Substrate matters:The influences of substrate layers on the performances of thin- film composite reverse osmosis membranes☆
2017-05-30JieLiMingjieWeiYongWang
Jie Li,Mingjie Wei*,Yong Wang*
State Key Laboratory of Materials-Oriented Chemical Engineering,Jiangsu National Synergetic Innovation Center for Advanced Materials,College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
1.Introduction
Shortage of fresh water is one of the most acute challenges facing the world today.Energy-efficient approaches to converting seawater into fresh water could be of substantial benefit[1,2].Desalination is a process for removing salts existing in saline water and providing fresh water suitable for human consumption as well as industrial and agricultural purposes.It has been a well-accepted,reliable and effective approach to issues related to water scarcity[3,4].Among various membrane-based desalination processes,reverse osmosis(RO)is playing a leading role in desalination industry because of its synergetic advantages including high efficiency,relatively low energy consumption,and low capital costs[5-8].
The first demonstration of RO using synthetic membranes was pioneered by Loeb and Sourirajan in the 1960s through their invention of asymmetric cellulose acetate membranes[9],and the greatest progress in this field so far was obtained with the development of thin- film composite(TFC)RO membranes[10].Typically,TFC RO membranes are composed of a top ultrathin polyamide(PA)layer,a porous middle polysulfone(PSF)substrate,and a polyester non-woven fabric base[11].For the composite membranes,compared with asymmetric membranes made from one single type of polymer,the top active layer isin-situsynthesized on the substrate and therefore,their structures and performances as well as those of the substrates can be independently designed and prepared[12].Usually,the PA layer of a TFC RO membrane is formedviainterfacial polymerization(IP)of an aromatic polyamine monomer such asm-phenylenediamine(MPD)with one or more aromatic polyacyl halides such as trimesoyl chloride(TMC)[13].
It has long been considered that the performances of TFC RO membranes are determined by the active layers.Based on this consideration,most studies are focused on modifying the structure and chemistry of the active layers to improve the performances of the membranes.In this regard,new monomers were used to synthesize the active layer so that boron and salt resistance of RO membranes were improved[14,15].Moreover,monomers with less carboxylic groups were used and thus-prepared RO membranes exhibited enhanced water flux without sacrificing the salt rejection[16].Alternatively,some researchers performed surface modification on active layers and achieved either improved fouling resistance or enhanced saltrejection/water permeability[17-20].Recently,incorporating of nanoscale fillers into the active layers to form nanocomposites has received huge attentions because thus-produced mixed-matrix membranes are able to provide considerably improved water permeabilities[4,21-23].
It might be commonly considered that,for the TFC RO membranes,the function of the substrate layer is merely to provide mechanical support to the ultrathin PA layer.Recently,however,there have emerged a few works discussing how the structure and surface chemistry of substrates influence the formation and the corresponding performances of the active layer.Typically,ultra filtration(UF)membranes produced on the polyester nonwoven fabrics by the nonsolvent-induced phase separation(NIPS)process are employed as the substrates.As an inherent limitation to the macroscale phase separation,thus-produced UF membranes are featured with pores with sizes scattered in relatively wide range.Consequently,the solution for IP reactions would permeate into some large pores much more than into smaller pores,so that the thickness of the formed PA active layer would vary in thickness.Even worse,there would also form defects in the active layer.Hence,homoporous membranes(HOMEs)with monodispersed pores and controllable surface chemistry are expected to act as ideal substrates for TFC RO membranes on which homogenous and defect-free active layers are produced,leading to RO membranes with synergistically upgraded RO performances.
In this review,we pay main attention to look into studies on how the substrate influences the IP process and consequently the structure and chemistry of the active layer,and eventually the RO performances of the composite membranes.Also,perspectives on the limitations of the current research on the substrate effect are also provided.Moreover,we propose to use membranes with uniform pores including track-etched membranes or anodized alumina membranes as the model substrates to reliably correlate the pore sizes and surface hydrophilicities with the RO performances of the TFC membranes.We anticipate that homoporous membranes derived from block copolymers are promising substrates for high-performance TFC RO membranes.
2.The Effect of Substrate Materials
PSF UF membranes casted on polyester nonwoven are predominantly used as substrates for TFC RO membranes,and commercialized RO membranes exclusively use PSF substrates possibly because of the combined advantages in mechanical robustness,thermal and chemical stability,and affordable costs.However,recently a number of other polymeric membranes have also been explored to be used as substrates for TFC membranes targeted for different applications.Since the substrate requires sufficient mechanical strength,some new types of polymers instead of PSF were explored to improve the solvent resistance,pressure resistance as well as acid resistance.Jimenez-Solomonet al.selected polyimide(PI)and poly(ether ether ketone)(PEEK)to prepare ultra filtration substrate membranes and then the TFC membranes were formed by IP for the organic solvent nanofiltration membranes[24].Their results suggested that the distinct substrate of different polymers would result in various pore morphology and chemistry of active layers.Polyvinylidene fluoride(PVDF)membrane was also used as a substrate to prepare PA TFC RO membranes because of the chemical,thermal,and mechanical stability,as well as toughness and resistance to corrosion of PVDF[25].However,the surface hydrophilicity of PVDF membranes had to be improved(e.g.by plasma modification)to allow the formation of PA layers on top of the PVDF substrates by the conventional IP method.Weiet al.prepared thermally stable TFC membranes by using poly(phthalazinone ether sulfone ketone)s(PPESKs)as the substrate[26]because the thermal stability of TFC membranes was determined by both the substrate and the active layer.
The new type of substrate would certainly influence the preparation of active layers.Chaoet al.prepared TFC RO membranes using polytetra fluoroethylene(PTFE)with varying surface hydrophilicities as the substrates and investigated the effect of the surface properties of PTFE substrates on the formation of the PA active layers[27].They found that the growth of the PA film had a strong relationship with the surface property of the PTFE substrate.Positron annihilation lifetime spectroscopy(PALS)results showed that the highest dense structure was formed at the interface between the PA layer and the PTFE substrate for the composite membranes using hydrophilic PTFE as substrates;however,the highest dense structure was formed at the top surface of the PA active layer for the composite membranes using hydrophobic PTFE as substrates(Fig.1).Unfortunately,the authors did not experimentally check the RO performances of these membranes.Nevertheless,it is expected that the composite membranes with hydrophobic PTFE as the substrates would give higher permeation and lower salt rejection because its PA layer was thinner.Alternatively,Kimet al.used polypropylene(PP)membranes as substrates to produce RO membranes.Pristine PP membranes were too hydrophobic to be wetted by the aqueous MPD solutions in the IP process,and defect-free PA layers cannot be formed on them.To solve this problem,they treated the PP membranes with plasma to generate polar groups on the membrane surface.By optimizing plasma treatment,the resultant RO membrane with PP as the substrate exhibited an improvement in salt rejection from 11%to 87%[28].
