Coumarin-derived Fluorescent Chemosensor for Sulfide Anion: Displacement Approach and Bioimaging Application
2017-06-19CHENGXiaohongZHONGZhichengLIWangnan
CHENG Xiao-hong, ZHONG Zhi-cheng, LI Wang-nan
(HubeiKeyLaboratoryofLowDimensionalOptoelectronicMaterialsandDevices,HubeiUniversityofArtsandScience,Xiangyang441053,China)
Coumarin-derived Fluorescent Chemosensor for Sulfide Anion: Displacement Approach and Bioimaging Application
CHENG Xiao-hong*, ZHONG Zhi-cheng, LI Wang-nan
(HubeiKeyLaboratoryofLowDimensionalOptoelectronicMaterialsandDevices,HubeiUniversityofArtsandScience,Xiangyang441053,China)
We reported herein an ensemble C1-Cu2+as a new displacement-based fluorescent probe for sulfide anion detection. Upon the addition of S2-, it displayed marked fluorescence enhancement under aqueous conditions and the detection limit was determined to be as low as 90 nmol/L. In addition to its high selectivity for sulfide anion rather than other common anions, C1 was successfully applied to the detection of sulfide anion in HeLa cells with ‘Turn-On’ fluorescent methods.
displacement approach; fluorescence enhancement; sulfide anion; good solubility; bioimaging application
1 Introduction
In the field of supramolecular chemistry, the progress in receptors for anions has attracted considerable attention in recent decades due to the fact that a large number of chemical, biological, and environmental processes involve molecular recognition of anionic species[1-13]. Among these anions, sulfide anion is one of the toxic anions, which could irritate mucous membranes and even cause unconsciousness and respiratory paralysis upon continuous and high concentration exposure of sulfide anion. Once being protonated, it becomes even more toxic. Actually, sulfide anion can be found not only in industrial settings where it is either used as a reactant or produced as a by-product of manufacturing or industrial processes, but also due to the microbial reduction of sulfate by anaerobic bacteria or formed from the sulfurcontaining amino acids in meat proteins, for instance, conversion into sulfur and sulfuric acid, dyes and cosmetic manufacturing, production of wood pulp,etc.[14-19]. Thus, the detection of sulfide anion is becoming very important from industrial, environmental, and biological points of view. A variety of detection techniques have been developed for the determination of sulfide anion, in which fluorimetry has received considerable attention due to its high sensitivity, specificity, simplicity of implementation, and fast response times, offering application methods not only for in vitro assays but also for in vivo imaging studies[20-31].
Generally, in comparison with the relatively large number of cation chemosensors, the development of anion chemosensors is still a challenging area[32-35]. On the one hand, most of the organic receptors for anions have been designed based on the attachment of a dye to an anion-binding site; however, this mechanism does not always work well especially in aqueous media, owing to the strong hydration nature of anions, which weakens the interactions of the sensors with the target anions[36-41]. On the other hand, we know much about the rules of coordination chemistry between ligands and cations, but poor knowledge of the interactions between compounds and anions, leading to the comparatively scarce number of anion chemosensors as mentioned above. Recently, the development of metal-based receptors[42-46]has been the centre of interest as metal ions pre-organize the binding sites structurally for optimal anion-metal ion coordination, resulting in strong affinities over purely organic receptors and relatively easy design and convenient handling.
Over the past few years, Lietal. developed an indirect strategy to detect cyanide utilizing the affinity of this anion towards copper[47-51]. The key point is that the stability constant of the complex Cu2+-anion is larger than that of the complex Cu2+-receptors, and then the added anions could preferentially snatch copper ions in the Cu2+-receptors coordination to form stable species ([Cu(CN)x]n-). One of the most important advantages of sensors based on this displacement approach was that they could be operable in aqueous solutions. Continuously, considering that sulfide anion opposed even stronger affinity towards copper ions than cyanide, they further developed the displacement-based sensing strategy and successfully designed a series of fluorescence and colorimetric chemosensors towards sulfide anions[52-53]based on the good Cu2+-based receptors (see supplementary data
Fig. S1). Now, more and more excellent chemosensors for sulfide anion base on this displacement strategy were reported. However, biological studies of fluorescent imaging for sulfide anion in living cells, so far, are scarce[54-56].
Herein, we expected to broaden the displacement-based strategy for S2-detection by utilizing excellent chemosensors of copper ions in order to realize the application in bioimaging. With this idea in mind, we focused our eyes on compound C1 as a potential new sulfide sensor after checking the literatures and thinking of some candidates carefully. Coumarin derivative C1[57]appending 2-picolylamide enabled efficient tridentate complexation for Cu2+with the binding constant of 1 with Cu2+in aqueous solution was reported to be (1.17±0.29) × 105. It displayed high selectivity towards copper in preference to a variety of other common heavy and toxic metal ions. In a sense of water solubility, membrane permeability, and nontoxic nature, C1 could be successfully applied in the detection of Cu2+in living cells. Our experimental results confirmed the quite excellent sensing behavior of compound C1 towards copper ions: the fluorescence of the solution was almost quenched in stark only in the presence of copper ions at very low concentration (5 μmol/L). Excitingly, upon the addition of trace sulfide anions, the non-emission solution went back to strong green fluorescence immediately. In this work, we would like to describe the synthesis and the spectroscopic evaluation of the new fluorescent chemosensor towards sulfide anion in detail, featuring advantages such as relative good solubility in aqueous media, easy-to-make, rapid ‘turn-on’ fluorescence response, as well as the successful application in bioimaging of living cells.
