astroglial proliferation. J Cell Biol 101:2411-2415.
Golder FJ, Reier PJ, Bolser DC (2001a) Altered respiratory motor drive aer spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci 21:8680-8689.
Golder FJ, Reier PJ, Davenport PW, Bolser DC (2001b) Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol 91:2451-2458.
Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC (2003) Respiratory motor recovery aer unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23:2494-2501.
Golder FJ, Mitchell GS (2005) Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function aer chronic cervical spinal cord injury. J Neurosci 25:2925-2932.
Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS (2011) Breathing patterns aer mid-cervical spinal contusion in rats. Exp Neurol 231:97-103.
Goshgarian HG (1979) Developmental plasticity in the respiratory pathway of the adult rat. Exp Neurol 66:547-555.
Goshgarian HG, Rafols JA (1984)e ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol 13:85-109.
Goshgarian HG, Moran MF, Prcevski P (1986) Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH and respiratory rate in the adult rat. Exp Neurol 93:440-445.
Goshgarian HG, Yu XJ, Rafols JA (1989) Neuronal and glial changes in the rat phrenic nucleus occurring within hours aer spinal cord injury. J Comp Neurol 284:519-533.
Goshgarian HG, Guthl(1977) Demonstration of functionally ineffective synapses in the guinea pig spinal cord. Exp Neurol 57:613-621.
Goshgarian HG, Ellenburger HH, Feldman JL (1991) Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol 111:135-139.
Gould DJ, Goshgarian HG (1997) Glial changes in the phrenic nucleus following superimposed cervical spinal cord hemisection and peripheral chronic phrenicotomy injuries in adult rats. Exp Neurol 148:1-9.
Gould DJ, Goshgarian HG (1999)e effects of mitotic inhibition on the spinal cord response to the superimposed injuries of spinal cord hemisection and peripheral axotomy. Exp Neurol 158:394-402.
Gransee HM, Zhan WZ, Sieck GC, Mantilla CB (2013) Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity aer cervical spinal hemisection. PLoS One 8:e64755.
Gransee HM, Gonzalez Porras MA, Zhan WZ, Sieck GC, Mantilla CB (2017) Motoneuron glutamatergic receptor expression following recovery from cervical spinal hemisection. J Comp Neurol 525:1192-1205.
Gray PA, Rekling JC, Bocchiaro CM, Feldman JL (1999) Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286:1566-1568.
Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL (2001) Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4:927-930.
Greenwald BD, Seel RT, Cifu DX, Shah AN (2001) Gender-related differences in acute rehabilitation lengths of stay, charges, and functional outcomes for a matched sample with spinal cord injury: a multicenter investigation. Arch Phys Med Rehabil 82:1181-1187.
Guthl(1976) Functional plasticity in the respiratory pathway of the mammalian spinal cord. Exp Neurol 51:414-420.
Hadley SD, Walker PD, Goshgarian HG (1999a) Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol 160:433-445.
Hadley SD, Walker PD, Goshgarian HG (1999b) Effects of the serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4 h following C2 spinal cord hemisection. Exp Neurol 160:479-488.
Hains BC, Everhart AW, Fullwood SD, Hulsebosch CE (2002) Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Exp Neurol 175:347-362.
Hains BC, Willis WD, Hulsebosch CE (2003a) Serotonin receptors 5HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp Brain Res 49:174-186.
Hains BC, Willis WD, Hulsebosch CE (2003b) Temporal plasticity of dorsal horn somatosensory neurons after acute and chronic spinal cord hemisection in rat. Brain Res 970:238-241.
Handwerker HO, Iggo A, Zimmermann M (1975) Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain 1:147-165.
Henneman E, Somjen G, Carpenter DO (1965a) Excitability and inhibitibility of motoneurones of different sizes. J Neurophysiol 28: 599-620.
Henneman E, Somjen G, Carpenter DO (1965b) Functional significance of cell size in spinal motoneurones. J Neurophysiol 28:560-580.