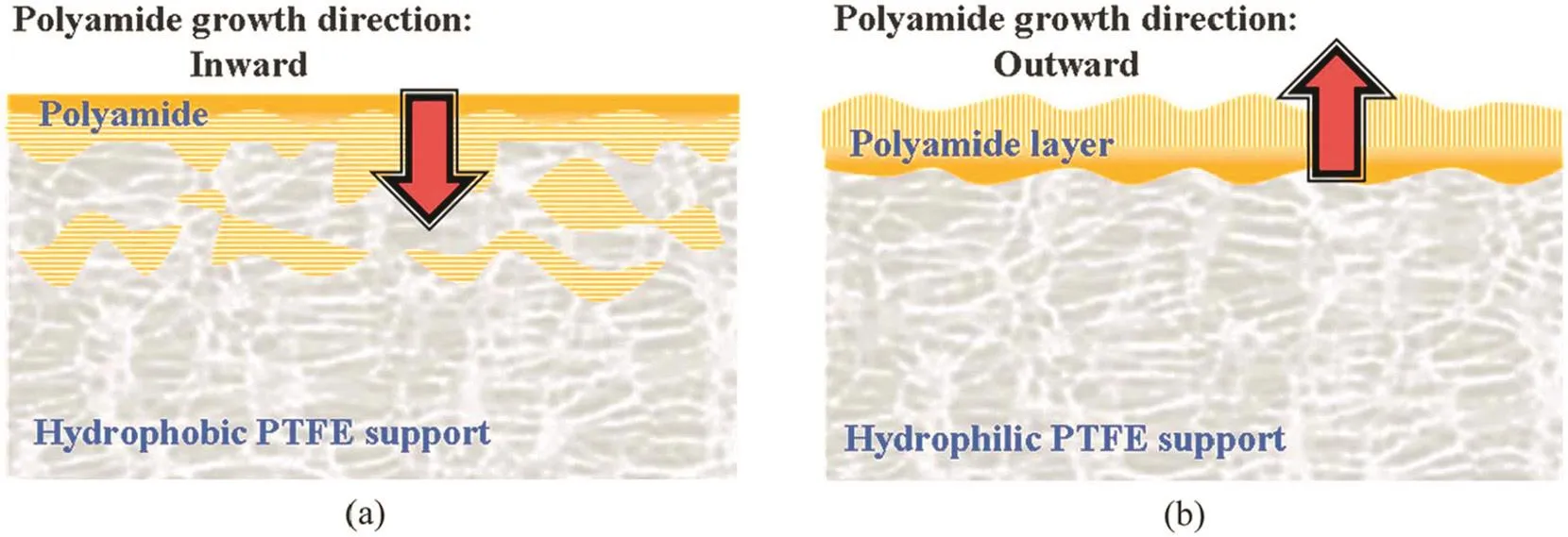
Fig.1.The growth of PA films in the IP process using(a)hydrophobic and(b)hydrophilic PTFE membranes as substrates.The first phase in which the PTFE substrate was immersed was the organic phase and aqueous phase,respectively for(a)and(b).Reproduced with permission from Ref.[27].Copyright 2012 Royal Society of Chemistry.
Considering most the efforts reported so far using UF membranes made of different polymers as substrates to produce TFC RO membranes,the predominant advantages achieved are the improvements in the chemical resistance and mechanical stability.The best RO performances,that is,good water permeability and very high salt rejection(>98%),are mainly observed on TFC membranes using PSF membranes as substrates.In addition to the selection of polymer material itself,incorporating additives into the substrate is another method to modify its properties.Poly(pyromellitic dianhydride-co-4,4-oxydianiline)with ZnCl2as additives had been developed as a substrate for a thermally stable composite reverse osmosis membrane[29].Zinc ions were able to interact with the carboxylic groups of polyamic acid so that an ionic cross-linking structure was formed.Correspondingly,the surface pores became smaller and the interior structure was turned from finger-like to sponge-like.Thus-prepared membranes exhibited smooth surfaces and improved mechanical strength.The authors also demonstrated that delamination of the PA active layer from such a smooth surface could be prevented by covalent bonding between the PA layer and the substrate polymer as a result of chemical reactions between the substrate polymer and diamines involved in the IP process.They showed that,by using 24%ZnCl2,the pure water flux of resultant TFC membranes was improved at least 30%while the salt rejection dropped from 97%from 61%.Namvar-Mahboub and Pakizeh prepared polyetherimide(PEI)nanocomposite membranes containing amino-functionalized silica nanoparticles and used them as the substrate to fabricate TFC nanofiltration membranes for organic solvents[30].The nanocomposite membranes were chemically stable in some organic solvents.When these nanocomposite membranes were used as substrates to prepare TFC membranes,the loading amount of silica nanoparticles influenced the IP processes.More nanoparticles were found on the top surface of the substrates containing 10%silica than for the substrate with 5%silica.The presence of silica nanoparticles in the polymer matrix impedes the TMC diffusion through substrates.Thus,for substrates with 10 wt%silica,the rate of TMC transport into surface pores was considerably decreased,producing nodular structures after immersing in MPD solution.Therefore,a less dense polyamide selective layer was formed.Consequently,higher flux(11.89 L·m-2·h-1)and lower oil rejection(86.82%)were obtained for the TFC membrane prepared on the substrate with 10 wt%silica while the flux and rejection of the membrane prepared on the substrate with 5 wt%silica were 10.4 L·m-2·h-1and 94.72%,respectively.Carbon nanotubes(CNTs)are extensively mixed into membrane-forming polymers to prepare mixed matrix membranes since CNTs are verified to enhance water permeance[31].Choiet al.[32,33]mixed functionalized CNTs into polyethersulfone(PES)substrate to prepare TFC RO membranes.They observed that the hydrophilicity,average pore width,total pore area and porosity of the membrane were improved after blending functionalized CNTs into the PES substrate(Fig.2).The PES substrates gained more negative charges on the surface as a result of the incorporation of negatively charged functionalized CNTs into the membrane.Consequently,thicker PA layers were formed on the substrates,indicating a less dense structure in the PA layer,and the water permeability was enhanced by 44%.On the other hand,due to the reduced surface zeta potential,the obtained TFC membrane exhibited an enhanced salt rejection(from 95%to 96.1%)simultaneously.Moreover,the TFC membranes also showed improved resistance to negatively charged foulants due to the enhanced repulsion force between the membrane surface and the anions present in water.
3.The Effect of Pore Size and Surface Hydrophilicity
As discussed in the previous section,researchers explored various polymeric materials and made some modification generally with the intention to prepare substrate membranes with proper pore sizes and surface chemistries.In this section,we will focus on the influence of pore size and surface chemistry of the substrate on the performances of the TFC RO membranes.
Ramonet al.theoretically investigated the effects of the pore size and porosity of substrates on diffusive transport through composite membranes by numerical modeling(Fig.3)[34].They used both 3D and 2D models to describe the relationship of the thickness of the PA layers as well as the permeability of TFC membranes with pore structure and hydrophilicities of the substrate.The modeling results suggested that the choice of substrates was also important in determining the performances of the composite membranes.In the case the permeability of the PA selective layer remained unchanged,the substrates with higher porosity and smaller surface pores would result in higher flux but lower salt rejection.
Singhet al.used two PSF membranes with the average pore size of 70 and 150 nm,respectively,as the substrates to prepare TFC RO membranes(Fig.4a)[35].The TFC membrane having the substrate with 70 nm pores exhibited much higher salt rejection(96%)than the membrane having the substrate with 150 nm pores(65%),but the flux of the former dropped from 85.75 to 47.25 L·m-2·h-1.The infrared characterizations suggested that a thicker active layer(200 nm)was formed on the substrate with 70 nmpores due to reduced penetration of PA into the pores in the PSF substrate.In the case of using PSF substrates with 150 nm pores,PA is produced inter deeper interior inside the pores because of the easy penetration of the monomer solutions into the large pores,leading to thinner PA layers(100 nm)formed on top of the PSF substrates.Thinner PA layers may lead to the possibility of higher degree of defects and,consequently,lower salt rejection efficiency.Similarly,Huanget al.prepared nylon 6,6 substrate membranes with a series of different pore sizes,i.e.25,100,200 and 450 nm,and then the PA selective layers were interfacially polymerized on them.It was found that the thicknesses of PA active layers were independent of the pore sizes of the substrates because the average thicknesses of the PA layers on all four substrates were measured to be nearly identical(~100 nm)[36].In addition,they argued that MPD was likely to diffuse out of the pore slowly due to the favorable hydrogen bonding interactions between MPD and amide groups on the pore walls in the nylon 6,6 membranes.This “dragging effect”on MPD slowed the diffusion rate down and allowed the PA formation to occur deeper inside the pore.Importantly,if the PA layers were prepared on substrates with large pores(e.g.450 nm),the polyamide layer tended to be damaged under the high pressure of RO process.The work of Tiraferriet al.supported theiropinions,in which an integral PA layer can be fabricated more easily when a thicker skin layer and smaller surface pores are present on the substrate[37].The salt rejection was improved from 95.8%to 97.2%as a thicker sponge-like stratum at the top of the support layer helps to minimize PA defects.However,the permeability of the PA active layer was obviously reduced by 70%because of the increased support skin layer thickness and decreased pore sizes in the substrate.