2 Experiments
2.1 Materials and Instrumentations
All reagents were of analytical reagent grade and used without further purification. Doubly distilled water was used in all experiments.
The1H and13C NMR spectra were measured on Varian Mercury300 spectrometer using tetramethylsilane (TMS,δ=0) as internal standard. The ESI mass spectra were measured on a Finnigan LCQ advantage mass spectrometer. Photoluminescence spectra were performed on a Hitachi F-4500 fluorescence spectrophotometer. UV-Vis spectra were obtained using a Shimadzu UV-2550 spectrometer. The pH values were determined by using a DELTA 320 PH dollar.
2.2 Synthesis of Compound C1
Compound C1 was readily synthesized in three steps according to the literature[57].1H NMR (300 MHz, CDCl3)δ: 9.53 (1H, s),8.56 (1H,s),8.51 (1H,s),7.63-7.65 (1H,t,J=3.0 Hz),7.32-7.34 (1H,d,J=6.0 Hz),7.14-7.17 (1H,m),6.94 (1H,s),4.76 (2H,s),3.25-3.27 (4H,t,J=3.0 Hz),2.81-2.82 (2H,d,J=3.0 Hz),2.69-2.72 (2H,d,J=4.5 Hz),1.91 (4H,m).13C NMR (75 MHz,CDCl3)δ:163.86,163.00,157.91,152.76,149.31,148.24,148.19,136.63,127.05,122.04,121.50, 119.61,109.00,108.27,105.73,67.98,50.25,49.84,45.29,27.47,25.62,21.17,20.23, 20.12. MS (ESI),m/z[M+H]+:376.4,calcd,376.2.
2.3 Preparation of Solutions of Anions
1 mmol of inorganic salt (Na2SO3,NaHSO3,Na2SO4,Na2S2O3·5H2O,NaN3,NaCN,KClO3,NaF,NaCl,KBr,KI,NaIO3,Na2HPO4·12H2O,Na3PO4,NaOAc·3H2O,Na2CO3,NaHCO3,NaSCN,NaNO2or Na2S) was dissolved in distilled water (10 mL) to afford 0.1 mol/L aqueous solution. The stock solutions were diluted to desired concentrations with distilled water prior to the experiment.
2.4 Fluorescence Titration of C1 with Cu2+Ions
A solution of C1 (2×10-6mol/L) was prepared inV(HEPES)∶V(DMSO)=9∶1 (10 mmol/L, pH=7.4). The solution of Cu(NO3)2·3H2O (1×10-3mol/L) was prepared in distilled water. A solution of C1 was placed in a quartz cell (10.0 mm width) and the fluorescence spectrum was recorded. The Cu2+ion solution was introduced in portions and fluorescence intensity changes were recorded at room temperature each time. Excitation wavelength: 420 nm.
2.5 Fluorescence Titration of C1-Cu2+with S2-Anions
A solution of C1 (2×10-6mol/L) was prepared inV(HEPES)∶V(DMSO)=9∶1 (10 mmol/L, pH=7.4). The solution of Cu2+with the concentration of 5×10-6mol/L was added to the above solution. The solution of Na2S (1×10-3mol/L) was prepared in distilled water. A solution of C1-Cu2+was placed in a quartz cell (10.0 mm width) and the fluorescence spectrum was recorded. The S2-anion solution was introduced in portions to the above C1-Cu2+solution and fluorescence intensity changes were recorded at room temperature each time. Excitation wavelength: 420 nm.
2.6 Fluorescence Titration of C1-Cu2+with Other Anions
A solution of C1 (2×10-6mol/L) was prepared inV(HEPES)∶V(DMSO)=9∶1 (10 mmol/L, pH=7.4). The solution of Cu2+with the concentration of 5×10-6mol/L was added to the above solution. A solution of C1-Cu2+was placed in a quartz cell (10.0 mm width) and the fluorescence spectrum was recorded. The solution of anion (S2-: 7×10-6mol/L; CN-and I-:1×10-5mol/L; other anions: 5×10-5mol/L) was introduced in portions to the above C1-Cu2+solution and fluorescence intensity changes were recorded at room temperature each time. Excitation wavelength: 420 nm.
2.7 Quantum Yield Changes of C1, C1-Cu2+and C1-Cu2++ S2-
Quantum yield was determined according to the equation as follows:
(1)
ΦFwas the fluorescence quantum yield,Awas the absorbance,Fwas the area under the corrected emission curve. Here, fluorescein was used as the standard; the quantum yield of fluorescein in 0.1 mol/L NaOH was 0.90[58].
