Polymer-based membranes for solvent-resistant nanofiltration:A review
2017-05-30SiowKeeLimKunliGohTaeHyunBaeRongWang
Siow Kee Lim ,Kunli Goh ,Tae-Hyun Bae ,Rong Wang *
1 Singapore Membrane Technology Center,Nanyang Environment and Water Research Institute,Nanyang Technological University,1 Cleantech Loop,637141 Singapore,Singapore
2 Nanyang Environment and Water Research Institute,Interdisciplinary Graduate School,Nanyang Technological University,637141 Singapore,Singapore
3 School of Chemical and Biomedical Engineering,Nanyang Technological University,62 Nanyang Drive,637459 Singapore,Singapore
4 School of Civil and Environmental Engineering,Nanyang Technological University,50 Nanyang Avenue,639798 Singapore,Singapore
1.Introduction
Solvent-resistant nanofiltration(SRNF),also known as organic solvent nanofiltration(OSN),is an emerging technology that allows for energy-efficient separation of molecules with molecular weights between 200 and 2000 g·mol-1in various organic solvents by employing a pressure gradient across a membrane[1,2].Industrially,SRNF is relevant for lowering the energy intensity of conventional separation processes,achieving process intensification and meeting the need for moderate processing conditions as well as minimizing waste production driven by the increased focus on sustainability and health[3].Since the late 20th century,various applications of SRNF have been realized,such as solvent recovery from lube oil filtrates[4,5],deacidification of vegetable oil[6],recovery of homogeneous catalysts[7-11]and solvent exchange[12,13]in multi-step chemical reactions.Progress in membrane research has enabled high-performance membranes with excellent chemical stability in a wide range of organic solvents including polar aprotic solvents[14]as well as tunable molecular separation properties that can be tailored for many target applications[15].As a result,the potential applications of SRNF have been expanded to include purification of active pharmaceutical ingredients(APIs)or high-value natural compounds(HVNCs)[16,17],solvent recovery in chemical processes[18,19],separation of ionic liquids[20],synthesis of peptide and oligonucleotide[21,22],and the membrane bioreactors for biotransformations[23].
SRNF offers many competitive advantages.First,it is a sustainable alternative for energy-intensive conventional processes such as distillation and evaporation as no phase transitions are involved,hence lowering energy consumption considerably[19,24].Second,the operating conditions of SNRF are milder than those of the conventional processes.This prevents thermal or other potential degradation of sensitive molecular products.Third,SRNF can strengthen the industry capacities to recycle solvents and reagents.This not only reduces the loss of valuable resources but also minimizes the generation of wastes that are produced in a large quantity in processes such as extractions,chromatography and recrystallizations.Lastly,SRNF can be installed as a continuous process due to the modularity nature to provide flexibility in combining with existing separation technologies to form hybrid processes[1].These advantages have empowered SRNF with the promise to apply in many different types of industries including petrochemical,food,pharmaceutical and fine chemical industries,serving as a green engineering process as well as an effective tool for process intensification(Fig.1).
A key component of SRNF is the membrane itself,of which there are many different types suitable for this application.As such,Section 2 of this article aims to provide a timely review of the current status of membranes development for SRNF,in particular those made from polymer-based materials.Section 3 highlights recent advancements in membrane engineering and progress in commercialization.Section 4 discusses the different types of applications of SRNF where special attention will be paid to focus on how these advancements are adapted for industrial applications.Finally,in Section 5,we present an outline for the future perspectives of SRNF.
2.Development of polymer-based SRNF membranes
The compatibility of membrane materials with a wide range of organic solvents is undoubtedly a paramount prerequisite in SRNF.Other desired membrane materials properties include film processability,mechanical strength,thermal resistance,material availability and cost[25].Inorganic membrane materials that are usually prepared from metal oxides such as alumina(Al2O3),zirconia(ZrO2)or titania(TiO2)can provide good selectivity as well as high chemical and thermal stabilities.However,the membranes are generally brittle and their large-scale membrane productions and module constructions are complicated and expensive[26].Furthermore,due to the intrinsic hydrophilicity of the materials,low permeation flux of non-polar solvents is commonly observed.As a result,inorganic membranes exhibit limited applications and remain less widespread than their polymeric counterpart despite several studies on inorganic membranes for SRNF[27-30].Polymeric materials,on the other hand,are widely used for membrane fabrication as they offer a great variety of different choices of polymers,cost effectiveness,excellent processability,good reproducibility and versatility in tailoring membrane properties for different targeted applications.The key drawbacks with polymeric materials are their limited solvent(chemical)and thermal stabilities,which continue to stifle their application in SRNF[31].There is also the challenge of overcoming the trade-off phenomenon between solvent permeation and solute rejection.Therefore,we focus our scope of this review on polymer-based membranes and the challenges they faced in SRNF.
Polymers that are preferentially used for fabricating SRNF membranes include polyacrylonitrile(PAN),polyimide(PI),polyamide(PA),polysulfone(PSf),poly(ether ether ketone)(PEEK)and polybenzimidazole(PBI).This is because such polymers contain structural elements like aromatic groups or imide bonds in the backbone which offer rudimentary solvent-resistance[1].To further strengthen the solvent-resistant ability of the membranes made from these polymers,post-treatments are usually carried out to chemically crosslink the polymer chains,giving rise to three dimensional(3-d)networks with higher rigidity and stability.This also helps to enhance the mechanical stability and the anti-swelling property of the polymeric membranes together with solvent stability to expand their capacities to take on harsh SRNF operating conditions.
Current state-of-the-art polymeric SRNF membranes are either integrally skinned asymmetric membranes(ISA)comprised of a skin layer on a more porous support layer of the same material,or thin film composite(TFC)membranes in which an ultrathin separation layer is deposited on a porous support made up of a different material[32].In TFC membranes,the separation layer and porous support can be optimized independently to meet the required membrane performance for a target application.The top separation layer is selective towards targeted solutes while the porous sublayer provides mechanical support and resistance against compaction.Recently,there is also an emerging trend in enabling nanomaterials for SRNF.In general,nanomaterials such as gold or silicon dioxide nanoparticles,zeolites,metal-organic frameworks(MOFs),multi-walled carbon nanotubes(MWCNTs)and graphene oxide(GO)nanosheets are integrated either in the polymer matrix or the separation layer to yield mixed matrix membranes(MMMs)or thin film nanocomposite(TFN)membranes,respectively(Fig.1).These discussions will be looked at in the following sections.
2.1.Integrally skinned asymmetric(ISA)membranes
ISA membranes are usually preparedviaa phase inversion process where a polymer solution is cast as a thin film and immersed into a non-solvent coagulation bath for inducing solvent/non-solvent exchange to for man asymmetric membrane structure[33].Sometimes,a short duration for solvent evaporation is introduced to increase the local polymer concentration on the membrane surface.Essentially,the final membrane morphology and its permeability and rejection performance are determined by the thermodynamics of the phase inversion and the kinetics of the solvent/non-solvent exchange.The principles governing the phase inversion process have been reviewed elsewhere[1,34].In this section,our discussion is focused on representative polymeric membranes,their chemical modifications,and the SRNF performance.
Polyimides(PIs)are one of the most widely used materials for ISA membranes owing to their excellent chemical resistance,good thermal stability and mechanical strength.Among them,some are soluble in organic solvents such as NMP,DMF,DMAc and DMSO,and hence they are suitable for ISA membranes fabrication using the phase inversion technique.Unfortunately,the resulting PImembranes are also susceptible to solvents interaction with the polymer chains and so these membranes should be further modified to improve their chemical stability towards organic solvents.
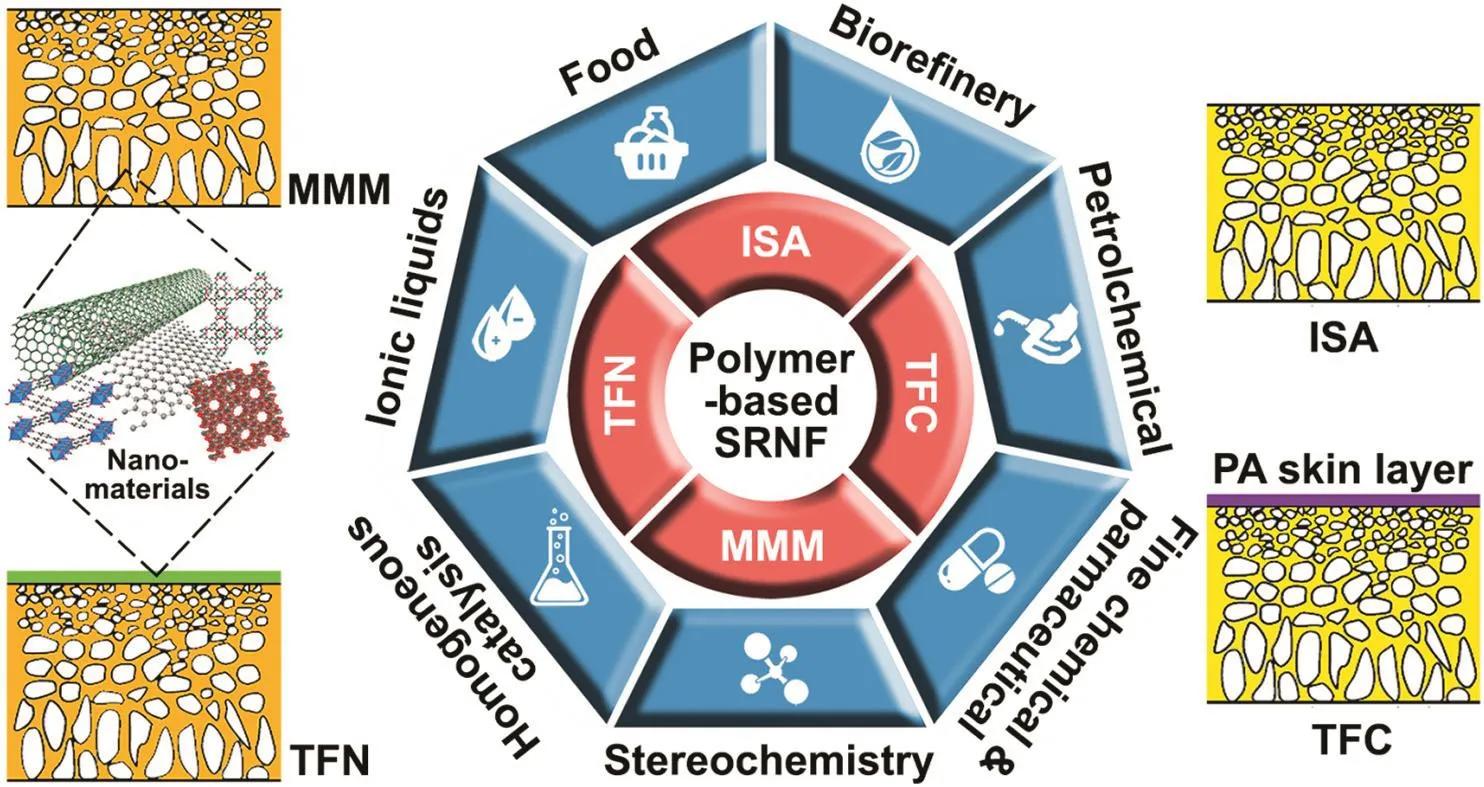
Fig.1.Fourapproaches to design SRNF membranes and their different types of industrial applications(ISA—integrally skinned asymmetric;MMM—mixed matrix membranes;TFC—thin film composite;TFN—thin film nanocomposite).
