White Long Persistent Phenomenon from Sr3Al2O5Cl2∶Eu2+, Tm3+Nano-fiber
2017-06-19ZHANGQiuhanTANHuiyingLIUYongchongZHANGYiyingWANGZhengliang
ZHANG Qiu-han, TAN Hui-ying, LIU Yong-chong, ZHANG Yi-ying, WANG Zheng-liang
(KeyLaboratoryofComprehensiveUtilizationofMineralResourceinEthnicRegions,SchoolofChemistry&Environment,YunnanMinzuUniversity,Kunming650500,China)
White Long Persistent Phenomenon from Sr3Al2O5Cl2∶Eu2+, Tm3+Nano-fiber
ZHANG Qiu-han, TAN Hui-ying, LIU Yong-chong, ZHANG Yi-ying, WANG Zheng-liang*
(KeyLaboratoryofComprehensiveUtilizationofMineralResourceinEthnicRegions,SchoolofChemistry&Environment,YunnanMinzuUniversity,Kunming650500,China)
White long persistent phosphors, Sr3Al2O5Cl2∶Eu2+, Tm3+,have been successfully prepared by the solid state reaction at 800 ℃. The as-prepared sample Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05 Tm3+shares single phase and regular nano-fiber morphology. This sample exhibits two broad emission bands located at ~ 448 nm and ~ 590 nm, respectively, and its white persistent duration is about 20 min (> 0.35 mcd/m2). At last, intense white light can be found from the white light-emitting diode with Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05 Tm3+.
long persistent properties; phosphors; photo-luminescent properties
1 Introduction
Long persistent phosphors (LPPs), which can absorb energy and then gradually emit visible light after the light source is removed, have attracted more and more interests, since they can find applications on the road signs, graphic arts, emergency lighting, and so on[1-3]. A lot of tri-color LPPs doped with rare earth ions (such as Eu2+, Pr3+and Eu3+) have been investigated in detail[4-7]. Then white LPPs can be obtained by mixing the currently available tri-color phosphors. However, this method to fabricate the white LPPs suffers from some shortcomings, such as, inconsistent color as white all the time, re-absorption of the blue light by green and red LPPs and the high manufacturing cost[8]. In order to resolve these problems, the white LPPs with white emission were developed from single phase host doped with single luminescent center. Such as, Dy3+can exhibit white long afterglow properties in many silicates[9-11]. However, these kind of LPPs share low emission efficiency due to the the f-f transitions of Dy3+with weak excitation peaks in UV regions, Therefore, it is of significance to develop novel highly efficient white LPPs.
Eu2+is an excellent luminescent center with the broad excitation and emission bands in many LPPs[12-14]. Such as Eu2+ions exhibits green and blue emitting in classical LPPs SrAl2O4∶Eu2+, Dy3+and CaAl2O4∶Eu2+, Dy3+[12-13]. Eu2+ion also shares long persistent in Sr3Al2O5Cl2co-doped with other rare earth ions, such as Ce3+, Tm3+, or Dy3+[15-17]. As we known, three kinds of sites of Sr2+exit in the structure of Sr3Al2O5Cl2[18]. Since the similar ion radii between Eu2+and Sr2+, Eu2+ions would be considered to occupy the sites of Sr2+with three kinds of sites in such LPPs. This means that Eu2+would exhibit different emissions in different crystal sites. For example, the white afterglow properties of Eu2+in Sr3Al2O5Cl2∶Eu2+, Dy3+were investigated in our recent work[18].
In current work, the LPPs Sr3Al2O5Cl2∶Eu2+, Tm3+were successfully prepared by the solid-state reaction at 800 ℃. Although the orange-yellow long persistent phenomenon of Sr3Al2O5Cl2∶Eu2+,Tm3+had been reported[17], the white long persistent properties in Sr3Al2O5Cl2∶Eu2+,Tm3+are not observed, according to our knowledge.
2 Experiments
The startingmaterials including SrCO3, SrCl2·6H2O, Al(OH)3, EuCl3·6H2O and Tm2O3are of analytical grade and without any purification prior to use. The preparation process of Sr2.96-xAl2O5Cl2∶0.04 Eu2+,xTm3+(x=0.03, 0.05, 0.07, 0.09) is similar with that of Sr3Al2O5Cl2∶Eu2+, Dy3+in our recent work[18]. These LPPs were obtained by firing the starting materials at 800 ℃ for 10 h in a reducing atmosphere (N2∶ H2=95∶5).