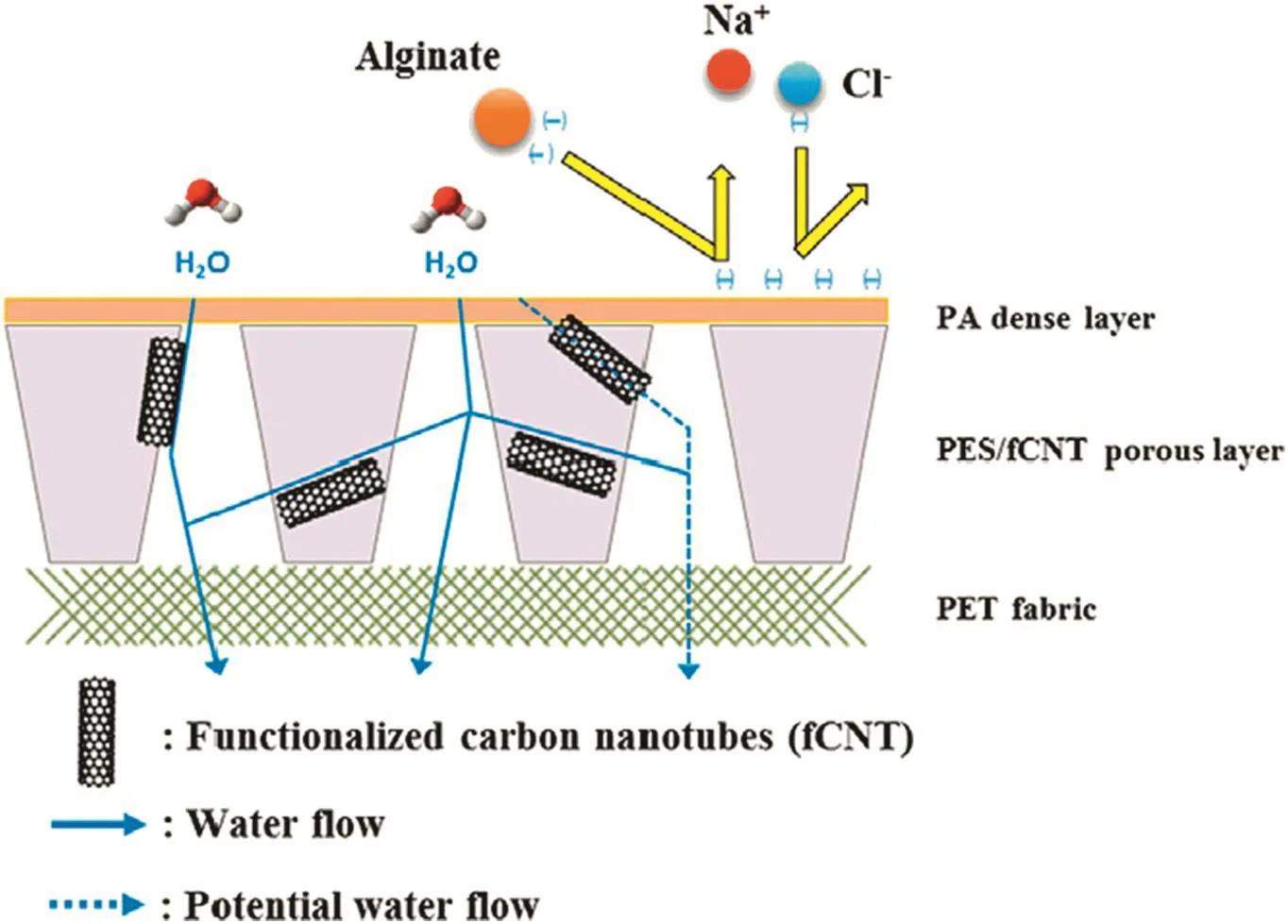
Fig.2.Illustration of the efficacy of CNTs positioning in the PES substrate on the performance of TFC membrane for desalination.Reproduced with permission from Ref.[32].Copyright2015 Elsevier B.V.
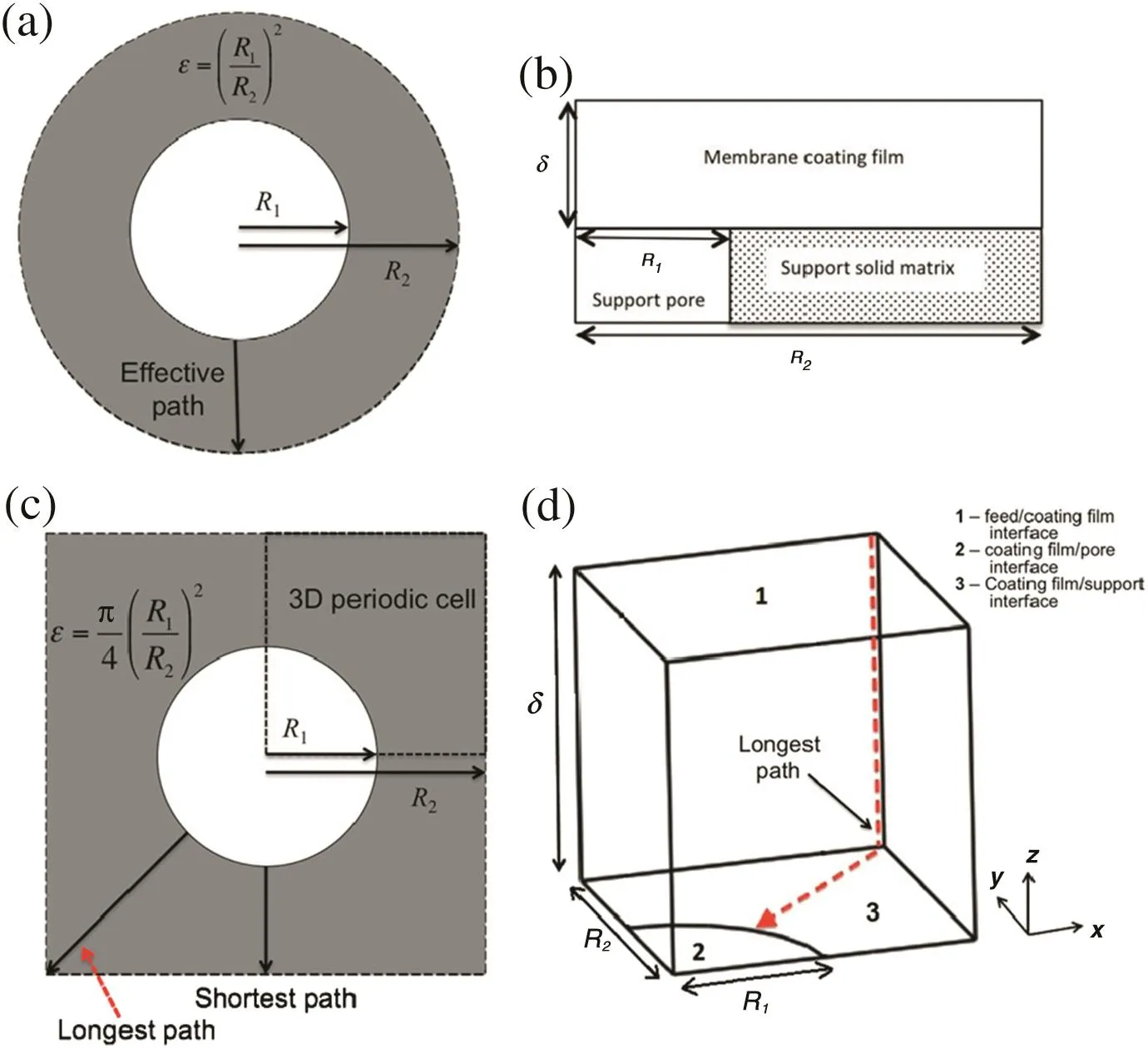
Fig.3.Schematic drawings illustrating the geometry used for the model calculations.(a)Top view of an axis-symmetric unit cell.(b)Aside view of the 2D cell.(c)Top view of a unit-cell in a square array.(d)The slice used for the 3D numerical model.Reproduced with permission from Ref.[34].Copyright 2012 El sevier B.V.