Current state-of-the-art PI membranes for SRNF are crosslinked by diamines either during phase inversion[35]or post-synthetically[14,36].The general reaction mechanism involves the breaking up of the imide rings in the polymer backbone to form amide bonds at both ends of the diamine,thus creating a crosslink between the two polymer chains(Fig.2)[37,38].For example,Lenzing P84®membranes showed good chemical stability in NMP,DMF,THF and DCM after being crosslinked by aliphatic diamines[14,39].An aromatic diamine,pxylylenediamine(XDA),was used as a crosslinkerto imbue an asymmetric Matrimid®membrane with good stability towards DMF,NMP,DMAc and DMSO[36].However,such post-synthetic crosslinking approach is not only time consuming but also consumes a large amount of extra solvent.To overcome this drawback,the crosslinker was directly added to the non-solvent coagulation bath so that phase inversion and chemical crosslinking can take place simultaneously[35].For example,Hendrixet al.applied this method using crosslinkers such as ethylenediamine(EDA),1,6-hexanediamine(HDA)and XDA to give diamine-crosslinked Matrimid®5218 membranes[40].It was found that XDA-crosslinked membranes exhibited excellent rejection(>95%)of Rose Bengal(RB)with low DMF permeability(<10 L·m-2·h-1·MPa-1).In contrast,HDA-crosslinked membranes showed high DMF permeability up to 100 L·m-2·h-1·MPa-1but with moderate rejections of ~85%(Table 1).Although chemical crosslinking with diamines improves the solvent stability of PIs membranes,re-imidization can occur when the membranes are subjected to high temperatures.See Tohet al.[14]found that Lenzing P84®membranes crosslinked with 1,8-octanediamine(ODA)were insoluble in DMF when annealed at 100°C.However,after a 150°C annealing,the membranes became soluble,indicating re-imidization of polymer chains.Thus,it is important for crosslinked PI membranes to operate below the imidization temperature.
In addition,the impact of fabrication parameters on the SRNF performance is also extensively investigated.Parameters such as polymer dope composition,choice of PI,solvent evaporation time and the effect of co-solvent have been studied to date[15,41-43].Key takeaways include low fluxes and rejections for membranes made from Matrimid®5218 and polyetherimide(PEI,Ultem®1000)as compared to that of P84®, flux decline without significant effect on the rejection when a solvent evaporation step was applied in the fabrication of a P84®/DMF/1,4-dioxane system,high rejection in the NF range when a membrane skin layer was formed through the addition of a co-solvent,and realizable defect-free membranes with sufficient mechanical strength when 35000 or higher molecular weight(MW)PIs were used.Nevertheless,these effects are specific to the polymer/solvent/non-solvent system and should not be over-generalized or taken for granted.
Apart from using diamines,the polyamide-imide(PAI,Torlon®)membranes were successfully crosslinked using diisocyanates[44].Specifically,commercial PAI membranes from SolSep B.V.were treated with hexamethylene diisocyanate(HMDI)to allow crosslinking between the amide group of the PAI polymer and the isocyanate group of HDMI(Fig.3).After crosslinking,the membranes were resistant to NMP and showed good mechanical properties.Re-imidization was not observed at 120°C although further investigations beyond this temperature are needed.In principle,chemical crosslinking is applicable to other polymers as long as the appropriate functional groups are present.Forexample,Hendrixetal.[45]utilized commercially available diphenolic acid and difluorobenzophenone as monomers to synthesize modified PEEK containing a valeric acid functional group(VAPEEK).The membranes derived from VAPEEK are capable of crosslinking with HDA to give promising membrane selectivity up to 90%RB rejection.However,the IPA and acetone permeances were generally low and these were comparable to the IPA permeance(0.9 L·m-2·h-1·MPa-1)of commercial DuraMem® 500(Table 1).
Contrary to chemical crosslinking,other means of crosslinking ISA membranes have also been reported.Thermal crosslinking of polyaniline(PANi)membranes[Fig.4(a)],by treating them at 180°C in air to induce crosslinking of the polymeric chains as well as the oxidation of PANi,were reported to strengthen the stability of membranes in solvents such as acetone,MeOH,DMF,NMP and THF[46-49].However,crosslinking the membranes thermally can reduce the membrane porosity,resulting in a decrease in flux.Alternatively,chemical crosslinking with α,α′-dichloro-p-xylene(DCX)or glutaraldehyde(GA)[Fig.4(b)]can achieve the same solvent stability while enhancing the compaction resistance of the crosslinked PANi membranes to give higher solvent fluxes as compared to the thermally crosslinked ones.Besides thermal crosslinking,UV curing is also employed to improve the chemical stability of PI and PSf membranes[50].In the case where penta-acrylate was used as a crosslinker for PSf membrane,higher solvent stability was obtained at the expense of the IPA permeability[51].The IPA permeability decreased significantly from 3.3 to 0.7 L·m-2·h-1·MPa-1after UV irradiation induced penta-acrylate crosslinking with the PSf polymer chains.This was attributed to the densification of the membrane top layer.
Compared to PIs,polymers belonging to the sulfone family such as PSf and polyethersulfone(PES)have not yet been studied extensively

Fig.2.Mechanism of crosslinking of Matrimid®with XDA[38].
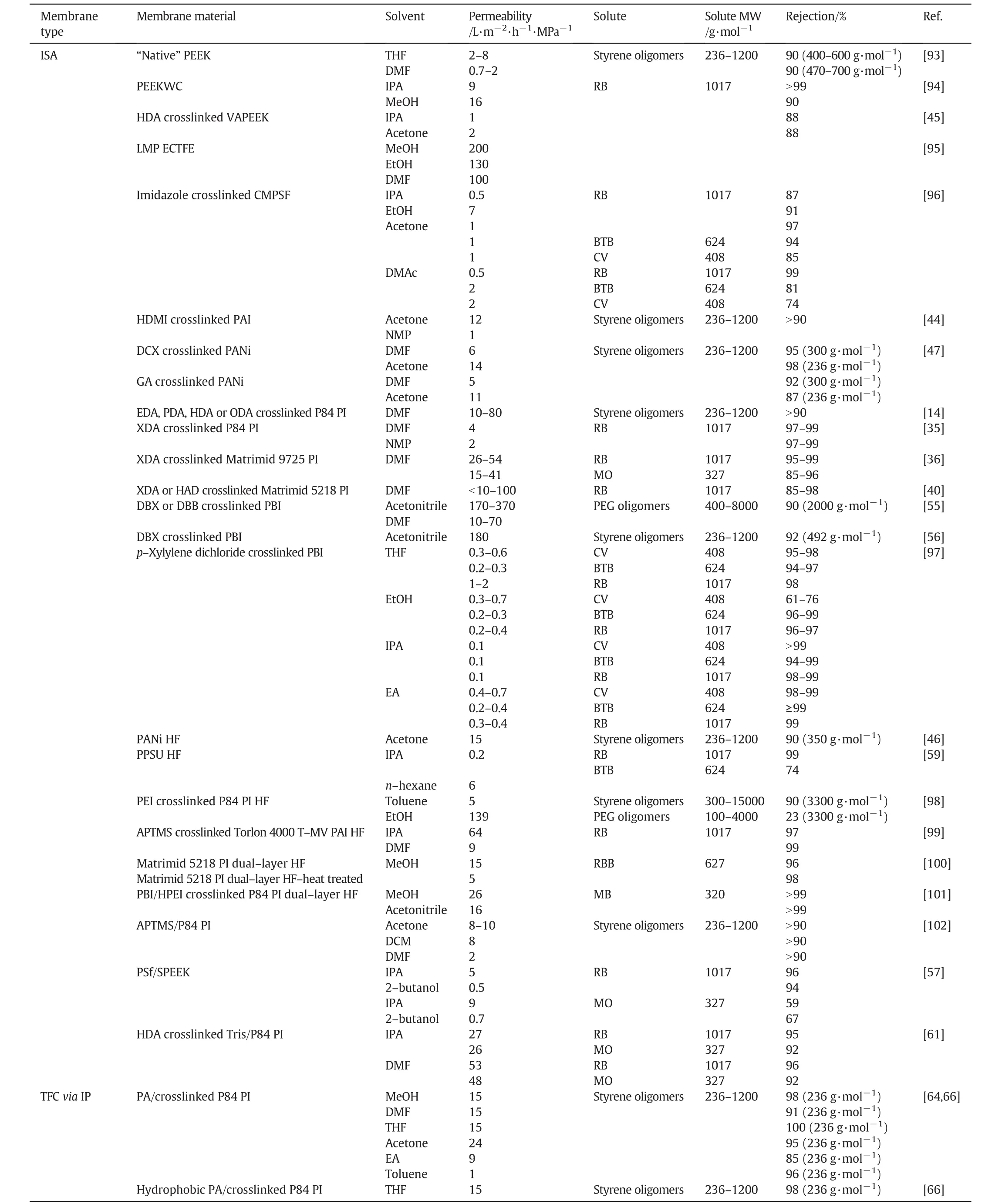
Table 1Overview of the SRNF performances of ISA and TFC membranes from representative literature results
(continued on next page)

Table 1(continued)
(continued on next page)as materials for SRNF despite being well-established polymers for membranes in aqueous phase separations.Recently,Hołda and co-workers investigated the effects of various parameters,such as solvent evaporation time,choice of solvent,and the addition of different MW additives on the SRNF performances of ISA PSf membranes[52-54].The results showed that a longer solvent evaporation time led to decreased permeation flux while the solute rejection increased and stabilized beyond a certain evaporation time.In addition,improved rejections and lower fluxes were obtained when a small amount of high MW polyethylene glycol(PEG)(5 wt%)was added into the casting solution.On the otherhand,low MW additives such as PEG dimethyl ether,diethylene glycol diethyl ether,acetone,2-butanol and acetic acid need to be added at high concentrations(up to 25 wt%)to give membranes with at least 90%RB rejections.Another promising candidate for SRNF membrane fabrication is polybenzimidazole(PBI).Recent works by Valtchevaet al.[55,56]have shown that PBI membranes crosslinked with α,α′-dibromo-p-xylene(DBX)were stable not only in polar aprotic solvents but also in strongly acidic and basic environments which opened up opportunities for membrane separations under corrosive conditions.

Table 1(continued)

Fig.3.Crosslinking reaction between PAI and HMDI[44].