Crystal structure of these phosphors was initially characterized by powder X-ray diffraction (D8 Advance,Bruker, Germany) equipped with graphite monochromatized Cu Kα radiation (λ=0.154 06 nm). The SEM image was obtained from field emission scanning electron microscopy (FEI Nova NanoSEM450). Excitation and emission spectra were examined on a Cary Eclipse FL1011M003 (Varian) spectrofluorometer, and the xenon lamp was used as the excitation source. The persistent brightness was measured on a ST-86LA brightness meter. The luminescence decay curve was obtained from a PR305 phosphorophotometer. The performance of light-emitting diode (LED) was recorded on a high accurate array spectrometer (HSP6000).
3 Results and Discussion
The XRD patterns of these LPPs doped with different amounts of Tm3+are displayed in
Fig.1. All the diffraction peaks of each phosphor can be well indexed to the corresponding JCPDS card 80-0564 [Sr3Al2O5Cl2]. This result indicates that the as-prepared LPPs have a single phase with an orthorhombic structure (space groupP212121,a=b=c=0.942 2 nm). A little co-doping of Eu2+and Tm3+ions does not induce any significant structure change. Since there are three kinds of Sr2+sites in Sr3Al2O5Cl2, Eu2+would share three different crystal sites in these LPPs.

Fig.1 XRD patterns of Sr2.96-xAl2O5Cl2∶0.04Eu2+,xTm3+(x= 0.03, 0.05, 0.07, 0.09).
Fig.2 is the SEM images of the sample Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05Tm3+. Obviously, the obtained phosphor is composed of a number of regular nano-fiber. The diameter of the nano-fiber is about 500 nm according to the closer view of these nano-fibers in
Fig.2(b). Moreover, smooth surfaces can be observed from these nano-fibers, indicating that this sample is in good crystallization.

Fig.2 SEM images of the obtained Sr2.91Al2O5Cl2∶ 0.04Eu2+, 0.05Tm3+
The photo-luminescent (PL) spectra of Sr2.96-x-Al2O5Cl2∶ 0.04Eu2+,xTm3+(x=0.03, 0.05, 0.07, 0.09) are shown in
Fig.3(a). The broad excitation band at ~340 nm with a full width at half maximum (FWHM) of 100 nm is due to the 4f-5d transitions of Eu2+. All samples exhibit similar excitation spectra with different excitation intensity. From the emission spectra in
Fig.3(a), two broad emission bands can be observed at blue region around 448 nm and orange-red region around 590 nm. The white emission composed of these two emission bands would originate from the 4f65d1→ 4f7transition of Eu2+at different crystal sites. In current work, the blue emission of Eu2+is obviously improved. This phenomenon also can be observed in Sr3Al2O5Cl2∶Eu2+, Dy3+prepared with EuCl3·6H2O[18]. As we know, the luminescent properties of Eu2+are highly influenced by the crystal field of the surrounding ions. The coordination environment of Eu2+prepared with EuCl3·6H2O as raw materials is different from that with Eu2O3. So the distribution of three kinds of Eu2sites in these phosphors would lead to the different emission intensity of Eu2+.
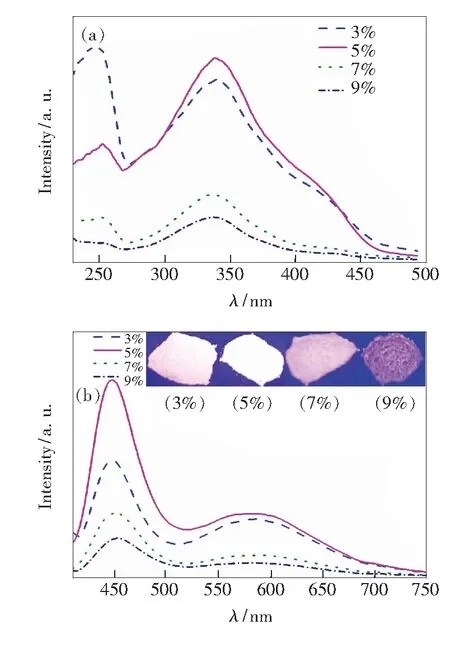
Fig.3 Excitation (a) and emission (b) spectra of Sr2.96-x-Al2O5Cl2∶ 0.04Eu2+,xTm3+(x=0.03, 0.05, 0.07, 0.09)
With the increasing content of Tm3+, the intensities of the blue emission and orange emission are enhanced in these phosphors. When the mole fraction of Tm3+is 0.05, the sample Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05Tm3+shows the strongest white emission among these phosphors, and bright white light can be observed from this phosphor under 365 nm light. The corresponding CIE coordinates according the emission spectrum of Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05Tm3+are calculated to be:x=0.268,y=0.231.
