Positively charged nanofiltration membrane fabricated by poly(acid-base)complexing effect induced phase inversion method for heavy metal removal☆
2017-05-30ChunliLiuWeihuiBiDongliangChenSuoboZhangHongchaoMao
Chunli Liu ,Weihui Bi,Dongliang Chen ,Suobo Zhang ,3,*,Hongchao Mao ,*
1 Key Laboratory of Ecomaterials,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China
2 University of Chinese Academy of Sciences,Beijing 100039,China
3 Jiangsu National Synergetic Innovation Center for Advanced Materials(SICAM),Nanjing 211816,China
1.Introduction
Nanofiltration is a pressure driving membrane filtration process that features low energy cost in water softening,food chemistry and pharmaceutical manufacturing and so on[1].Nanofiltration membrane is defined to have pore diameters in the range of1-2 nm,and molecular weight cut off(MWCO)for neutral solutes in the range of 150 to 2000,which would penetrate appreciable monovalent ions,but reject specific divalent or multivalent ions that possess the similar charges as the membrane has,especially with substantially higher rejection for multivalent ions[2].Actually,most of the commercially available nanofiltration membranes are polyamide membranes fabricated by interfacial polymerization,which are sensitive and unstable in acidic/basic conditions,as well as to chlorine dissolved in water.Besides,considering the energy consumption and separation efficiency in membrane filtration process,it's of imperative need to develop nanofiltration membranes that are relatively stable and have high performance.Therefore,preparing high performance membranes by other methods rather than interfacial polymerization is one of the approaches for improving membrane efficiency and reducing operation cost.
Up to the present,various methods are developed for the fabrication of high performance nanofiltration membranes,such as nonsolvent induce phase inversion[3,4],polymer deposition[5],single layer coating or layer-by-layer coating[6],surface grafting[7]and so on.Among them,nonsolvent induced phase inversion(NIPS)is the most convenient approach to prepare integrally skinned asymmetric membranes.In the NIPS process,the solvent-nonsolvent exchange between polymer film and coagulation bath occurs immediately upon the immersion of the film into the coagulants,subsequently the polymer precipitates out and a membrane with asymmetric structure can be formed.Membrane formation parameters[8]that are related to the NIPS process including polymer concentration[9],evaporation time and relative humidity[10],solvent and co-solvent type[11],coagulation bath properties[12-14],and so on.Those parameters could profoundly impact the separation performance of the membrane by adjusting the thermal and/or kinetic aspects of the phase inversion process.Among them,altering the coagulation bath properties is the most feasible and contributable method to prepare high performance membranes,which consequently produces several novel derivative phase inversion processes that have already been developed by researches,including the salt-induced phase inversion process[15],alkaline-induced phase inversion process[16],and phase inversion process modified by chemical reaction[17,18].Jin and coworkers fabricated two kinds of high performance superhydrophilic micro filtration membranes by using the same poly-(acrylic acid)-grafted PVDF(PAA-g-PVDF)polymer casting solution to take the one-step phase inversion process in two coagulation bathes,which are sodium chloride aqueous solution and sodium hydroxide aqueous solution,where salt-induced phase inversion process and alkaline-induced phase inversion process occurred,respectively[15,16].Both of those membranes exhibit outstanding filtration performance towards oil and water emulsions.Gaoet al.added acetic acid as a nonsolvent additive in polymer casting solution,and used sodium carbonate aqueous solution as coagulant,then chemical reaction modified phase inversion process occurred between the additive and coagulant.It was found that the involved chemical reaction increased membrane permeability and mean pore size while it maintained the relatively narrow pore size distribution of the membrane[17].The distinguishing feature of the above methods is that the phase inversion process involved in is a perfect combination of this process with chemical reaction between polymers in casting solution and reactants in the coagulation bath.What effect would the reaction between macromolecular polymers in casting solution and polymers in coagulation bath have on membrane performance and morphology?It is a meaningful topic for study.
Herein,carboxylated cardo poly(arylene ether ketone)s(PAEKCOOH)was employed as membrane fabrication material and polyethylenimine(PEI)as reactive polyelectrolyte in the coagulation bath.Carboxylated PAEK-COOH is an acidic polyelectrolyte,and PEI is a versatile,basic,and hydrophilic polymer[19]that has been widely used in preparing water process membranes[5,20,21].Then PAEKCOOH/N-methyl pyrrolidone(NMP)/1,4-dioxane(DO)polymer solution and PEI solution were employed as casting solution and coagulant,respectively.When the polymer film is immersed in the coagulant,the poly(acid-base)complexation reaction induced phase inversion process occurs,and membranes with denser and thinner separation skin layer were obtained.Moreover,the PEI chelated on membrane surface not only made the membrane positively charged,but also increased the surface charge density and membrane rejection towards divalent cations.
2.Materials and Methods
2.1.Materials and reagents
2-[Bis(4-hydroxyphenyl)methyl]benzoic acid was obtained by reducing phenolphthalein(PPH).4,4′-Bis fluorodiphenylketone,purchased from Sinopharm Group Chemical Reagent,was dried at 80°C for 12 h before polymerization.PEIs with molecular weight of 1800,10000 and 60000(50 wt%in water)were obtained from Alfa Aesar.All other chemicals were used as they were received.Heavy metal salts,zinc acetate dihydrate,nickel acetate tetrahydrate,and lead acetate dihydrate,were used as received.
2.2.Synthesis of carboxylic cardo poly(arylene ether ketone)
Carboxylated cardo poly(arylene ether ketone)s(PAEK-COOH)was synthesized by condensation polymerization of 2-[bis(4-hydroxyphenyl)methyl]benzoic acid and 4,4′-bis fluorodiphenylketone in DMSO;detailed information was provided in previous paper[22].The Ubbelohde viscosity and IEC of PAEK-COOH are 0.5 dL·g-1and 2.0056 mmol·g-1,respectively.The structure of PAEK-COOH is shown in Fig.1.
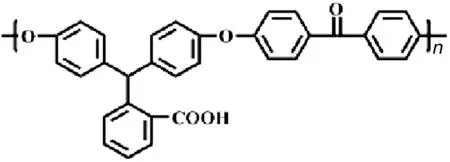
Fig.1.The structures of PAEK-COOH.
2.3.Fabrication of nanofiltration membrane
Polymer PAEK-COOHwas dissolved in solventpair ofNMP and DOat 60°C,stirred for 5 h to obtained a transparent and homogeneous polymer solution,and it was degassed and aged for12 h priorto casting film.Then the polymer solution was cast on a nonwoven fabric with a casting knife gap of 150 μm at room temperature of 30 °C and relative humidity of 20%.Finally,the cast polymer film was immersed in PEI solution for the phase inversion process to take place for 10 min.The phase separated membranes were transferred to deionized water and stored in that for 1 h to remove the residual solvents.Obtained membrane samples were kept in deionized water at 4°C before testing.
2.4.Characterization of membrane
X-ray photoelectron spectroscopy(XPS,ESCALAB 280 system)with Al/Ka(h=1486.6 eV)as radiation source was employed to illustrate the chemical composition of membrane surface.Drop Shape Analysis Dataphysics OCA 20 was used to measure the water contact angle of membrane.Membrane for cross-section morphology observation was fractured in liquid nitrogen and the cross-section morphology was observed by Field Emission Scanning Electron Microscopy(FESEM,XL 30 ESEM FEG,FEI Company).SurPASS electrokinetic analyzer(SurPASS,Anton Paar GmbH,Austria)was used to characterize the charged surface property of nanofiltration membrane,using Ag/AgCl electrodes in a 0.01 mol·L-1KCl background electrolyte solution.The surface zeta potential ζ was calculated by the Helmholtz-Smoluchowski equation[23]:

where ΔEis the streaming potential of membrane surface;ΔPis the driving pressure between two membrane sample;ε is the dielectric constant of the streaming solution;ε0is the vacuum permittivity;η is the solution viscosity,and κ is conductivity of bulk solution.
2.5.Evaluation of membrane filtration performance
The filtration performance of membranes was performed on a lab-scale cross- flow filtration system[24].All membranes were precompacted at 0.4 MPa and(25± 2)°C for 2 h by deionized water prior to the measurement of membrane flux.The permeations were collected for 5 min to measure the flux and rejection of membranes.The permeation flux was measured using Eq.(2).