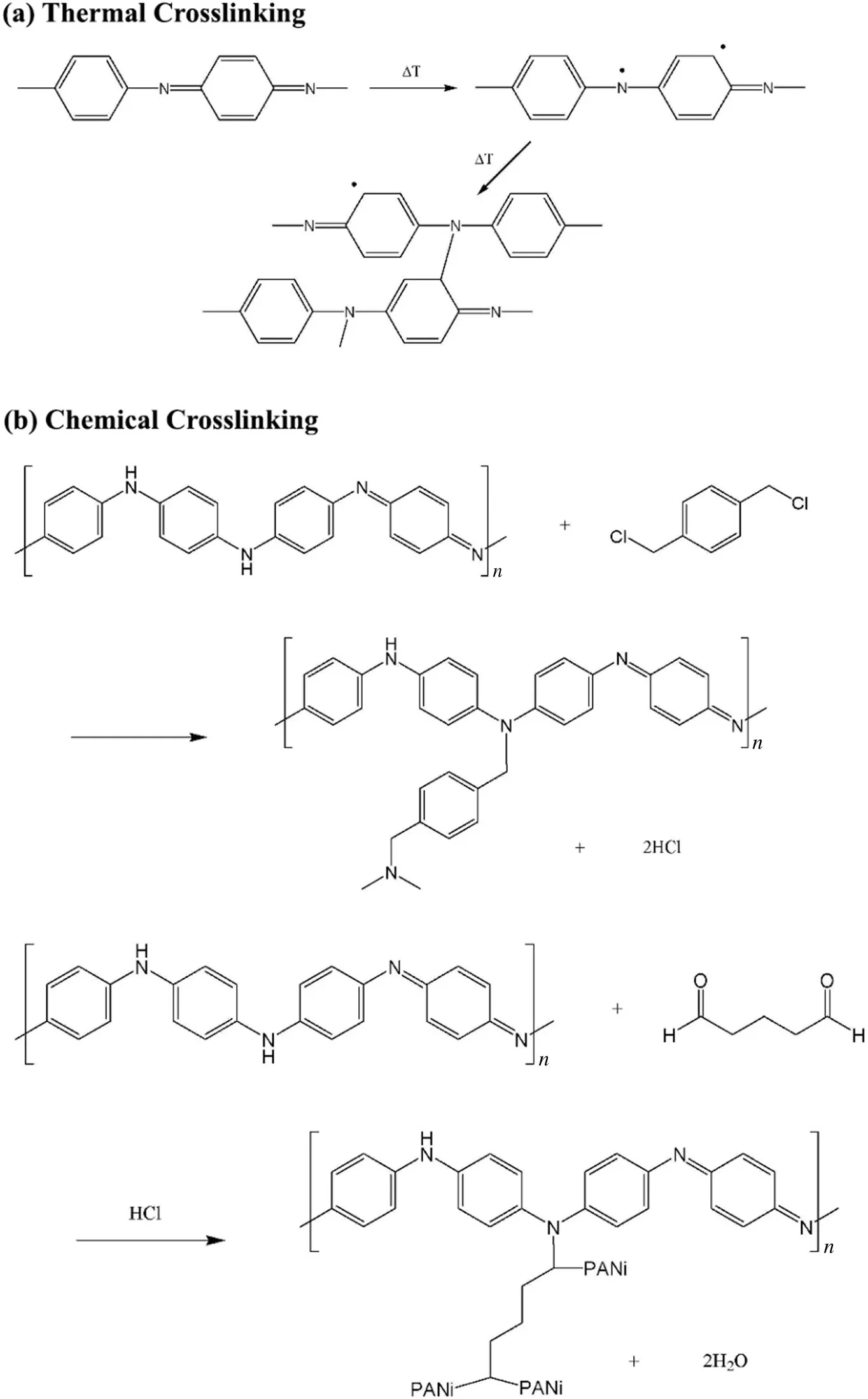
Fig.4.Crosslinking reactions of PANi:(a)thermal crosslinking and(b)chemical crosslinking with DCX and GA[47].
In the quest for novel materials for SRNF membranes,polymer blending has been explored as a relatively simple and reproducible strategy to chemically modify or tailor polymers[25].For example,charged membranes prepared by blending sulfonated poly(ether ether ketone)(SPEEK)with PSf were used for separating dyes in alcohol-based solutions such as MeOH,EtOH,IPA and 2-butanol[57].Neat polyphenylsulfone(PPSU)has also been used to fabricate SRNF membranes[58,59]owing to its higher impact and chemical resistance than PSf and PEI[60].They are stable in alcohols and alkanes but not resistant to acetone,toluene and methyl ethylketone.Blending PPSU with Matrimid®5218 improved the solvent stability of the membranes significantly[25].This can be attributed to the difference in solubility parameters between PPSU and Matrimid such that the resultant membrane has a different structure consisting of two co-continuous phases with nanoscale gaps in the interface.Another approach involved blending a hydrophilic monoamine,Tris (hydro-xymethyl)aminomethane(Tris),with PI[61].The resultant membranes showed close to three times higher IPA permeance compared to the original PI membrane.Recently,Kohet al.[62]fabricated carbon molecular sieve(CMS)hollow fiber(HF)membranes with solvent resistance towards organic solvents such as benzene,toluene and various isomers of ethylbenzene and xylene.Although CMS is not polymer-based,it is noteworthy that the membranes were derived from a polymeric poly(vinylidene fluoride)(PVDF)counterpart.Interestingly,they proposed a XDA crosslinking method similar to that as aforementioned to prevent the micro and mesopores from collapsing when the HF membranes were subjected to pyrolysis at temperature ranging from 450 to 550°C[Fig.5(a)].As a result,the CMS HF membranes exhibited excellent SRNF performances with order-of-magnitude increase in flux(relative to zeolite membranes)while maintaining para-xylene/ortho-xylene selectivity of~100 with separation factors approaching 4.3[Fig.5(b)].
2.2.Thin film composite(TFC)membranes
TFC membranes,which comprise of a thin selective skin layer on a porous support,are distinct from ISA membranes.The key advantage of TFC membranes lies in the versatility of tuning the chemistry of the selective layer and porous substrate independently to optimize the overall membrane performance[32].As such,TFC membranes can potentially achieve better performance than ISA membranes by forming much thinner skin layers through means such as interfacial polymerization(IP),plasma polymerization,in situ polymerization,solvent casting,dip-or spin-coating and layer-by-layer(LBL)deposition[32].These methods will be elaborated in the following sections.
2.2.1.Interfacial polymerization(IP)
IP is a common technique used to prepare the skin layer of TFC membranes[32].In a typical process,a polymeric support layer is first impregnated in an aqueous solution of amine monomers,such as m-phenylamine(MPD),piperazine,polyethyleneimine or triethanolamine(TEOA),before it is placed in an organic phase containing the crosslinker,trimesoyl chloride(TMC)[63].The monomers quickly crosslink at the interface of the two phases to yield the TFC membrane with a thin selective layer of PA network on the surface of the membrane support.By carefully selecting the solvents and reactive monomers as well as controlling conditions such as monomer concentrations and reaction time,the skin layer can be tailored.IP is a technique used in fabricating reverse osmosis(RO)and NF membranes for aqueous phase separations and is also commonly used for SRNF membranes due to the wide range of solvent stability of the crosslinked PA.
Jimenez Solomonet al.[64]prepared TFC membranes by IP on a crosslinked P84 PI support.The membranes showed higher permeability in polar aprotic solvents including DMF,acetone and THF than the commercial SRNF membrane DuraMem®150(Table 1)at comparable solute rejections.It was found that solvent fluxes could increase further by impregnating the crosslinked PI support with PEG before the IP reaction and post-treating the membrane with DMF or DMSOafter the IP reaction.PEG served to prevent pore collapse in the support membrane and reduce the intrusion of the aqueous amine solution.The support also became more hydrophilic and this affected the diffusion rate of amine into the organic phase.Here,DMF or DMSO was used to remove some of the loose PAstructures in the top layer so as to reduce the resistance of the PAlayer.Sunet al.also improved the flux ofTFC membranes in solvents such as methanol,DMF and DMSO by adding triethylamine/camphorsulfonic acid into the MPD solution and post-treating the membranes with a glycerol/sodium dodecyl sulfate solution followed by filtering with DMSO[65].The addition of additives in the aqueous phase facilitated the IP reaction and increased the membrane surface roughness,while the post-treatment preserved the membrane porosity and increased the free volume of the top layer.In anotherstudy,the TFC membranes prepared by IP were hydrophobized by capping the unreacted acyl chloride groups residing on the membrane surface with hydrophobic reagents containing fluorine and silicon[66].The results showed significant increase in the permeabilities of non-polar solvents such as toluene and EA(Table 1).This suggests that surface chemistry plays an important role in governing the mass transport through the PA selective layer.As an attestation,Zhangetal.[67]formed a hydrophilic-hydrophobic hybrid layer over a PAN substrate.The substrate was first dip-coated with aqueous polyethyleneimine before bringing into contact a solution containing hydroxyl terminated tri fluoride polydimethylsiloxane(PDMS)and TMC.Polyethyleneimine and PDMS were crosslinked by TMC to afford TFC membranes with good resistance to both polar and non-polar solvents,including IPA,butanone,EA andn-heptane,as evidenced by negligible swelling of the membranes in the solvents.
Besides the chemistry of the PA layer,the thickness plays a more direct role in determining the mass transport through the selective layer.Recently,Karanet al.[68]synthesized freestanding PA nano films with thickness less than 10 nm using a sacrificial layer of cadmium hydroxide nanostrands[Fig.6(a)].The results showed high MeOH permeability in the range of 110-120 L·m-2·h-1·MPa-1(Table 1).When the concentration of MPD increased,crumpling of the nano film occurred which further improved MeOH permeability up to ~520 L·m-2·h-1·MPa-1without compromising the membrane selectivity(Table 1).This was attributed to the larger surface area of the crumpled nano film.Similarly,Jimenez Solomonet al.[69]created ultrathin polyarylate nanofilms down to the thickness of 20 nm on the top of crosslinked P84 PI supports.They reported MeOH permeability of 60-80 L·m-2·h-1·MPa-1when using monomers such as cardo-structured 9,9-bis(4-hydroxyphenyl)fluorene(BHPF)and spiro-structured 5,50,6,60-tetrahydroxy-3,3,30,30-tetramethylspirobisindane(TTSBI)(Table 1).In addition to the low thickness,the high permeability was accredited to the use ofcontorted monomers which introduced intrinsic microporosity and interconnectivity within the nanofilms[Fig.6(b)].

Fig.5.(a)Schematic illustrating the fabrication of the CMS membrane from XDA crosslinked PVDF HF membrane;(b)p-xylene/o-xylene permselectivity as a function of the p-xylene flux[62].
2.2.2.Solution coating
TFC membranes can also be prepared by coating a polymer solution on a support,either with a casting knife or spread over a tilted support by pouring or dip-coating[3].PDMS is one of the most studied coating materials for preparing TFC SRNF membranes[70-73].Cross-linked PDMS has been reported to be chemically stable towards some organic solvents and its low polarity makes it preferred for use in apolar solvents.The thickness and crosslinking density of the PDMS selective layer are the two major parameters for controlling solvent flux and membrane selectivity.A major problem with PDMS is the swelling of the polymer in apolar solvents.However,this can be reduced by the addition of fillers which will be described in Section 2.3.Other polymers such as polyurethane[74],polypyrrole(PPy)[75,76]and poly[1-(trimethylsilyl)-1-propyne](PTMSP)[77]have also been studied as coating materials on polymeric supports.
Polymers of intrinsic microporosity(PIMs),a new class of polymers containing interconnected micropores formed by rigid and contorted polymer chains,have shown a good potential for SRNF[78,79].TFC membranes of PIM-1 and PIM copolymers on PAN supports were synthesized with promising separation performances especially for n-heptane with permeability as high as 180 L·m-2·h-1·MPa-1and rejection of hexaphenylbenzene up to 97%[80,81](Table 1).The solvent stability of these TFC PIM/PAN membranes can also be enhanced by chemical or thermal crosslinking.Another promising class of materials is the block copolymers that can be self-assembled into various well-defined nanostructures[82].For example,polystyrene-blockpoly(ethylene oxide)(PS-b-PEO)diblock copolymer blended with poly(acrylic acid)(PAA)homopolymer can be deposited as a selective layer on a porous support by dip-or spin-coating[83].Following which,UV irradiation can be used to crosslink the PS phase to form an interpenetrated network structure that is resistant to solvents such as THF,DMF and DCM.On top of that,polymeric homogeneous composite(PHC)membranes,with selective and support layers made from the same material,can be optimized independently for SRNF.Using the same material implies a high affinity between the two layers and reduces the possibility of delamination.As a result,PHC membranes synthesized by coating a P84 PI solution on a diamine-crosslinked P84 support can yield membranes that were completely stable in NMP for over 96 h[84].