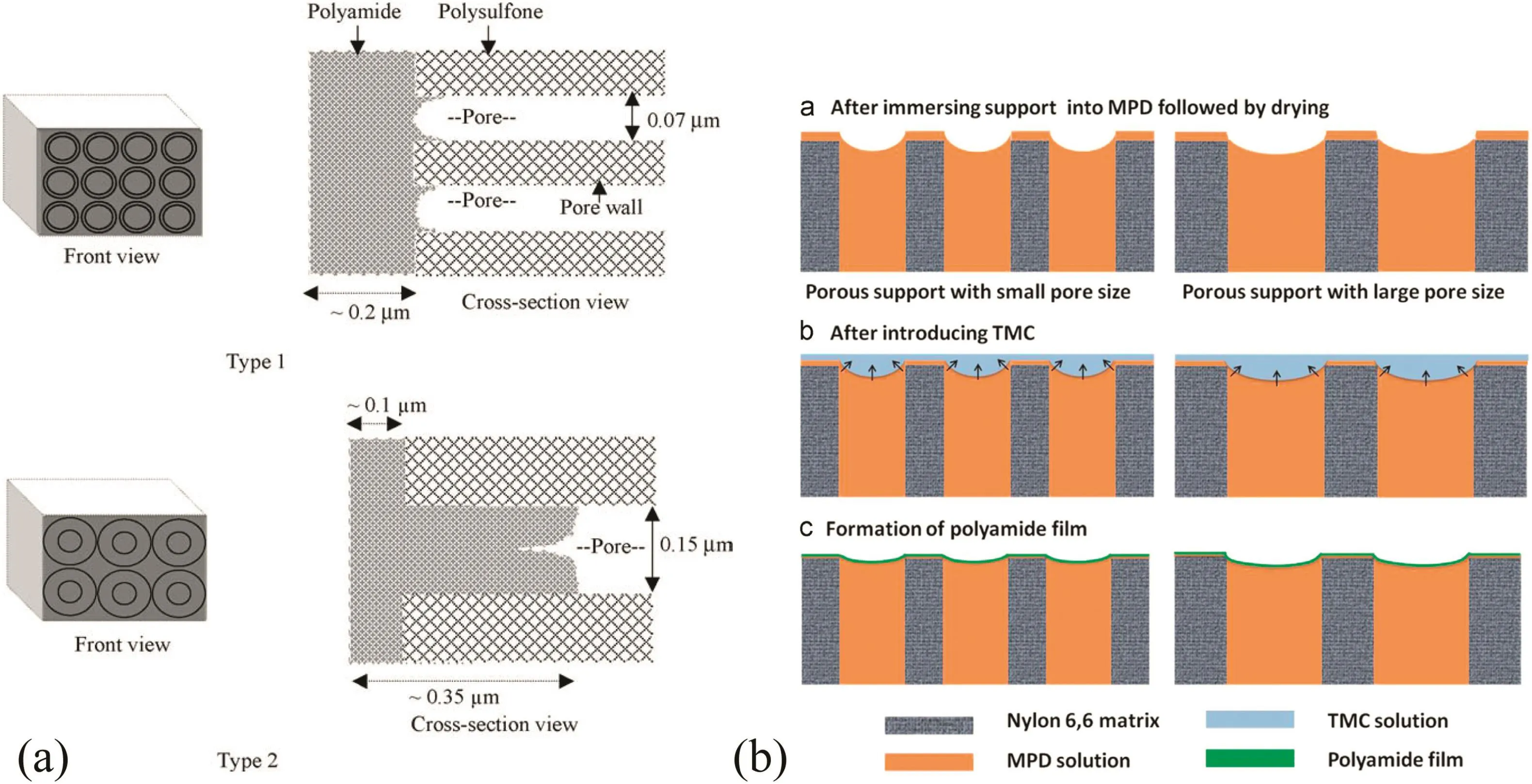
Fig.4.The schematic diagram by(a)Singh et al.and(b)Huang et al.showing the penetration of monomer solutions into the pores in substrates and the formation of PA layers on top of the substrates proposed.Reproduced with permission from Refs.[35,36].Copyright 2006 and 2015 El sevier B.V.
Recently,Hoek and coworkers prepared porous PSF membranes with different physical and chemical properties and used them as substrates to synthesize TFC RO membranes[38].The PSF substrate membranes exhibited a wide range of pure water permeability,dextran rejections,water contact angles,and surface roughnesses.Each substrate resulted in a different pore morphology and surface chemistry of the PA layer.They found that the substrate with the largest pores and the most hydrophobic and rough surface produced composite membranes with highest water permeability.The substrates with the same hydrophilicity but smaller pores exhibited similar water permeability and salt rejection,suggesting that the hydrophilicity of substrate is more important rather than the pore sizes.However,it should be noted that the pore sizes investigated in this work were ranged from 60 to 140 nm,which are obviously smaller compared to the case of Huanget al.[36]discussed above.The hydrophilic substrates(with polyethylene glycol or polyvinyl pyrrolidone additives)resulted in thicker and denser PA layers forming inside the pores and consequently reduced water flux.The authors explained that the diffusion rate of MPD from pore interior to membrane interface would be certainly affected by the presence of hydrophilic additives.Therefore,they suggested that more and large hydrophobic pores in the substrates would produce more permeable and rougher composite membranes because less PA were formed inside the substrate pores(Fig.5)and the salt rejection would not be sacrificed due to the intact and relatively thick PA layer over the surface of such substrate.
However,Fathizadehet al.had different observations and understandings.They used PES substrates to prepare TFC RO membranes[39].The authors illustrated the effect of lag time between adding the MPD and TMC solution on the IP process and its relationship with the pore size and hydrophilicities of the substrates.They suggested that the penetration of the MPD solution into the pores of hydrophobic PES substrates would be limited,so the IP reactions occurred near the surface of substrates rather than the interior of pores.Thus obtained PA layers were thicker.When the lag time was increased from 0 min to 8 min,more MPD would penetrate into pores of substrates,resulting in a thinner PA film because PA preferred to form in the pores.For the hydrophilic substrates,the MPD would penetrate into pores of substrates more easily,so that the obtained PA film was thinner and smoother outside but thicker inside the pores with large lag time.If the surface pore size was small,the MPD and TMC diffusion rate would be limited when IP reaction occurred,therefore the PA layer inside the pores would be thinner.After all,the authors concluded that a more hydrophilic substrate membrane with smaller pore size would produce a better RO membrane because more MPD solution would penetrate into PES substrate so as to form a thinner,more hydrophilic and less rough PA layer.Moreover,Yakavalangiet al.[40]demonstrated that RO membranes prepared on substrates with bigger pores had more “leaf-like”folds on their surface,while nodular morphology was observed on the membrane using substrates with smaller pores.When the pores in the substrate are big enough,more MPD solution will be absorbed into the substrate,and more MPD will diffuse into the oil phase to react with TMC,leading to “leaf-like”structures.The IP of PA on PES substrates modified with multi-wall CNTs also supported this opinion[41].In that work,the authors explained that the substrate with smaller pores would help to prepare a much smoother PA layer,and they produced obviously improved water flux in FO.Although the authors did not produce RO membranes on these substrates,it can be expected that they will also offer higher permeance in RO.