Hernandez-Torres V, Gransee HM, Mantilla CB, Wang Y, Zhan WZ, Sieck GC (2017) BDNF effects on functional recovery across motor behaviors aer cervical spinal cord injury. J Neurophysiol 117:537-544.
Hilaire G, Monteau R, Errchidi S (1989) Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res 485:325-332.
Hoskin R, Duffin J (1987a) Excitation of upper cervical inspiratory neurons by inspiratory neurons of the nucleus tractus solitarius in the cat. Exp Neurol 95:126-141.
Hoskin RW, Duf fin J (1987b) Excitation of upper cervical inspiratory neurons by inspiratory neurons of the nucleus retroambigualis in the cat. Exp Neurol 98:404-417.
Houle JD (1991) Demonstration of the potential for chronically injured neurons to regenerate axons into intraspinal peripheral nerve gras. Exp Neurol 113:1-9.
Houle JD, Schramm P, Herdegen T (1998) Trophic factors modulation of c-Jun expression in supraspinal neurons aer chronic spinal cord injury. Exp Neurol 154:602-611.
Hoy KC Jr, Alilain WJ (2015) Acute theophylline exposure modulates breathing activity through a cervical contusion. Exp Neurol 271:72-76.
Hsu SH, Lee KZ (2015) Effects of serotonergic agents on respiratory recovery aer cervical spinal injury. J Appl Physiol 119:1075-1087.
Huang Y, Goshgarian HG (2009a) Postnatal conversion of cross phrenic activity from an active to latent state. Exp Neurol 219:66-73.
Huang Y, Goshgarian HG (2009b)e potential role of phrenic nucleus glutamate receptor subunits in mediating spontaneous crossed phrenic activity in neonatal rat. Int J Dev Neurosci 27:477-483.
Huang Y, Goshgarian HG (2009c) Identification of the neural pathway underlying spontaneous crossed phrenic activity in neonatal rats. Neuroscience 163:1109-1118.
Iscoe S, Duf fin J (1996) Effects of stimulation of phrenic afferents on cervical respiratory interneurons and phrenic motoneurons in cats. J Physiol 497:803-812.
Jankowska E (2001) Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol 533:31-40.
Jenkins R, TetzlaffW, Hunt SP (1993) Differential expression of immediate early genes in rubrospinal neurons following axotomy in rat. Eur J Neurosci 5:203-209.
Kajana S, Goshgarian HG (2008a) Spinal activation of the cAMP-PKA pathway induces respiratory motor recovery following high cervical spinal cord injury. Brain Res 1232:206-213.
Kajana S, Goshgarian HG (2008b) Administration of phosphodiesterase inhibitors and an adenosine A1 receptor antagonist induces phrenic nerve recovery in high cervical spinal cord injured rats. Exp Neurol 210:671-680.
Kajana S, Goshgarian HG (2009) Systemic administration of rolipram increases medullary and spinal cAMP and activates a latent respiratory motor pathway aer high cervical spinal cord injury. J Spinal Cord Med 32:175-182.
Kastner A, Gauthier P (2008) Are rodents an appropriate pre-clinical model for treating spinal cord injury? Examples from the respiratory system. Exp Neurol 213:249-256.
Kerr FW (1961) Structural relation of the trigeminal spinal tract to upper cervical roots and the solitary nucleus in the cat. Exp Neurol 4:134-148.
Knoll P (1885) Beiträge zur Lehre von der Athmungsinnervation. Zur Lehre vom Einfluss des centralen Nervensystems auf die Athmung. Sitzungsberichte d. math.-naturwiss. Classe d. kais. Akad. d. Wissenschaen. Wien, XCII. 3 Abth., 328-344.
Knoll P (1888) Beiträge zur Lehre von der Athmungsinnervation. Ueber die Lage des Athemcentrums. Sitzungsberichte d. math.-naturwiss. Classe d. kais. Akad. d. Wissenschaen. Wien, XCVII. 3 Abth., 163-180.
Kong FJ, Berger AJ (1986) Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl Physiol 61:1999-2004.
Kusurkar RA (2004) Sir Charles Sherrington (1857 - 1952). J Postgrad Med 50:238-239.
Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ (2008) Cervical prephrenic interneurons in the normal and lesioned spinal cord of adult rat. J Comp Neurol 511:692-709.
Lane MA, Jones AL, O’Steen BE, Hunsaker FL, Vavrousek J, Salazar K, Fuller DD, Reier PJ (2009a) Pre-phrenic interneurons as an anatomical substrate for plasticity following cervical spinal cord injury (SCI) in the adult rat. FASEB J 23:5.
Lane MA, Lee KZ, Fuller DD, Reier PJ (2009b) Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 169:123-132.
Lane MA, Lee KZ, Salazar K, O’Steen BE, Bloom DC, Fuller DD, Reier PJ (2012) Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol 235:197-210.
LangendorffO (1880) Ueber die spinalen Centren der Athmung. Archiv für Physiologie 1880:518-549.
Lee KZ (2016) Phrenic motor outputs in response to bronchopulmonary C-fibre activation following chronic cervical spinal cord injury. J Physiol 594:6009-6024.
Lee KZ, Chang YS (2014) Recovery of the pulmonary chemoreflex and functional role of bronchopulmonary C-fibers following chronic cervical spinal cord injury. J Appl Physiol 117:1188-1198.
Lee KZ, Huang YJ, Tsai IL (2014) Respiratory motor outputs following unilateral midcervical spinal cord injury in the adult rat. J Appl Physiol 116:395-405.
Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD (2010) Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats. J Appl Physiol 109:377-387.
Lee KZ (2016) Phrenic motor outputs in response to bronchopulmonary C-fibre activation following chronic cervical spinal cord injury. J Physiol 594:6009-6024.
Lee KZ, Dougherty BJ, Sandhu MS, Lane MA, Reier PJ, Fuller DD (2013) Phrenic motoneuron discharge patterns following chronic cervical spinal cord injury. Exp Neurol 249:20-32.
Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD (2015) Hypoxia triggers short term potentiation of phrenic motoneuron discharge aer chronic cervical spinal cord injury. Exp Neurol 63:314-324.
Lee KZ, Hsu SH (2017) Compensatory function of the diaphragm following high cervical hemisection in the rat. J Neurotrauma doi:10.1089/neu.2016.4943.
Lewis LJ, Brookhart JM (1951) Significance of the crossed phrenic phenomenon. Am J Physiol 166:241-254.
Li Y, Field PM, Raisman G (1997) Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277:2000-2002.
Li Y, Field PM, Raisman G (1998) Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci 18:10514-10524.
Li Y, Decherchi P, Raisman G (2003) Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci 23:727-731.
Ling L, Bach KB, Mitchell GS (1994) Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res 101:35-43.
Liou WW, Goshgarian HG (1994) Quantitative assessment of the effect of chronic phrenicotomy on the induction of the crossed phrenic phenomenon. Exp Neurol 127:145-153.
Liou WW, Goshgarian HG (1997)e superimposed effects of chronic phrenicotomy and cervical spinal cord hemisection on synaptic cytoarchitecture in the rat phrenic nucleus. Exp Neurol 145:258-267.
Lipski J, Duf fin J, Kruszewska B, Zhang X (1993) Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res 95:477-487.
Lipski JX, Zhang B, Kruszewska B, Kanjhan R (1994) Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640:171-184.
Liu D, Xu GY, Pan E, McAdoo DJ (1999) Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience 93:1383-1389.
Loveridge BM, Dubo HI (1990) Breathing pattern in chronic quadriplegia. Arch Phys Med Rehabil 71:495-499.
Loveridge B, Sanii R, Dubo HI (1992) Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia 30:479-488.
Lu F, Qin C, Foreman RD, Farber JP (2004) Chemical activation of C1-C2 spinal neurons modulates intercostals and phrenic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 286:R1069-1076.
Macleod IB (1974) The effects of vagal section. J R Coll Surg Edinb 19:257-262.
Majczyński H, Sławińska U (2007) Locomotor recovery aer thoracic spinal cord lesions in cats, rats and humans. Acta Neurobiol Exp 67:235-257.
Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC (2007) Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal inactivity. Neuroscience 146:178-189.
Mantilla CB, Gransee HM, Zhan WZ, Sieck GC (2013) Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol 247:101-109.
Mantilla CB, Greising SM, Stowe JM, Zhan WZ, Sieck GC (2014) TrkB kinase activity is critical for recovery of respiratory function after cervical spinal cord hemisection. Exp Neurol 261:190-195.
Marchenko V, Ghali MG, Rogers RF (2015)e role of spinal gabaergic circuits in the control of phrenic nerve motor output. Am J Physiol 308:R916-926.
Marchenko V, Ghali MG, Rogers RF (2012) Motoneuron firing patterns underlying fast oscillations in phrenic nerve discharge in the rat. J Neurophysiol 108:2134-2143.
Martínez-Gálvez G, Zambrano JM, Diaz Soto JC, Zhan WZ, Gransee HM, Sieck GC, Mantilla CB (2016) TrkB gene therapy by adeno-associated virus enhances recovery after cervical spinal cord injury. Exp Neurol 276:31-40.
Mason MR, Lieberman AR, Anderson PN (2003) Corticospinal neurons upregulate a range of growth associated genes following intracortical, but not spinal, axotomy. Eur J Neurosci 18:789-802.
Mateika JH, Duf fin J (1989)e connections from botzinger expiratory neurons to upper cervical inspiratory neurons in the cat. Exp Neurol 104:138-146.
Mercier LM, Gonzalez-Rothi EJ, Streeter KA, Posgai SS, Poirier AS, Fuller DD, Reier PJ, Baekey DM (2017) Intraspinal microstimulation and diaphragm activation aer cervical spinal cord injury. J Neurophysiol 117:767-776.
Minic Z, Wilson S, Liu F, Sankari A, Mao G, Goshgarian H (2017) Nanoconjugate-bound adenosine A1 receptor antagonist enhances recovery of breathing following acute cervical spinal cord injury. Exp Neurol 292:56-62.
Minor KH, Akison LK, Goshgarian HG, Seeds NW (2006) Spinal cord injury-induced plasticity in the mouse — the crossed phrenic phenomenon. Exp Neurol 200:486-495.
Miyata H, Zhan WZ, Prakash YS, Sieck GC (1995) Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol 79:1640-1649.
Moreno DE, Yu XJ, Goshgarian HG (1992) Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol 116:219-228.
Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG (1999) Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Rep 13:225-234.
Nantwi KD, El-Bohy AA, Goshgarian HG (1996) Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol 140:53-59.
Nantwi KD, Goshgarian HG (1998a) Effects of chronic systemic theophylline injections on recovery of hemidiaphragmatic function aer cervical spinal cord injury in adult rats. Brain Res 789:126-129.
Nantwi KD, Goshgarian HG (1998b)eophylline-induced recovery in a hemidiaphragm paralyzed by hemisection in rats. Contribution of adenosine receptors. Neuropharmacology 37:113-121.
Nantwi KD, Goshgarian HG (2001) Alkylxanthine-induced recovery of respiratory function following cervical spinal cord injury in adult rats. Exp Neurol 168:123-134.
Nantwi KD, Goshgarian HG (2002) Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol 29:915-923.
Nantwi KD, Basura GJ, Goshgarian HG (2003a) Effects of long-term theophylline exposure on recovery of respiratory function and expression of adenosine A1 mRNA in cervical spinal cord hemisected adult rats. Exp Neurol 182:232-239.
Nantwi KD, Basura GJ, Goshgarian HG (2003b) Adenosine A1 receptor mRNA expression and the effects of systemic theophylline administration on respiratory function 4 months after C2 hemisection. J Spinal Cord Med 26:364-371.
Nattie E, Li A (2006) Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol 101:1596-1606.
Navarrete-Opazo AA, Vinit S, Mitchell GS (2014) Adenosine 2A receptor inhibition enhances intermittent hypoxia-induced diaphragm but not intercostal long-term facilitation. J Neurotrauma 31:1975-1984.