2.2.3.Layer-by-layer deposition of polyelectrolytes
Layer-by-layer(LBL)deposition of polyelectrolytes(PE)provides a versatile and inexpensive approach to create selective layer of SRNF membranes[85].This is typically done by successive deposition of oppositely charged PE on a charged membrane substrate resulting in a multilayered polyelectrolyte complex(PEC)selective layer stabilized by strong electrostatic interactions.The key advantage of LBL electrostatic self-assembly technique is the ability to offer precise control of the PEC layer thickness by varying the number of deposited bilayers[86].Through the careful selection of individual PEC constituents and manipulation of conditions such as the ionic strength and pH of the PE solutions,the properties of the resulting PEC are tunable for different targeted SRNF applications.

Fig.6.Schematic illustrations of the IP reaction to give(a)nanofilm of sub-10 nm in thickness using sacrificial cadmium hydroxide nanostrand layer[68];(b)nanofilm using contorted monomers such as BHPF and TTSBI leading to microporosity and interconnectivity within the PA nanofilm[69].
Liet al.[87]reported the first use of PEC membranes in SRNF.Multilayered poly(diallyldimethylammonium chloride)(PDDA)/SPEEK membrane prepared on a hydrolyzed PAN(PAN-H)support exhibited an excellent solvent stability in IPA,THF and DMF.It was found that the IPA permeability gradually increased from 0.6 to 9.8 L·m-2·h-1·MPa-1as the concentration of NaCl added to the PE solutions increased from 0 to 0.5 mol·L-1[88](Table 1).The addition of salt made the PE conformation more “loopy”or“tailed”,resulting in a thicker and looser PEC layer.This increased the solvent flux while maintaining a high selectivity.On the other hand,Ahmadiannaminiet al.[89]utilized PDDA with PAA to form the PEC layer.In addition,they paired PDDA with two different polyanions,namely poly(sodium styrene sulfonate)(PSS)or poly(vinyl sulfate)(PVS),each either in Naor H-form,to study the influence of polyanion type and cationic counter ion on the SRNF performance[90].Higher permeabilities and rejections were obtained when polyanions in H-form were used,which were attributed to a loopier PEC layer and higher surface charges.Chen[91]reported the deposition of PDDA/SPEEK bilayers on a Si/PAN-H composite support.The rejection ofRB in IPA increased from 50%to 70%after introducing SiO2into the PAN-H support.Upon deposition of the PDDA/SPEEK bilayers,the rejection was further increased up to 99%.Furthermore,the membranes also showed good RB rejections in solvents like DMF and THF(Table 1).This indicates that the multilayered PDDA/SPEEKcan compensate for the defects introduced by the inorganic fillers in the support membrane and thus improve the SRNF performance.Ilyaset al.prepared PEC membranes using weak polyelectrolytes such as PAA and poly(allylamine hydrochloride)(PAH)and investigated the effect of pH conditions on the membrane performance[92].
2.3.Mixed matrix membranes(MMMs)
The advantages of polymeric membranes such as excellent processability,cost-effectiveness and good mechanical stability are highly attractive.However,they suffer from problems such as membrane compaction which causes flux decline over time[102],and a typical trade-off between membrane permeability and selectivity,which suggests that membranes with high throughput also have low selectivity.On the other hand,inorganic membrane made from materials such as ceramic and zeolite can provide higher permeability and selectivity but the membranes are difficult to fabricate and scale-up owing to their poor processability[107].Thus,the concept of mixed matrix membranes(MMMs)where inorganic fillers are incorporated into polymer matrices has been proposed to synergistically combine the merits of these materials.To date,MMMs are widely used for gas separation[108],pervaporation[109],desalination[110,111]and have been employed for SRNF applications.Some of the common fillers include metal oxides,metal nanoparticles,zeolites,MWCNTs,MOFs,and GO nanosheets[112].However,difficulties in reproducing MMMs and non-idealities such as poor adhesion between the continuous polymer phase and the fillers resulted in interfacial defects,which pose a major challenge to their commercial implementations[113,114].
PDMS and PI are common polymers for MMM preparation.As discussed earlier,PDMS has a tendency to swell especially in nonpolar solvents,leading to an increase in the free space between the polymer chains and compromising solute rejection[115].To overcome this problem,Geverset al.[71,116]added ZSM-5 zeolites into PDMS solution before coating it on a PAN support.This decreased the swelling of the PDMS network as the large surface area of ZSM-5 facilitated a crosslinking between the surface silanol groups with the PDMS polymer chains.As shown in Fig.7,the membranes showed no defects in the top layer.The addition of zeolite decreased the free volume of the PDMS matrix and thus the zeolite- filled membranes showed higher rejection values of the Wilkinson catalyst in various solvents than neat PDMS membrane(Table 2).Interestingly,the membranes with ZSM-5 showed higher permeability than neat PDMS membrane,indicating that the pores of zeolites provide an additional route for transport of solvent molecules.In order to reduce the mass transfer resistance of conventional zeolite fillers,Vanhercket al.[117]incorporated micron-sized hollow spheres of silicalite-1 crystals into PDMS membranes on crosslinked PI supports.The MMMs containing the silicalite-1 hollow spheres showed higher solvent permeabilities than that of the regular silicalite-1 while maintaining similar solute rejections(Table 2).
TiO2nanoparticles can also be dispersed in the polymer dope solution to prepare composite organic-inorganic crosslinked PI membranes[118].Morphological changes in the polymer matrix were brought about by the incorporation of TiO2.As observed by Soroko and Livingston[118],the macrovoids in the sublayer of the PI membranes were completely suppressed when TiO2loading was greater than 3 wt%.Consequently,this enhanced the compaction resistance of the membranes significantly without affecting the solvent flux and the solute rejection.In addition,incorporating SiO2nanoparticles modified by aminopropyldiethoxymethylsilane(APDEMS)within a PEI polymer matrix was found to increase the solvent stability as well as mechanical and thermal resistances of the support membrane[119].After IP on the support,the TFC membranes exhibited a 95%rejection of lube oil from a solvent mixture of toluene and MEK at 5 wt%SiO2loading(Table 2).More importantly,solvent permeability remained relatively constant when the pressure increased from10 to 2.0 MPa due to an enhancement in the compact resistance of the membranes.In another study,Siddiqueet al.[102]used 3-aminopropyl trimethoxysilane(APTMS)as a crosslinker to prepare Lenzing P84®PI membranes.The APTMS also served as an organosilicone precursor to generate a homogeneous inorganic Si-O-Si network throughout the PI membranes,including the skin layer(Fig.8).It was found that membrane compaction was negligible(<2%)after treatment with APTMS as the polymerchains were rigidified due to the strong inorganic network.However,the solvent permeability was penalized with acetone,DCM and DMF permeabilities below 10 L·m-2·h-1·MPa-1(Table 1)as a result of the diminished fractional free volume of the polymer matrix.
MOFs are a new class of nanoporous crystalline materials composed of metal ions connected by various organic ligandsviastrong coordinative bonds to create highly porous structures[120].They have attracted much research attention due to their high porosity and tunable physicochemical properties as compared to other nanoporous materials such as zeolites[121].As such,MMMs using various MOFs have been

Fig.7.Cross-section of zeolite- filled PDMS membrane on PAN support[71].

Table 2Overview of the different types of nanomaterials used for MMMs and TFN membranes and their SRNF performances from representative literature results
(continued on next page)extensively studied for gas separation[122]and SRNF applications.For example,Basuet al.[123]incorporated various MOFs including HKUST-1,MIL-47,MIL-53(Al)and ZIF-8 into PDMS membranes formed on PI supports.Morphological observation revealed the presence of voids at the MOF/polymer interface.To improve the compatibility between the MOFs and PDMS matrix,the surface of the MOF crystals was modified usingN-methyl-N-(trimethylsilyl)-trifluoroacetamide(MSTFA)as a coupling agent to react with the functional groups on the MOF surfaces(Fig.9).Cross-sectional images of the PDMS MMMs showed good compatibility,especially at lower loadings,after MSTFA modification(Fig.10).As a result,the rejections of RB improved from 87%to 95%-98%without compromising on the IPA flux(Table 2).

Table 2(continued)
2.4.Thin film nanocomposite(TFN)membranes
Preparation of the TFN membranes by IP is a concept that was first used by Jeonget al.to develop RO membranes[124].It involves the integration of nanoparticles into the top PA layer by introducing them through the monomer solutions during the IP reaction.Sorribaset al.[125]reported the first use of MOFs as fillers in designing TFN membranes for SRNF.Various MOFs including ZIF-8,MIL-53(Al),NH2-MIL-53(Al)and MIL-101(Cr)with size ranging from 50 to 150 nm were dispersed in the TMC organic phase and subsequently embedded into the thin PA layer.SRNF performance evaluations revealed that the MeOH permeability was enhanced when the pore size and porosity of the MOF increased,indicating that the pore channels in the MOFs created additional flow paths for the solvent.Besides that,the presence ofnanoparticles in the selective layer is highly effective in tuning the free volume of the PA matrix for improving the transport properties of the TFN membranes.Peyraviet al.[126]developed a TFN membrane by functionalizing TiO2nanoparticles with either amine or chloride compounds and respectively dispersing them in an aqueous or organic phase prior to IP reaction.Aminated TiO2nanoparticles were found to have a uniform dispersion throughout the PA layer,resulting in high MeOH permeabilities of 24.0-260 L·m-2·h-1·MPa-1at more than 90%rejection of crystal violet(CV)solute.The presence of TiO2nanoparticles also lowered the chain mobility of the PA matrix,hence improving membrane stability in aggressive solvents like DMF and reducing membrane swelling.To better control the incorporation of MOFs in the membrane top layer,Van Goethemet al.recently developed an evaporation controlled filler positioning method to deposit a layer of ZIF-8 particles at the interface before IP reaction[127].In the filtration of NaCl,the resultant membranes showed more than two times increase in permeance over unfilled membranes without sacrificing rejection.The optimal performance was reported at a low ZIF-8 loading of 0.005%(w/v),greatly reducing the amount of filler required as compared to previous studies.This could potentially lower the cost of production and increase the commercial viability of preparing TFN membranes.
Graphene-based nanomaterials are another emerging class of materials which is promising for SRNF owing to their two-dimensional(2-d)structure as well as the chemical and thermal stability of the carbon nanomaterials[128].In particular,GO nanosheets offer competitive advantages of easy processability in aqueous medium to obtain films with lamellar microstructures and well-defined nanopores formed by the interlayer spacing between the nanosheets[Fig.11(a)][129].GO membranes have been reported to be stable towards acetone,ethanol,toluene andn-hexane[130].As such,Shaoet al.[76]incorporated GO nanosheets by dispersing them into a PPy solution before inducing a polymerization reaction to form a selective layer over a PAN-H support.Due to the 2-D structure of the GO nanosheets,a thinner selective layer was made possible,leading to a significant increase in the permeabilities of MeOH,EtOH and IPA without compromising on the RB rejections(Table 2).To afford better selectivity,GO membranes were reduced to give reduced graphene oxide(rGO)membranes with smaller interlayer spacing[131].Huanget al.thermally reduced GO membranes to give rGO membranes with higher selectivity towards acetone,toluene,p-xylene,naphthalene and pyrene solutes[Fig.11(a)and(b)].Moreover,with enhanced membrane selectivity,the thickness of the rGO selective layer can be further lowered to reduce mass transfer resistance through the layer.As a result,a sub-20 nm ultrathin rGO membrane exhibited high MeOH permeabilities of over 700 L·m-2·h-1·MPa-1[132](Table 2).Despite being ultrathin,the solvent resistance of the rGO membrane remained excellent with good stability towards acidic and basic corrosive environment[Fig.11(c)].GO nanosheets were also incorporated into a polyethyleneimine matrix and dip-coated on a PAN support[133].Another class of nanofiller,MXenes,shows great promise in preparing TFN membranes for SRNF due to their layered structure[134].