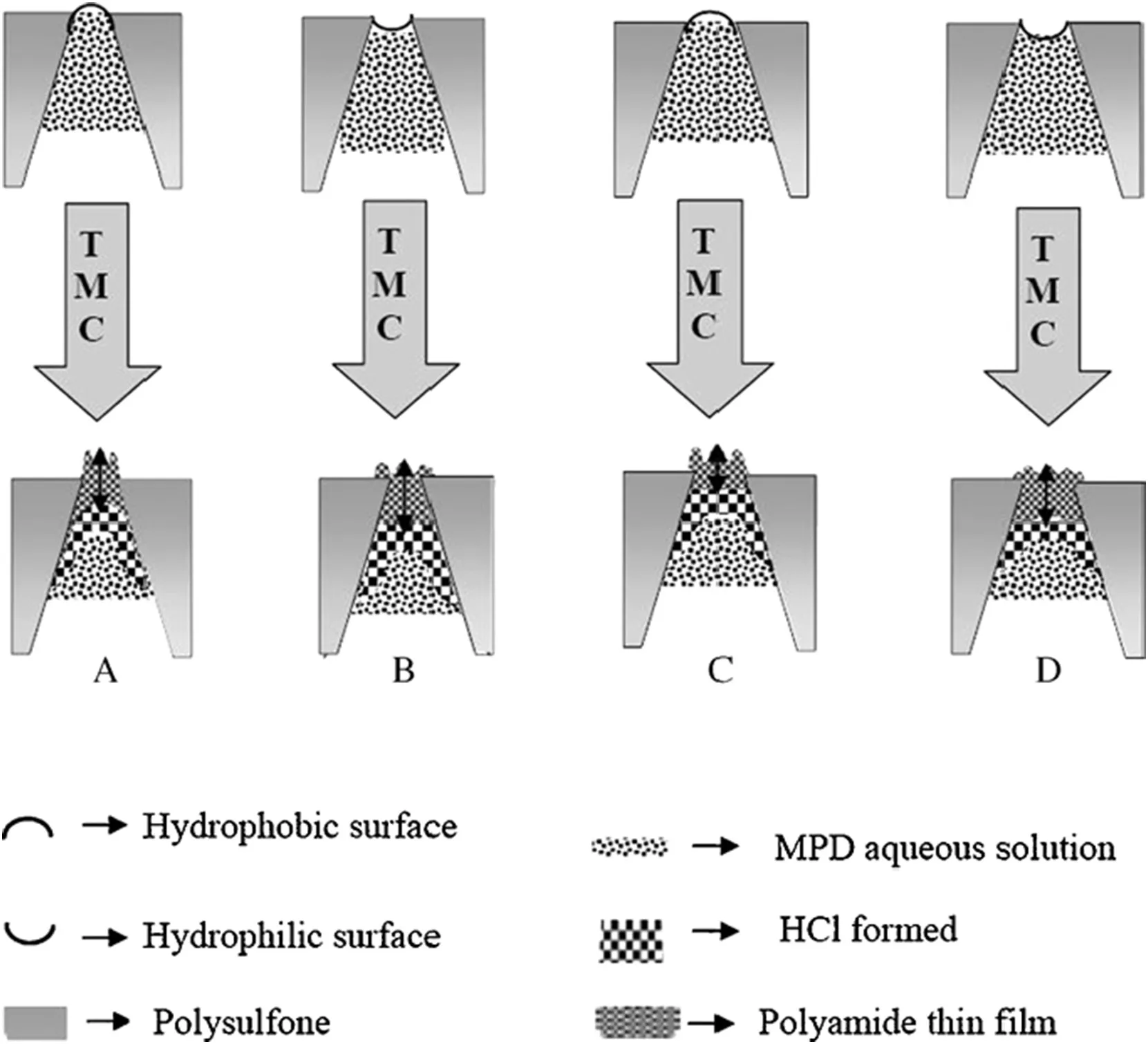
Fig.5.The schematic diagram showing the effect of pore structure and chemistry of PSF substrate membranes on the IP process and the formation of PA layers.Reproduced with permission from Ref.[38].Copyright 2009 Elsevier B.V.
Although the transport resistance in the RO process mainly comes from the dense PA layer.However,the porous nature of the substrates has been demonstrated to also play a role in influencing the water transport resistance of the composite RO membranes.Yanet al.found that,by their unique secondary pore-forming method(adding SiO2nanoparticles during phase inversion of substrate and then removing the nanoparticles with alkali treatment),the surface porosity of the substrate was significantly improved,so that the transport resistance of substrate was reduced.Consequently they observed simultaneously improved flux(from 35.4 to 55.0 L·m-2·h-1)and rejection(from 98.74%to 99.10%)of TFC RO membrane[42].They evidenced that TFC membranes with thinner active layers but lower surface porosity of substrate exhibited inferior water flux and salt rejection.Some works on nanofiltration and FO membranes that are also in the form of TFC with PA selective layers produced by interfacial polymerization have discussed the role of the substrate layer on mass transport resistance of the whole membrane,which is helpful for us to understand the case of RO membranes.PSF membranes after annealing treatment were used as substrates for the preparation of nanofiltration membranes[43].Pore size and porosity in the skin layer of the annealed PSF membranes were well-promoted while those in the bulk of the membranes were suppressed.Itwas demonstrated that PSF membranes with larger pores and higher porosity in the skin layer were favorable for higher water permeance in nanofiltration while the rejection to MgSO4was not sacrificed at all.Yasukawaet al.revealed that there was a good correlation between the FO flux and the partial morphology at the dense part in the substrate,whereas the hydraulic breaking ressure is strongly dependent on the thickness of the entire substrate and the presence of large voids[44].In the theoretical work of Ramonet al.,it was also demonstrated that the substrates with more pores and higher permeability would help to reduce the mass transport resistance[34].
Moreover,the topography of the substrates should also have an effect on the IP process and the RO properties of thus-produced composite membranes.Recently,Dinget al.purposely developed sub-micron patterns PES substrate membranes by hot imprinting(Fig.6).They managed to perform IP on the patterned substrates,and obtained composite membranes.With the presence of sub-micron patterns on the substrate surface,the composite membranes exhibited appreciable advantages in decreasing concentration polarization and scaling[45].Their preliminary filtration tests suggested that their membranes composited on patterned surfaces showed slightly increased water permeability than counterparts composited on flat substrates because of larger surface areas of the former.The salt rejections of two membranes remained comparable[46].The improvement in permeability may be further enhanced if patterns with smaller features down to the nanometer scale can be densely developed on the substrates.
4.Perspectives
Previous works discussed above clearly evidence that the substrates have an effect,which is typically stronger than expected,on the performances of TFC RO membranes.The porous nature(pore size,size distribution,and porosity)and the surface properties(hydrophilicity and roughness)of the substrates influence the IP process,and the thickness and morphology of the PA layers formed on top of the substrates are correspondingly changed,consequently leading to variation of the performances of the composite RO membranes including water permeability,salt rejection,fouling resistance,etc.