2.8 Cell Culture
HeLa cells were seeded to the 24-well plates, the cells with an initial density of 5 × 104cells/well in 24-well plates were routinely maintained at 37 ℃ in a humidified 5% CO2atmosphere using DMEM (Dulbecco’s modified eagle’s medium) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin for 24 h.
2.9 Fluorescence Imaging
Fluorescence cell imaging was performed with an OLYMPUS IX73 scanning microscopy with a 40×objective lens. Fluorescence images of HeLa cells were monitored at 460-490 nm for green channel. The data were analyzed using software package provided by OLYMPUS instruments. Cell imaging was then carried out after washing cells with phosphate-buffered saline (PBS, 10 mmol/L, pH 7.02).
3 Results and Discussion
3.1 Sensing Properties Towards Cu2+Ions
Compound C1 emitted strong green fluorescence with the maximum emission wavelength centered at about 490 nm. Then, we tried to add Cu2+ions into the diluted solution of compound C1, and investigated the sensing behavior of C1 towards copper ions carefully. As shown in
Fig.1, excitingly, the emission spectra displayed apparent decrease with the increasing of Cu2+ions. In fact, the emission intensity at 490 nm decreased immediately to about 90% of the original one at the concentration of Cu2+ions as low as 0.3 μmol/L. With the increasing of the concentration of Cu2+ions in the test system, the emission intensity decreased correspondingly. When the concentration of Cu2+was 5 μmol/L, the emission intensity at 490 nm was completely quenched (Quantum yield: 0.12). However, further increasing the concentration of Cu2+ions to 7 μmol/L, no big difference could be observed. To see the results more visually, we summarized the emission intensities at 490 nm as a function of copper concentrations. As demonstrated in the inset of
Fig.1, in the range of 0-5 μmol/L, there was a good linear relationship between the intensity change and the concentration of Cu2+ions. The association constant of C1 for Cu2+was calculated to be ~1.4×105mol-1·L using the equation in supplementary data Eq. S1[59]. It suggested that compound C1 could act as a ‘switching-off’ fluorescent probe for Cu2+ions.

Fig.1 Fluorescent emission spectra of C1 (2 μmol/L, inV(HEPES)∶V(DMSO)=9∶1(10 mmol/L,pH=7.4)) in the presence of different concentrations of Cu2+. The inset plot showed the fluorescence intensities at 490 nm of compound C1 with different concentrations of Cu2+(Excitation wavelength: 420 nm).
3.2 Sensing Propertiestowards S2-Anion
As mentioned in the introduction section, sulfide anion could form very stable complex with copper ions with the solubility-product constant (Ksp) of CuS was as low as 6.3×10-36. Meanwhile, the previous titration experiments demonstrated that compound C1 could act as a ‘switching-off’ chemosensor towards copper ions through the formation of C1-Cu2+complex with the association constant of 1.4×105mol-1·L. With the both considerations in mind, it was reasonable that the addition of sulfide anion could preferentially snatch copper ion from the above C1-Cu2+complex to form stable CuS species (Scheme 1). As a result, the fluorescence of C1 was fully revived by the transformation from C1-Cu2+(quenched, ‘off’) to free C1 (revived, ‘on’). Then, we added the aqueous solution of sodium sulfide into the C1-Cu2+complex and investigated its indirect sensing response to S2-.

Scheme 1 Speculated sensing process of C1 towards Cu2+and S2-
Fortunately, the peak at 490 nm, which disappeared upon the addition of Cu2+, recovered, and its intensity increased with the concentrations of S2-becoming higher as expected (Fig.2). Encouraged by this result, the recovering fluorescence behaviour of the complex C1-Cu2+was studied in detail. It was reasonable that when more Cu2+ions were added, more S2-anions were needed to coordinate with the copper ions added in the first step to give an obvious optical signal. Therefore, we fixed the concentration of C1 at 2 μmol/L and the added Cu2+ions as 5 μmol/L considering that the addition of 5 μmol/L of Cu2+ions induced the emission intensity reaching the minimum during the fluorescent titration experiment. Under this condition, we presented the response of complex C1-Cu2+towards S2-anion to inspect the sensitivity of the mixture system. As demonstrated in
Fig.2, the emission intensity at 490 nm increased immediately to about 6-fold of the original one at the concentration of S2-anions as low as 0.2 μmol/L. Finally, the detection limits of complex C1-Cu2+for S2-anion was found to be 90 nmol/L (when the lowest fluorescence increase equals to three fold of the instrument noise). When the added concentration of S2-was 7 μmol/L, the fluorescent intensity increased to ~125-fold of the original one (Quantum yield: 0.65), and this intensity was strong enough to be used as ‘turn-on’ sensor to probe trace S2-by the naked eye (the difference between the ‘off’ and ‘on’ state was also shown in the inset in
Fig. 2). Thus, by applying a ‘turn-off-turn-on’ circle, compound C1 could act as a sensitive chemosensor towards S2-anions.

Fig.2 Emission spectra of C1-Cu2+(C1: 2 μmol/L, Cu2+: 5 μmol/L, inV(HEPES)∶V(DMSO)=9∶1) in the presence of different concentrations of S2-(Excitation wavelength: 420 nm). Inset: fluorescent photograph of C1 (a), C1-Cu2+(b) and C1-Cu2++ S2-(c).