Newsom Davis J, Goldman M, Loh L, Casson M (1976) Diaphragm function and alveolar hypoventilation. Q J Med 45: 87-100.
O’Hara TE Jr, Goshgarian HG (1991) Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals aer spinal cord injury. Exp Neurol 111:244-250.
Oo T, Watt JW, Soni BM, Sett PK (1999) Delayed diaphragm recovery in 12 patients aer high cervical spinal cord injury. A retrospective review of the diaphragm status of 107 patients ventilated aer acute spinal cord injury. Spinal Cord 37:117-122.
Polentes J, Stamegna JC, Nieto-Sampedro M, Gauthier P (2004) Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells. Neurobiol Dis 16:638-653.
Prakash YS, Miyata H, Zhan WZ, Sieck GC (1999) Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve 22:307-319.
Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC (2000) Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol 89:563-572.
Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, Lipp HP, Aguzzi A, Wagner EF, Behrens A (2004)e AP-1 transcription factor c-Jun is required for ef ficient axonal regeneration. Neuron 43:57-67.
Ramón-Cueto A, Plant GW, Avila J, Bunge MB (1998) Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci 18:3803-3815.
Ridyard JA, Stewart RM (1976) Regional lung function in unilateral diaphragmatic paralysis.orax 31:438-442.
Rosenblueth A, Ortiz T (1936)e crossed respiratory impulses to the phrenic. Am J Physiol 117:495-513.
Rowley KL, Mantilla CB, Sieck GC (2005) Respiratory muscle plasticity. Resp Physiol Neurobiol 147:235-251.
Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD (2009) Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol 169:94-101.
Sandhu MS, Ross HH, Lee KZ, Ormerod BK, Reier PJ, Fuller DD (2017) Intraspinal transplantation of subventricular zone-derived neural progenitor cells improves phrenic motor output aer high cervical spinal cord injury. Exp Neurol 287:205-215.
Sapru HN, Krieger AJ (1979) Cardiovascular and respiratory effects of some anesthetics in the decerebrate rat. Eur J Pharmacol 53:151-158.
Sieck GC, Fournier M, Enad JG (1989) Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett 97:29-34.
Sieck GC, Zhan WZ, Prakash YS, Daood MJ, Watchko JF (1995) SDH and actomyosin ATPase activities of different fiber types in rat diaphragm muscle. J Appl Physiol 79:1629-1639.
Sipski ML, Jackson AB, Gómez-Marín O, Estores I, Stein A (2004) Effects of gender on neurologic and functional recovery aer spinal cord injury. Arch Phys Med Rehabil 85:1826-1836.
Skagerberg G, Bjorklund A (1985) Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience 15:445-480.
Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254:726-729.
Song A, Tracey DJ, Ashwell KW (1999) Development of the rat phrenic nerve and the terminal distribution of phrenic afferents in the cervical cord. Anat Embryol 200:625-643.
Song A, Ashwell KW, Tracey DJ (2000) Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat Embryol 202:159-177.
Sperry MA, Goshgarian HG (1993) Ultrastructural changes in the rat phrenic nucleus developing within 2 h aer cervical spinal cord hemisection. Exp Neurol 120:233-244.
Sprague JM, Hongchien HA (1964)e terminal fields of dorsal root fibers in the lumbosacral spinal cord of the cat, and the dendritic organization of the motor nuclei. Prog Brain Res 11:120-154.
St-John WM, Paton JF (2004) Role of pontile mechanisms in the neurogenesis of eupnea. Respir Physiol Neurobiol 143:321-332.
Steward O, Zheng B, Tessier-Lavigne M, Hofstadter M, Sharp K, Yee KM (2008) Regenerative growth of corticospinal tract axons via the ventral column aer spinal cord injury in mice. J Neurosci 28:6836-6847.
Tian GF, Duffin J (1996a) Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 111:178-186.
Tian GF, Duffin J (1996b) Connections from upper cervical inspiratory neurons to phrenic and intercostals motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 110:196-204.