Fig.4 is the decay curve of Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05Tm3+sample. This decay curve is fitted into the following double-exponential decay equation:
(1)
whereIis the luminescent intensity of the samples,I1andI2are constants,tis the time,τ1andτ2are the decay constants. According to the above equation,τ1andτ2values are 0.49 s and 2.89 s, respectively. And the persistent duration was measured to be 20 min (> 0.35 mcd/m2). Prior to the measurement for persistent brightness, the selected sample was excited for 5 min using 365 nm UV radiation standard lamp.

Fig.4 Decay curve of Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05Tm3+
In order to further investigate the luminescent properties of Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05Tm3+, the white LED was fabricated by coating this phosphor on a InGaN chip (~395 nm). Its electro-luminescent spectrum (EL) is displayed in
Fig.5. Two intense emission of this phosphor can be found in this EL spectrum, and bright white light can be observed from this white LED with the appropriate CIE value of (0.396, 0.319), which is close to the National Television Standards Committee (NTSC)
standard value for white (0.33,0.33). The other performance of this white LED is as follows: color rendering index is 87.9, color temperature is 3 011 K, and luminous efficiency is 7.31 lm/W.

Fig.5 EL spectrum of white LED based on Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05Tm3+under 20 mA drive current
4 Conclusion
In summary, white LPPs Sr3Al2O5Cl2∶Eu2+, Tm3+were preparedviathe solid state reaction with EuCl3·6H2O as raw materials. The sample Sr2.91-Al2O5Cl2∶0.04Eu2+, 0.05Tm3+exhibits regular nano-fiber morphology and intense white-emitting under UV light excitation. Intense white light can be observed from the white LED based on this single phosphor with the appropriate CIE value of (0.396, 0.319).
[1] LI Y, ZHOU S F, LI Y Y,etal.. Long persistent and photo-stimulated luminescence in Cr3+-doped Zn-Ga-Sn-O phosphors for deep and reproducible tissue imaging [J].J.Mater.Chem. C., 2014, 2:2657-2663.
[2] VAN DEN EECKHOUT K, POELMAN D, SMET P F. Persistent luminescence in non-Eu2+-doped compounds: a review [J].Materials, 2013, 6(7):2789-2818.
[3] MALDINEY T, LECOINTRE A, VIANA B,etal.. Controlling electron trap depth to enhance optical properties of persistent luminescence nanoparticles forinvivoimaging [J].J.Am.Chem.Soc., 2011, 133(30):11810-11815.
[4] JIN Y H, HU Y H, CHEN L,etal.. A novel orange emitting long afterglow phosphor Ca3Si2O7∶Eu2+and the enhancement byR3+ions (R=Tm, Dy and Er) [J].Mater.Lett., 2014, 126:75-77.
[5] WU H Y, HU Y H, CHEN L,etal.. Enhancement on the afterglow properties of Sr2MgSi2O7∶Eu2+by Er3+codoping [J].Mater.Lett., 2011, 65(17-18):2676-2679.
[6] ZHANG S A, HU Y H, CHEN L,etal.. Luminescence properties of the pink emitting persistent phosphor Pr3+-doped La3GaGe5O16[J].RSCAdv., 2015, 5(47):37172-37179.
[7] SOM S, DUTTA S, KUMAR V,etal.. CaTiO3∶Eu3+, a potential red long lasting phosphor: Energy migration and characterization of trap level distribution [J].J.AlloyCompd., 2015, 622:1068-1073.
[8] CHEN Y H, CHENG X R, LIU M,etal.. Comparison study of the luminescent properties of the white-light long afterglow phosphors: CaxMgSi2O5+x∶Dy3+(x=1, 2, 3) [J].J.Lumin., 2009, 129(5):531-535.
[9] LIU Y L, LEI B F, SHI C S. Luminescent properties of a white afterglow phosphor CdSiO3∶Dy3+[J].Chem.Mater., 2005, 17(8):2108-2113.
[10] XU X H, HE Q L, YAN L T,etal.. White-light long persistent and photo-stimulated luminescence in CaSnSiO5∶Dy3+[J].J.AlloyCompd., 2013, 574:22-26.
[11] WEI R P, JU Z H, MA J X,etal.. A novel white afterglow phosphorescent phosphor Ca3SnSi2O9∶Dy3+[J].J.AlloyCompd., 2009, 486(1-2):L17-L20.
[12] CHEN R, WANG Y H, HU Y H,etal.. Modification on luminescent properties of SrAl2O4∶Eu2+, Dy3+phosphor by Yb3+ions doping [J].J.Lumin., 2008, 128(7):1180-1184.
[13] LIU B, SHI C S, QI Z M. Potential white-light long-lasting phosphor: Dy3+-doped aluminate [J].Appl.Phys.Lett., 2005, 86(19):191111-1-3.
[14] GUO H J, CHEN W B, ZENG W,etal.. A long-lasting phosphor Ba3P4O13∶Eu2+[J].ECSSolidStateLett., 2015, 4(1):R1-R3.
[15] CHEN R, HU Y H, CHEN L,etal.. Luminescent properties of a novel afterglow phosphor Sr3Al2O5Cl2∶Eu2+, Ce3+[J].Ceram.Int., 2014, 40(6):8229-8236.
[16] DUTCZAK D, RONDA C, MEIJERINK A,etal.. Red luminescence and persistent luminescence of Sr3Al2O5Cl2∶Eu2+, Dy3+[J].J.Lumin., 2013, 141:150-154.
[17] LI Y Q, WANG Y H, GONG Y,etal.. Design, synthesis and characterization of an orange-yellow long persistent phosphor: Sr3Al2O5Cl2∶Eu2+, Tm3+[J].Opt.Express, 2010, 18(24):24853-24858.
[18] ZHANG Q H, RONG M Z, TAN H Y,etal.. Luminescent properties of the white long afterglow persistent phosphors: Sr3Al2O5Cl2∶Eu2+, Dy3+[J].J.Mater.Sci.Mater.Electron., 2016, 27(12):13093-13098, doi: 10.1007/s10854-016-5453-x.

张秋函(1992-),女,云南昆明人, 硕士研究生,2014年于云南民族大学获得学士学位,主要从事发光材料的研究。

E-mail: 272299028@qq.com汪正良 (1977-),男,安徽东至人,博士,教授,2006年于中山大学获得博士学位,主要从事发光材料的研究。
E-mail: wangzhengliang@foxmail.com
2016-11-28;
2017-02-06
国家自然科学基金(21261027) 资助项目 Supported by National Natural Science Foundation of China (21261027)
纳米纤维状Sr3Al2O5Cl2∶Eu2+, Tm3+的白色长余辉性能
张秋函, 谭慧英, 刘永冲, 张艺颖, 汪正良*
(云南民族大学 化学与环境学院民族地区矿产资源综合利用重点实验室, 云南 昆明 650500)
采用高温固相法在800 ℃下制备出系列白色长余辉荧光粉Sr3Al2O5Cl2∶Eu2+, Tm3+,并研究了它们的结构、形貌及发光性能。样品Sr2.91Al2O5Cl2∶0.04Eu2+, 0.05 Tm3+具有单一晶相和纳米纤维结构。该样品在紫外光激发下表现出两个很强的宽带发射(分别位于~448 nm和~590 nm)。它的余辉寿命大约是20 min。利用此种荧光粉所制作出的白光LED器件表现出很强的白光发射。
长余辉性能; 荧光粉; 发光性能
1000-7032(2017)06-0697-05
O482.31 Document code: A
10.3788/fgxb20173806.0697
*CorrespondingAuthor,E-mail:wangzhengliang@foxmail.com