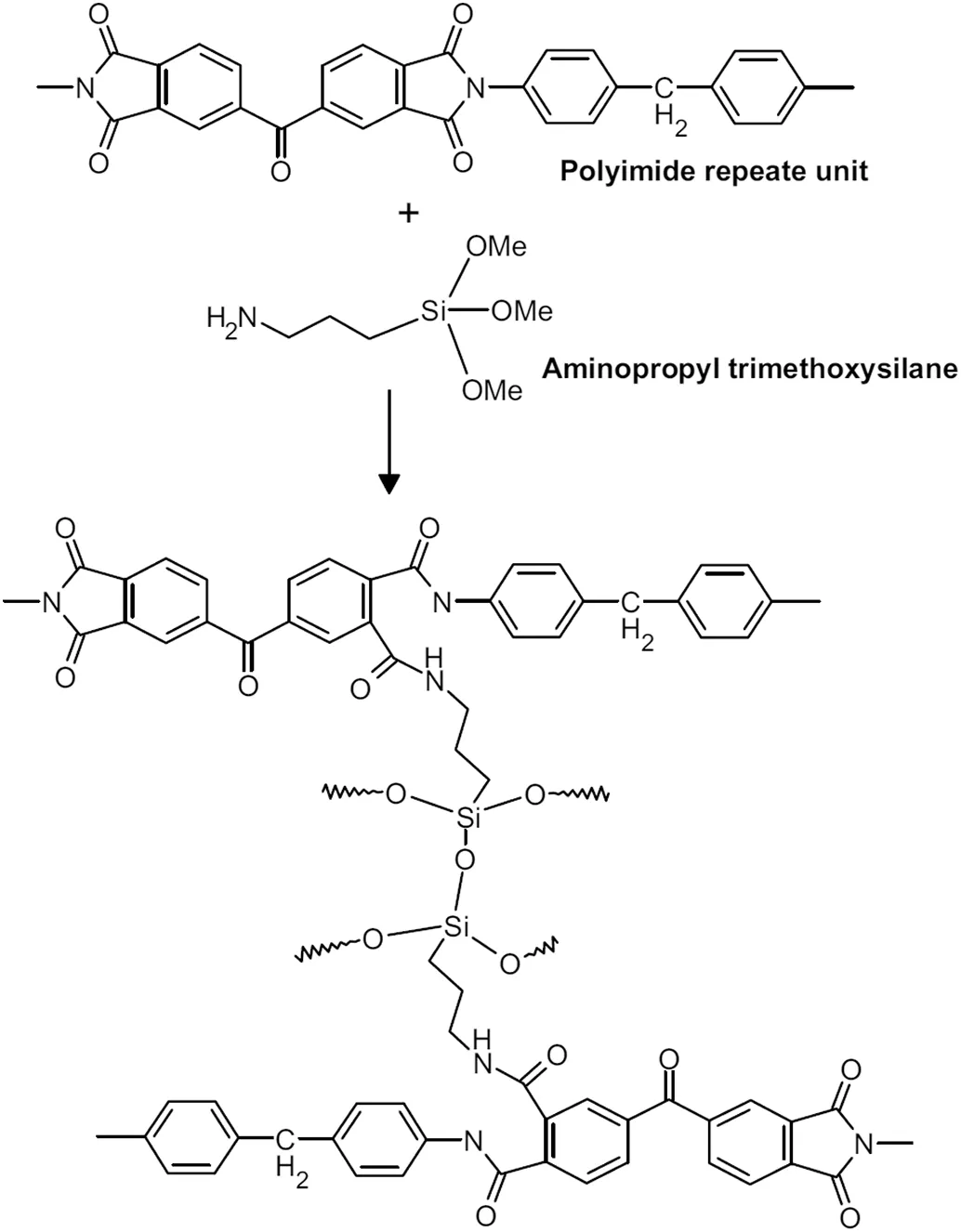
Fig.8.Crosslinking of P84 polyimide with APTMS[102].
Current SRNF membranes tend to be more suitable for either polar or non-polarsolvents depending on the affinity between the membrane material and solvent.However,Henget al.demonstrated an interesting approach that aimed to create dual-transfer pathways in the membrane active layer such that the permeation of polar and non-polar solvents through the membrane was tunable[135].Cyclodextrins were incorporated into a polyethyleneimine matrix and the permeation of non-polar solvents could be controlled by changing their cavity size.On the other hand,the transport of polar solvents was influenced by adjusting the free fractional volume of polyethyleneimine.
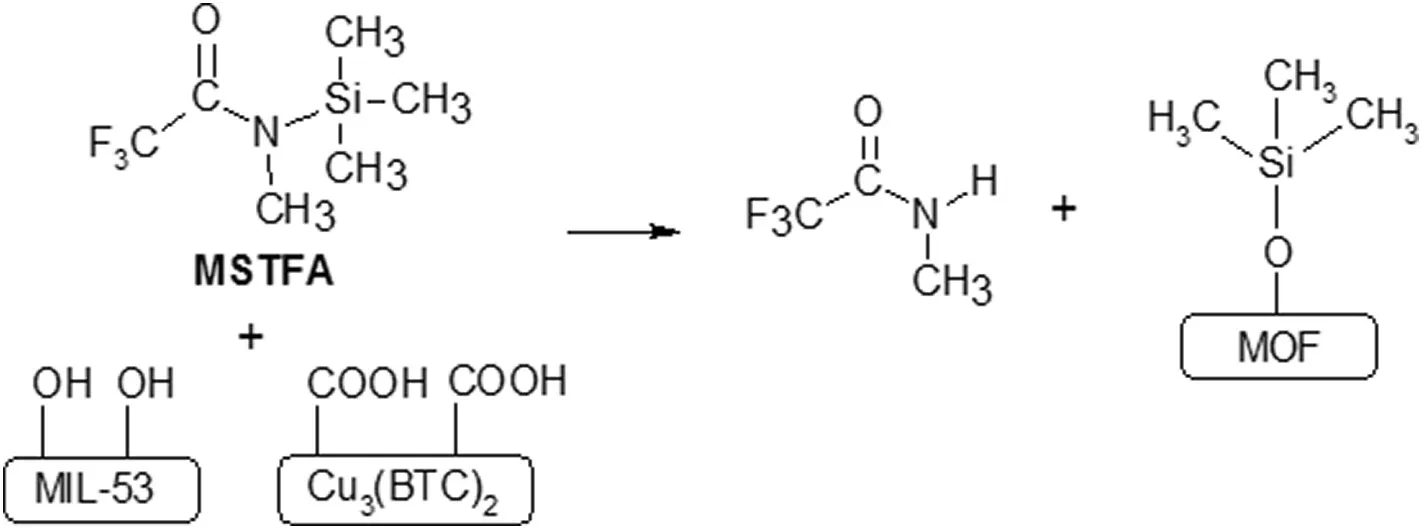
Fig.9.Possible reaction between MSTFA and surface functional groups of MOFs,e.g.MIL-53(-OH-)and HKUST-1(-COOH-)groups[123].
3.Membrane con figurations and commercialization
At present,most lab-scale studies are carried out on flat sheet membranes while commercial SRNF membranes are limited to just a few types of flat sheet and spiral-wound modules(Section 3.3).Although in-house fabricated membranes have shown enhanced performances over commercial SRNF membranes,the studies were usually not carried out at the process level.There remain many unknown factors regarding membrane con figurations and how they affect fluid dynamics and mass transfer characteristics in SRNF[150].
3.1.Spiral-wound modules
Spiral-wound module allows a large area to volume packing and this requires the membrane to be in a flat sheet configuration,cast on a support and has good mechanical properties for easy handling[34].Spiral wound modules of chemically and thermally crosslinked PANi membranes were applied in SRNF[49].The PANi membranes with MWCO ranging from 150 to 300 g·mol-1achieved stable fluxes and good separation performance using PS solutes in acetone,THF and DMF.Furthermore,the membrane module was reusable even after drying out and showed a potential for the large-scale commercial applications.In addition,Lenzing P84 PI membranes crosslinked with APTMS were tested in spiral wound modules and obtained similar rejections of PS but lower acetone flux compared to the flat sheet membranes[102].Nevertheless,the scaled-up production of the membrane was successfully demonstrated without the need for post-treatment using conditioning agents.Without this extra step,manufacturing of membrane modules at industrial scale is expected to be simpler and more cost-effective,leading to a high potential of commercialization.
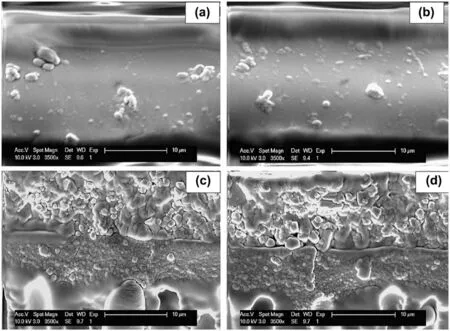
Fig.10.Cross-section morphology of PDMS membranes containing(a)5 wt%,(b)10 wt%,(c)15 wt%,(d)20 wt%MIL-53(Al)nanoparticles modified with MSTFA[123].
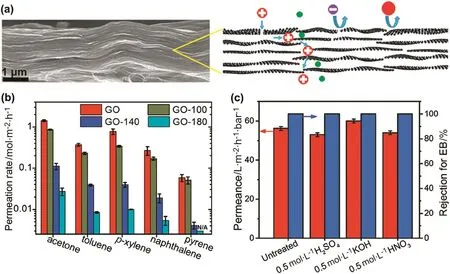
Fig.11.(a)Cross-sectional morphology of rGO membranes and schematic illustration of the well-de fined nanopores formed by the interlayer spacing between the nanosheets;(b)permeation rates of different solutes in EtOH for rGO membranes thermally reduced at 100,140 and 180°C;(c)stability of the rGO membrane in acidic and basic environment[130,132](1 bar=105 Pa).
3.2.Hollow fiber(HF)configuration
In general,HF membrane con figuration can provide several advantages over flat sheet membranes,such as a high area to module volume ratio,removal of the need for spacers which are necessary in spiral wound modules and the relative ease in using different hydrodynamics on the membranes[63].On the other hand,due to their self-supporting structure,hollow fibers cannot withstand as high a pressure as that of flat sheets.Despite these advantages,the use of SRNF HF membranes remains relatively scarce.