Titmus MJ, Faber DS (1990) Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol 35:1-55.
Tsai IL, Lee KZ (2014) Attenuation of the pulmonary chemoreflex following acute cervical spinal cord injury. J Appl Physiol 116:757-66.
Vinit S, Gauthier P, Stamegna JC, Kastner A (2006) High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma 23:1137-1146.
Vinit S, Stamegna JC, Boulenguez P, Gauthier P, Kastner A (2007) Restorative respiratory pathways aer partial cervical spinal cord injury: role of ipsilateral phrenic afferents. Eur J Neurosci 25:3551-3560.
Vinit S, Darlot F, Stamegna JC, Sanchez P, Gauthier P, Kastner A (2008) Long-term respiratory pathways reorganization aer partial cervical spinal cord injury. Eur J Neurosci 27:897-908.
Vinit S, Kastner A (2009) Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir Physiol Neurobiol 169:115-122.
Vinit S, Darlot F, Stamegna JC, Gauthier P, Kastner A (2009) Effect of cervical spinal cord hemisection on the expression of axon growth markers. Neurosci Lett 462:276-280.
Vulpian A (1866) Leçons sur la physiologie générale et comparée du système nerveux, faites en 1864 au Musée d’Histoire Naturelle. Paris: Gerner-Baillière.
Webber CL, Pleschka K (1976) Structural and functional characteristics of individual phrenic motoneurons. Pflügers Arch 364:113-121.
Weidner N, Ner A, Salimi N, Tuszynski MH (2001) Spontaneous corticospinal axonal plasticity and functional recovery aer adult central nervous system injury. Proc Natl Acad Sci U S A 98:3513-3518.
Wertheimer E (1886) Recherches expérimentales sur les centres respiratoires de la moelleépinière. J de l’anat et de la physiol 22:458-507.
Winslow C, Rozovsky J (2003) Effect of spinal cord injury on the respiratory system. Am J Phy Med Rehabil 82:803-814.
Yaksh TL, Wilson PR (1979) Spinal serotonin terminal system mediates antinociception. J Pharmacol Exper 208:446-453
Yu XJ, Goshgarian HG (1993) Aging enhances synaptic efficacy in a latent motor pathway following spinal cord hemisection in adult rats. Exp Neurol 121:231-238.
Zabka AG, Mitchell GS, Behan M (2006) Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol 576:903-912.
Zhan WZ, Miyata H, Prakash YS, Sieck GC (1997) Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol 82:1145-1153.
Zhou SY, Goshgarian HG (2000) 5-Hydroxytryptophan-induced respiratory recovery aer cervical spinal cord hemisection in rats. J Appl Physiol 89:1528-1536.
Zhou SY, Basura GJ, Goshgarian HG (2001) Serotonin(2) receptors mediate respiratory recovery aer cervical spinal cord hemisection in adult rats. J Appl Physiol 91:2665-2673.
Zimmer MB, Goshgarian HG (2005) Spontaneous crossed phrenic activity in the neonatal respiratory network. Exp Neurol 194:530-540.
Zimmer MB, Goshgarian HG (2006) Spinal activation of serotonin 1A receptors enhances latent respiratory activity aer spinal cord injury. J Spinal Cord Med 29:147-155.
Zimmer MB, Goshgarian HG (2007a) GABA, not glycine, mediates inhibition of latent respiratory motor pathways aer spinal cord injury. Exp Neurol 203:493-501.
Zimmer MB, Goshgarian HG (2007b) Spinal cord injury in neonates alters respiratory motor output via supraspinal mechanisms. Exp Neurol 206:137-145.
How to cite this article: Ghali MG (2017)e crossed phrenic phenomenon. Neural Regen Res 12(6):845-864.
*Correspondence to:
Michael George Zaki Ghali, Ph.D., M.S., mgg26@drexel.edu.
orcid:
0000-0001-7660-620X
(Michael George Zaki Ghali)
10.4103/1673-5374.208539
Accepted: 2017-05-15
杂志排行
中国神经再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease