酿酒酵母细胞中内质网应激与未折叠蛋白反应的研究进展
2017-06-01赵运英蒋伶活
赵运英, 王 頔, 袁 凡, 蒋伶活
(江南大学生物工程学院 粮食发酵工艺与技术国家工程实验室, 江苏 无锡 214122)
酿酒酵母细胞中内质网应激与未折叠蛋白反应的研究进展
赵运英, 王 頔, 袁 凡, 蒋伶活*
(江南大学生物工程学院 粮食发酵工艺与技术国家工程实验室, 江苏 无锡 214122)
内质网应激激活的未折叠蛋白反应(Unfolded protein response,UPR)途径在酿酒酵母和哺乳动物细胞中是非常保守的。内质网(Endoplasmic reticulum,ER)是蛋白质合成、折叠和修饰的细胞器,也是贮存钙的主要场所之一。酵母细胞内质网钙平衡与UPR的作用是相互的;两个MAPK途径——HOG途径和CWI途径都是细胞应答内质网应激压力时生存所必需的;重金属镉离子能够激活UPR途径,它通过激活钙离子通道Cch1/Mid1进入细胞影响钙离子的功能。本文结合最新研究进展对酿酒酵母细胞中的两个MAPK途径、镉离子和钙离子稳态与内质网应激激活的UPR途径之间相互关系进行综述。
未折叠蛋白反应;内质网应激;MAPK;钙离子信号途径;酿酒酵母
细胞应激涉及线粒体、内质网和细胞核的应激。内质网(Endoplasmic reticulum,ER)是蛋白质合成、折叠和修饰的细胞器,也是贮存钙的主要场所之一。内质网功能的紊乱会导致未折叠蛋白在内质网腔内积累及其腔内钙稳态的破坏。缺氧、营养缺乏、氧化还原状态的破坏、异常的钙离子调节、病毒感染、蛋白翻译后修饰发生故障或者内质网中未折叠或错误折叠蛋白质的积累均可导致内质网应激(Endoplasmic reticulum stress,ER stress)[1-5]。内质网应激与许多人类疾病的发生和发展密切相关,如癌症、糖尿病、炎性疾病以及神经系统、肾脏、肺和心血管疾病等[6-10]。许多研究证明癌细胞可以抵抗极端的环境压力,可能是由于癌细胞改变了内质网应激反应的正常状态[11]。酿酒酵母作为一种模式生物,被广泛用于科学研究。内质网应激激活的未折叠蛋白反应(Unfolded protein response,UPR)途径在酿酒酵母和哺乳动物细胞中是非常保守的。本文将论述酿酒酵母细胞中内质网应激与未折叠蛋白反应的最新研究进展。
1 酵母细胞中内质网应激和UPR概述
1.1 酵母细胞和哺乳动物细胞的UPR组分
内质网压力是指未折叠蛋白在内质网内积累,从而诱导一种可适应性程序称作未折叠蛋白反应。内质网应激激活的UPR反应是1988年由Kozutsumi等人观察到的非折叠蛋白反应[12]。后来通过酵母遗传学研究发现了决定UPR反应的蛋白因子,UPR这一独特的细胞反应才得到普遍的认可[13-14]。酿酒酵母内质网膜上的跨膜蛋白Ire1(Inositol requiring enzyme 1)是一种核酸内切酶。在无内质网应激条件下,Ire1的内质网腔内结构域与内质网内的分子伴侣蛋白Grp38结合[15]。当错误折叠或者未折叠蛋白聚集时,Grp38转而与这些蛋白结合,Ire1得到释放并激活,形成聚合体,非常规地剪接胞质内无翻译活性的HAC1 mRNA[16-17](图1a,表1)。剪接后的HAC1 mRNA被翻译为转录因子蛋白Hac1,Hac1进入细胞核中结合靶基因启动子上的UPR元件,诱导靶基因的表达,以缓解错误折叠或者未折叠蛋白造成的内质网应激压力。这些靶基因的启动子上都含有UPR元件,包括KAR2、PDI1、EUG1、FKB2、LHS1、AIM17、ERJ5、FIT2、FIT3、FRE1、GAS5、HXT9、KEG1、LDB17、MTR10、SIL1、TAD2和VPS17等内质网应答相关基因[18-20]。
在哺乳动物的内质网膜上,有三种跨膜蛋白:IRE1、PERK (Protein kinase receptor-like ER kinase)和ATF6 (Activating transcription factor 6)[21]。三者在内质网腔内的结构域均与内质网分子伴侣Grp78(Glucose-regulated protein 78)结合而处于非活化状态。内质网中的未折叠蛋白或错误折叠蛋白增加时,这三种跨膜蛋白与Grp78分离而被激活。哺乳动物有酵母Ire1的同源体IRE1α 和IRE1β,但尚未发现酵母Hac1的同源体。但是,哺乳动物细胞的ATF6与酵母Hac1具有序列同源性[21]。哺乳动物UPR的启动依赖于IRE1α 把XBP1u mRNA剪接为XBP1s mRNA,后者的翻译产物有转录因子的功能;同时,还依赖于高尔基体的两个蛋白酶S1P和S2P分步酶切ATF6,使其成为有活性的转录因子(图1b和1d,表1)。ATF6激活UPR基于其结合靶基因的顺式作用元件[22]。PERK可以磷酸化翻译起始因子eIF2,导致细胞整体翻译水平的下调,从而降低内质网中的蛋白负载量;同时,诱导转录因子ATF4的翻译(图1c,表1)。

表1 酵母细胞和哺乳动物细胞的UPR组分比较
UPR在酵母和哺乳动物细胞中普遍存在,并且两者UPR激活的分子机制都存在明显的相同点[25-26]。在酵母和哺乳动物细胞中,许多条件能够诱导UPR,如抑制糖基化反应(衣霉素)、干扰二硫键的正常形成(DTT,β-巯基乙醇)以及破坏钙离子体内平衡等能够引起内质网未折叠/错误折叠蛋白积累的条件。在酵母中,这些条件激活Ire1,从而激活HAC1 mRNA(在哺乳动物细胞中是XBP1 mRNA)的剪切,形成有功能的转录因子Hac1(XBP1)。在酵母细胞中HAC1 mRNA和哺乳动物细胞中XBP1 mRNA的剪切被认为是内质网压力的典型标志。除了UPR,内质网压力也能触发其他反应,尤其是钙离子的内流,这对细胞存活是很重要的[27],这一过程能够帮助蛋白质折叠。钙离子的内流过程通过Slt2 MAPK途径被激活,但不依赖于UPR[27-28]。
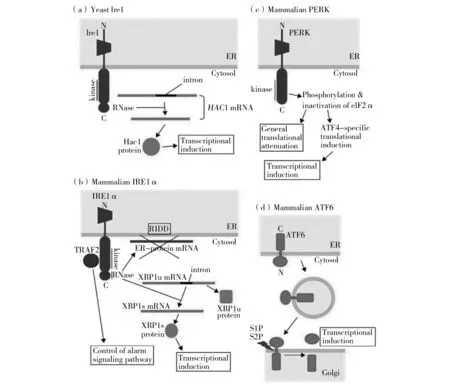
图1 酵母细胞(a)和哺乳动物细胞(b~d)中内质网应激的感受器和UPR反应[23-24]Fig.1 ER-stress sensors and responses in yeast and mammalian cells[23-24]
1.2 酵母细胞中UPR的类型

2 内质网应激与有丝分裂原蛋白激酶(Mitogen-activated protein kinase, MAPK)途径的关系
2.1 内质网应激与高渗透压甘油形成途径(High Osmolarity Glycerol, HOG)的关系
当未折叠蛋白积聚于内质网引起内质网压力时,未折叠蛋白反应迅速响应诱导转录程序以缓解压力。然而,在极端条件下,当UPR激活不足以减轻内质网压力时,内质网压力可能会持续较长时间。在不能通过立即激活UPR解决内质网压力的情况下,细胞是如何反应来抵抗内质网压力的还不清楚。研究表明,酿酒酵母细胞中的MAP激酶-Hog1在内质网应激阶段后期被磷酸化并且帮助内质网恢复动态平衡[32]。在内质网应激反应后期,Hog1磷酸化移位进入细胞核,调节基因表达。随后,Hog1返回细胞质,在细胞质中其磷酸化水平仍然很高,这有助于细胞自噬的激活,其中Atg8是一个关键的自噬蛋白。因此,Hog1在抵抗内质网压力上有多方面的功能。
在酵母细胞中,UPR的激活依赖于IRE1和HAC1两个基因。内质网应激可以诱导Hog1的磷酸化,但UPR的诱导表达并不依赖于HOG途径。UPR通过一种特殊的机制调节Hog1的磷酸化:内质网应激条件下,UPR可以促进Hog1的磷酸化,Hog1的磷酸化需要IRE1和HAC1,以及HOG途径的Ssk1支路。高渗胁迫条件下,UPR对Hog1的磷酸化没有影响;CWI途径条件下,UPR可以抑制Hog1的磷酸化。研究表明[32-33],在内质网应激条件下,HOG途径中相关基因的缺失株表现出生长缺陷,这说明HOG途径对内质网应激反应是必需的。但是,HOG途径在内质网应激反应中的作用机制并不清楚。
2.2 内质网应激与细胞壁完整性途径(Cell Wall Integrity, CWI)的关系
酵母细胞壁是一种依赖于分泌蛋白和膜蛋白构成其组分的胞外结构,而内质网具有运输新合成的分泌蛋白和膜蛋白的功能。蛋白质量控制机制在细胞壁完整性方面作用的研究表明,未折叠蛋白反应(UPR)以及内质网相关的蛋白质降解(Endoplasmic Reticulum-Associated Degradation, ERAD)途径对于构成细胞壁组分是必需的[34]。IRE1的无意突变株、ERAD组分hrd1和ubc7的双缺失株以及ire1缺失株或错误折叠蛋白的表达都与细胞壁蛋白的突变株表现出相似的表型,包括对破坏细胞壁化合物的高度敏感、对细胞壁蛋白层的改变、减少细胞壁的厚度以及增加细胞凝集度。与在细胞壁完整性中的重要作用一致,在细胞壁胁迫或未受胁迫生长状态下,UPR可以被细胞壁完整性途径的MAP激酶信号途径激活。细胞壁胁迫和本底UPR活性都受到Swi6p的调节,Swi6p是一种细胞循环和细胞壁胁迫基因转录的调节子,以一种不依赖于已知的共调节分子的方式进行调节。另外,Mpk1在应对内质网胁迫过程中被磷酸化而激活,并且在内质网胁迫条件下直接或间接激活Cch1-Mid1 Ca2+通道[28]。能引起内质网胁迫的各种试剂也会导致G2/M期的Swe1依赖性延迟或停止。然而,大多数细胞在任何条件下,Mpk1和钙调磷酸酯酶对于G2/M期的延迟都不是必需的。反过来,Swe1对于CCS途径的运行不是必需的。在酵母中,MPK1激酶信号到达Cch1-Mid1 Ca2+通道是应对内质网胁迫最主要的和最基本的方式[28]。
IRE1的缺失或错误折叠蛋白的表达能够导致细胞对CWI途径压力敏感。在CWI途径压力作用下,UPRE-lacZ的表达活性被诱导,HAC1的mRNA的剪接和UPRE-lacZ的表达诱导依赖于Mpk1、Ire1和CWI途径中细胞膜感受器Mid2及下游转录因子Swi6[34]。然而,内质网应激条件下,Mpk1可以被磷酸化而激活但不依赖于Ire1,HAC1的mRNA的剪接也不依赖于Mpk1[35]。内质网应激反应主要是通过CWI途径中细胞膜感受器Wsc1进行信号传导,从而激活Mpk1磷酸化。表型分析也表明,WSC1和MPK1基因的缺失可以导致酵母细胞对内质网应激压力敏感。
3 镉离子与UPR的关系
镉(Cd)是一种重要的重金属环境污染物。空气中的镉主要来自家庭或工业产生的废气、汽车尾气、金属加工行业、电池或油漆制造业以及废物的处理过程。镉可以从污染源地随着空气传播,污染食物或水质。烟叶本身可以积累较高浓度的镉,因而吸烟是镉污染的重要来源之一[36]。近来的流行病学案例研究进一步证实镉具有致癌性。肺癌、前列腺癌、胰腺癌和肾癌的发生都与长期接触镉有关[37-39]。镉离子是一种毒性很强的金属离子,可以在不同的细胞水平引起许多毒害作用。首先,镉是一种能够诱导细胞突变的化合物。镉离子引起的超突变主要是通过抑制DNA修复系统中相关酶的活性完成的[40-41]。主要的机制有两种,一是通过高亲和力结合到蛋白活性位点的半胱氨酸残基上,抑制酶的活性[41];二是通过替换金属蛋白酶结合的锌离子和钙离子抑制酶的活性[42-44]。其次,镉可以引起氧化胁迫(ROS)。镉能够提高细胞内的ROS水平从而提高脂类的过氧化作用和ROS相关的DNA损伤[45-48]。第三,镉也可以引起酵母细胞和许多哺乳动物细胞类型的凋亡[45,49-50]。这个过程涉及到caspase-依赖的细胞凋亡和caspase-不依赖的细胞凋亡[51-53]。最新的研究表明,对于许多细胞而言,镉依赖的细胞凋亡是内质网应激反应应答的结果[54-55]。到目前为止,镉的细胞和分子生物学毒性机制尚未清楚。研究表明[56],内质网应激反应应答途径中的功能基因IRE1和HAC1的缺失株对镉耐受性是必需的,镉能够通过诱导UPR和HAC1的mRNA剪接造成酿酒酵母细胞中内质网压力,因此在酵母细胞中内质网是镉离子毒性的靶目标。在哺乳动物细胞中,镉离子是诱导内质网压力的典型标志[54,57-58]。在肾小管细胞中,镉离子激活哺乳动物UPR的三个主要分支(PERK/eIF2α、IRE1/XBP1 和 ATF6 途径)[54];在纤维细胞中镉离子至少能激活IRE1/XBP1途径[58]。这表明在哺乳动物细胞中,内质网可能是镉离子毒性的靶位点。因此,镉在内质网积累的结果也可以直接导致诱导内质网应激反应和镉毒性。另外,扰乱钙离子的内平衡是镉离子毒性的另一个重要方面,镉离子不能够抑制内质网蛋白质二硫键的形成,但是能够扰乱钙离子代谢:镉离子激活钙离子通道Cch1/Mid1刺激镉离子进入细胞[56]。
本实验室最近通过对酿酒酵母非必需基因缺失株文库基因组规模的筛选,发现了106个基因缺失株对镉离子敏感,其中包括编码两个MAP激酶途径——HOG途径和CWI途径的相关组分。进一步研究发现,HOG途径中Sho1和Sln1分支都参与了镉胁迫下对Hog1磷酸化的激活[59]。在 CWI 途径中,镉激活Slt2的磷酸化是通过膜感受器Mid2p将信号通过 GEFs-Rom1p传递到Rho1,进而激活PKC途径中的MAP激酶[60]。在这些研究基础上,将进一步研究镉离子诱导的UPR途径与HOG途径和CWI途径的关系。
4 内质网应激与钙离子信号途径的关系
酵母细胞内质网钙平衡与UPR的作用是相互的,酵母细胞中内质网中钙的排空能够激活依赖于Ire1和Hac1的未折叠蛋白反应信号途径[27]。内质网的排空刺激也可以使钙通过细胞膜上的钙通道Cch1-Mid1和另外一个未知系统(转运蛋白X和M)流入细胞内。内质网上错误折叠蛋白激活钙输入系统的能力是不依赖于Ire1p和Hac1p的,并且钙的流入和信号因子也不是起始UPR信号途径所必须的。在内质网应激反应条件下,CWI途径中的Mpk1被磷酸化而激活[35]。而Mpk1可以直接或间接激活位于细胞膜上的钙通道Mid1和Cch1,从而使钙流入细胞内。钙通道、钙调蛋白、钙调磷酸酯酶和其他因子的激活是细胞应答内质网压力时所必需的。
哺乳动物细胞的内质网通过内质网上高Ca2+亲和力的钙泵Ca2+-ATPase (sarco-endoplasmic reticulum Ca2+ATPase,SERCA)家族的激活积累高浓度的钙[61-62]。这个钙库对蛋白质的迁移、折叠、糖基化、二硫键的形成和通过内质网滞留分子伴侣来分选分泌蛋白是非常重要的[63]。引起内质网内钙排空的药物,如钙载体和螯合剂、SERCA泵的抑制剂和钙释放通道的激活剂等,都可以影响内质网中蛋白的折叠效率,从而激活UPR信号途径[64]。酵母细胞缺少SERCA-型钙泵的同源物,但是可以表达哺乳动物分泌途径Ca2+-ATPases(SPCAs)的同源物Pmr1[65]。Pmr1定位在高尔基体上,为高尔基复合体上高效糖基化、蛋白加工和分选反应提供需要的钙离子和锰离子。与用SERCA抑制剂处理的哺乳动物细胞一样,pmr1缺失株表现为通过高亲和力钙通道Cch1和Mid1高效的流入钙,并表现出对内质网应激反应敏感的表型[66]。钙离子稳态的破坏有助于癌症、老年痴呆症和心血管疾病的发生和加重[67-68]。编码人体钙泵蛋白SERCA的基因ATP2A2突变导致毛囊角化病, 而ATP2A2的活性下降会妨碍钙的吸收,这是心脏衰竭的标志[69-70]。这些钙离子稳态相关的人体疾病与内质网钙离子稳态失衡造成的内质网应激相关。
阐明钙在酵母细胞内质网中的作用是非常困难的。酵母细胞的钙结合蛋白在没有钙的情况下也可以发挥功能。另外,酵母细胞中内质网的钙浓度比哺乳动物低10~100倍[62]。缺失Pmr1的突变株积累正常内质网中一半的钙离子,但是钙离子浓度的降低并不能激活UPR信号途径,这说明酵母细胞内质网腔内的高钙离子浓度对蛋白质的折叠并不是必须的[71]。由于SERCA同源物已经在动物、植物、无脊椎动物以及其他真菌中发现[72],在芽殖酵母进化过程中SERCA-样酶的缺失反应了钙离子在内质网折叠反应中作用的降低。另外,其他钙离子-ATPases,例如Pmr1p、Pmc1p或者新发现的P型-ATPase Cod1/Spf1[73],都补偿了酵母细胞中原有SERCA的缺失。文献报道[27],内质网中蛋白质折叠或者麦角固醇合成的抑制剂都会刺激钙离子的流入(通过Cch1-Mid1钙通道和其他途径)和/或激活细胞存活所必须的钙信号途径。这种钙离子细胞存活途径在其他治病酵母菌——白色念珠菌和光滑假丝酵母菌中可以被激活[74]。
前期工作中,通过基因组规模的遗传学筛选,我们发现120个基因的缺失导致酿酒酵母细胞对钙敏感[75],包括7个ESCRT基因(SNF7、SNF8、VPS20、STP22、VPS25、VPS28和VPS36)的缺失株。内质网/高尔基体膜上的钙泵Pmr1是维持内质网钙浓度所必须的,PMR1的缺失可以导致对内质网应激反应敏感。我们进一步发现,7个ESCRT基因通过Rim101/ Nrg1这条负向调控信号途径来调控PMR1的表达(图2)[76]。在上述研究的基础上,对以前我们发现的120个钙敏感基因缺失株进行内质网应激反应敏感筛选,找出对钙和内质网应激压力均敏感的基因缺失株,进而研究钙在内质网应激反应应答过程中的作用。

图2 PMR1的正调控和负调控Fig.2 Positive and negative regulation of PMR1 gene
5 结论与展望
除了作为蛋白质合成、折叠和翻译后修饰的部位,内质网还是细胞内钙主要的存储位置,并通过多个集成系统维持钙离子稳态。内质网内的钙离子由钙泵蛋白SERCA从胞质内摄入,降低SERCA表达会导致内质网内的钙离子储存耗竭和内质网应激相关的细胞凋亡,而SERCA的过度表达则可以减轻内质网应激程度。因此,内质网内的钙离子稳态直接与内质网应激相关。阐明内质网应激过程的功能和调控机制对进一步了解以上人体相关疾病的发生机理有十分重要的理论意义。酿酒酵母作为一种模式生物,被广泛用于科学研究。因此,研究酿酒酵母细胞内的UPR应答机制将为了解哺乳动物细胞的相关机制提供重要线索。
镉可以诱导酵母和动物细胞凋亡,而镉诱导的细胞凋亡是内质网应激的结果,内质网是镉的靶细胞器。目前,镉的细胞毒性作用已有很多报道,但其作用靶点及毒性机理并不十分明确。镉可以影响DNA复制和修复、细胞周期进程、生长和分化以及凋亡。镉能干扰细胞生长所必需的钙、锌、铁等离子的稳态。虽然镉本身没有氧化还原活性,不能与DNA直接作用,但它可以间接诱导氧化胁迫,破坏基因组的完整性。而细胞内的氧化胁迫是诱导内质网应激的因素之一。镉可以激活两个MAPK激酶Hog1和Slt2的磷酸化,而这两个MAPK与细胞内的各种应激反应是密切相关的。因此镉和内质网应激的关系值得深入研究。
[1]Xu C,Bailly-Maitre B,Reed JC.Endoplasmic reticulum stress:cell life and death decisions[J].Journal of Clinical Investigation,2005,115(10):2656-2664.
[2]Cao SS,Kaufman RJ.Endoplasmic reticulum rtress and oxidativestress in cell fate decision and human disease[J].Antioxidants and Redox Signaling,2014,21(3):396-413.
[3]He B.Viruses,endoplasmic reticulum stress,and interferon responses[J].Cell Death and Differentiation,2006,13(3):393-403.
[4]Han J,Back SH,Hur J.ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death[J].Nature Cell Biology,2013,15(5):481-490.
[5]Malhotra JD,Miao H,Zhang K.Antioxidants reduce endoplasmic reticulum stress and improve protein secretion[J].Proceedings of the National Academy of Sciences of the United States of America,2008,105(47):18525-18530.
[6]Mekahli D,Bultynck G,Parys JB,et al.Endoplasmic reticulum calcium depletion and disease[J].Cold Spring Harbor Perspectives in Biology,2011,3(6).pii:a004317.doi:10.1101/cshperspect.a004317.
[7]Wang S,Kaufman RJ.The impact of the unfolded protein response on human disease[J].Journal of Cell Biology,2012,197(7):857-867.
[8]Yoshida H.ER stress and diseases[J].Febs Journal,2007,274(3):630-658.
[9]Oyadomari S,Araki E,Mori M.Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells[J].Apoptosis,2002,7(4):335-345.
[10]Liang CP,Han S,Li G,et al.Impaired MEK signaling and SERCA expression promote ER stress and apoptosis in insulin-resistant macrophages and are reversed by exenatide treatment[J].Diabetes, 2012,61(10):2609-2620.
[11]Cui J,Kaandorp JA,Sloot PM,et al.Calcium homeostasis and signaling in yeast cells and cardiac myocytes[J].FEMS Yeast Research,2009,9(8):1137-1147.
[12]Kozutsumi Y,Segal M,Normington K,et al.The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins[J].Nature,1988,332(6163):462-464.
[13]Sidrauski C,Chapman R,WALTER P.The unfolded protein response:an intracellular signalling pathway with many surprising features[J].Trends in Cell Biology,1998,8(6):245-249.
[14]Ron D,Walter P.Signal integration in the endoplasmic reticulum unfolded protein response[J].Nature Reviews Molecular Cell Biology,2007,8(7):519-529.
[15]Ma Y,Hendershot LM.The unfolding tale of the unfolded protein response[J].Cell,2001,107(7):827-830.
[16]Kimata Y,Ishiwata-Kimata Y,Ito T,et al.Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins[J].Journal of Cell Biology,2007,179(1):75-86.
[17]Aragon T,van Anken E,Pincus D,et al.Messenger RNA targeting to endoplasmic reticulum stress signalling sites[J].Nature,2009,457(7230):736-740.
[18]Mori K,Ogawa N,Kawahara T,et al.Palindrome with spacer of one nucleotide is characteristic of the cis-acting unfolded protein response element inSaccharomycescerevisiae[J].The Journal of Biological Chemestry,1998,273(16):9912-9920.
[19]Hu Z,Killion PJ,Iyer VR.Genetic reconstruction of a functional transcriptional regulatory network[J].Nature Genetics,2007,39(5):683-687.
[20]MacIsaac KD,Wang T,Gordon DB,et al.An improved map of conserved regulatory sites forSaccharomycescerevisiae[J].BMC Bioinformatics,2006,7:113.
[21]Ye J,Rawson RB,Komuro R,et al.ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs[J].Molecular Cell,2000,6(6):1355-1364.
[22]Lee K,Tirasophon W,Shen X,et al.IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response[J].Genes & Development,2002,16(4):452-466.
[23]Kimata Y, Kohno K. Endoplasmic reticulum stress-sensing mechanisms in yeast and mammalian cells[J].Current Opinion in Cell Biology,2011,23(2):135-142.
[24]Boyce M,Yuan J.Cellular response to endoplasmic reticulum stress: a matter of life or death[J].Cell Death and Differentiation,2006,13(3):363-373.
[25]Bernales S,Papa FR,Walter P.Intracellular signaling by the unfolded protein response[J].Annual Review of Cell and Developmental Biology,2006,22:487-508.
[26]Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease[J]. Neurology, 2006, 66 (2 Suppl 1): S102-109.
[27]Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress[J]. EMBO Journal, 2002, 21(10): 2343-2353.
[28]Bonilla M, Cunningham KW. Mitogen-activated protein kinase stimulation of Ca2+signaling is required for survival of endoplasmic reticulum stress in yeast[J]. Molecular and Cellular Biology, 2003, 14(10): 4296-4305.
[29]Mori K, Ogawa N, Kawahara T, et al. Palindrome with spacer of one nucleotide is characteristic of the cis-acting unfolded protein response element inSaccharomycescerevisiae[J]. The Journal of Biological Chemistry, 1998, 273(16): 9912-9920.
[30]Carrara M, Prischi F, Nowak PR, et al. Crystal structures reveal transient PERK luminal domain tetramerization in endoplasmic reticulum stress signaling[J]. EMBO Journal, 2015, 34(11):1589-1600.
[32]Bicknell AA, Tourtellotte J, Niwa M. Late phase of the endoplasmic reticulum stress response pathway is regulated by Hog1 MAP kinase[J]. The Journal of Biological Chemistry, 2010, 285(23): 17545-17555.
[33]Torres-Quiroz F, Garcia-Marques S, Coria R, et al. The activity of yeast Hog1 MAPK is required during endoplasmic reticulum stress induced by tunicamycin exposure[J]. The Journal of Biological Chemistry, 2010, 285(26): 20088-20096.
[34]Scrimale T, Didone L, de Mesy Bentley KL, et al. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity inSaccharomycescerevisiae[J]. Molecular and Cellular Biology, 2009, 20(1): 164-175.
[35]Babour A, Bicknell AA, Tourtellotte J, et al. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance[J]. Cell, 2010, 142(2): 256-269.
[36]Ashraf MW. Concentrations of cadmium and lead in different cigarette brands and human exposure to these metals via smoking[J]. Journal of Arts, Science & Commerce, 2011, 2: 140-147.
[37]Nawrot T, Plusquin M, Hogervorst J, et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study[J]. The Lancet Oncology, 2006, 7(2): 119-126.
[38]Kellen E, Zeegers MP, Hond ED, et al. Blood cadmium may be associated with bladder carcinogenesis: the Belgian case-control study on bladder cancer[J]. Cancer Detection and Prevention, 2007, 31(1): 77-82.
[39]Gallagher CM, Chen JJ, Kovach JS. Environmental cadmium and breast cancer risk[J]. Aging (Albany NY), 2010, 2(11): 804-814.
[40]Jin YH, Clark AB, Slebos RJC, et al. Cadmium is a mutagen that acts by inhibiting mismatch repair[J]. Nature Genetics, 2003, 34(3): 326-329.
[41]Bravard A, Vacher M, Gouget B, et al. Redox regulation of human OGG1 activity in response to cellular oxidative stress[J]. Molecular and Cellular Biology, 2006, 26: 7430-7436.
[42]Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions[J]. Free Radical Biology and Medicine, 1995, 18(2): 321-336.
[43]Schutzendubel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization[J]. Journal of Experimental Botany, 2002, 53(372): 1351-1365.
[44]Faller P, Kienzler K, Krieger-Liszkay A. Mechanism of Cd2+toxicity: Cd2+inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+site[J]. Biochimica et Biophysica Acta, 2005, 1706(1-2): 158-164.
[45]Pulido MD, Parrish AR. Metal-induced apoptosis: mechanisms[J]. Mutatation Reserach, 2003, 533(1-2): 227-241.
[46]Howlett NG, Avery SV. Induction of lipid peroxidation during heavy metal stress inSaccharomycescerevisiaeand influence of plasma membrane fatty acid unsaturation[J]. Applied and Environmental Microbiology, 1997, 63(8): 2971-2976.
[47]Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage[J]. Current Topics in Medicinal Chemistry, 2001, 1(6): 529-539.
[48]Lopez E, Arce C, Oset-Gasque MJ, et al. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture[J]. Free Radical Biology and Medicine, 2006, 40(6): 940-951.
[49]Nargund AM, Avery SV, Houghton JE. Cadmium induces a heterogeneous and caspase-dependent apoptotic response inSaccharomycescerevisiae[J]. Apoptosis, 2008, 13(6): 811-821.
[50]Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review)[J]. Biochimie, 2006, 88(11): 1549-1559.
[51]Li M, Kondo T, Zhao Q L, et al. Apoptosis induced by cadmium in human lymphoma U937 cells through Ca2+-calpain and caspase-mitochondria-dependent pathways[J]. The Journal of Biological Chemistry, 2000, 275(50): 39702-39709.
[52]Lemarie A, Lagadic-Gossmann D, Morzadec C, et al. Cadmium induces caspase-independent apoptosis in liver Hep3B cells: role for calcium in signaling oxidative stress-related impairment of mitochondria and relocation of endonuclease G and apoptosis-inducing factor[J]. Free Radical Biology and Medicine, 2004, 36(12): 1517-1531.
[53]Coutant A, Lebeau J, Bidon-Wagner N, et al. Cadmium-induced apoptosis in lymphoblastoid cell line: involvement of caspase-dependent and-independent pathways[J]. Biochimie, 2006, 88(11): 1815-1822.
[54]Yokouchi M, Hiramatsu N, Hayakawa K, et al. Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response[J]. Cell Death and Differentiation, 2007, 14(8): 1467-1474.
[55]Yokouchi M, Hiramatsu N, Hayakawa K, et al. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response[J]. The Journal of Biological Chemistry, 2008, 283(7): 4252-4260.
[56]Gardarin A, chedin S, lagniel G, et al. Endoplasmic reticulum is a major target of cadmium toxicity in yeast[J]. Molecular Microbiology, 2010, 76(4): 1034-1048.
[57]Liu F, Inageda K, Nishitai G, et al. Cadmium induces the expression of Grp78, an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial cells[J]. Environmental Health Perspectives, 2006, 114(6): 859-864.
[58]Biagioli M, Pifferi S, Ragghianti M, et al. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis[J]. Cell Calcium, 2008, 43(2): 184-195.
[59]Jiang L, Cao C, Zhang L, et al. Cadmium-induced activation of high osmolarity glycerol pathway through its Sln1 branch is dependent on the MAP kinase kinase kinase Ssk2, but not its paralog Ssk22, in budding yeast[J]. FEMS Yeast Research, 2014, 14(8): 1263-1272.
[60]Xiong B, Zhang L, Xu H, et al. Cadmium induces the activation of cell wall integrity pathway in budding yeast[J]. Chemico Biological Interacttions, 2015, 240: 316-323.

[62]Tadic V, Prell T, Lautenschlaeger J, et al. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis[J]. Frontiers in Cellular Neuroscience, 2014, 8:147.
[63]Nurbaeva MK, Eckstein M, Snead ML, et al. Store-operated Ca2+entry modulates the expression of enamel genes[J]. Journal of Dental Research, 2015, 94(10):1471-1477.
[64]Azzimonti B, Dell'oste V, Borgogna C, et al. The epithelial-mesenchymal transition induced by keratinocyte growth conditions is overcome by E6 and E7 from HPV16, but not HPV8 and HPV38: characterization of global transcription profiles[J]. Virology, 2009, 388(2):260-269.
[65]Strayle J, Pozzan T, Rudolph HK. Steady-state free Ca2+in the yeast endoplasmic reticulum reaches only 10 microM and is mainly controlled by the secretory pathway pump Pmr1[J]. EMBO Journal, 1999, 18(17): 4733-4743.
[66]Locke EG, Bonilla M, Liang L, et al. A homolog of voltage-gated Ca2+channels stimulated by depletion of secretory Ca2+in yeast[J]. Molecular and Cellular Biology, 2000, 20(18): 6686-6694.
[67]Camandola S, Mattson MP. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease[J]. Biochimica et Biophysica Acta, 2011, 1813(5): 965-973.
[68]Varghese J, Rich T, Jimenez C. Benign familial hypocalciuric hypercalcemia[J]. Endocrine Practice, 2011,Suppl 1, 13-17.
[69]Huo J, Liu Y, Ma J, et al. A novel splice-site mutation of ATP2A2 gene in a Chinese family with Darier disease[J]. Archives Dermatological Research, 2010, 302(10): 769-772.
[70]Kho C, Lee A, Jeong D, et al. SUMO1-dependent modulation of SERCA2a in heart failure[J]. Nature, 2011, 477(7366): 601-605.
[71]Devasahayam G, Burke DJ, Sturgill TW. Golgi manganese transport is required for rapamycin signaling inSaccharomycescerevisiae[J]. Genetics, 2007, 177(1):231-238.
[72]Cyert MS, Philpott CC. Regulation of cation balance inSaccharomycescerevisiae[J]. Genetics, 2013 , 193(3):677-713.
[73]Cronin SR, Khoury A, Ferry DK, et al. Regulation of HMG-CoA reductase degradation requires the P-type ATPase Cod1p/Spf1p[J]. Journal of Cell Biology, 2000, 148(5): 915-924.
[74]Brand A, Lee K, Veses V, et al. Calcium homeostasis is required for contact-dependent helical and sinusoidal tip growth in Candida albicans hyphae[J]. Molecular Microbiology, 2009,71(5):1155-1164.
[75]Zhao Y, Du J, Zhao G, et al. Activation of calcineurin is mainly responsible for the calcium sensitivity of gene deletion mutations in the genome of budding yeast[J]. Genomics, 2013, 101(1): 49-56.
[76]Zhao Y, Du J, Xiong B, et al. ESCRT components regulate the expression of the ER/Golgi calcium pump genePMR1 through the Rim101/Nrg1 pathway in budding yeast[J]. Journal of Molecular Cell Biology, 2013, 5(5): 336-344.
Advances in Endoplasmic Reticulum Stress and Unfolded Protein Response inSaccharomycescerevisiae
ZHAO Yun-ying, WANG Di, YUAN Fan, JIANG Ling-huo
(TheNat’lEngin.Lab.forCerealFerment’nTechnol.,Schl.ofBiotech.,JiangnanUni.,Wuxi214122)
Unfolded protein response (UPR) signaling pathway activated by endoplasmic reticulum stress is highly conserved in bothSaccharomycescerevisiaeand mammalian cells. Reticulum Endoplasmic (ER) is an organelle for protein synthesis, folding and modification as well as one of the main places for storage Ca2+.The homeostasis of Ca2+and UPR are interrelated and interact on each other. The two MAPK pathways, HOG pathway and CWI pathway are all necessary for cell survival under ER stress treated conditions. And ion of heavy metal cadmium is also able to activate the UPR pathway and it enters into the cells through activating the calcium channel Cch1/Mid1 to affect the function of calcium ion. The interactions between two MAPK kinase pathways, cadmium or calcium ion homeostasis and UPR signaling activated by endoplasmic reticulum stress inS.cerevisiaecells are all summarized in this review.
unfolded protein response; endoplasmic reticulum stress; MAPK; calcium signaling pathway;Saccharomycescerevisiae
国家自然科学基金项目(81371784,31301021);中国博士后科学基金第55批资助项目(5924130201140280)
赵运英 女,讲师。从事酵母遗传学和分子生物学研究工作。E-mail:yunying1213@hotmail.com
* 通讯作者。男,教授,博士生导师。从事酵母和丝状真菌遗传学与分子生物学研究工作。E-mail:linghuojiang@jiangnan.edu.cn
2016-03-25;
2016-08-31
Q93
A
1005-7021(2017)02-0098-09
10.3969/j.issn.1005-7021.2017.02.017