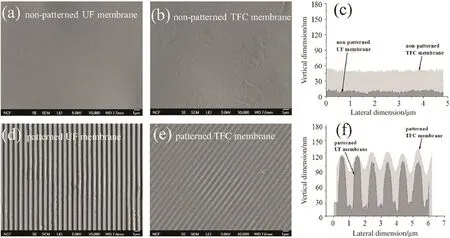
Fig.6.Morphological characterization of the patterned and non-patterned TFC membranes and UF substrate membranes.Representative top-surface SEM images of(a)the non-patterned PES UF substrate membrane,(b)non-patterned TFC membrane,(d)patterned UF membrane substrate,and(e)patterned TFC membrane.Images(c)and(f)are representative cross-sectional pro files for non-patterned and patterned TFC membranes obtained from AFM scans.Note that the overlay of the TFC layer on the patterned UF membrane shown in panel(f)is a schematic representation rather than an actual pro file,as it is challenging to isolate the barrier layer in this system.Reproduced with permission from Ref.[46].Copyright 2014 Elsevier B.V.
However,the observations and understandings on the effect of substrates from different works are not very consistent and sometimes are even contradictory.The main reason lies in that the substrates they used are highly scattering in terms of both pore sizes and surface properties.So far,the substrates used for TFC RO membranes are exclusively UF membranes prepared by the conventional process of NIPS.As the pores are developed from the polymer-lean phases,which are kinetically controlled,these membranes have a strong variation in pore sizes and pore density on the membrane surface.These individual pores with varying sizes behave differently in absorbing monomer solutions and accommodating the formation of PA.However,researchers have to treat them as“uniform pores”with average pore sizes when considering their effect on IP and PA layers.There is a big risk because one average pore size can come from numerous different combinations of pores with changing sizes.Moreover,the hydrophilicity of the membranes can only be unambiguously tuned within narrow regions by changing the dosages of polar additives in the NIPS process.Even worse,the surface hydrophilicity of a membrane is commonly determined by the tests of water contact angles(WCAs).WCAs are a macroscopic parameter determined by both the inherent chemistry of the material and the surface roughness in the area of micrometer scale[47,48].Therefore,WCAs reflect the wettability of the membrane to water droplets with a microliter volume,and they do not necessarily represent the wettability of the pores with sizes in the range from a few nanometers to a few of tens of nanometers.
To have a clear understanding on the substrate effect,substrates with uniform pores and controllable surface chemistry are required.To this end,we suggest to perform in-depth investigations using HOMEs as substrates.HOMEs are membranes composed of uniform and straightpores[49].Specifically,track-etched polymeric membranes[50]and anodized alumina membranes[51]may be the first choice because of their relatively narrow pore size distribution,availability of pore sizes down to~20 nm,and easy chemical functionalization to the pore walls.Molecules with different hydrophilicities can be chemically attached to the pore walls to precisely tune the wettability of membrane pores within a wide range.Detailed studies on the IP process,the PA morphology,and the performances of RO membranes composited on these substrates with various pore sizes and pore wall chemistries are expected to reliably reveal the relationships between the pore size and hydrophilicity of the substrates and the water permeability and salt rejection.
However,because of the extremely low porosities of track-etched membranes and the poor mechanical robustness of alumina membranes the two membranes can only be used as model systems to investigate the substrate effect.Considered from the angle of practical applications,they neither are appropriate.Alternatively,we suggest using HOMEs derived from block copolymers(BCPs)as substrates to produce high-performance TFC RO membranes.BCPs are hybrid macromolecules tending to microphase separate,yielding ordered,periodic structures with feature sizes in the range of~10-50 nm.By converting the dispersed phases in the phase-separated BCP films into empty voids,one obtains membranes with extremely high pore uniformity.Recently,many efforts have been investigated to develop BCP membranes.A number of methods have been demonstrated to produce BCP-derived HOMEs with adjustable pores,including selective removal of the labile blocks or additives pre-incorporated into the minority blocks,[52-54]NIPS of BCP solutions[55,56]and selective swelling of pre-aligned amphiphilic BCP films[49,57].In addition to cylindrical pores perpendicular to the substrate surface,the selective swelling method is also able to produce slit-like pores parallel to the substrate surface(Fig.7).Membranes with these slitted pores are featured as more than doubled surface porosities compared to membranes with perpendicular pores[58].Therefore,TFC membranes using BCP membranes with slitted pores as substrates are expected to exhibit significantly improved water permeability at no or little expense in loss of salt rejection.It is predicted that using BCP HOMEs as substrates will be a general and efficient approach to evidently upgrade the performances of other TFC membranes in addition to RO membranes.Therefore,more efforts both from academia and industry should be strongly desired to advance the research and development of BCP HOMES,especially to overcome the obstacles hindering their upscaling and real applications.
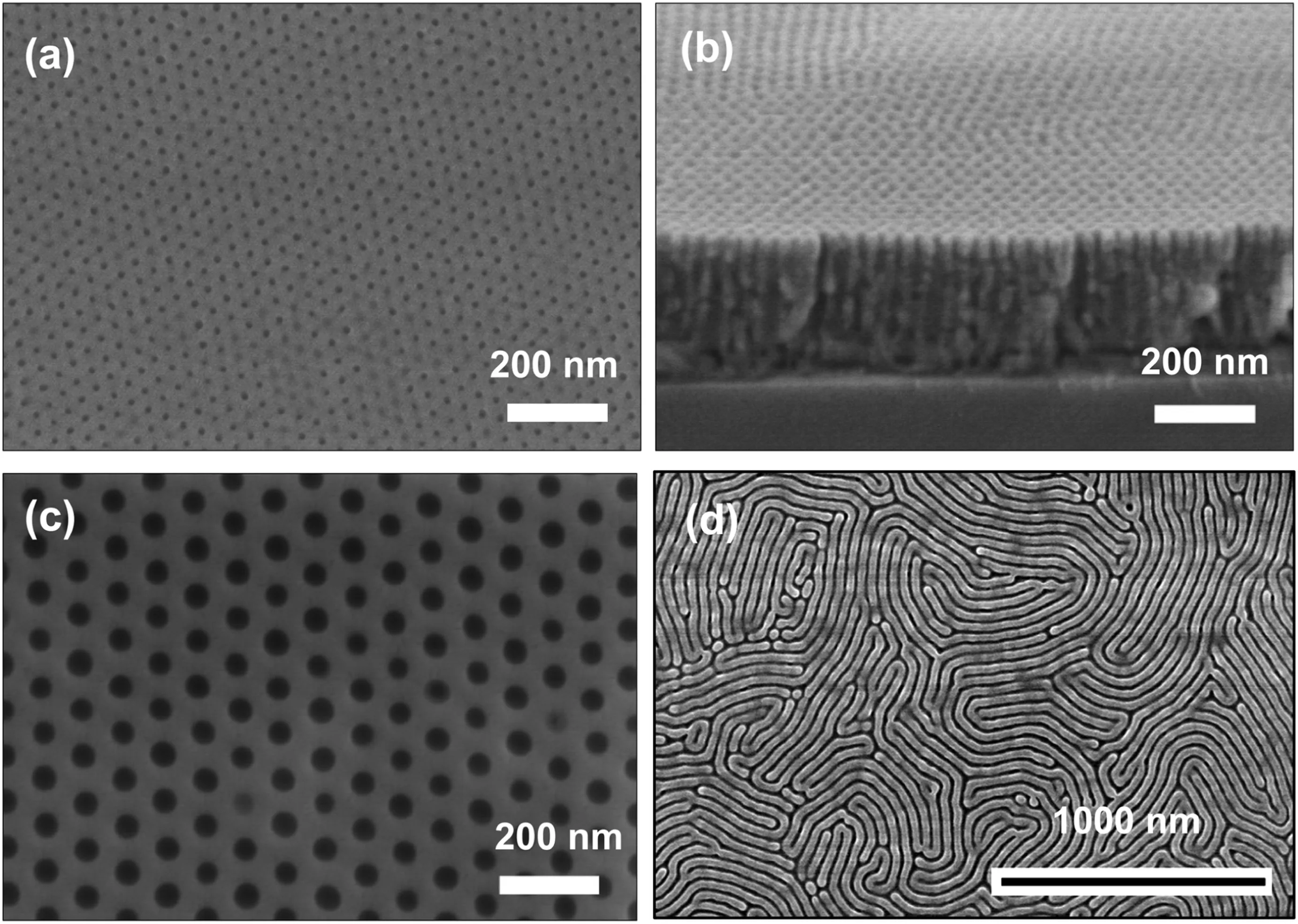
Fig.7.Homoporous membranes with pores oriented in the perpendicular(a-c)and in-plane(d)direction prepared by selective swelling of solvent-annealed PS-b-PVP films.(a,c)Surface SEM images of perpendicular pores with diameters of 12 and 46 nm,respectively.(b)Cross-sectional SEM image of perpendicular pores with a diameter of 12 nm.(d)Surface SEM image of in-plane oriented pores with a pore width of 12 nm.Reproduced with permission from Ref.[57].Copyright 2016 American Chemical Society.