To see the sensing process more visually, we summarized the intensity changes at 490 nm as a function of S2-anion concentrations. As shown in
Fig.3, it was easily seen that the intensity change was almost linear with the concentrations of S2-in the concentration range of 0-6 μmol/L. Actually, this fluorescence difference for the solution of complex C1-Cu2+before and after the addition of S2-anion could be easily distinguished by the naked eyes. As displayed in the inset of
Fig.3, under a normal UV lamp, the solution changed from non-fluorescence to strongly green fluorescence with 21.5-fold enhancement in the quantum yield (from 0.12 to 0.65). Although not so sensitive as measured by the fluorescent spectrometer, we could easily distinguish the fluorescent color change upon the addition of as little as 10 μmol/L of S2-(the inset of
Fig.3, c). Thus, these results indicated that upon the addition of S2-, the S2--promoted displacement reaction of C1-Cu2+ensemble really occurred as expected, and the liberated C1 was formed step by step.
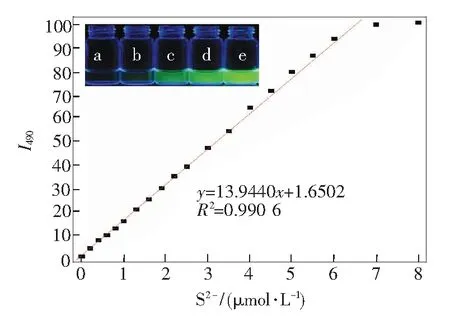
Fig.3 Plot of fluorescent intensities at 490 nm of C1-Cu2+(C1: 2 μmol/L, Cu2+: 5 μmol/L, inV(HEPES)∶V(DMSO)=9∶1) in the presence of different concentrations of S2-(Excitation wavelength: 420 nm). Inset: fluorescent photograph of C1-Cu2+to various concentration of S2-, from a to e: 0, 5, 10, 20, 50 μmol/L.
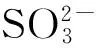
Fig.4 Emission intensities of C1-Cu2+in the presence of different anions (S2-: 7 μmol/L, CN-and I-: 10 μmol/L, the others: 50 μmol/L). Inset: fluorescent emission spectra of C1-Cu2+, C1-Cu2++ Mixture of other anions, and C1-Cu2++ mixture of other anions + S2-(C1: 2 μmol/L, Cu2+: 5 μmol/L, S2-: 7 μmol/L, other anions: 10 μmol/L).
In addition to the different fluorescent behavior caused by theaddition of sulfide anion, the UV spectra change was another apparent property. As shown in Fig.5, with the increasing concentrations of sulfide anion, the maximum absorption wavelength gradually shifted from 465 to 450 nm with a similar molar absorption coefficient compared to the original one. The addition of 10 μmol/L of S2-produced a 15 nm blue shift of the absorption maximum, resulting in a perceived color change from dark yellow to green-yellow. Competition experiments revealed that the absorbance changes induced by sulfide anions were retained in the presence of other anions (see supplementary data
Fig.S3).
The application of complex C1-Cu2+to track intracellular S2-level was also investigated via a scanning microscopy. Firstly, the HeLa cells were treated with Cu2+(20 μmol/L, 20 min) followed by subsequent staining with C1 (15 μmol/L, 20 min) and then washed three times with phosphate-buffered saline (PBS, 10 mmol/L, pH 7.02). As shown in
Fig.6, fluorescence was little observable in the cells after incubation prior to the S2-anion treatment (Fig.6, control). Afterwards, S2-level dependent increases of fluorescence intensity were observed inside the cells after the S2-anion treatment. As expected, green fluorescence recovered inside of the cells correspondingly with the increasing of S2-concentrations. Importantly, obvious fluorescence enhancement could be observed even with the concentration of S2-as low as 50 μmol/L. The bright-field images confirmed that the cells were viable throughout the imaging experiments (Fig.6, DIC pictures). The fluorescence imaging results were in consistent with the observations in titration experiments, and demonstrated that probe C1 could readily detect the presence of sulfide anions in cells with switching-on fluorescent methods and through the displacement approach.
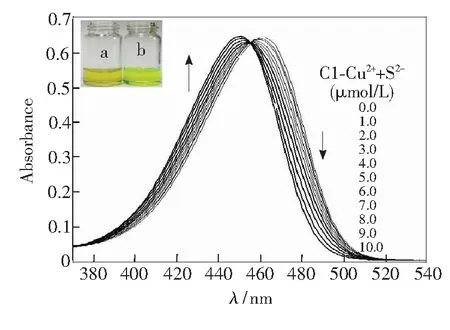
Fig.5 UV-Vis absorption spectra of C1-Cu2+(C1: 7 μmol/L, Cu2+: 10 μmol/L, inV(HEPES)∶V(DMSO)=9∶1) in the presence of different concentrations of S2-. Inset: photograph of C1-Cu2+(a), and C1-Cu2++ S2-(b).

Fig.6 Scanning microscopic images of HeLa cells in the presence of C1-Cu2+. The fluorescence images were acquired after 30 min of treatment of S2-(0, 50, 100, 150 μmol/L) in HeLa cells.
4 Conclusion
In summary,we demonstrated that an ‘ensemble’-based chemosensor C1-Cu2+can selectively probe the sulfide anion in aqueous media with respect to a marked fluorescence enhancement over other anionic species, by utilizing the displacement method. The preliminary results demonstrated that the fluorescent and colorimetric chemosensor C1 could be used to sense sulfide anion indirectly, featuring advantages such as relative good solubility in aqueous media, easy-to-make, rapid ‘turn-on’ fluorescence response, as well as the successful application in bioimaging of living cells. It was believed that more excellent metal-based receptors could be utilized as optimal anion-recognition chemosensors by virtue of the famous displacement strategy.
[1] BEER P D, HAYES E J. Transition metal and organometallic anion complexation agents [J].Coord.Chem.Rev., 2003, 240:167-189.
[2] CHRISTIANSON D W, LIPSCOME W N. Carboxypeptidase A [J].Acc.Chem.Res., 1989, 22:62-69.
[3] GALEP P D. Anion recognition and sensing: the state of the art and perspectives [J].Angew.Chem.Int.Ed.Engl., 2001, 40:486-516.
[4] LINARES J M, POWELL D, BOWMAN-JAMES K. Ammonium based anion receptors [J].Coord.Chem.Rev., 2003, 240:57-75.
[5] CAROLAN J V, BUTLER S J, JOLLIFFE K A. Selective anion binding in water with use of a zinc(II) dipicolylamino functionalized diketopiperazine scaffold [J].J.Org.Chem., 2009, 74:2992-2996.
[6] KIM Y K, LEE Y H, LEE H Y,etal.. Molecular recognition of anions through hydrogen bonding stabilization of anion-ionophore adducts: a novel trifluoroacetophenone-based binding motif [J].Org.Lett., 2003, 5:4003-4006.
[7] JIANG X, VIEWEGER M C, BOLLINGER J C,etal.. Reactivity-based fluoride detection: evolving design principles for spring-loaded turn-on fluorescent probes [J].Org.Lett., 2007, 9:3579-3582.
[8] SUN Z, WANG H, LIU F,etal.. BODIPY-based fluorescent probe for peroxynitrite detection and imaging in living cells [J].Org.Lett. 2009, 11:1887-1890.
[9] XU Z, KIM S K, YOON J. Revisit to imidazolium receptors for the recognition of anions [J].Chem.Soc.Rev., 2010, 39: 1457-1466.
[10] DE SILVA A P, GUNARATNE H Q N, GUNNLAUGSSON T,etal.. Signaling recognition events with fluorescent sensors and switches [J].Chem.Rev., 1997, 97:1515-1566.
[11] THOMAS S W, JOLY G D, SWAGER T M. Chemical sensors based on amplifying fluorescent conjugated polymers [J].Chem.Rev., 2007, 107:1339-1386.
[12] YOON J, KIM S K, SINGH N J,etal.. Imidazolium receptors for the recognition of anions [J].Chem.Soc.Rev., 2006, 35:355-360.
[13] SCHMIDTCHEN F P, BERGER M. Artificial organic host molecules for anions [J].Chem.Rev., 1997, 97: 1609-1646.
[14] BALASUBRAMANIAN S, PUGALENTHI V. A comparative study of the determination of sulphide in tannery waste water by ion selective electrode (ISE) and iodimetry [J].WaterRes., 2000, 34:4201-4206.
[15] SILVA M S P, GALHARDO C X, MASINI J C. Application of sequential injection-monosegmented flow analysis (SI-MSFA) to spectrophotometric determination of sulfide in simulated waters samples [J].Talanta, 2003, 60:45-52.
[16] SILVA M S P, DA SILVA I S, ABATE G,etal.. Spectrophotometric determination of acid volatile sulfide in river sediments by sequential injection analysis exploiting the methylene blue react [J].Talanta, 2001, 53: 843-850.
[17] MAYA F, ESTELA J M, CERDV. Improving the chemiluminescence-based determination of sulphide in complex environmental samples by using a new, automated multi-syringe flow injection analysis system coupled to a gas diffusion unit [J].Anal.Chim.Acta. 2007, 601:87-94.
[20] ZHANG J, ZHOU Y, YOON J,etal.. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions) [J].Chem.Soc.Rev., 2011, 40:3416-3429.
[21] KIM J S, QUANG D T. Calixarene-derived fluorescent probes [J].Chem.Rev., 2007, 107:3780-3799.
[22] SINKELDAM R W, GRECO N J, TOR Y. Fluorescent analogs of biomolecular building blocks: design, properties, and applications [J].Chem.Rev., 2010, 110:2579-2619.
[23] DE SILVA A P, GUNARATNE H Q N, GUNNLAUGSSON T,etal.. Signaling recognition events with fluorescent sensors and switches [J].Chem.Rev., 1997, 97:1515-1566.
[24] KIM H N, LEE M H, KIM H J,etal.. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions [J].Chem.Soc.Rev., 2008, 37:1465-1472.