whereVis membrane permeate volume of an effective membrane areaAover a filtration period oft.Protein rejection(R)is calculated as Eq.(3):whereCpandCfare the concentrations of salts or dyes in the permeate and feed solution,respectively.Salt concentration of the permeation and feed solution were measured by a conductance meter(Elmeiron conductivity meter CC-501,Poland);concentration of heavy metal ions in aqueous solution was determined by ion chromatography(ICP-1000 spectrometer),and that of dyes was measured by a UV-vis spectrophotometer at the biggest absorption peak of a dye.
2.6.Molecular weight cut off(MWCO)and pore size distribution
A series of neutral polyethylene glycols(PEGs)having an average molecular weight of 200,400,600,1000 and 1500 were employed to estimate the MWCO of membranes;glycerol(92)was also used.Similaras the filtration process for salts,PEGs were filtrated at 0.4 MPa with concentration of 100 mg·L-1.The concentration of neutral PEGs was measured by a total organic carbon(TOC)at TOC-VCPHSHIMADZU,and its rejection was determined by Eq.(3).The MWCO of membrane has been defined as the molecular weight of PEG having a 90%rejection rate,and the mean effective pore size(μp)has been defined as the Stokes diameter of a neutral solute at 50%rejection rate.The Stokes radius(ds)of PEG could be calculated on the basis of its average molecular weight:

The relationship between pore size distribution and solute Stokes radius was mathematically fitted by an exponential probability density function on the hypothesis that there are no hydrodynamic and electrostatic interactions between neutral solutes and membrane pores.
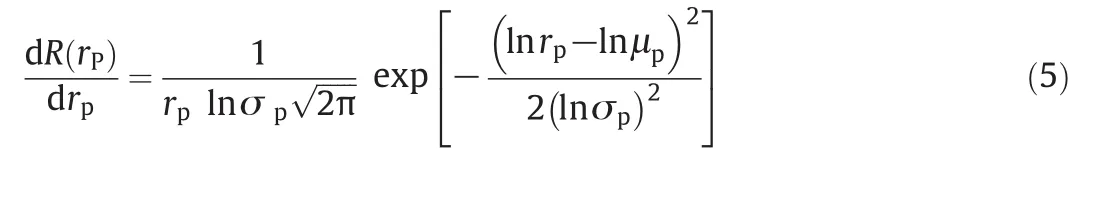
whererpis the pore radius that could be explained by the Stokes radius(ds)of PEG,μpis the mean effective pore size,and σpis the geometric standard deviation,which is the ratio ofrpat 84.13%rejection rate over that at 50%.
3.Results and Discussion
3.1.Preparation of membrane
A positively charged nanofiltration membrane for heavy metal removal has been fabricated by the poly(acid-base)complexing effect induced phase inversion process,in which the PAEK-COOH with carboxylic acid groups acts as weak polyacids and PEI dissolved in water acts as weak polybases,then the acid-base complexation reaction would occur when the polymer casting solution been immersed in the coagulants.Nanofiltration membranes obtained by coagulating PAEK-COOH polymer film in PEI aqueous solution were named as PAEK-COOH-PEI series chelated membranes.Membrane preparation conditions,such as polymer concentration,co-solvent content,and the effect of coagulation bath properties,were investigated.
3.1.1.The influence of co-solvent content
As shown in Fig.2,when polymer concentration is 22.0 wt%and PEI concentration is 1.5 wt%in the coagulation bath,membrane performance is greatly related to co-solvent content in casting solution.The water flux of membrane decreases while the rejection increases with the increasing of DO content.The introduction of co-solvent into polymer casting solution in phase inversion process is expected to form a dense skin layer[25];therefore,membrane flux decreases and rejection increases with the increasing of DO content.At DO content of 50%,membrane has reasonable water flux and Mg Cl2rejection.
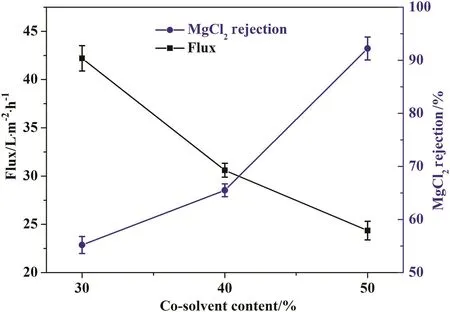
Fig.2.Membrane performance with respect to co-solvent content.
3.1.2.The influence of polymer concentration
In order to investigate the relationship between membrane performance and polymer concentration in casting solution,nanofiltration membranes with a polymer concentration of 20 wt%,21 wt%and 22 wt%were prepared at co-solvent content of 50%and PEI concentration of 1.5 wt%in the coagulation bath.Increasing polymer concentration in casting solution would result in membrane with dense and thick separation skin layer[9].Seen from Fig.3,the water flux of membrane decreases while the rejection increases with the increasing of polymer concentration,and membrane that makes from polymer concentration of 22 wt%has the best performance.
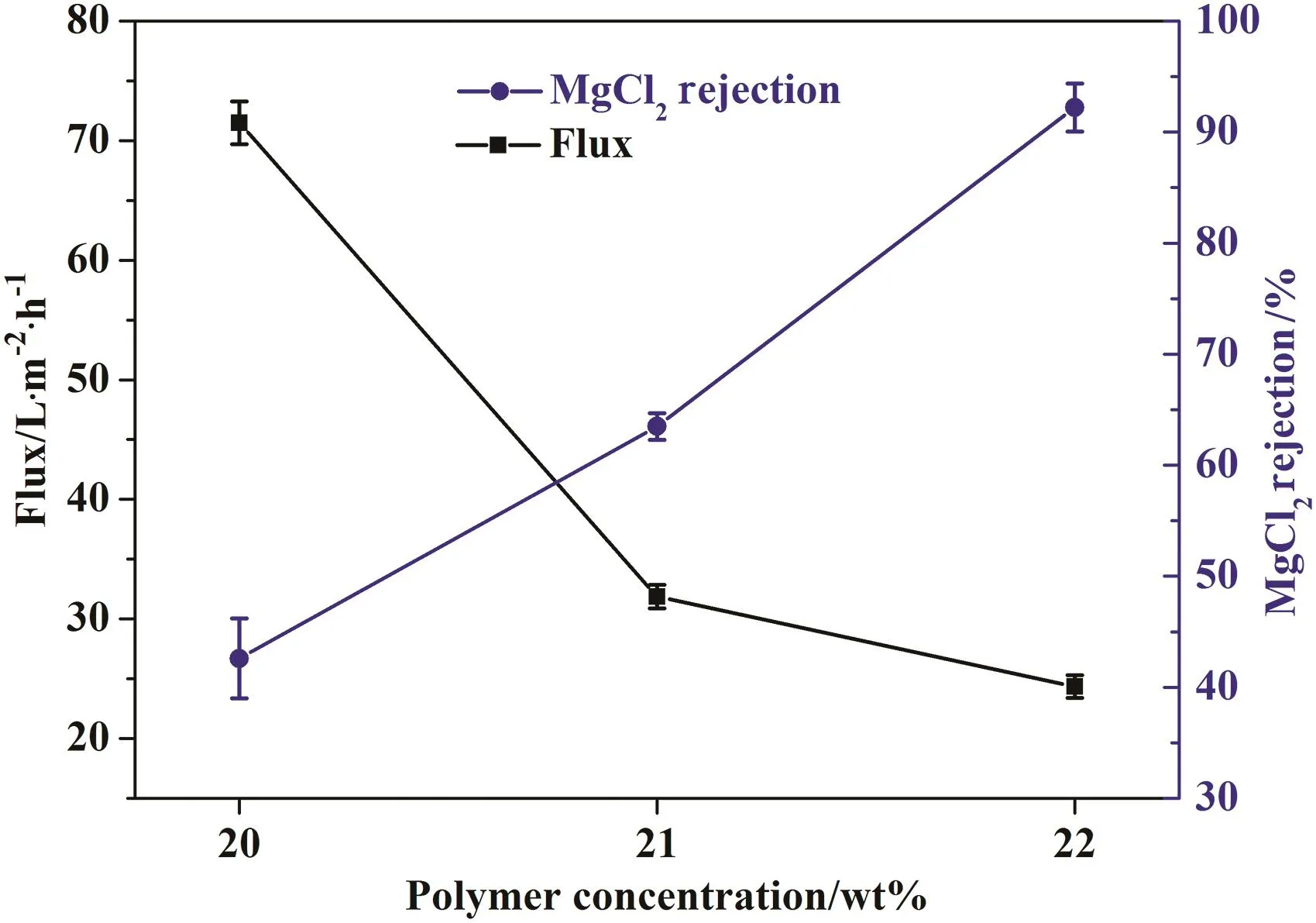
Fig.3.Membrane performance with respect to polymer concentration.
3.1.3.The influence of coagulation bath temperature
The influence of coagulation bath temperature on membrane performance was studied in the temperature range of 20-60°C at co-solvent(DO)content of 50%and polymer concentration of 22 wt%.Increasing the temperature of coagulation bath would result in membrane with high water flux and low salt rejection.Higher temperature of coagulation bath leads to membrane with looser structure,which exhibits relatively thinner skin layer and higher porosity[26].Membrane has best performance at coagulation bath temperature of 20°C,which is determined as the optimal temperature.(See Fig.4.)
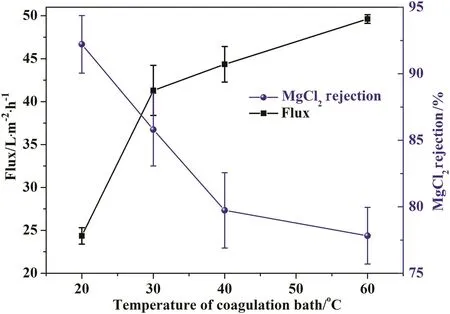
Fig.4.The effect of coagulation bath temperature on membrane performance.
3.1.4.The influence of PEI concentration
The influence of PEI concentration on membrane permeability and MgCl2rejection is shown in Fig.5.The PAEK-COOH membrane has almost no water permeability and very low salt rejection at operation pressure of 0.4 MPa.When PEIs are added in the coagulation bath,not only the water flux does increase,but also MgCl2rejection increases.The rejection of MgCl2has greatly increased from 40.2%to 94.1%as the concentration of PEI in the coagulation bath increased from 0 to 2.0 wt%.While the water flux of membrane initially increased from 1.5 L·m-2·h-1to 27.0 L·m-2·h-1as the PEI concentration increased from 0 to 1.0 wt%,then decreased to 22.1 L·m-2·h-1when the concentration of PEI increased to 2.0 wt%.At the PEI concentration of 1.5 wt%,the obtained membrane has reasonable performance with pure water flux of(24.35 ± 0.96)L·m-2·h-1and MgCl2rejection of(92.21±2.16)%.It is interesting to find that the rejection and water flux of membrane have different change tendency when it comes to PEI concentration in the coagulant.
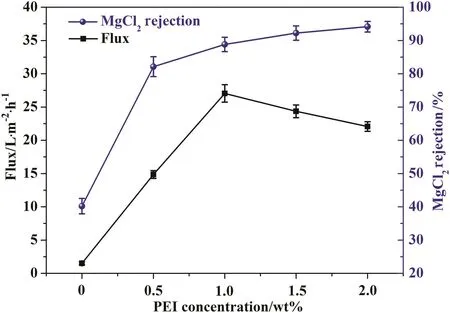
Fig.5.The effect of PEI concentration in the coagulation bath on membrane performance.
In order to investigate the relationship between membrane performance and PEI concentration in the coagulant,the cross-section morphology of membranes with respect to PEI concentrations in the coagulation bath is illustrated in Fig.6.From the overall prospects,the cross section morphology of nanofiltration membranes is dependent on the coagulation bath properties.The cross-section morphology of control PAEK-COOH membrane is composed by dispersed irregular finger-like pores in the sponge matrix of membrane,and has a dense and thick skin layer.When there are PEIs in the coagulation bath,the cross-section morphology of the membrane is composed of regular finger-like pores that stretch to the bottom of the membrane,resulting in a membrane with a more loose structure.Furthermore,the skin layer thickness on the upper bottom of the membrane is correlated to the concentration of PEIs.The enlarged SEM images of the membrane upper bottom are demonstrated in Fig.6(b,d,f,h,and j),which suggest that the thickness of membrane skin layer decreases from 1.45 μm to 151 nm,then increases to 209 nm as the concentration of PEI in coagulation bath increases from 0 to 1.5 wt%,then to 2.0 wt%.There is a negative correlation between the thickness of membrane skin layer and PEI concentration in the coagulant at PEI concentration of 0 to 1.5 wt%,and the thickness increases with PEI concentration increase to 2.0 wt%.At PEI concentration of 1.5 wt%,the skin layer thickness is the smallest.Those results demonstrate that the complexation reaction between polyelectrolytes(shown in Eq.(6))would affect the properties of membrane skin layer,including skin layer thickness and compactness.
The complexation reaction between PAEK-COOH and PEI,PEI is written as PEI-NRH:
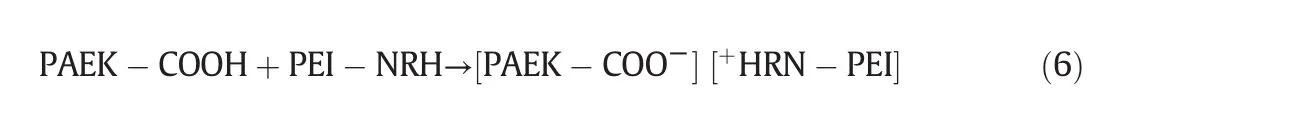
The influence of PEI concentration on skin layer thickness of membrane is a reflex of kinetic aspects of the phase inversion process.Two factors that determine the skin layer thickness of membrane in liquid liquid phase separation process:the delay time for liquid-liquid phase separation and the interfacial polymer concentration at the onset of the liquid-liquid phase separation process[27].The chelating behavior induced instantaneous precipitation of polymer leads to the decrease of delay time of phase separation.As the concentration of PEI in the coagulant increases,the delay time for liquid-liquid phase separation decreases while the interfacial polymer concentration at the onset of the liquid-liquid phase separation increases,the former could decrease,while the latter could increase the skin layer thickness.Those two kinds of effects have opposite influence on membrane skin layer thickness,and come to their balance state at PEI concentration of 1.5 wt%,at which membrane has smallest skin layer thickness.Meanwhile,the polymer concentration at the interface determines membrane skin layer compactness,the higher the polymer concentration,the compacter the skin layer.Then the skin layer thickness and compactness in turn would determine the flux and solute rejection of membrane,so membrane flux firstly increases and then decreases as the skin layer becomes thinner and compacter,while the rejection keeps increasing.The chelated membranes have dense and thinner skin layer at PEI concentration of 1.5 wt%,thus,having best membrane performance.
The influence of PEI concentration on membrane cross section morphology also could be interpreted by the kinetics of phase inversion process.In the phase inversion process,the complexation reaction between PAEK-COOH and PEI takes place instantaneously upon immersion,leading to the formation of polyelectrolyte complex([PAEK-COO-][+HRN-PEI])on membrane skin layer,then which would inhibit the relative exchange rate of solvent and nonsolvent.Furthermore,the viscosity of coagulation bath increases when PEI is introduced.The generated polyelectrolyte complex and increased viscosity of nonsolvent would slowdown the in-diffusion rate of nonsolvent profoundly while the out-diffusion rate of solvent decreases slightly.Thus,out-diffusion rate of solvent through the interface over weighs the in diffusion rate of nonsolvent,which results in fewer nuclei in sublayer and is possible to form a finger-like structure referring to the nucleation and growth mechanism[26].
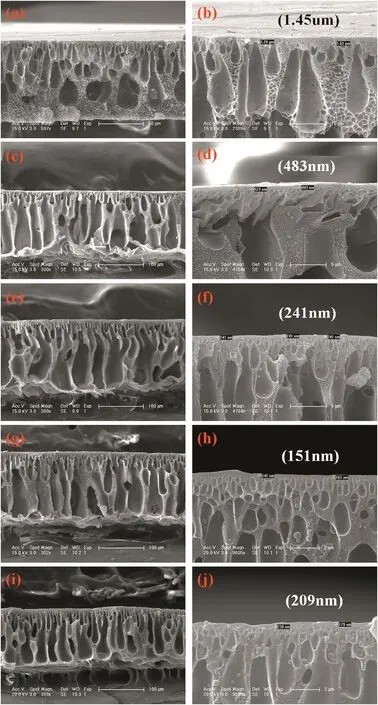
Fig.6.Cross-section morphology of asymmetric membrane coagulated in PEIs(10000)with the following concentration:0(a)and(b),0.5 wt%(c)and(d),1.0 wt%(e)and(f),1.5 wt%(g)and(h)and 2.0 wt%(i)and(j).
Based on the results of PEI concentration with respect to membrane performance and cross-section morphology,a hypothesized mechanism for the poly(acid-base)complexation effect induced phase inversion process is proposed.Fig.7 illustrates membrane formation processes.
3.1.5.The influence of PEI molecular weight
In Section 3.1.4,the optimal PEI concentration(PEI,MW=10000)for membrane preparation is determined to be 1.5 wt%in the coagulant.Herein,the influence of PEI molecular weight on membrane performance and morphology is studied.PEIs with molecular weight of 1800,10000 and 60000 are employed as solutes.As shown in Fig.8,when the molecular weight of PEI increases from 1800 to 60000,the flux of membrane decreases while the rejection of MgCl2firstly increases,then slightly decreases.As a result,the membrane coagulated in PEI solution with molecular weight of 10000 has best performance.The influence of PEI molecular weight on membrane cross-section morphology is shown in Fig.9,which suggests that the overall morphology is similar while the skin layer thickness is dependent on PEI molecular weight.The skin layer thickness increases when PEI molecular weight increases from 1800 to 10000,then decreases when the molecular weight increases to 60000.PEIs with lower molecular weight produce membrane with thinner,but less compact skin layer when compared with PEI having higher molecular weight,therefore the membrane has the highest flux and lowest rejection.PEIs with higher molecular weight have longer branched polymer chains and higher degree of polymer chain entanglement and interpenetration,which results in lower molecule migration rates in aqueous solution,in turn leading to poor chelating degree between PAEK-COOH and PEI during phase inversion process,thus a thicker but less compact skin layer being formed,and the membrane having lower flux and rejection.Hence,the membrane with a PEI of 10000 in the coagulant has the best performance.
To conclude,the optimal membrane preparation conditions are:co-solvent(DO)content of 50%and polymer concentration of 22 wt%of polymer solution and PEI concentration of 1.5 wt%and molecular weight of 10000 in the coagulant with temperature of 20°C.At optimized conditions,the final performance of nanofiltration membrane is:pure water flux of(24.35 ± 0.96)L·m-2·h-1and MgCl2rejection of(92.21±2.16)%.

Fig.8.The effect of PEI molecular weight on membrane performance.
3.2.Surface characterization and hydrophilic properties of membranes
XPS is a sensitive technique to measure the chemical composition of material surface.As shown in Table 1,the relative amount of elements of chelated membrane is different from that of the control membrane,the amount of C and O decreases,while that of N increases.
Fig.10 shows the XPS spectra of membrane.PAEK-COOH membrane mainly has three kinds of elements(C,H,and O)on the surface,while the PAEK-COOH-PEI chelated membrane has four kinds of elements(C,H,O,and N)on the surface.Moreover,seen in Fig.10(b),the nitrogen element on membrane surface has two binding types,NR3and NR4+,of which the NR4+binding type nitrogen originated from the amine groups of PEI that has complexed with carboxylic acid groups of PAEK-COOH.Those results indicate the successful complexation of PEIs on PAEKCOOH-PEI chelated membrane surface.
The dynamic water contact angles of membranes are shown in Fig.11.The water contact angle of membrane would reflect its hydrophilicity to some extent,and which in turn determines the permeation of membrane[28,29].The original PAEK-COOH and chelated PAEKCOOH-PEI nanofiltration membranes have initial contact angles of 64.7°and 58.5°,which then decrease to their equilibrium state of 61.5°and 57.2°respectively within 3 s.These results demonstrate that the surface hydrophilicity of chelated PAEK-COOH-PEI membrane is higher than the original PAEK-COOH membrane.The polyelectrolyte complex([PAEK-COO-][+HRN-PEI])formed between carboxylic acid groups and polyethylenimines leads to the increment of hydrophilicity of membrane surface.
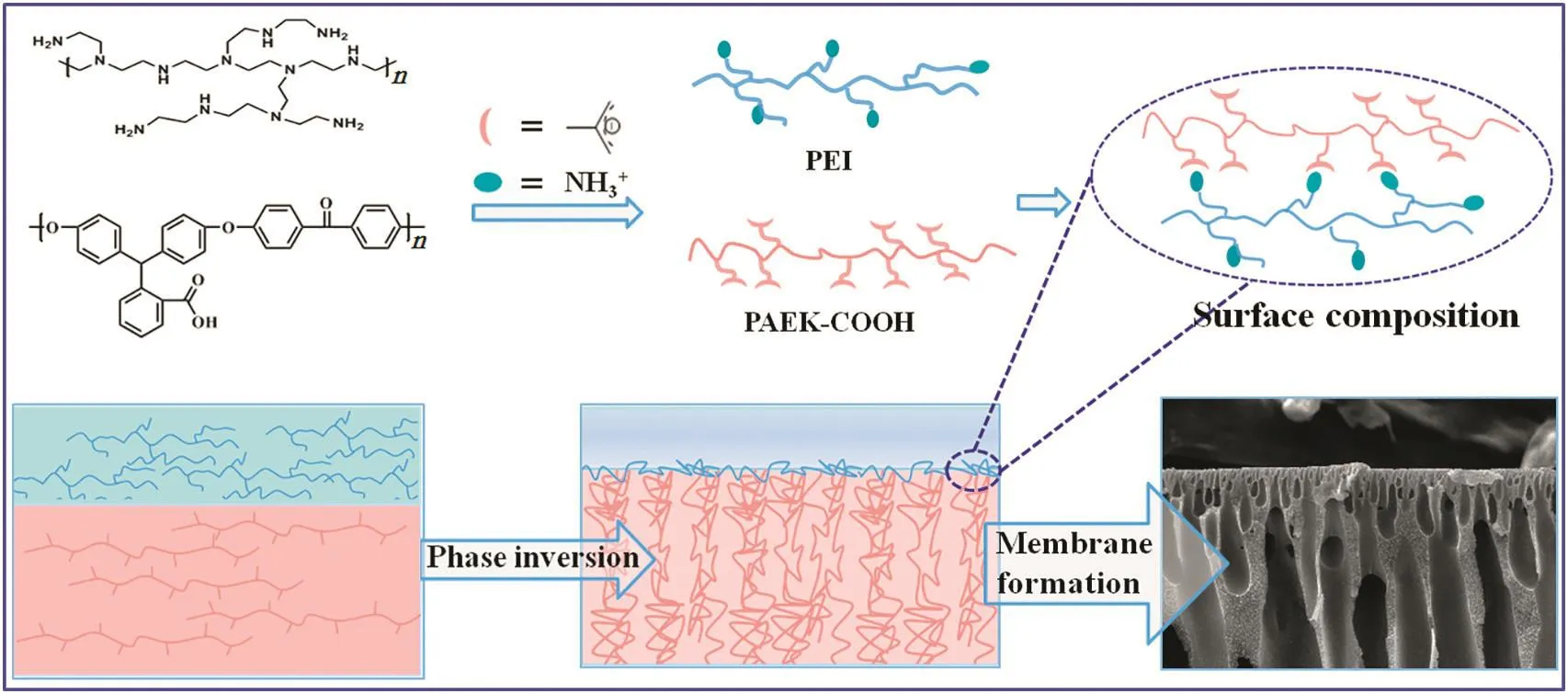
Fig.7.Formation procedure of chelated PAEK-COOH-PEI positively charged nanofiltration membrane by the poly(acid-base)complexing effect induced phase inversion process.
The zeta potential of membrane surface is shown in Fig.12.In the pH range of 3 to 10,the zeta potential of membrane surface decreases from 65 mV to-31 mV.The chelated membrane has high isoelectric point(about pH=8),so it would acquire more positive charges at the lower tested pH(65 mV,pH=3)[30].At pH=7,the zeta potential is 20.3 mV,indicating that the membrane is positively charged in a neutral solution.Therefore,the membrane could be applied to remove divalent and multivalent cations from water.
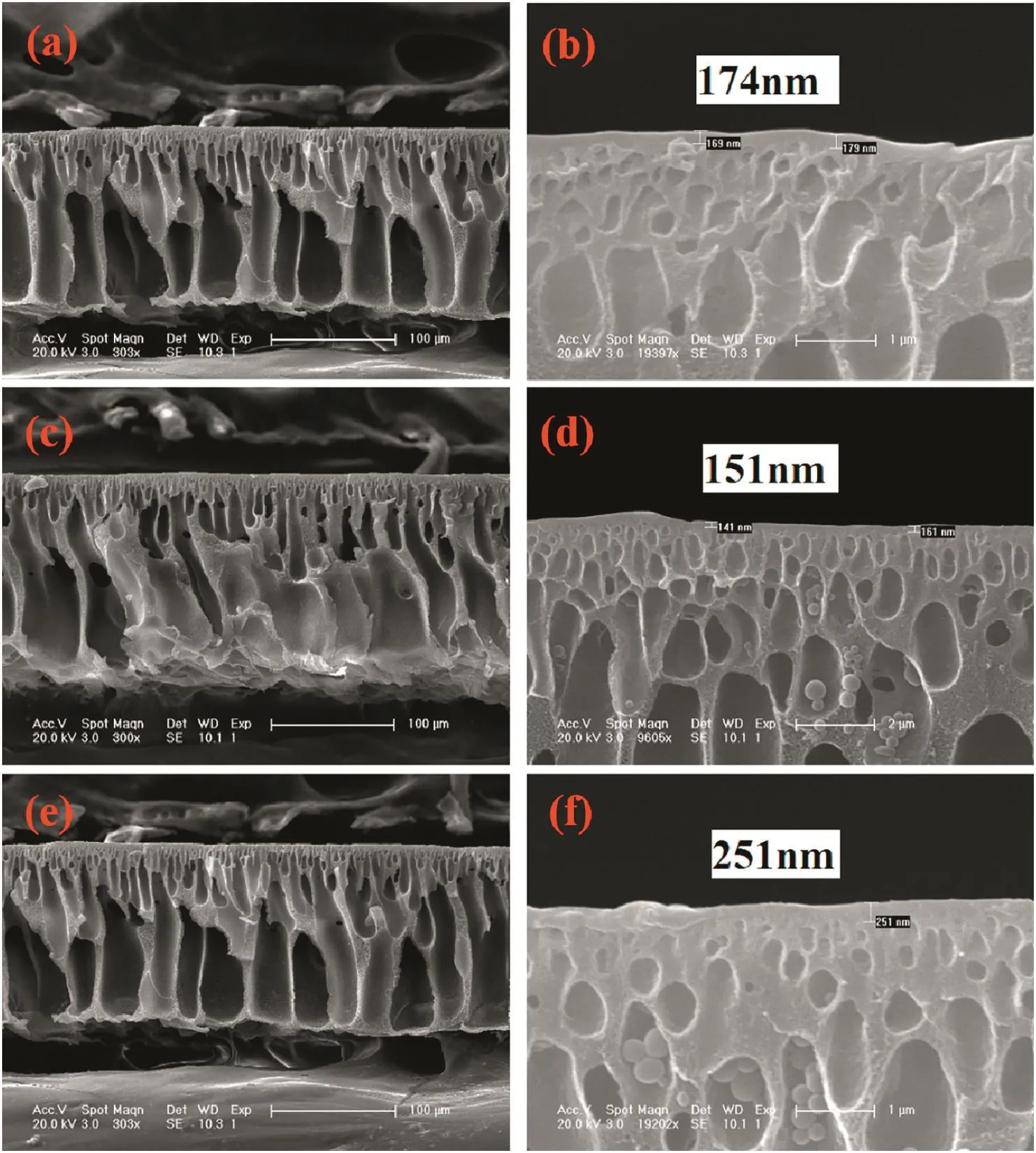
Fig.9.Cross-section morphology of asymmetric membrane coagulated in PEIs(1.5 wt%)with the following molecular weight:1800(a)and(b),10000(c)and(d)and 60000(e)and(f).
3.3.Molecular weight cut off(MWCO)and pore size of membranes
Fig.13 demonstrates the molecular weight cut off(MWCO)[Fig.13(a)]and pore size distribution[Fig.13(b)]of the chelated nanofiltration membrane.The membrane has a MWCO of 1110 and a mean effective pore size of 0.283 nm,which lies in the MWCO and pore size range of nanofiltration membrane.Probability density function shows that the membrane has a geometric standard deviation(σp)of 2.229,a relatively wide pore size distribution.The pores with larger pore size account for a relatively large proportion(pores with radius larger than 0.8 nm,counts for 32.6%),which results in membrane with high pure water flux and monovalent salt permeability.
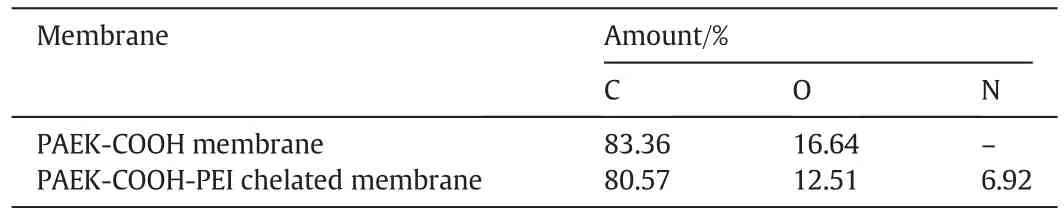
Table 1The surface element composition of membrane
3.4.Filtration performance of membrane
The separation mechanism for nanofiltration membrane is a combination of the steric hindrance and Donnan exclusion effect[31,32].The membrane has pores with radius larger than 0.8 nm counting for 32.6%,exceeding the diameter of heavy metal ions,which indicates that Donnan exclusion effect is the dominating mechanism for separation metal ions.As shown in Fig.14,the salts rejection of membrane follows the order of NiAc2>PbAc2>ZnAc2>CaCl2>MgCl2>MgSO4>Na2SO4,and a total rejection for positively charged organic dyes Methylviolet6B and Methyl green(details of inorganic salts and dyes seen in supporting information).The rejection of membrane for salts of different valence follows the order of MgCl2>MgSO4>Na2SO4,which is a typical character of the positively charged nanofiltration membrane.The positive charges on membrane surface originate from the protonated amine groups of PEIs that has been chelated on membrane surface by complexing reaction,which provides the membrane with higher surface charge density,as well as higher salt rejection due to the electrostatic-repulsion effect between membrane surface and divalent cations.The electrostatic-repulsion mechanism in nanofiltration process makes the chelated membrane has high rejection for AB2type(A:cation,B:anion)salts,and membrane rejection for five kinds of tested salts(NiAc2,PbAc2,ZnAc2,CaCl2,and MgCl2)are higher than 92%.Thus,the PAEK-COOH-PEI chelated membrane could be applied for heavy metal removal from wastewater.
The separation performance of the chelated membrane,other literature reported,and commercial nanofiltration membranes are listed in Table 2.In comparison with the performance of two kinds of positively charged membrane:dopamine/PEI deposited membrane and PAN/PEI crosslinked membrane,the PAEK-COOH-PEI chelated membrane features higher water permeability and moderate salt rejection.When compared with the commercial thin film composite(TFC)polyamide membrane NTR7450 and composite NF hollow fiber membrane,PEK-C flat-sheet TFC nanofiltration membrane,the PAEK-COOH-PEI chelated membrane has lower water permeability and salt rejection,but this kind of membrane has strong acid resistance than the polyamide membranes.
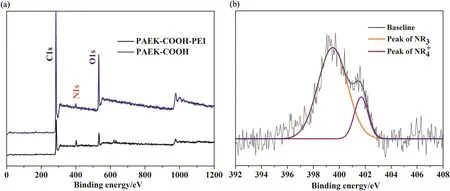
Fig.10.XPS spectra of PAEK-COOH-PEI chelated nanofiltration membrane.(the overall spectra(a)and N1s spectra(b)).
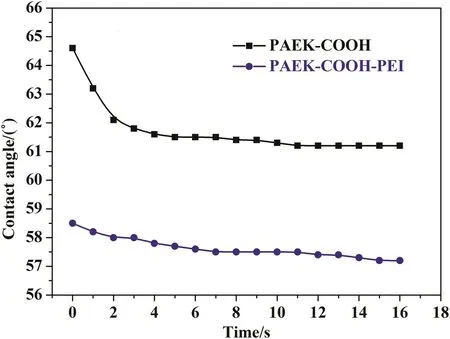
Fig.11.Dynamic water contact angles of original PAEK-COOH and chelated PAEK-COOHPEI nanofiltration membranes.
3.5.Operation conditions of membrane
Fig.15 shows the performance of PAEK-COOH-PEI chelated membrane with respect to the pH of operation solution.The water flux of membrane is almost stable in the pHrange of1 to 9,and then drastically increases when the pH increases to 11.While the rejection of membrane for MgCl2decreases with the increase of solution pH.In acidic condition,the rejection of membrane is a little higher than that of membrane in neutral solution.This is a consequence of the increased membrane surface charge density caused by the protonation of unchelated amine groups on membrane surface,which lead to the increase of Donnan exclusion effect between membrane and cations,so the rejection for MgCl2is much higher in acidic solutions.In weak basic conditions(pH<9),as the pH increases,the zeta potential of membrane surface decreases from 20.3 mV to-19.3 mV,i.e.,the charging type of membrane surface changes from positively charged to negatively charged,which leads to the decrease of electrostatic repulsion between membrane and cations,thus membrane rejection towards MgCl2decreases while membrane flux maintains stable.In strong basic conditions(pH>9),the hydroxide anions in solution would destroy the complexing reaction between carboxylic acid groups and polyethylenimines on membrane surface,leading to the release of PEI molecules from chelated membrane surface,therefore the rejection of membrane has a sharp decrease while the flux has a big increase.Thus,the operation pH range for the chelated nanofiltration membrane is 1-9.
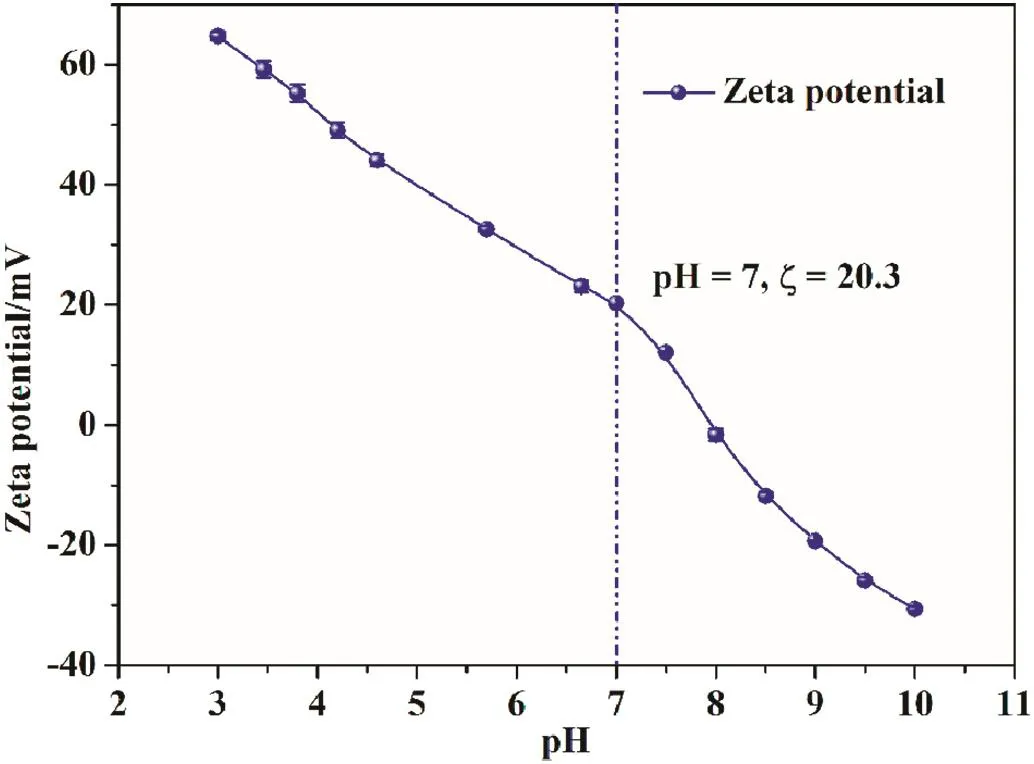
Fig.12.Zeta potential of PAEK-COOH-PEI nanofiltration membrane.
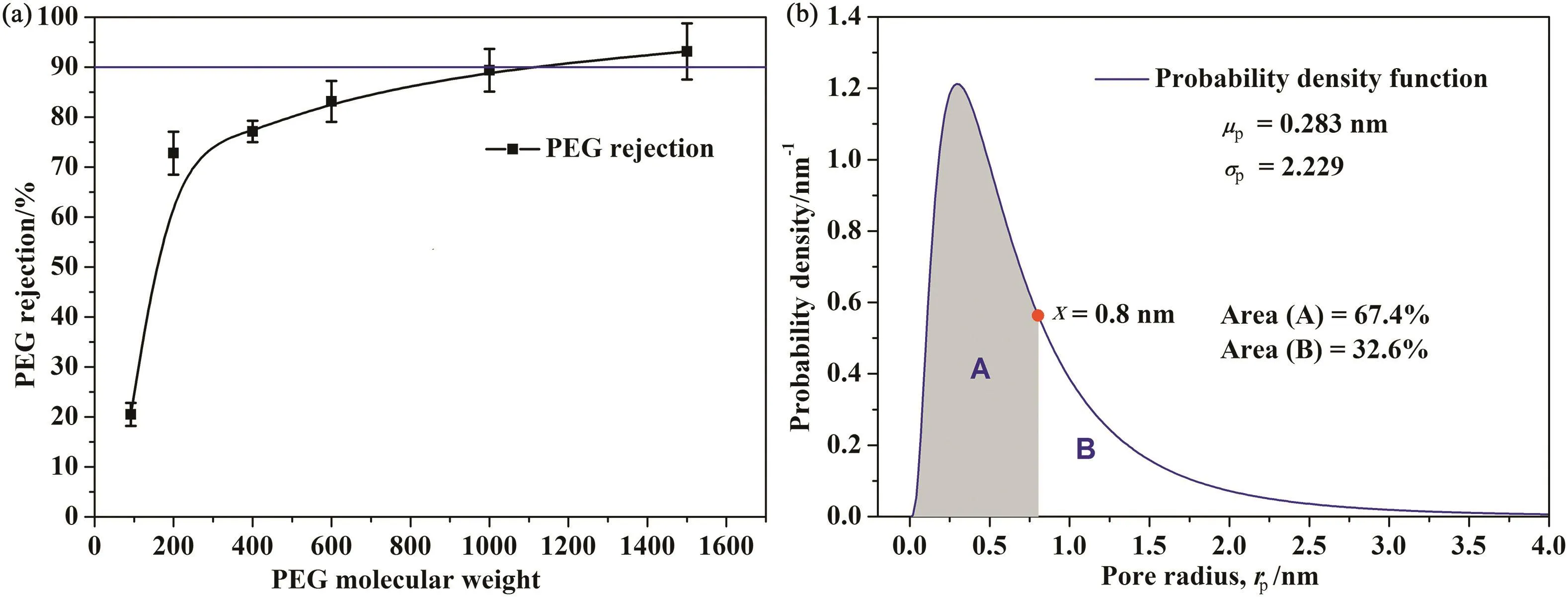
Fig.13.The MWCO and pore size distribution of nanofiltration membrane.
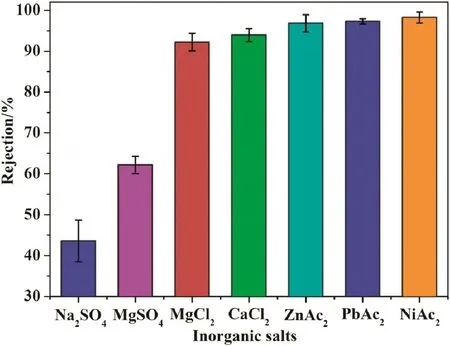
Fig.14.Heavy metal rejection of nanofiltration membrane.
4.Conclusions
In this paper,PAEK-COOH/NMP/DO mixture was employed as polymer casting solution and aqueous solution of PEI was used as coagulation bath,then a positively charged high permeable nanofiltration membrane for heavy metal removal was fabricated by the poly(acid-base)complexation effect induced phase inversion method.The complexation effect between polyacids and polybases accelerated the phase inversion rate in the coagulant step,an instantaneous phase inversion process took place,and a membrane with denser and thinner separation skin layer was obtained.Furthermore,the pore size of the membrane also decreased through the chelating of PEI on the surface.The results of water contact angle and zeta potential measurement demonstrated that the chelating of PEI on membrane surface would increase membrane hydrophilicity and provide positive charges on membrane surface.The optimal membrane preparation conditions are polymer concentration of 22 wt%,co-solvent content of 50%and PEI molecular weight of 10000 and concentration of 1.5 wt%.At those conditions,the PAEK-COOH-PEI nanofiltration membrane has pure water flux of 24.3 L·m-2·h-1and MgCl2rejection of 92.2%.The obtained positively charged membrane possesses high rejection towards divalent cations,such as Mg2+,Ca2+,Pb2+,Ni2+,Zn2+,etc.,which could be used for heavy metal removal from polluted water.
Nomenclature
AThe effective membrane area,cm2
CfConcentrations of solute in the feed solution,mg·L-1
CpConcentrations of solute in the permeate,mg·L-1
dsStokes radius,nm
ΔEStreaming potential of membrane,mV
FPure water flux,L·m-2·h-1
RSolute rejection,%
tFiltration time,h
VThe permeate volume,L
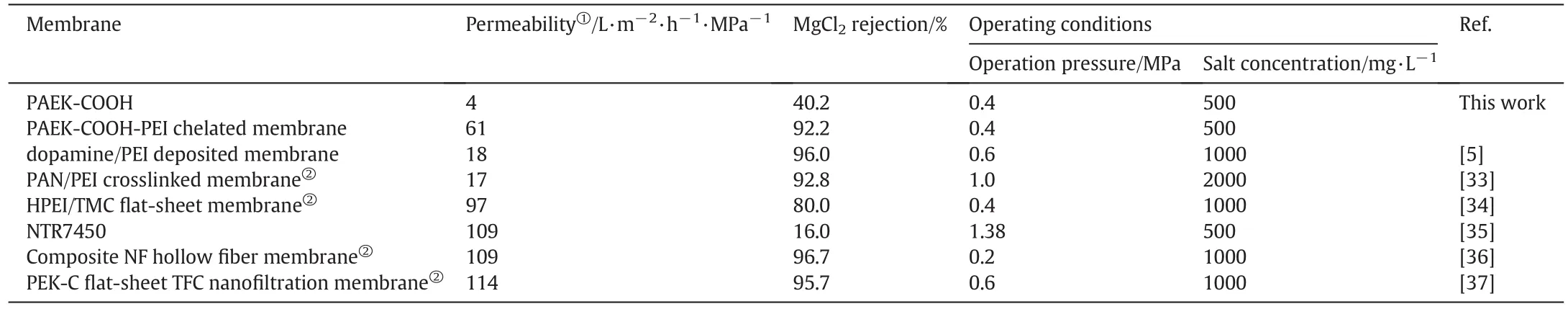
Table 2Comparisons of membrane filtration performance with that of other literature reported nanofiltration membranes
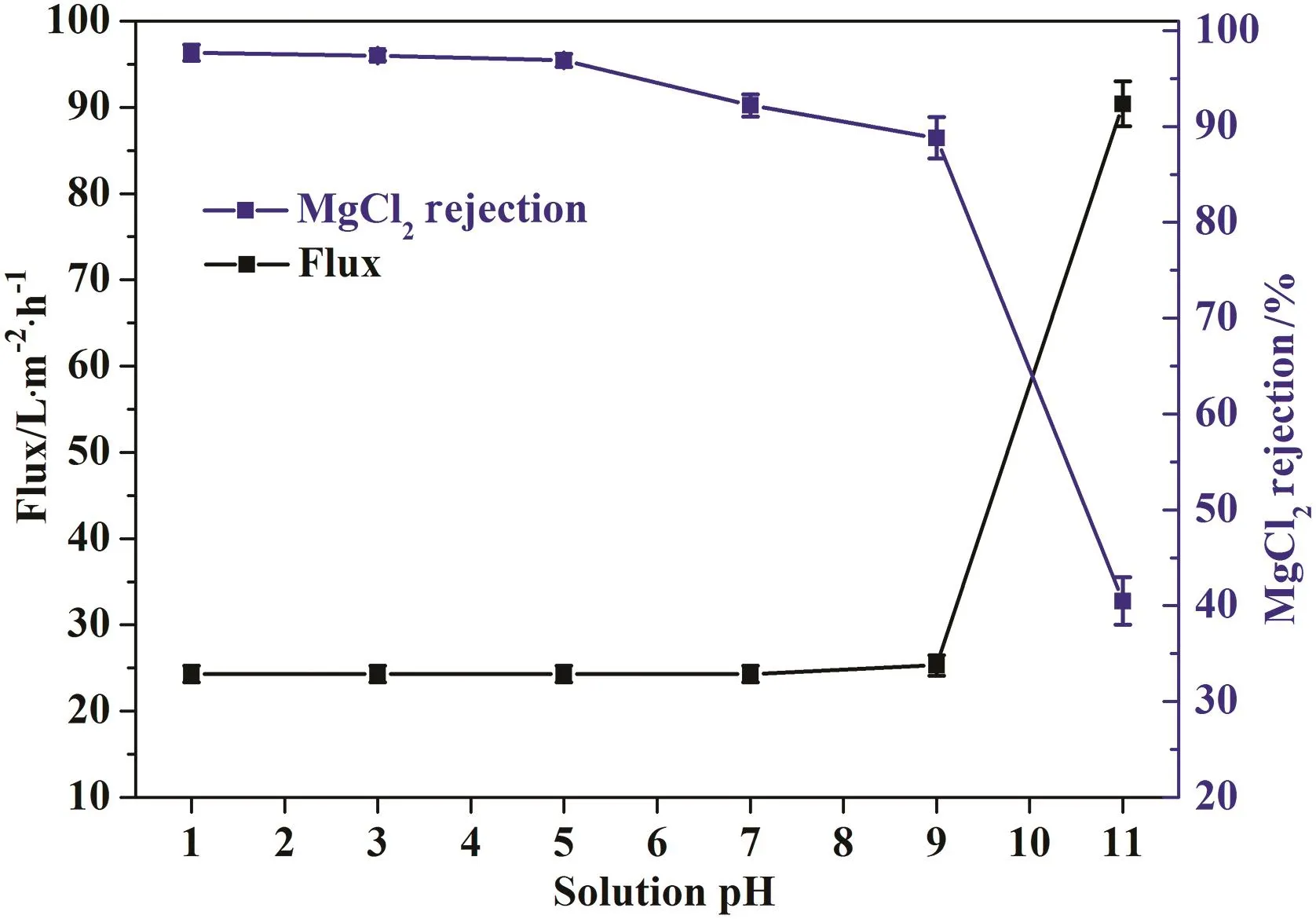
Fig.15.The effect of solution pH on membrane performance.
ε Dielectric constant of streaming solution
ε0Vacuum permittivity,F·m-1
ζ Zeta potential,mV
η Solution viscosity
κ Conductivity of bulk solution,mS
μpMean effective pore size,nm
σpGeometric standard deviation
Supplementary Material
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2017.06.001.
[1]Z.Cui,H.S.Muralidhara,Membrane Technolog,1st edition Elsevier,UK,2010.
[2]M.Paul,S.D.Jons,Chemistry and fabrication of polymeric nanofiltration membranes:A review,Polymer103(2016)417-456.
[3]A.K.Hołda,I.F.J.Vankelecom,Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part A:Influence of high molecular weight additives,J.Membr.Sci.450(2014)512-521.
[4]A.K.Hołda,I.F.J.Vankelecom,Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part B:Influence of low molecular weight additives,J.Membr.Sci.450(2014)499-511.
[5]Y.Lv,H.-C.Yang,H.-Q.Liang,L.-S.Wan,Z.-K.Xu,Nano filtration membranes via codeposition of polydopamine/polyethylenimine followed by cross-linking,J.Membr.Sci.476(2015)50-58.
[6]L.Krasemann,B.Tieke,Selective ion transport across self-assembled alternating multilayers of cationic and anionic polyelectrolytes,Langmuir16(2000)287-290.
[7]F.Liu,B.-r.Ma,D.Zhou,L.-J.Zhu,Y.-Y.Fu,L.-x.Xue,Positively charged loose nanofiltration membrane grafted by diallyl dimethyl ammonium chloride(DADMAC)via UV for salt and dye removal,React.Funct.Polym.86(2015)191-198.
[8]Y.H.See-Toh,F.C.Ferreira,A.G.Livingston,The influence of membrane formation parameters on the functional performance of organic solvent nanofiltration membranes,J.Membr.Sci.299(2007)236-250.
[9]A.K.Hołda,B.Aernouts,W.Saeys,I.F.J.Vankelecom,Study of polymer concentration and evaporation time as phase inversion parameters for polysulfone-based SRNF membranes,J.Membr.Sci.442(2013)196-205.
[10]L.Gao,B.Tang,P.Wu,An experimental investigation of evaporation time and the relative humidity on a novel positively charged ultra filtration membrane via drywet phase inversion,J.Membr.Sci.326(2009)168-177.
[11]K.Hendrix,G.Koeckelberghs,I.F.J.Vankelecom,Study of phase inversion parameters for PEEK-based nanofiltration membranes,J.Membr.Sci.452(2014)241-252.
[12]J.Xu,Y.Tang,Y.Wang,B.Shan,L.Yu,C.Gao,Effect of coagulation bath conditions on the morphology and performance of PSf membrane blended with a capsaicin-mimic copolymer,J.Membr.Sci.455(2014)121-130.
[13]S.Zhao,Z.Wang,J.Wang,S.Wang,The effect of pH of coagulation bath on tailoring the morphology and separation performance of polysulfone/polyaniline ultra filtration membrane,J.Membr.Sci.469(2014)316-325.
[14]Y.Termonia,Molecular modeling of phase-inversion membranes:effect of additives in the coagulant,J.Membr.Sci.104(1995)173-180.
[15]W.Zhang,Y.Zhu,X.Liu,D.Wang,J.Li,L.Jiang,J.Jin,Salt-induced fabrication of superhydrophilic and underwater superoleophobic PAA-g-PVDF membranes for effective separation of oil-in-water emulsions,Angew.Chem.53(2014)856-860.
[16]S.Gao,J.Sun,P.Liu,F.Zhang,W.Zhang,S.Yuan,J.Li,J.Jin,A robust polyionized hydrogel with an unprecedented underwater anti-crude-oil-adhesion property,Adv.Mater.28(2016)5307-5314.
[17]M.Wang,L.Wu,C.Gao,The influence of phase inversion process modified by chemical reaction on membrane properties and morphology,J.Membr.Sci.270(2006)154-161.
[18]B.Tang,T.Xu,M.Gong,W.Yang,A novel positively charged asymmetry membranes from poly(2,6-dimethyl-1,4-phenylene oxide)by benzyl bromination and in situ amination:Membrane preparation and characterization,J.Membr.Sci.248(2005)119-125.
[19]S.Roesler,F.P.Koch,T.Schmehl,N.Weissmann,W.Seeger,T.Gessler,T.Kissel,Amphiphilic,low molecular weight poly(ethylene imine)derivatives with enhanced stability for efficient pulmonary gene delivery,J.Gene Med.13(2011)123-133.
[20]T.Wang,H.Qiblawey,E.Sivaniah,A.Mohammadian,Novel methodology for facile fabrication of nanofiltration membranes based on nucleophilic nature of polydopamine,J.Membr.Sci.511(2016)65-75.
[21]J.Dasgupta,M.Singh,J.Sikder,V.Padarthi,S.Chakraborty,S.Curcio,Response surface-optimized removal of reactive red 120 dye from its aqueous solutions using polyethyleneimine enhanced ultra filtration,Ecotoxicol.Environ.Saf.121(2015)271-278.
[22]C.Liu,H.Mao,J.Zheng,S.Zhang,Tight ultra filtration membrane:Preparation and characterization of thermally resistant carboxylated cardo poly(arylene ether ketone)s(PAEK-COOH)tight ultra filtration membrane for dye removal,J.Membr.Sci.530(2017)1-10.
[23]M.Hadidi,A.L.Zydney,Analysis of the effects of electrostatic interactions on protein transport through zwitterionic UF membranes using protein charge ladders,J.Appl.Polym.Sci.41540(2015)1-8.
[24]Y.Zhao,Z.Zhang,L.Dai,S.Zhang,Preparation of high water flux and antifouling RO membranes using a novel diacyl chloride monomer with a phosphonate group,J.Membr.Sci.536(2017)98-107.
[25]I.Soroko,M.Makowski,F.Spill,A.Livingston,The effect of membrane formation parameters on performance of polyimide membranes for organic solvent nanofiltration(OSN).Part B:Analysis of evaporation step and the role of a co-solvent,J.Membr.Sci.381(2011)163-171.
[26]J.Peng,Y.Su,W.Chen,Q.Shi,Z.Jiang,Effects of coagulation bath temperature on the separation performance and antifouling property of poly(ether sulfone)ultra filtration membranes,Ind.Eng.Chem.Res.49(2010)4858-4864.
[27]P.Radovanovic,S.W.Thiel,S.-T.Hwang,Formation of asymmetric polysulfone membranes by immersion precipitation.Part II.The effects of casting solution and gelation bath compositions on membrane structure and skin formation,J.Membr.Sci.65(1992)231-246.
[28]J.Liu,L.Yu,Y.Zhang,Fabrication and characterization of positively charged hybrid ultra filtration and nanofiltration membranesviathe in-situ exfoliation of Mg/Al hydrotalcite,Desalination335(2014)78-86.
[29]J.Zhu,Y.Zhang,M.Tian,J.Liu,Fabrication of a mixed matrix membrane with in situ synthesized quaternized polyethylenimine nanoparticles for dye purification and reuse,ACS Sustain.Chem.Eng.3(2015)690-701.
[30]A.E.Childress,M.Elimelech,Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes,J.Membr.Sci.119(1996)253-268.
[31]A.W.Mohammad,Y.H.Teow,W.L.Ang,Y.T.Chung,D.L.Oatley-Radcliffe,N.Hilal,Nano filtration membranes review:Recent advances and future prospects,Desalination356(2015)226-254.
[32]A.E.Yaroshchuk,Rejection mechanisms of NF membranes,Membr.Technol.100(1998)9-12.
[33]C.Feng,J.Xu,M.Li,Y.Tang,C.Gao,Studies on a novel nanofiltration membrane prepared by cross-linking of polyethyleneimine on polyacrylonitrile substrate,J.Membr.Sci.451(2014)103-110.
[34]Y.-C.Chiang,Y.-Z.Hsub,R.-C.Ruaan,C.-J.Chuang,K.-L.Tung,Nano filtration membranes synthesized from hyperbranched polyethyleneimine,J.Membr.Sci.326(2009)19-26.
[35]Q.Chen,P.Yu,W.Huang,S.Yu,M.Liu,C.Gao,High- flux composite hollow fiber nanofiltration membranes fabricated through layer-by-layer deposition of oppositely charged crosslinked polyelectrolytes for dye removal,J.Membr.Sci.492(2015)312-321.
[36]W.Fang,L.Shi,R.Wang,Interfacially polymerized composite nanofiltration hollow fiber membranes for low-pressure water softening,J.Membr.Sci.430(2013)129-139.
[37]L.Lianchao,W.Baoguo,T.Huimin,C.Tianlu,X.Jiping,A novel nanofiltration membrane prepared with PAMAM and TMC by in situ interfacial polymerization on PEK-C ultra filtration membrane,J.Membr.Sci.269(2006)84-93.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Substrate matters:The influences of substrate layers on the performances of thin- film composite reverse osmosis membranes☆
- Polymer-based membranes for solvent-resistant nanofiltration:A review
- Recent developments in nanofiltration membranes based on nanomaterials☆
- Mass transfer model,preparation and applications of zeolite membranes for pervaporation dehydration:A review☆
- Manipulation of confined structure in alcohol-permselective pervaporation membranes☆
- Monovalent cation perm-selective membranes(MCPMs):New developments and perspectives☆