Several TFC HF membranes for SRNF were reported by Korikovet al.[151]and Kosaraju and Sirkar[152].The membranes were prepared by IP on the lumen side of a microporous polypropylene(PP)HF support.Solute rejections of88%and 43%were obtained for brilliant blue R and safranin O,respectively,in MeOH solutions[152].The membranes were also stable in toluene.Lohet al.[46]reported the fabrication of ISA PANi HF membranes using a novel method of adding maleic acid into the PANi dope solution before spinning.The acid was then removed from the membranes after spinning in order to induce nanoporosity.After thermal crosslinking,the HF was found to be stable in acetone,MeOH,THF,DMF and NMP with a low MWCOof~350 g·mol-1.Kopećetal.[153]explored a novel HF fabrication method called “chemistry in a spinneret”that combined membrane fabrication and crosslinking in a single step.Such a process was accomplished by using a bore fluid containing polyethyleneimine as the crosslinker.Subsequently,Dutczaket al.[98]explored this method for preparing P84 PI solvent-resistant ultra filtration membranes using the same crosslinker in the bore fluid.The interplay between the phase inversion and chemical crosslinking during the spinning process was systematically studied by varying the composition of polyethyleneimine,NMP and water in the bore fluid.The MWCO of the membranes was in the range of2500-3500 g·mol-1and toluene permeability was 2-11 L·m-2·h-1·MPa-1.As the membranes were more hydrophilic due to crosslinking with polyethyleneimine,they were more suited for separation in alcohol systems as evidenced by the higher EtOH permeability of 146-172 L·m-2·h-1·MPa-1.The membranes were partially stable in NMP,with the most stable one maintaining 80%of its mass after 11 days in NMP.Further studies are required to develop membranes that are suitable for polar organic solvents.Nevertheless,in contrast to post-synthesis crosslinking,this new method can be seen as simpler and less time-consuming.
Composite capillary membranes comprising of a PDMS top layer coated on the inside of a commercial α-alumina capillary support showed stable performance of over 40 h in toluene,with permeability of16 L·m-2·h-1·MPa-1and MWCO of500[154].The ceramic support offered advantages such as high chemical,mechanical and thermal stability over conventional polymer supports.This initial study may provide opportunities for developing high packing density membrane module synthesized by coating stable selective layers on tailor-made HF membranes.
3.3.Commercial SRNF membranes
The various commercial membranes are listed in Table 3.Currently,most of the commercial SRNF membranes are made from neatpolymeric materials and available in either flat sheet or spiral-wound con figurations.ISA membranes are typically made of PI while PDMS has been widely used as the top layer.The Inopor®series of ceramic membranes made from either SiO2or TiO2and having mean pore size of 0.9-1.0 nm are also included for comparison[155].
4.Potential Applications
4.1.Food industry
In the food industry,production of vegetable oil is a complex multistage process in which organic solvents are extensively used.For example,the extraction of vegetable oils from plant seeds involves a combination of solvent and mechanical extraction or solely solvent extraction wheren-hexane is used.The oil fraction contains over 95%of triacylglycerides with the remainder consisting of phospholipids,free fatty acids(FFA),pigments,sterols,carbohydrates,proteins and their degradation products[1].The processes where membrane separations can play a part include the removal of phospholipids from the extracted crude oil(degumming)[156,157],the recovery of extraction solvents[158]and the removal of FFAs from crude oil fraction(deacidification)[6,159].The use of SRNF membranes in the vegetable oilindustry allows for the possibility to separate molecules in a customized manner,minimize thermal damage,recycle solvents and reduce oil losses and waste while maintaining lower energy consumption[3].
Conventional vegetable oil processing utilizes evaporation to separate the oil-solvent mixture after oil extraction,such that the process not only requires large amounts of energy but also possesses potential hazards from the release of explosive vapors[160].SRNF is an attractive alternative to retain the oil fraction by removing the solvent partially and concentrating the oil solution before final purification by distillation.To demonstrate the potential,a tailor-made crosslinked PDMS membrane formed on a PAN support was applied in the separation of sun flower oil fromn-hexane and showed rejections greater than 90%[158].Meanwhile,a zeolite- filled PDMS membrane prepared on a PVDF support achieved 96%rejection of soybean oil in hexane[161].Since no phase change is involved,new solvents such as EtOH or acetone[162,163]can be introduced for oil extraction,resulting in a more environmentally friendly and safer process.For example,commercial SRNF membranes STARMEM 122 and SOLSEP NF030306 showed good separation performance of soybean oil in various solvents including EtOH,IPA and acetone[164].
The removal of FFAs from vegetable oil is another stage in which membrane technology has a potential to replace conventional methods[165].SRNF membranes can be used to partially remove FFAs during the process of concentrating vegetable oil[166],extract FFAs using alcohols and subsequently separate and recycle the solvent,as well as to directly separate triglycerides and FFAs.The key challenge is to obtain acceptable solvent fluxes and high selectivity between triglycerides and FFAs.
Spurred by an increasing interest in new techniques for extraction,fractionation and purification of biologically active natural compounds,SRNF processes have also been developed for separating compounds such as xanthophylls from corn[167],different flavonoids from propolis[168],γ-oryzanol from rice bran oil[169]and fatty acid ethyl esters from fish oil[170].An area of interest is the concentration of polyphenols from different plant extracts while recovering residual solvent used as extractant.In this process,NF has been combined with other separation techniques(adsorption,precipitation and crystallization)as reviewed elsewhere[171].Phenolic acids have been identified as one of the main compounds responsible for high antioxidant activity of aromatic herbs and spices such as rosemary,sage,thyme,spearmint,oregano and many more[172].Peshevet al.used a semi-batch cross flow diafiltration process to concentrate fresh rosemary extracts in EtOH solvent.Particularly,DuraMem®200 membrane showed almost complete rejection of rosemarinic acid and reasonable flux[172].No significant loss of antioxidant effect was measured in the retentate during the filtration and the level of extract concentration achieved may allow the retentate to be directly applied as ingredient in the food,cosmetics,nutraceuticals or medicines.The possibility of separating monophenolic acids(e.g.caffeic acid)from higher MW antioxidant compounds(e.g.rosmarinic acid)using selected DuraMem®membrane has also been suggested.Meanwhile,Gilmer and Bowden[170]utilized epoxy NF membranes to separate two different omega-3 fatty acid ethyl esters,namely eicosapentaenoic ethyl ester(EPA-EE)and docosahexaenoic acid ethyl ester(DHA-EE).Separating these esters is highly challenging due to the small difference in their molecular weights of only 26 g·mol-1and their similar chemical structures.However,by varying the choice of amine and epoxide monomers,the crosslink density of the epoxy membranes can be engineered to successfully separate EPA-EE from DHA-EE with a selectivity of~1.4.
4.2.Biorefinery
The production of biofuels utilizing lignocellulosic materials as a feedstock has attracted a great amount of interests,as the effort to secure renewable energy sources is gradually escalating.Other than biofuel,the various products,such as foods,pharmaceuticals,biomaterials and biochemicals,can also be produced in biore fineries by applying different chemical processes.However,the processing efficiency should be significantly improved for this technology to become economically feasible[173].In this context,the various processes employing membrane technology have been investigated for biore finery applications in the past decade as reviewed elsewhere[173].A few studies about the applications of SRNF membranes have also been reported so far.For instance,commercial SRNF membranes were used to separate the methyl estersrich ef fluent(permeant)from impurities such as glycerides and excess MeOH(retentate)in the product mixture after the transesterification reaction[174].Among the membranes tested,SolSep 030705 membrane showed the highestpermeate flux of the transesterification productwhile achieving 99.8%rejection oftriglycerides and highestrejection of MeOH at75%Meanwhile,in the recovery of monomeric lignin oxidation products from the solution mixture comprising higher MW reaction products and EA,PuraMem S380 membrane gave the best results by allowing a rapid transport of the monomeric products while maintaining a high rejection of the polymeric products[175].
4.3.Petrochemical industry
The application of large-scale membrane systems can provide significant benefits in the petrochemical industry,since re fining processes are extremely energy intensive,involve complex separations,and use large amounts of liquids.The first large-scale industrial SRNF plant was installed in 1998 at Exxon Mobil's Beaumont(Texas)re finery for the recovery of dewaxing solvents from lube oil filtrates using asymmetric Matrimid 5218 PI membranes in spiral wound modules[4,5,176].The process,trademarked as MAX-DEWAX™,was integrated with existing process units and allowed a 99%pure solvent mixture,usually MEK and toluene,to be obtained at refrigeration temperature and directly recycled to the chilled feed stream.With just a third of the capital investment,the membrane process reduced energy consumption per product unit by 20%,increased average base oil production by 25 vol%and led to substantial reductions in VOC emissions and the use of cooling water.In a recent study using a TFN membrane prepared by incorporating amino-functionalized UZM-5 zeolite nanoparticles into the PA top layer,MEK/toluene solvent permeability of 9.2 L·m-2·h-1·MPa-1and lube oil rejection of 96%were achieved at ambient temperature with optimal loading of 0.02%(w/v)of USM-5[138].
SRNF membranes were also applied in the enrichment of aromatics such as benzene,toluene,or xylenes in re finery feed streams.For instance,in the toluene disproportionation process where toluene is catalytically converted top-xylene and benzene,a purge stream is used to remove non-aromatic impurities from the toluene feedstock before recycling it back to the processing unit.By integrating Lenzing P84 PI membrane modules into the purge stream to allow the selective permeation of toluene while rejecting the non-aromatics,a large fraction of toluene(>50%)can be recovered and returned to the main reactor loop[177,178].In the reforming process,the membrane system can be combined with the reformer or distillation units to increase product yield or quality[5].Examples of other refinery processes that can potentially benefit from SRNF include aromatic isomerization,aromatic hydrogenation,aromatic disproportionation,aromatic alkylation and dealkylation[178].
4.4.Fine chemical and pharmaceutical industry
Control of impurities present in the final API or drug product is a critical challenge in the pharmaceutical industry where a small impurity content can affect the end-product quality adversely[16].Existing technologies to isolate or purify APIs rely on recrystallization or preparative column chromatography,both of which suffer from certain disadvantages such as difficulty in process control and high consumption of solvents[16].Particularly in the case of recrystallization,the loss of API in the mother liquor may lead to an increase in the production cost[179].To achieve more efficient separations,SRNF can be integrated with upstream and downstream processes to concentrate and purify target molecules at low temperatures,eliminating the risk of thermal degradation of many thermolabile APIs and HVNCs[16].Szekelyet al.[180]compared the performance efficiency and environmental impact of three different processes,namely recrystallization, flash chromatography and SRNF,in the degenotoxification of APIs.The loss of API was the lowest for SRNF.Furthermore,a solvent recycling stage in the process coupled with SRNF was not only economically feasible but also environmentally favorable as overall solvent consumption was reduced.Conventionally,waste organic solvents are either discharged or recovered through energy intensive distillation or evaporation processes.Their unsustainability makes SRNF an attractive option to reduce the volume of waste solvent streams at lower energy intensity[18].Rundquistet al.[19]studied the feasibility of using SRNF as an alternative to distillation for solvent recovery.Isopropyl acetate was able to be recovered from crystallization mother liquors containing API with more than 40 different organic impurities.With SRNF,the energy required per liter of recovered solvent was estimated to be 25 times less than distillation.Even by adopting a hybrid approach of SRNF and distillation,energy consumption can be lowered 9-folds than with just distillation alone.
Sereewatthanawutet al.[16]have successfully applied DuraMem spiral-wound modules for the purification of APIs/HVNCs in DMF and THF using a dual membrane dia filtration(DMD)process.The DMD process combines a purification stage and a solvent recovery stage in order for the recovered solvent to return to the purification stage for further purification(Fig.12).As a result,fresh solvent consumption was reduced.99%of the impurities were removed from API-INT,an intermediate of a new drug candidate.This allowed the impurity level to drop below the allowed limit of 3%while recovering more than 99%of API-INT.