[25] QUANG D T, KIM J S. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens [J].Chem.Rev., 2010, 110: 6280-6301.
[26] MCDONAGH C, BURKE C S, MACCRAITH B D. Optical chemical sensors [J].Chem.Rev., 2008, 108:400-422.
[27] KOBAYASHI H, OGAWA M, ALFORD R,etal.. New strategies for fluorescent probe design in medical diagnostic imaging [J].Chem.Rev., 2010, 110:2620-2640.
[28] BOBACKA J, IVASKA A, LEWENSTAM A. Potentiometric ion sensors [J].Chem.Rev., 2008, 108:329-351.
[29] KIM H N, REN W, KIM J S,etal.. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions [J].Chem.Soc.Rev., 2012, 41:3210-3244.
[30] LV J, WANG F, QIANG J,etal.. Enhanced response speed and selectivity of fluorescein-based H2S probeviathe cleavage of nitrobenzene sulfonyl ester assisted by ortho aldehyde groups [J].Biosens.Bioelectron., 2017, 87:96-100.
[31] ZHOU G, WANG H, MA Y,etal.. An NBD fluorophore-based colorimetric and fluorescent chemosensor for hydrogen sulfide and its application for bioimaging [J].Tetrahedron, 2013, 69:867-870.
[33] GALE P A. Anion receptor chemistry: highlights from 2008 and 2009 [J].Chem.Soc.Rev., 2010, 39:3746-3771.
[34] CLAUDIA R C, FRANCISCO J C. Application of flow injection analysis for determining sulphites in food and beverages: a review [J].FoodChem., 2009, 112:487-493.
[35] ITO A, ISHIZAKA S, KITAMURA N. A ratiometric TICT-type dual fluorescent sensor for an amino acid [J].Phys.Chem.Chem.Phys., 2010, 12:6641-6649.
[36] LODEIRO C, PINA F. Luminescent and chromogenic molecular probes based on polyamines and related compounds [J].Coord.Chem.Rev., 2009, 253:1353-1383.
[37] WU J, ZHOU J, WANG P,etal.. New fluorescent chemosensor based on exciplex signaling mechanism [J].Org.Lett., 2005, 7: 2133-2136.
[38] CHEN W B, ELFEKY S A, NONNE Y,etal.. A pyridinium cation-π interaction sensor for the fluorescent detection of alkyl halides [J].Chem.Commun., 2011, 47:253-255.
[39] HUANG Y, JIANG Y, BULL S D,etal.. Diols and anions can control the formation of an exciplex between a pyridinium boronic acid with an aryl group connected via a propylene linker [J].Chem.Commun., 2010, 46:8180-8182.
[40] CALLAN J F, DE SILVA A P, MAGRI D C. Luminescent sensors and switches in the early 21st century [J].Tetrahedron, 2005, 61:8551-8588.
[42] LOU X, OU D, LI Q,etal.. An indirect approach for anion detection: the displacement strategy and its application [J].Chem.Commun., 2012, 48:8462-8477.
[43] CHEN X, NAM S-W, KIM G-H,etal.. A near-infrared fluorescent sensor for detection of cyanide in aqueous solution and its application for bioimaging [J].Chem.Commun., 2010, 46:8953-8955.
[44] QIANG J, CHANG C, ZHU Z,etal.. A dinuclear-copper(Ⅱ) complex-based sensor for pyrophosphate and its applications to detecting pyrophosphatase activity and monitoring polymerase chain reaction [J].Sens.Actuat. B, 2016, 233:591-598.
[45] WANG H, ZHOU G, CHEN X. An iminofluorescein-Cu2+ensemble probe for selective detection of thiols [J].Sens.Actuat. B, 2013, 176:698-703.
[46] YU W, QIANG J, YIN J,etal.. Ammonium-bearing dinuclear Copper(Ⅱ) complex: a highly selective and sensitive colorimetric probe for pyrophosphate [J].Org.Lett., 2014, 16:2220-2223.
[47] LI Z, LOU X, YU H,etal.. An imidazole-functionalized polyfluorene derivative as sensitive fluorescent probe for metal ions and cyanide [J].Macromolecules2008, 41:7433-7439.
[48] LOU X, ZHANG L, QIN J,etal.. An alternative approach to develop a highly sensitive and selective chemosensor for the colorimetric sensing of cyanide in water [J].Chem.Commun., 2008, 44:5848-5850.
[49] LOU X, QIANG L, QIN J,etal.. A new rhodamine-based colorimetric cyanide chemosensor: convenient detecting procedure and high sensitivity and selectivity [J].ACSAppl.Mater.Interf., 2009, 1:2529-2535.
[50] LOU X, QIN J, LI Z. Colorimetric cyanide detection using an azobenzene acid in aqueous solutions [J].Analyst, 2009, 134:2071-2075.
[51] ZENG Q, CAI P, LI Z,etal.. An imidazole-functionalized polyacetylene: convenient synthesis and selective chemosensor for metal ions and cyanide [J].Chem.Commun., 2008, 44:1094-1096.
[52] LOU X, MU H, GONG R,etal.. Displacement method to develop highly sensitive and selective dual chemosensor towards sulfide anion [J].Analyst, 2011, 136:684-687.
[53] ZHANG L, LOU X, YU Y,etal.. A new disubstituted polyacetylene bearing pyridine moieties: convenient synthesis and sensitive chemosensor toward sulfide anion with high selectivity [J].Macromolecules, 2011, 44:5186-5193.
[54] CHOI M G, CHA S, LEE H,etal.. Sulfide-selective chemosignaling by a Cu2+complex of dipicolylamine appended fluorescein [J].Chem.Commun., 2009, 45:7390-7392.
[55] CAO X, LIN W, HE L. A near-infrared fluorescence turn-on sensor for sulfide anions [J].Org.Lett., 2011, 13:4716-4719.
[56] HOU F, HUANG L, XI P,etal.. A retrievable and highly selective fluorescent probe for monitoring sulfide and imaging in living cells [J].Inorg.Chem., 2012, 51:2454-2460.
[57] JUNG H S, KWON P S, LEE J W,etal.. Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells [J].J.Am.Chem.Soc., 2009, 131:2008-2012.
[58] WILLIAMS A T R, WINFIELD S A, MILLER J N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer [J].Analyst, 1983, 108:1067-1071.
[59] LIU L, DONG X, XIAO Y,etal.. Two-photon excited fluorescent chemosensor for homogeneous determination of copper(Ⅱ) in aqueous media and complicated biological matrix [J].Analyst, 2011, 136:2139-2145.
[60] FU Y, LI H, HU W,etal.. Fluorescence probes for thiol-containing amino acids and peptides in aqueous solution [J].Chem.Commun., 2005, 41:3189-3191.
[61] HAO W, MCBRIDE A, MCBRIDE S,etal.. Colorimetric and near-infrared fluorescence turn-on molecular probe for direct and highly selective detection of cysteine in human plasma [J].J.Mater.Chem., 2011, 21:1040-1048.
[62] HUO F, YANG Y, SU J,etal.. Indicator approach to develop a chemosensor for the colorimetric sensing of thiol-containing water and its application for the thiol detection in plasma [J].Analyst, 2011, 136:1892-1897.
[63] GAO C, ZHANG L, YANG Y,etal.. Application of rhodamine salicylidene hydrazone-Cu2+complex probe to the detection of cysteine [J].Petrochem.Technol., 2016, 45:1375-1379.
Supplementary Data
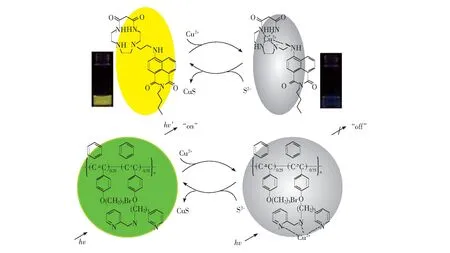
Fig. S1 Displacement-based chemosensors for S2-anions reported by Lietal.
Eq. S2 The equation for the calculation of association constant:
I0is the fluorescence intensity of C1,I∞is the intensity measured with excess amount of Cu2+,Iis the intensity measured with different amount of Cu2+,Kis the association constant, and [Cu2+] is the concentration of Cu2+ion added.

Fig.S2 Fluorescence spectra of C1-Cu2+(C1: 2 μmol/L, Cu2+: 5 μmol/L, inV(HEPES)∶V(DMSO)=9∶1) in the presence of different concentrations of Cys or Hcy.
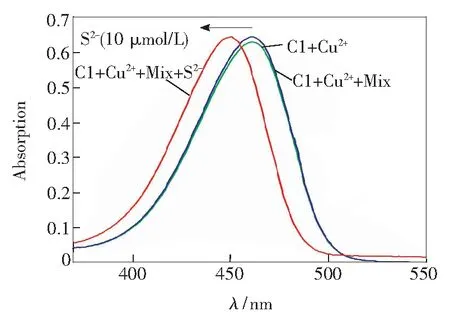
Fig.S3 Absorption spectra of C1-Cu2+, C1-Cu2++ mixture of other anions, and C1-Cu2++ mixture of other anions + S2-(C1: 7 !μmol/L, Cu2+: 10 μmol/L, S2-: 10 μmol/L, other anions: 20 μmol/L).
