卵清蛋白诱导的过敏小鼠模型中多不饱和脂肪酸代谢变化
2017-07-31武玉凤黎海芪
武玉凤 黎海芪
·论著·
卵清蛋白诱导的过敏小鼠模型中多不饱和脂肪酸代谢变化
武玉凤 黎海芪
目的 观察卵清蛋白(OVA)诱导的过敏小鼠模型中多不饱和脂肪酸(PUFA)代谢的变化。方法 以缺乏n-3 PUFA饲料饲养雌鼠的仔鼠(21日龄)24只,随机分为空白组、OVA组和PBS组,各8只。实验第1 d,空白组心脏采血处死。实验第1 和15 d,OVA组腹腔注射含50 μg OVA和1.3 mg氢氧化铝凝胶的PBS 0.2 mL,PBS组腹腔注射等量PBS。实验第29~39 d,OVA组以含50 mg OVA的PBS 0.3 mL隔日灌胃,共6次;PBS组予等量PBS灌胃。实验第39 d末次灌胃后,OVA组和PBS组均心脏采血处死,取空肠组织和脾组织。 OVA过敏小鼠模型成功建立的判断标准:观察灌胃后小鼠腹泻情况,对空肠组织切片进行形态学观察(HE染色)和肥大细胞计数(甲苯胺蓝染色),血清sIgE水平检测(ELISA 法),脾组织中IL-4和IFN-γ检测(ELISA法)。提取各组小鼠血清中的脂肪酸,采用液相色谱法检测α-亚麻酸(ALA)、二十碳五烯酸(EPA)、二十二碳六烯酸(DHA)、花生四烯酸(AA)和亚油酸(LA)水平。结果 ①成功建立OVA诱导过敏小鼠模型:与PBS组比较,OVA组在灌胃后发生急性腹泻;小肠绒毛有大量炎症细胞浸润,潘氏细胞脱颗粒,固有层中肥大细胞聚集脱颗粒,肥大细胞数目增加(P<0.05);血清OVA-sIgE 水平、脾细胞培养上清中IL-4和IL-4/IFN-γ升高(P<0.05)。②PBS组血清DHA、EPA和LA水平较空白组降低,AA水平升高,差异均有统计学意义,OVA组较PBS组血清DHA水平降低。结论 OVA过敏小鼠出现以DHA降低为主的脂肪酸代谢紊乱。
n-6多不饱和脂肪酸; 卵清蛋白; 食物过敏
多不饱和脂肪酸(PUFA)按照不饱和双键位置的不同,分为n-3PUFA和n-6 PUFA。 n-3 PUFA主要包括α-亚麻酸(ALA)、二十碳五烯酸(EPA)和二十二碳六烯酸(DHA)等,在去饱和酶和延长酶等的作用下,ALA可转化为EPA和DHA,EPA可转化为DHA; n-6 PUFA主要包括亚油酸(LA)和花生四烯酸(AA)等,LA可在去饱和酶和延长酶等的作用下转化为AA[1]。n-3 PUFA对机体免疫系统有保护作用[2],可能参与免疫相关疾病的调节,如哮喘和炎症性肠病等[3, 4]。2016年Tressou的调查显示,仅14.6%的法国人饮食中的DHA达推荐摄入量[5]。
过敏性疾病是基因、免疫、环境和营养等多因素诱发的疾病,近年来发现PUFA可能参与其中。然而,不同研究结果不一致。有研究[6]显示,哮喘和过敏性皮炎患者血清LA水平增加,AA水平降低。另有研究[7]发现,有过敏症的学龄前儿童AA水平增加, n-3PUFA水平显著降低,n-6PUFA/n-3PUFA增加。
本研究给予小鼠n-3PUFA缺乏饲料,模拟人类脂肪酸缺乏的情况,并参照文献[8]建立卵清蛋白(OVA)诱导的食物过敏模型,探讨体内PUFA水平受过敏反应的影响。
1 方法
1.1 动物 重庆医科大学实验动物中心提供的SPF级 Balb/c 小鼠,连续传2代后随机选择第3代雌鼠6只,雄鼠3只,按雌雄比例2∶1合笼饲养,阴栓出现日确定为受孕时间。雌鼠妊娠第10 d起予富含n-6PUFA饲料(n-3PUFA缺乏)饲养,取其仔鼠(予相同饲料饲养)进行实验。n-6PUFA饲料配方:每100 g饲料含玉米淀粉30 g,麦麸10 g,豆粨15 g,次粉15 g,脱脂鱼粉10 g,氯化钠0.5 g,脱脂奶粉8 g,酵母粉2 g,骨粉2 g,中华复合维生素预混料0.5 g,葵花籽油7 g。
1.2 实验设计和方法 将24只仔鼠随机分为空白组、OVA组和PBS组,实验流程见图1。OVA组小鼠腹腔注射含50 μg OVA(V级,美国Sigma公司)和1.3 mg氢氧化铝凝胶的PBS 0.2 mL;PBS组腹腔注射等量PBS。OVA组小鼠以含50 mg OVA的PBS 0.3 mL灌胃,隔日1次,共6次;PBS组予等量PBS灌胃。
1.3 过敏模型成功建立的判断
1.3.1 腹泻症状的观察 每次灌胃后30~60 min观察小鼠大便情况,若排出糊状或水样便,判定为发生过敏性腹泻。

图1 实验流程图
注 PUFA:多不饱和脂肪酸;OVA:卵清蛋白
1.3.2 空肠形态学观察和肥大细胞计数 末次灌胃后1 h心脏穿刺取血处死仔鼠,取0.5 cm空肠,固定,HE染色后进行形态学观察,甲苯胺蓝染色后进行肥大细胞计数。如观察发现空肠黏膜水肿、炎性细胞浸润、潘氏细胞脱颗粒和肥大细胞数目增加,提示小鼠发生肠道过敏。
1.3.3 血清sIgE水平检测 采用ELISA 法(试剂盒购于北京四正柏公司),以每孔 100 μL OVA(浓度10 μg·mL-1)包被 96 孔酶标板。将血清样品1∶1稀释加样。结合反应时HRP 标记的大鼠抗小鼠 IgE 二抗以 1∶100 比例稀释, 3,3',5,5'-四甲基联苯(TMB)显色后终止反应,用全自动酶标仪在450 nm 波长下测OD 值。若血清sIgE水平显著增加,提示小鼠发生速发型超敏反应,食物过敏模型建立成功。
1.3.4 脾组织中IL-4和IFN-γ检测 无菌条件下取小鼠脾脏,用小弯剪在200目不锈钢筛网上剪碎,PBS冲洗,制成单细胞悬液,与等量 PBS 混匀。以密度梯度离心法分离单个核细胞。台盼兰染色活细胞计数应>95%。将细胞与含10%小牛血清和OVA(终浓度 1 mg·mL-1)的RPMI 1640培养基加入 24 孔培养板,细胞浓度为2×106·mL-1,在37℃、5%CO2条件下培养48 h后,离心收集上清液,置-70℃储存。采用ELISA法检测培养上清中IFN-γ和 IL-4水平,按照试剂盒说明书操作。若IL-4/IFN-γ比例增加,提示机体Th2/Th1比例失衡,进一步证实食物过敏模型建立成功。
1.4 血清PUFA的检测 脂肪酸提取和衍生化参照文献[9],取20 μL衍生化后的脂肪酸溶液注入液相色谱HPLC仪,用依利特Hypersil BDS C18 (4.6 mm×150 mm,5 μm)色谱柱,在乙腈∶水为81∶19的流动相、流速2 mL·min-1、波长242 nm、柱温45℃下分析,记录峰面积,绘制标准曲线,以外标法测定ALA、EPA、DHA、AA和LA。

2 结果
2.1 成功建立过敏模型 ①OVA 第3次灌胃后6/8的仔鼠在30~60 min排出糊状或水样便,第5次灌胃后8只仔鼠均出现持续 1 h 左右的急性腹泻。PBS组无仔鼠发生腹泻。②空肠组织HE染色(图2A、B)显示,OVA组仔鼠小肠绒毛有嗜酸性粒细胞和淋巴细胞等大量炎症细胞浸润,潘氏细胞脱颗粒,固有层渗出水肿。空肠组织甲苯胺蓝染色(图2C、D)显示,肥大细胞颗粒呈红紫色,胞核蓝色,OVA暴露仔鼠肥大细胞聚集,且多数胞膜破裂,轮廓不清,并向外排出颗粒;PBS组肥大细胞数目较少,轮廓清楚,仅少数出现脱颗粒现象。OVA组肥大细胞数目较PBS组差异有统计学意义(P=0.002,表1)。③血清OVA-sIgE 水平,OVA组高于PBS组,差异有统计学意义(表1)。④脾细胞培养上清中IL-4水平和IL-4/IFN-γ,OVA组较PBS组增高(P<0.05)。综合以上结果,认为成功建立OVA诱导小鼠过敏模型。
2.2 血清PUFA水平 表2显示,PBS组血清DHA、EPA和LA水平较空白组降低,AA水平升高,差异均有统计学意义。OVA组较PBS组血清DHA水平降低,差异有统计学意义; 两组ALA、EPA、 LA和n-6PUFA/n-3PUFA差异无统计学意义。
3 讨论
虽然目前尚缺乏统一的n-3PUFA摄入标准,但调查显示人群中普遍存在摄入严重不足。2003年比利时健康委员会推荐PUFA摄入量为5.3%~10.0%E(E,总能量),其中n-6PUFA摄入量为4.0%~8.0%E,n-3PUFA摄入量为1.3%~2%E[10]。2006年Sioen[10]报道,比利时妇女PUFA摄入量为6.0%E,其中LA占5.3%E,ALA、EPA和DHA分别占0.6%E、0.04%E和 0.06%E,提示饮食中n-3PUFA缺乏。2016年调查结果显示,仅14.6%的法国人饮食中DHA达推荐摄入量[5]。2015年Eilander[11]综合欧洲24个国家的饮食调查结果发现,近半数国家饮食中PUFA未达到WHO推荐摄入量(6%~11%E),且PUFA主要为植物油中的LA,缺乏n-3PUFA。
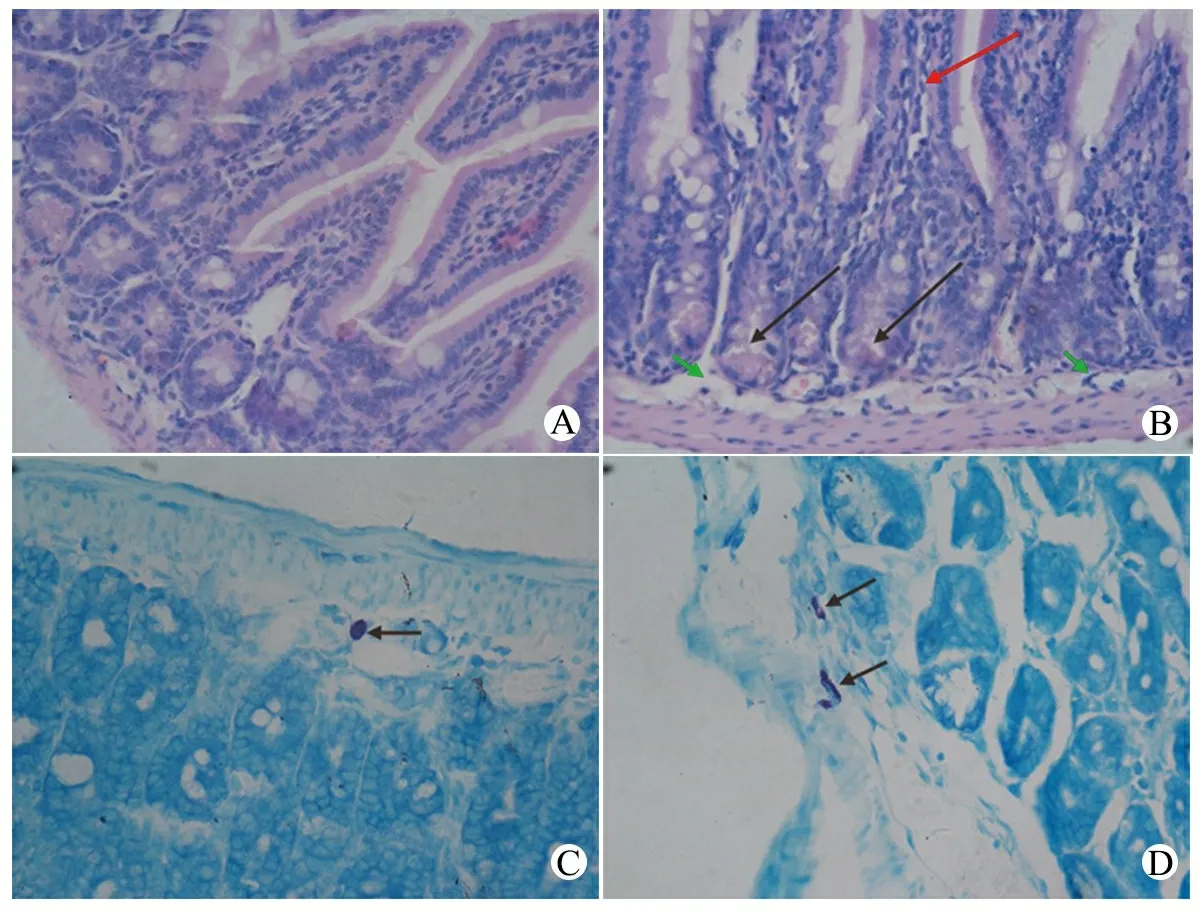
图2 空肠组织切片染色
注 A、B:HE 染色,×400,A为PBS组,绒毛形态正常;B为OVA组,大量炎症细胞浸润(红色箭头),黏膜下水肿(绿色箭头),潘氏细胞脱颗粒(黑色箭头)。C、D:甲苯胺蓝染色,×400, C为PBS组;D为OVA组,空肠肥大细胞聚集、脱颗粒
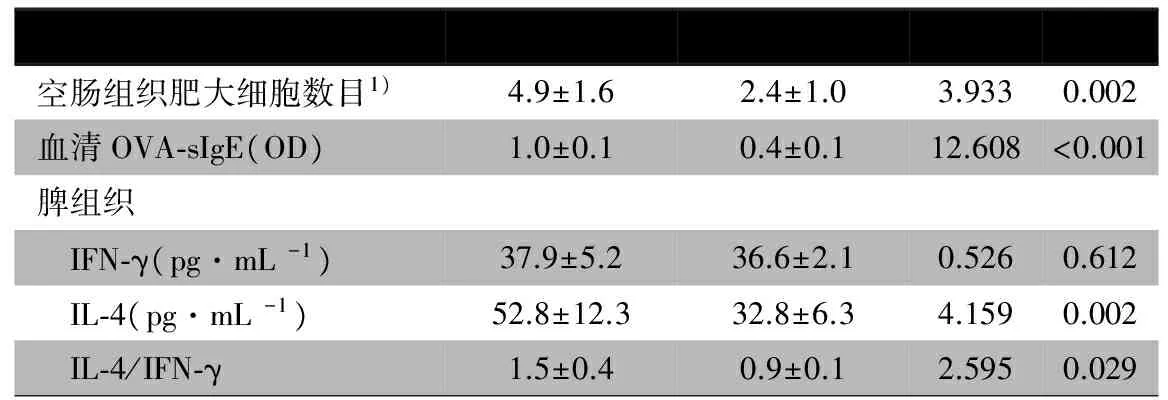
表1 OVA组和PBS组各项指标比较
注 1)个/10个连续高倍视野

表2 各组小鼠血清PUFA水平比较
注t1、P1:PBS组与空白组比较;t2、P2:OVA组与PBS组比较
PUFA中生物学活性较高的主要有AA、DHA和EPA。在环氧化酶和脂氧合酶的作用下,AA代谢生成前列腺素(PG)E2和白三烯(LT)B4等炎症因子,EPA代谢生成炎症活性较弱的PGE3和LTB5等,DHA代谢产生Resolvins和Protectins等抗炎因子[12]。AA、DHA和EPA与细胞膜上的PPAR-γ受体结合完成细胞内信号转导。体内AA、 DHA和EPA代谢所需的酶和受体相同,因此存在竞争性抑制,推测n-3PUFA摄入不足时,其代谢产物PGE3、LTB5、Resolvins和resolvins等减少,炎症因子产生增多,从而与过敏反应的发生有关。
机体内DHA和EPA的来源包括食物供给和ALA转化。本研究采用n-3PUFA缺乏饲料喂养小鼠,模拟现代人类社会饮食PUFA的摄入状况,并建立OVA诱导的过敏模型,以了解体内PUFA水平受过敏反应的影响。结果显示,不同生长时期小鼠PUFA构成不同,21日龄小鼠(空白组,断奶时)血清n-3PUFA(DHA和EPA)水平较高,59日龄小鼠(PBS组)血清n-3PUFA水平较低。因n-3PUFA尤其DHA是大脑和视网膜中脂肪酸的主要成分,对大脑和视网膜的发育有促进作用[13, 14],提示生命早期n-3PUFA参与生长过程消耗。
OVA组较PBS组血清DHA降低,AA虽有下降趋势但差异无统计学意义(P=0.064),EPA、ALA和LA差异无统计学意义,提示OVA多次暴露使仔鼠体内脂肪酸合成代谢失衡,以消耗DHA为主,与Hwang等[7]和Yu等[15]的研究结果基本一致,但他们的研究中AA水平增加,原因有待进一步研究。过敏反应是一系列炎症放大反应,生成大量炎性和抗炎介质。食物过敏发生过程中的一系列炎症反应与机体PUFA的代谢相关,如PGE2和LTB4是由 AA在体内分别与环氧化酶和脂氧合酶作用产生。2015年van den Elsen等[16]报道,增加LA的摄入可致AA代谢产生的PGE2增加,血清IgE水平升高;同时,DHA代谢产生Resolvins和Protectins等保护性抗炎介质。2007年Levy研究[17]发现,OVA介导呼吸道炎症,促进肺组织中DHA转化为Protectin D1,Protectin D1可降低肺泡灌洗液中炎症细胞数目和气道高反应性,哮喘发生过程中消耗Resolvin E1和Protectin D1,进而消耗DHA。因此,本研究中OVA暴露仔鼠DHA水平降低,可能与OVA过敏消耗Resolvin E1和Protectin D1有关,且喂养缺乏n-3PUFA饲料,从食物中得不到DHA补充,加速体内DHA水平下降。本文结果提示,或许可通过补充DHA改善机体脂肪酸代谢紊乱,缓解食物过敏症状,值得进一步研究。
[1]Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci, 2014,35(6):284-292
[2]Khaddaj-Mallat R, Morin C, Rousseau E. Novel n-3 PUFA monoacylglycerides of pharmacological and medicinal interest: Anti-inflammatory and anti-proliferative effects. Eur J Pharmacol, 2016, 792: 70-77
[3]Muley P, Shah M, Muley A. Omega-3 Fatty Acids Supplementation in Children to Prevent Asthma: Is It Worthy?—A Systematic Review and Meta-Analysis. J Allergy (Cairo), 2015,2015:312052
[4]Marion-Letellier R, Savoye G, Beck PL, et al. Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflamm Bowel Dis, 2013,19(3):650-661
[5]Tressou J, Moulin P, Vergès B, et al. Fatty acid dietary intake in the general French population: are the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) national recommendations met. Br J Nutr, 2016,116(11):1966-1973
[6]Yen CH, Dai YS, Yang YH, et al. Linoleic acid metabolite levels and transepidermal water loss in children with atopic dermatitis. Ann Allergy Asthma Immunol, 2008,100(1):66-73
[7]Hwang I, Cha A, Lee H, et al. N-3 polyunsaturated fatty acids and atopy in Korean preschoolers. Lipids, 2007,42(4):345-349
[8]Brandt EB, Strait RT, Hershko D, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest, 2003,112(11):1666-1677
[9]Mehta A, Oeser AM, Carlson MG. Rapid quantitation of free fatty acids in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl, 1998,719(1-2):9-23
[10]Sioen IA, Pynaert I, Matthys C, et al. Dietary intakes and food sources of fatty acids for Belgian women, focused on n-6 and n-3 polyunsaturated fatty acids. Lipids, 2006,41(5):415-422
[11]Eilander A, Harika RK, Zock PL. Intake and sources of dietary fatty acids in Europe: Are current population intakes of fats aligned with dietary recommendations. Eur J Lipid Sci Technol, 2015,117(9):1370-1377
[12]Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int, 2015,64(1):27-34
[13]Moon K, Rao SC, Schulzke SM, et al. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev, 2016,12: CD000375
[14]Harris WS, Baack ML. Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity. J Perinatol, 2015,35(1):1-7
[15]Yu G, Björkstén B. Polyunsaturated fatty acids in school children in relation to allergy and serum IgE levels. Pediatr Allergy Immunol, 1998,9(3):133-138
[16]van den Elsen LW, van Esch BC, Dingjan GM, et al. Increased intake of vegetable oil rich in n-6 PUFA enhances allergic symptoms and prevents oral tolerance induction in whey-allergic mice. Br J Nutr, 2015,114(4):577-585