In general,the separation is highly efficient for solutes with high MW difference,especially if the feed solution already contains 90%of the product.Thus,the real challenge lies in separating solutes with MW that are very close.Furthermore,as complete rejection(100%)of most commercial APIs has yet to be achieved,the purification of APIviaa single-stage diafiltration process will result in a trade-off between yield and purity[181].Higher API purities can be obtained by increasing the number of filtration cycles but at the expense of greater API loss and lower process yield.To overcome these limitations,the concept of membrane cascades may be applied.Siewet al.[181]were the first to experimentally validate the use of membrane cascades in SRNF for solute fractionation.They found that one of the biggest challenges in cascade implementation was the delicate control of fluid flow since inadequate control can reduce separation efficiency.Hence,Kimet al.[182]proposed a simplified membrane cascade process that was powered by a single high-pressure pump and without any buffer tank between membrane stages.The schematic diagrams of a single-stage and a two-stage cascade diafiltration are shown in Fig.13.A binary solution of PEG-400 and PEG-2000 in acetonitrile was tested.It was found that with the two-stage cascade process,the yield of PEG-2000 increased from 59%to 94%while maintaining the purity at 98%.
The cost analysis was also performed to determine the optimum number of stages and module size.A combined process comprising two-stage membrane cascade employing PBI membranes and an adsorptive solvent recovery unit containing charcoal was proposed for the purification of an API product from two different classes of genotoxic impurities[183].Generally,product yield increased from 58%to 95%with significant cost savings up to 92%as compared to the conventional single-stage dia filtration.The study also identified solvent recovery as a crucial component necessary for membrane diafiltration processes to stay economically competitive.
In the pharmaceutical industry,the synthesis of drugs often involves multi-step chemical reactions in different solvents.Solvent exchange is therefore common and ideally the temperature should be maintained below acceptable limits to prevent thermal degradation of the products.For this purpose,distillation is conventionally used by the industry.However,it is limited when the solvent for the next step has a lower boiling point,the solvents form an azeotropic mixture,or the products are thermally labile.As an alternative approach,SRNF can be employed to carry out solvent exchanges at ambient temperatures allowing us to avoid the aforementioned problems[12].Fig.14 shows the sequence of solvent exchange using SRNF.In the first step,the solvent permeates through the membrane while the product with high MW is retained.When 70-90%of the initial solvent is removed,the second solvent is added and the mixture filtered again.The NF/dilution cycles can be repeated until the concentration of the first solvent in the final mixture decreases to a target level,which is typically less than 1%.This concept was demonstrated by Livingstonet al.using STARMEM 122 membranes for a methanol-toluene system[12].After 5 filtration runs,the solvent composition changed from 100%toluene to 99.7%MeOH with rejection of tetraoctylammonium bromide exceeding 99%.
SRNF was also coupled with counter-current chromatography(CCC)to improve the efficiency of API recovery from a waste stream.The process solvent was exchanged into the mobile phase for CCC to decrease impurity concentration in CCC feed to a sufficient level.Subsequently,the mobile phase can be recovered and recycled for the next CCC operation while reducing solvent consumption by 56%[184].
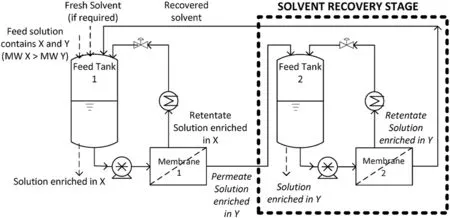
Fig.12.Dual membrane diafiltration(DMD)process[16]:Membrane 1 separates compound Y(lower MW)from X(higher MW)in the primary stage while Membrane 2 retains compound Y in the secondary stage,allowing solvent to be recycled back.
4.5.Stereochemistry
The preparation of enantiomerically enriched compounds is of great importance in the pharmaceutical and biopharmaceutical industries to extract the right enantiomer with the desired biological activity[185].Therefore,the development of separation processes to obtain pure enantiomers is crucial.In this context,SRNF has been applied in various areas such as elucidation of mechanism of chiral selectivity[186],dynamic kinetic resolution[187],enantiomeric separation[188],and asymmetric synthesis[189,190].Ghazaliet al.reported the coupling of enantioselective inclusion complexation(EIC)and SRNF to achieve enantiomer purification as well as directrecycling of the chiral host at ambient temperature and high concentrations[188].SRNF was also employed in the recycling of a homogeneous enantiomerically selective quinidine-based organocatalyst used in the synthesis of a drug product.High enantioselectivity and membrane rejection allowed for process intensification by recycling the catalyst as well as reduction of solvent usage by 96%[190].

Fig.13.Single-stage and two-stage cascade dia filtration.For the cascade,the permeate from the firststage is connected to the feed of the second stage[182].The symbols V i and V2 are feed tank plus first-stage volume and second-stage volume(L),respectively;Fi,F1,F2 and F3 are flow-rates of the feed solution,permeate 1,2 and retentate 2(L·h-1),respectively;C P1,C R i and C R2 are concentrations of species i in permeate 1,rententate 1 and 2(g·L-1),respectively.
4.6.Homogeneous catalysis
NF is an attractive approach to realize process intensification in various catalytic reactions involving enzyme,organo-and homogeneous catalysts by the recovery/recycling of soluble catalysts[191].Since no phase transition or biphasic operation is involved,advantages in terms of economic feasibility and process efficiency can be reaped with a continuous process that is integrated with catalyst recovery by SRNF[192].An integrated process,such as a continuous flow membrane reactor,not only reduces total capital costs and energy consumptions but also achieves higher reaction rates and cleaner product streams since product is continuously removed.However,the operation becomes more complex and thus the design of the reactor and the interpretation of reaction kinetics become crucial[191].
Homogeneous organometallic catalysts which consist of transition metal complexes and organic ligands are often used in multi-step reactions for the synthesis of drugs or other complex organic molecules.SRNF membranes have been successfully used in the recovery and reuse of palladium catalysts in Suzuki and Heck coupling reactions[193-196],gold metal colloidal catalysts in oxidation reactions[197],cobalt-Jacobsen catalysts[11,198],and polyoxometalate catalysts[199,200].In these studies,the catalysts were successfully retained by the membranes.In addition,several studies on the recovery of catalysts used in ole fin metathesis were also reported[201-203].Rabiller-Baudryet al.[204]evaluated the performance of two types of NF membrane reactors,one in semi-continuous mode and the other in continuous mode.Separation of the rhodium catalysts by Starmem membranes was not only useful in preventing catalyst deactivation but also achieving an excellent catalyst recovery efficiency of>99%[205].This enhanced the catalyst productivity in the hydroformylation of ole fin.
Recently,SRNF has been applied in the separation and recycling of organocatalysts,comprised of pure organic compound without any metal constituent[206].Commercial DuraMem membranes showed high rejections(>96%)of a quinine-based organocatalyst in Henry post-reaction mixtures[207].In addition,99%rejection of phosphorus-based organocatalyst was achieved using DuraMem 300 membrane.The catalyst and membrane were able to be reused in four consecutive reactions without any loss of activity and selectivity,respectively[208].
4.7.Separation of ionic liquids
Ionic liquids(IL)are a class of non-volatile organic salts which are usually liquid at room temperature and poorly coordinating.They offer good solubility to many metal complex catalysts and some are immiscible with water and many organic solvents[209].When used in chemical reactions,some ILs allow reaction products to be extracted from a post-reaction IL phase to an organic solvent with the catalyst being retained in the IL phase for recycling[210].However,the unavoidable dissolution of a certain amount of IL and catalyst in the organic phase poses problems such as the loss of IL and the contamination of the product which have to be addressed.SRNF thus provides an opportunity to separate ILs from reaction products and solvent for subsequent recycling.Promising results were obtained in some of the earlier studies involving the use of STARMEM membranes[189,195,210].High rejections(>95%)of CYPHOS IL 101 and ECOENG 500 in MeOH or EA were obtained by STARMEM 120 and 122 membranes[210].
Separation of Suzukireaction products from IL and homogeneous transition metal catalyst is an area of interest as Suzuki cross-coupling uses expensive palladium complex catalysts in organic solvent media.For this reason,Wonget al.[195]employed a 1:1 mixture of EA and IL as the medium for the Suzuki coupling between 4-bromoacetophenone and phenylboronic acid to form 4-acetyl-biphenyl.The post reaction mixture was then processed with STARMEM 122 membranes to recover IL and palladium catalyst which were recycled in subsequent reactions.Compared to reactions in pure organic solvent,the addition of ILs improved the stability of the palladium catalyst and provided high reaction yields over consecutive recycles.In the Ru-BINAP catalyzed hydrogenation of dimethylitaconate in a homogeneous CYPHOS IL 101-methanol system,the enantioselectivity was enhanced by the addition of IL while the Ru-BINAP catalyst and IL were successfully collected by STARMEM 122 membrane with recovery rates of 95%and 92%,respectively[189].
5.Conclusion and future outlook
The feasibility of SRNF technology in various applications has been demonstrated in lab-scale ventures which provide a good starting point for larger scale process development.In order to incorporate SRNF technology into established processes and take advantages of its benefits,one challenge is to have more extensive demonstrations of the technology in relevant pilot testing so as to convince potential end-users[211].Therefore,it is important to increase the availability and ease of access to SRNF pilot test facilities.Recently,a large-scale mobile pilot installation with capability ranging from proof of principle testing to pilot scale production has been designed by a Belgian research institution,VITO(Flemish Institute for Technological Research).This allows potential end-users with a well-equipped platform to carry out independent pilot-testing[211].Furthermore,the pilot system,which is available to customers on a rental basis,allows testing with both polymeric and ceramic membranes in virtually all organic solvents.
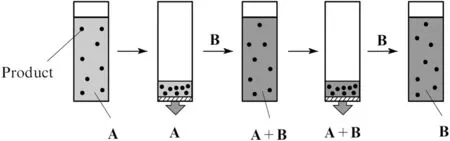
Fig.14.Schematic diagram of solvent exchange using SRNF:solvent A is replaced by solvent B without significant loss of the product[165].
In addition,to further improve the adoption rate and lower the market entry barrier of SRNF membranes,it is necessary to consider the environmental impact involved in the manufacturing of SRNF membranes.The discharge of hazardous chemicals as waste during SRNF membrane production inevitably offsets the environmental advantages of SRNF to some extent.Fortunately,novel methodologies to produce SRNF membranes without compromising the performance of membranes have been successfully developedviamodifying the dope composition or using milder membrane post-treatment conditions.For example,Sorokoet al.[212]managed to replace DMF and 1,4-dioxane in the dope solution for preparing PI membrane with more environmentally friendly solvents like DMSO and acetone.They also used water instead of IPA as the crosslinking medium.In another study by Xinget al.[213],the IL,1-ethyl-3-methylimidazolium acetate([EMIM]OAc),was used as the solvent to dissolve PBI polymer and thus crosslinking of the PBI membrane with GA can be carried out at room temperature in aqueous solution.In the preparation of TFC membranes by IP,Marienet al.substituted the conventional hexane in the organic phase with an ionic liquid[214].Furthermore,the MPD concentration could be lowered and the use of additives was not necessary.Such efforts to explore environmentally friendly routes for preparation of membranes are expected to bring huge benefits in the field of membrane separations and should be an ongoing and major focus of research.