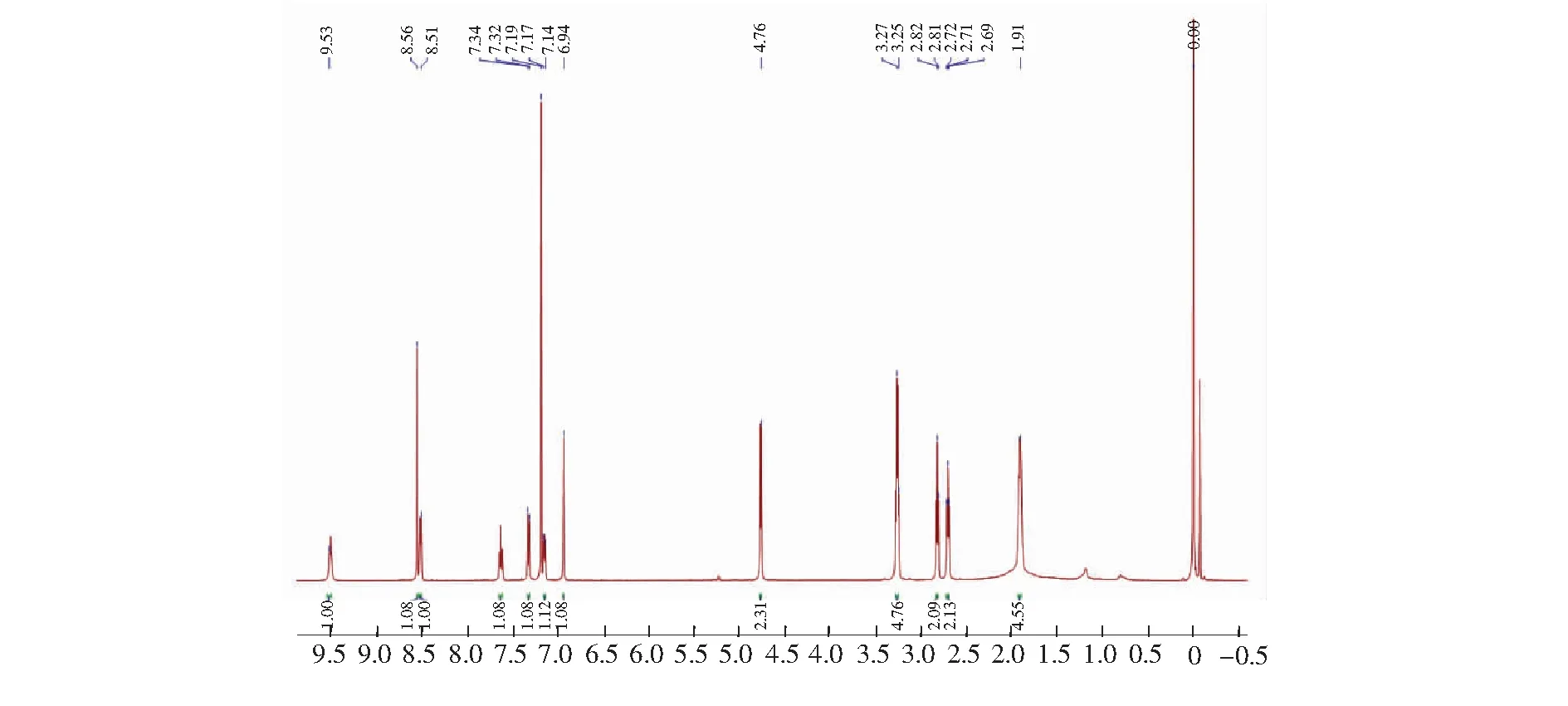
Fig.S4 1H NMR spectra of compound C1
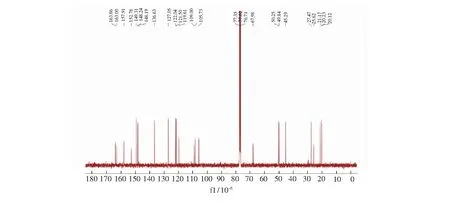
Fig.S5 13C NMR spectra of compound C1

程晓红(1986-),女,河北邯郸人,博士,讲师,2013年于武汉大学获得博士学位,主要从事新型化学/生物传感器的研究。
Email: chengxiaohong0807@126.com
2017-01-01;
2017-03-15
国家自然科学基金(21502047); 湖北省光电子协同创新中心基金资助项目 Supported by National Natural Science Foundation of China (21502047); Partially Supported by Hubei Provincial Collaborative Innovation Center for Optoelectronics
基于置换法的硫离子荧光传感器的研究及其在生物细胞成像中的应用
程晓红*, 钟志成, 李望南
(湖北文理学院 低维光电材料与器件湖北省重点实验室, 湖北 襄阳 441053)
报道了一种基于香豆素衍生物与铜离子的络合物(C1-Cu),可通过间接的方法检测硫离子。络合物C1-Cu的溶液中加入硫离子后,表现出明显的荧光增强响应,检测灵敏度高,检出限低达90 nmol/L;响应速度快,可实现硫离子的实时检测;荧光强度变化与硫离子浓度呈现良好的线性关系,能准确地定量分析硫离子浓度。上述络合物C1-Cu对硫离子的检测具有超高的选择性,即使在其他阴离子存在下也能高效地识别出硫离子;得益于香豆素良好的水溶性和生物相容性,上述络合物C1-Cu可实现生物细胞中硫离子的荧光成像。除荧光光谱响应之外,该体系对硫离子亦有紫外-可见光谱响应,无需借助任何仪器便可实现对硫离子方便、快捷的“裸眼”检测。
置换法; 荧光增强; 硫离子; 水溶性; 生物成像
1000-7032(2017)06-0768-12
O622.4 Document code: A
10.3788/fgxb20173806.0768
*CorrespondingAuthor,E-mail:chengxiaohong0807@126.com