5.Conclusions
Compared to extensive studies on the PA selective layers,the influence of substrates on the performances of TFC RO membranes is paid less attention.Reported works suggest that the porous nature as well as the surface properties of the substrates significantly influences the RO performances including water permeability and salt rejection of the composite membranes by changing the thickness and morphology of the PA layers synthesized on the substrates.However,due to the limitation of available substrate membranes with uniform pores and surface chemistry the observations and understandings from different researchers on the substrate effect are not consistent and even contradictory.To clearly elucidate the substrate effect comprehensive and systematic studies based on homoporous membranes with uniform pore sizes and pore wall wettabilities are highly desired.Furthermore,we expect that homoporous membranes derived from block copolymers hold great potential to be used as substrates in the manufacturing of high-performance TFC RO membranes.
Nomenclature
BCPs block copolymers
CNTs carbon nanotubes
FO forward osmosis
HOMEs homoporous membranes
IP interfacial polymerization
MPDm-phenylenediamine
NIPS nonsolvent induced phase separation
PA polyamide
PALS positron annihilation lifetime spectroscopy
PEEK poly(ether ether ketone)
PEI polyetherimide
PES polyethersulfone
PI polyimide
PP polypropylene
PPESKs poly(phthalazinone ether sulfone ketone)s
PSF polysulfone
PTFE polytetra fluoroethylene
PVDF polyvinylidene fluoride
RO reverse osmosis
TFC thin- film composite
TMC trimesoyl chloride
UF ultra filtration
WCAs water contact angles
[1]S.J.Kim,S.H.Ko,K.H.Kang,J.Han,Direct seawater desalination by ion concentration polarization,Nat.Nanotechnol.5(4)(2010)297-301.
[2]M.Elimelech,W.A.Phillip,The future of seawater desalination:Energy,technology,and the environment,Science333(6043)(2011)712-717.
[3]L.F.Greenlee,D.F.Lawler,B.D.Freeman,B.Marrot,P.Moulin,Reverse osmosis desalination:Water sources,technology,and today's challenges,Water Res.43(9)(2009)2317-2348.
[4]D.Li,Y.Yan,H.Wang,Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes,Prog.Polym.Sci.61(2016)104-155.
[5]M.A.Shannon,P.W.Bohn,M.Elimelech,J.G.Georgiadis,B.J.Marinas,A.M.Mayes,Science and technology for water purification in the coming decades,Nature452(7185)(2008)301-310.
[6]T.Humplik,J.Lee,S.C.O'Hern,B.A.Fellman,M.A.Baig,S.F.Hassan,M.A.Atieh,F.Rahman,T.Laoui,R.Karnik,Nanostructured materials for water desalination,Nanotechnology22(29)(2011)292001.
[7]K.P.Lee,T.C.Arnot,D.Mattia,A review of reverse osmosis membrane materials for desalination—Development to date and future potential,J.Membr.Sci.370(1-2)(2011)1-22.
[8]S.Burn,M.Hoang,D.Zarzo,F.Olewniak,E.Campos,B.Bolto,O.Barron,Desalination techniques—A review of the opportunities for desalination in agriculture,Desalination364(2015)2-16.
[9]M.Safarpour,A.Khataee,V.Vatanpour,Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2with improved desalination performance,J.Membr.Sci.489(2015)43-54.
[10]J.E.Cadotte,Evolution of composite reverse osmosis membranes,Materials Science of Synthetic Membranes,ACS symposium series no.269,American Chemical Society,Washington,D.C.1985,pp.273-294.
[11]G.-R.Xu,J.-N.Wang,C.-J.Li,Strategies for improving the performance of the polyamide thin film composite(PA-TFC)reverse osmosis(RO)membranes:Surface modifications and nanoparticles incorporations,Desalination328(2013)83-100.
[12]M.Liu,Z.Chen,S.Yu,D.Wu,C.Gao,Thin- film composite polyamide reverse osmosis membranes with improved acid stability and chlorine resistance by coating N-isopropylacrylamide-co-acrylamide copolymers,Desalination270(1-3)(2011)248-257.
[13]R.J.Petersen,Composite reverse osmosis and nanofiltration membranes,J.Membr.Sci.83(1)(1993)81-150.
[14]M.Liu,S.Yu,J.Tao,C.Gao,Preparation,structure characteristics and separation properties of thin- film composite polyamide-urethane seawater reverse osmosis membrane,J.Membr.Sci.325(2)(2008)947-956.
[15]S.Yu,M.Liu,X.Liu,C.Gao,Performance enhancement in interfacially synthesized thin- film composite polyamide-urethane reverse osmosis membrane for seawater desalination,J.Membr.Sci.342(1-2)(2009)313-320.
[16]Y.Zhao,Z.Zhang,L.Dai,H.Mao,S.Zhang,Enhanced both water flux and salt rejection of reverse osmosis membrane through combining isophthaloyl dichloride with biphenyl tetraacyl chloride as organic phase monomer for seawater desalination,J.Membr.Sci.522(2017)175-182.
[17]J.Wu,Z.Wang,Y.Wang,W.Yan,J.Wang,S.Wang,Polyvinylamine-grafted polyamide reverse osmosis membrane with improved antifouling property,J.Membr.Sci.495(2015)1-13.
[18]J.Meng,Z.Cao,L.Ni,Y.Zhang,X.Wang,X.Zhang,E.Liu,A novel salt-responsive TFC RO membrane having superior antifouling and easy-cleaning properties,J.Membr.Sci.461(2014)123-129.
[19]L.Ni,J.Meng,X.Li,Y.Zhang,Surface coating on the polyamide TFC RO membrane for chlorine resistance and antifouling performance improvement,J.Membr.Sci.451(2014)205-215.
[20]Y.Pan,L.Ma,S.Lin,Y.Zhang,B.Cheng,J.Meng,One-step bimodel grafting via a multicomponent reaction toward antifouling and antibacterial TFC RO membranes,J.Mater.Chem.A4(41)(2016)15945-15960.
[21]H.Dong,L.Zhang,H.L.Chen,C.J.Gao,Mixed-matrix membranes for water treatment:Materials,synthesis and properties,Prog.Chem.26(12)(2014)2007-2018.
[22]J.Yao,H.Wang,Zeolitic imidazolate framework composite membranes and thin films:Synthesis and applications,Chem.Soc.Rev.43(13)(2014)4470-4493.
[23]Y.Zhang,Y.Wei,Z.Cao,H.Zhang,Y.Yao,Progress and prospect in the development of reverse osmosis membrane technology,Chem.Ind.Eng.32(5)(2015)8-19.
[24]M.F.Jimenez-Solomon,P.Gorgojo,M.Munoz-Ibanez,A.G.Livingston,Beneath the surface:Influence of supports on thin film composite membranes by interfacial polymerization for organic solvent nanofiltration,J.Membr.Sci.448(2013)102-113.
[25]E.-S.Kim,Y.J.Kim,Q.Yu,B.Deng,Preparation and characterization of polyamide thin- film composite(TFC)membranes on plasma-modified polyvinylidene fluoride(PVDF),J.Membr.Sci.344(1-2)(2009)71-81.