[17]Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol, 2007,178(1): 496-502
(本文编辑:张崇凡,孙晋枫)
Changesofpolyunsaturatedfattyacidmetabolisminovalbumin-inducedallergicmice
WUYu-feng,LIHai-qi
(DepartmentofChildCare,Children'sHospitalofChongqingMedicalUniversity,MinistryofEducationKeyLaboratoryofChildDevelopmentandDisorders,ChinaInternationalScienceandTechnologyCooperationbaseofChildDevelopmentandCriticalDisorders,ChongqingKeyLaboratoryofPediatrics,Chongqing400014,China)
Corresponding Author:LI Hai-qi, E-mail: haiqili2010@hotmail.com
ObjectiveTo observe the changes of polyunsaturated fatty acid metabolism in Balb/C mice model of ovalbumin(OVA)-induced allergy.MethodsAt 21 day old, 24 baby Balb/C mice, whoes mother had a diet of n-3PUFA deficiency, randomly divided into 3 groups, blank, OVA and PBS groups. There were 8 offsprings in each group. Weaned BALB/c offsprings in group h, m and l were sensitized with 50 μg of OVA (grade V, Sigma) in the presence of 1.3 mg of aluminum hydroxide gel (Sigma) as an adjuvant by intraperitoneal injection twice at 3 and 5 weeks old. Half of the offsprings delivered from the mice of the control group were sensitized with OVA as positive control. And remainders of the offsprings were injected with PBS as negative control.The sensitized BALB/c mice with 7 weeks of life were fed with 50 mg of OVA dissolved in 0.3ml of sterile saline by intragastric needles at interval day for six times. One hour after the last oral administration, all BALB/c mice were sacrificed with excessive carbon dioxide and blood samples were collected by cardiac puncture.At the first day of the test, blood samples of baby mice in blank group were collected by cardiac puncture. The offsprings in group OVA were sensitized with 50 g of OVA dissolved in 0.2 mL PBS in the presence of 1.3 mg of aluminum hydroxide gel by intraperitoneal