[26]J.Wei,X.Jian,C.Wu,S.Zhang,C.Yan,Influence of polymer structure on thermal stability of composite membranes,J.Membr.Sci.256(1-2)(2005)116-121.
[27]W.-C.Chao,Y.-H.Huang,W.-S.Hung,Q.An,C.-C.Hu,K.-R.Lee,J.-Y.Lai,Effect of the surface property of poly(tetra fluoroethylene)support on the mechanism of polyamide active layer formation by interfacial polymerization,Soft Matter8(34)(2012)8998-9004.
[28]H.I.Kim,S.S.Kim,Plasma treatment of polypropylene and polysulfone supports for thin film composite reverse osmosis membrane,J.Membr.Sci.286(1-2)(2006)193-201.
[29]C.Ba,J.Economy,Preparation of PMDA/ODA polyimide membrane for use as substrate in a thermally stable composite reverse osmosis membrane,J.Membr.Sci.363(1-2)(2010)140-148.
[30]M.Namvar-Mahboub,M.Pakizeh,Development of a novel thin film composite membrane by interfacial polymerization on polyetherimide/modified SiO2support for organic solvent nanofiltration,Sep.Purif.Technol.119(2013)35-45.
[31]R.Das,M.E.Ali,S.B.A.Hamid,S.Ramakrishna,Z.Z.Chowdhury,Carbon nanotube membranes for water purification:A bright future in water desalination,Desalination336(2014)97-109.
[32]M.Son,H.-g.Choi,L.Liu,E.Celik,H.Park,H.Choi,Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin- film composite membrane for desalination,Chem.Eng.J.266(2015)376-384.
[33]M.Son,H.Park,L.Liu,H.Choi,J.H.Kim,H.Choi,Thin- film nanocomposite membrane with CNT positioning in support layer for energy harvesting from saline water,Chem.Eng.J.284(2016)68-77.
[34]G.Z.Ramon,M.C.Y.Wong,E.M.V.Hoek,Transport through composite membrane,part 1:Is there an optimal support membrane?J.Membr.Sci.415-416(2012)298-305.
[35]P.S.Singh,S.V.Joshi,J.J.Trivedi,C.V.Devmurari,A.P.Rao,P.K.Ghosh,Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions,J.Membr.Sci.278(1-2)(2006)19-25.
[36]L.Huang,J.R.McCutcheon,Impact of support layer pore size on performance of thin film composite membranes for forward osmosis,J.Membr.Sci.483(2015)25-33.
[37]A.Tiraferri,N.Y.Yip,W.A.Phillip,J.D.Schiffman,M.Elimelech,Relating performance of thin- film composite forward osmosis membranes to support layer formation and structure,J.Membr.Sci.367(1-2)(2011)340-352.
[38]A.K.Ghosh,E.M.V.Hoek,Impacts of support membrane structure and chemistry on polyamide-polysulfone interfacial composite membranes,J.Membr.Sci.336(1-2)(2009)140-148.
[39]M.Fathizadeh,A.Aroujalian,A.Raisi,Effect of lag time in interfacial polymerization on polyamide composite membrane with different hydrophilic sub layers,Desalination284(2012)32-41.
[40]M.Ehsan Yakavalangi,S.Rimaz,V.Vatanpour,Effect of surface properties of polysulfone support on the performance of thin film composite polyamide reverse osmosis membranes,J.Appl.Polym.Sci.134(6)(2017)http://dx.doi.org/10.1002/app.44444.
[41]Y.Wang,R.Ou,Q.Ge,H.Wang,T.Xu,Preparation of polyethersulfone/carbon nanotube substrate for high-performance forward osmosis membrane,Desalination330(2013)70-78.
[42]W.Yan,Z.Wang,J.Wu,S.Zhao,J.Wang,S.Wang,Enhancing the flux of brackish water TFC RO membrane by improving support surface porosityviaa secondary pore-forming method,J.Membr.Sci.498(2016)227-241.
[43]S.Liang,G.Xu,Y.Jin,Z.Wu,Z.Cai,N.Zhao,Z.Wu,Annealing of supporting layer to develop nanofiltration membrane with high thermal stability and ion selectivity,J.Membr.Sci.476(2015)475-482.
[44]M.Yasukawa,S.Mishima,Y.Tanaka,T.Takahashi,H.Matsuyama,Thin- film composite forward osmosis membrane with high water flux and high pressure resistance using a thicker void-free polyketone porous support,Desalination402(2017)1-9.
[45]S.H.Maruf,A.R.Greenberg,Y.Ding,Influence of substrate processing and interfacial polymerization conditions on the surface topography and permselective properties of surface-patterned thin- film composite membranes,J.Membr.Sci.512(2016)50-60.
[46]S.H.Maruf,A.R.Greenberg,J.Pellegrino,Y.Ding,Fabrication and characterization of a surface-patterned thin film composite membrane,J.Membr.Sci.452(2014)11-19.
[47]P.G.de Gennes,Wetting:Statics and dynamics,Rev.Mod.Phys.57(3)(1985)827-863.
[48]G.Bhutani,K.Muralidhar,S.Khandekar,Determination of apparent contact angle and shape of a static pendant drop on a physically textured inclined surface,1(1)(2013)29-49.
[49]Y.Wang,W.Xing,N.Xu,Homoporous membranes,CIESC J.67(1)(2016)27-40(in Chinese).
[50]Y.Fang,J.Leddy,Surface diffusion in microstructured,ion-exchange matrixes:Na fion/neutron track-etched polycarbonate membrane composites,J.Phys.Chem.99(16)(1995)6064-6073.
[51]W.Lee,R.Ji,U.Gosele,K.Nielsch,Fast fabrication of long-range ordered porous alumina membranes by hard anodization,Nat.Mater.5(9)(2006)741-747.
[52]W.A.Phillip,M.A.Hillmyer,E.L.Cussler,Cylinder orientation mechanism in block copolymer thin films upon solvent evaporation,Macromolecules43(18)(2010)7763-7770.
[53]W.A.Phillip,B.O'Neill,M.Rodwogin,M.A.Hillmyer,E.L.Cussler,Self-assembled block copolymer thin films as water filtration membranes,ACS Appl.Mater.Interfaces2(3)(2010)847-853.
[54]S.Y.Yang,I.Ryu,H.Y.Kim,J.K.Kim,S.K.Jang,T.P.Russell,Nanoporous membranes with ultrahigh selectivity and flux for the filtration of viruses,Adv.Mater.18(6)(2006)709-712.
[55]K.-V.Peinemann,V.Abetz,P.F.W.Simon,Asymmetric superstructure formed in a block copolymer via phase separation,Nat.Mater.6(12)(2007)992-996.
[56]V.Abetz,Isoporous block copolymer membranes,Macromol.Rapid Commun.36(1)(2015)10-22.
[57]Y.Wang,Nondestructive creation of ordered nanopores by selective swelling of block copolymers:Toward homoporous membranes,Acc.Chem.Res.49(7)(2016)1401-1408.
[58]L.Guo,L.Wang,Y.Wang,Stretched homoporous composite membranes with elliptic nanopores for external-energy-free ultra filtration,Chem.Commun.52(42)(2016)6899-6902.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Positively charged nanofiltration membrane fabricated by poly(acid-base)complexing effect induced phase inversion method for heavy metal removal☆
- Polymer-based membranes for solvent-resistant nanofiltration:A review
- Recent developments in nanofiltration membranes based on nanomaterials☆
- Mass transfer model,preparation and applications of zeolite membranes for pervaporation dehydration:A review☆
- Manipulation of confined structure in alcohol-permselective pervaporation membranes☆
- Monovalent cation perm-selective membranes(MCPMs):New developments and perspectives☆