injection at 1 and 15 day. From 29 to 39 day, the offsprings in OVA group were fed wiht 50 mg of OVA dissolved in 0.3 mL of PBS by intragastric needles at interval day for six times. The offsprings in PBS group were given equivalent PBS. After the last oral administration, all BALB/c mice were sacrificed by cardiac puncture and blood, jejunum, spleen samples were collected. To establish the model of OVA-induced allergy, diarrhea was observed after OVA challenge; histological examinations of jejunum were performed by HE staining and the mast cells in jejunum were observed by toluidine blue staining; and the levels of OVA-sIgE in serum, IL-4 and IFN-γ in spleen cell culture supernatants were measured by ELISA. Fatty acids were extracted from serum in each group. The linoleic acid (ALA), inolenic acid (LA), arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexenoic acid (DHA) levels of serum were analyzed by high performance liquid chromatography.Results①The model of OVA-induced allergy in Balb/C mice was established: compared with group PBS, after the oral OVA challenge allergic diarrhea, gathered phenomenon of inflammatory cells in jejunum villus,aggregation of mast cells in lamina propria(P<0.05),increasing of the levels of OVA-specific IgE in serum and IL-4, IL-4/IFN-γ in spleen cells(P<0.05) were observed in the offspring of OVA group. ②The levels of DHA, EPA and LA of mice in group PBS were statistically lower than those in blank group. Compared with PBS group, the levels of DHA decreased in mice of OVA group.ConclusionBalb/C mice,repeatedly exposed with OVA, exerted a fatty acid metabolic disorder, mainly a reduction of DHA.
n-6 polyunsaturated fatty acid; Food allergy; Ovalbumin
重庆医科大学附属儿童医院儿保科,儿童发育疾病研究教育部重点实验室,儿童发育重大疾病国家国际科技合作基地,儿科学重庆市重点实验室 重庆 400014
黎海芪,E-mail: haiqili2010@hotmail.com
10.3969/j.issn.1673-5501.2017.03.009
2017-06-12
2017-06-22)