Meanwhile,more efforts should be devoted to improving the properties and performance of membranes.Continuing research to develop new MMMs and TFN membranes with excellent stability in harsh environments including polar aprotic solvents and extreme pH conditions is important.This is because novel MMMs and TFN membranes have a better potential to overcome the trade-off phenomenon in ISA and TFC membranes.For instance,Sorribaset al.[125]have demonstrated significant improvement in the solvent flux without sacrificing solute rejection by loading MOF crystals into the PA layer of a TFN membrane.With precise engineering of the MOF/polymer interfacial morphology and tuning of the physicochemical properties of the MOF,there is plenty of room for improving the separation performances of these TFN membranes[32].Also,SRNF membrane featuring a sharp molecular discrimination at a target MWCO level is highly desirable to perform fractionation of molecules with similar MW.In the development of polymeric SRNF membranes,a key challenge is to make polymeric membranes more resistant to compaction and swelling.The recentwork by Livingston's group[102]involving the use of an organic-inorganic crosslinker has shown promising results in producing MMMs that are not only more thermally resistant but also less susceptible to membrane compaction and swelling.Moreover,fabrication of MMMs eliminates the need for additional membrane post-treatment and has great potential for scaled-up fabrication at the industrial level[215].Nevertheless,the use of nanomaterials will incur additional costs.Detailed economic analysis has to be carried out to carefully study the payback duration and overall cost benefits of using MMMs and TFN membranes.
From the standpoint of the chemical and petrochemical industries,high solvent permeabilities would contribute to improved energy efficiency in the recovery of solvents.The key is to make the membrane selective layer as thin as possible with materials having high affinity to organic solvents.For example,skin layers made of diamond-like carbon(DLC)nanosheets with thicknesses ranging 35-50 nm demonstrated extremely high solvent permeabilities while maintaining considerable mechanical strength[216].The solvent permeabilities can be up to three orders-of-magnitude greater than that of commercially available membranes.However,similar to efforts in fabricating ultrathin PA layers as aforementioned in Section 2.2,it remains a challenge to produce such membranes on an industrial scale.Thus,further research is needed on nanoscale engineering approaches to prepare ultrathin separation layers that possess long-term stability under realistic membrane operations to afford permeability and selectivity beyond any existing SRNF membranes.
Nomenclature
AF Acid fuchsin
APDEMS Aminopropyldiethoxymethylsilane
API Active pharmaceutical ingredient
APTMS 3-aminopropyl trimethoxysilane
BB Brilliant blue
BF Basic fuchsine
BHPF 9,9-bis(4-hydroxyphenyl) fluorene
BINAP-Ru(II)2,2’-bis(diphenylphosphino)-1,1’-binaphthyl)ruthenium(II)complex
BTB Bromothymol blue
BY Brilliant yellow
CSG Chrysoidine G
CMA 1,2,4-benzenetricarboxylic anhydride
CMPSF Chloromethyl polysulfone
CV Crystal violet
DBB 1,4-dibromobutane
DBX α,α’-dibromo-p-xylene
DCM Dichloromethane
DCX α,α’-dichloro-p-xylene
DMAcN,N-dimethylacetamide
DMFN,N-dimethylformamide
DMSO Dimethyl sulfoxide
DR Disperse red 1
EA Ethyl acetate
EB Evans blue
EDA 1,2-ethylenediamine
EtOH Ethanol
FCl Penta fluorooctanoyl chloride
GA Glutaraldehyde
GO Graphene oxide
HDA 1,6-hexanediamine
HPB Hexaphenylbenzene
HVNC High-value natural compound
HNSA 6-hydroxy-2-naphthalenesulfonic acid sodium salt
IL Ionic liquid
IPA Isopropanol
LMP ECTFE Low melting point(ethylene-chlorotri fluoroethylene)
LSPC Lotus seed proanthocyanidins
MEK Methyl ethyl ketone
MeOH Methanol
MI Matrimid®
MMM Mixed matrix membrane
MO Methyl orange
MOF Metal organic framework
MPDm-phenylenediamine
MSTFAN-methyl-N-(trimethylsilyl)-trifluoroacetamide
MW Molecular weight
MWCO Molecular weight cut-off
NB Naphthalene brown
ODA 1,8-octanediamine
PA Polyamide
PAI Poly(amide-imide)
PAR Polyarylate
PANi Polyaniline
PAA Poly(acrylic acid)
PAN-H Hydrolyzed polyacrylonitrile
PAN Polyacrylonitrile
PBI Polybenzimidazole
PDA 1,3-propanediamine
PDDA Poly(diallyldimethylammonium chloride)
PDMS Polydimethylsiloxane
PEC Polyelectrolyte complex
PEEK Poly(ether ether ketone)
PEEKWC Poly(oxa-p-phenylene-3,3-phtalido-p-phenylenxoxa-pphenylenoxoxy-p-phenylene)with Cardo group
PEG Polyethylene glycol
PEI Polyetherimide
PES Polyethersulfone
PHC Polymeric homogeneous composite
PI Polyimide
PIM Polymer of intrinsic microporosity
P(NIPAM-HEMA)Poly(N-isopropylacrylamide-ethyl methacrylate)
PP Polypropylene
PP IX DME Protoporphyrin IX dimethyl ester
PPSU Polyphenylsulfone
PPy Polypyrrole
PS Polystyrene
PSf Polysulfone
PSS Poly(sodium styrene sulfonate)
PTMSP Poly[1-(trimethylsilyl)-1-propyne]
PVS Poly(vinyl sulfate)
Py Pyridyl group
RB Rose bengal
RBB Remazol brillant blue
RES 1,3-benzenediol
rGO Reduced graphene oxide
RO16 Reactive orange 16
SPEEK Sulfonated poly(ether ether ketone)
SPESS Sulfonated poly(ether sul fide sulfone)
SY 7 Solvent yellow 7
TFC Thin film composite
TFN Thin film nanocomposite
THF Tetrahydrofuran
TMC Trimesoyl chloride
Tris Tris(hydroxymethyl)aminomethane
TTSBI 5,5’,6,6’-tetrahydroxy-3,3,3’,3’-tetramethylspirobisindane
VAPEEK PEEK with valeric acid group
XDAp-xylylenediamine
Acknowledgements
We would like to thank funding support from the Singapore Economic Development Board to Singapore Membrane Technology Center.
[1]P.Vandezande,L.E.Gevers,I.F.Vankelecom,Solvent resistant nanofiltration:Separating on a molecular level,Chem.Soc.Rev.37(2008)365-405.
[2]P.Marchetti,M.F.Jimenez Solomon,G.Szekely,A.G.Livingston,Molecular separation with organic solvent nanofiltration:A critical review,Chem.Rev.114(2014)10735-10806.
[3]A.I.Schäfer,A.G.Fane,T.D Waite,Nano filtration,Principles and Applications,Elsevier Advanced Technology,Oxford,2005.
[4]L.S.White,A.R.Nitsch,Solvent recovery from lube oil filtrates with a polyimide membrane,J.Membr.Sci.179(2000)267-274.
[5]L.S.White,Development of large-scale applications in organic solvent nanofiltration and pervaporation for chemical and re fining processes,J.Membr.Sci.286(2006)26-35.
[6]B.M.Bhosle,R.Subramanian,K.Ebert,Deacidification of model vegetable oils using polymeric membranes,Eur.J.Lipid Sci.Tech.107(2005)746-753.
[7]K.De Smet,S.Aerts,E.Ceulemans,I.F.J.Vankelecom,P.A.Jacobs,Nanofiltration coupled catalysis to combine the advantages of homogeneous and heterogeneous catalysis,Chem.Commun.(2001)597-598.
[8]I.F.Vankelecom,Polymeric membranes in catalytic reactors,Chem.Rev.102(2002)3779-3810.
[9]J.T.Scarpello,D.Nair,L.M.F.dos Santos,L.S.White,A.G.Livingston,The separation of homogeneous organometallic catalysts using solvent resistant nanofiltration,J.Membr.Sci.203(2002)71-85.
[10]S.S.Luthra,X.J.Yang,L.M.F.dos Santos,L.S.White,A.G.Livingston,Homogeneous phase transfer catalyst recovery and re-use using solvent resistant membranes,J.Membr.Sci.201(2002)65-75.
[11]S.Aerts,H.Weyten,A.Buekenhoudt,L.E.Gevers,I.F.Vankelecom,P.A.Jacobs,Recycling of the homogeneous Co-Jacobsen catalyst through solvent-resistant nanofiltration(SRNF),Chem.Commun.(2004)710-711.
[12]A.Livingston,L.Peeva,S.Han,D.Nair,S.S.Luthra,L.S.White,L.M.Freitas Dos Santos,Membrane separation in green chemical processing:solvent nanofiltration in liquid phase organic synthesis reactions,Ann.N.Y.Acad.Sci.984(2003)123-141.
[13]J.P.Sheth,Y.J.Qin,K.K.Sirkar,B.C.Baltzis,Nano filtration-based dia filtration process for solvent exchange in pharmaceutical manufacturing,J.Membr.Sci.211(2003)251-261.
[14]Y.H.See Toh,F.W.Lim,A.G.Livingston,Polymeric membranes for nanofiltration in polar aprotic solvents,J.Membr.Sci.301(2007)3-10.
[15]Y.H.See-Toh,M.Silva,A.Livingston,Controlling molecular weight cut-off curves for highly solvent stable organic solvent nanofiltration(OSN)membranes,J.Membr.Sci.324(2008)220-232.
[16]I.Sereewatthanawut,F.W.Lim,Y.S.Bhole,D.Ormerod,A.Horvath,A.T.Boam,A.G.Livingston,Demonstration of molecular purification in polar aprotic solvents by organic solvent nanofiltration,Org.Process.Res.Dev.14(2010)600-611.
[17]J.Vanneste,D.Ormerod,G.Theys,D.Van Gool,B.Van Camp,S.Darvishmanesh,B.Van der Bruggen,Towards high resolution membrane-based pharmaceutical separations,J.Chem.Technol.Biot.88(2013)98-108.
[18]S.Darvishmanesh,L.Firoozpour,J.Vanneste,P.Luis,J.Degreve,B.Van der Bruggen,Performance of solvent resistant nanofiltration membranes for purification of residual solvent in the pharmaceutical industry:Experiments and simulation,Green Chem.13(2011)3476-3483.
[19]E.M.Rundquist,C.J.Pink,A.G.Livingston,Organic solvent nanofiltration:A potential alternative to distillation for solvent recovery from crystallisation mother liquors,Green Chem.14(2012)2197-2205.
[20]C.Van Doorslaer,D.Glas,A.Peeters,A.Cano Odena,I.Vankelecom,K.Binnemans,P.Mertens,D.De Vos,Product recovery from ionic liquids by solvent-resistant nanofiltration:Application to ozonation of acetals and methyl oleate,Green Chem.12(2010)1726.
[21]S.So,L.G.Peeva,E.W.Tate,R.J.Leatherbarrow,A.G.Livingston,Membrane enhanced peptide synthesis,Chem.Commun.46(2010)2808-2810.
[22]S.U.So,L.G.Peeva,E.W.Tate,R.J.Leatherbarrow,A.G.Livingston,Organic solvent nanofiltration:A new paradigm in peptide synthesis,Org.Process.Res.Dev.14(2010)1313-1325.
[23]R.Valadez-Blanco,F.C.Ferreira,R.F.Jorge,A.G.Livingston,A membrane bioreactor for biotransformations of hydrophobic molecules using organic solvent nanofiltration(OSN)membranes,J.Membr.Sci.317(2008)50-64.
[24]P.Silva,L.G.Peeva,A.G.Livingston,Nano filtration in organic solvents,In:Advanced Membrane Technology and Applications,John Wiley&Sons,Inc,Hoboken,New Jersey,2008 pp.451-467.
[25]J.C.Jansen,S.Darvishmanesh,F.Tasselli,F.Bazzarelli,P.Bernardo,E.Tocci,K.Friess,A.Randova,E.Drioli,B.van der Bruggen,Influence of the blend composition on the properties and separation performance of novel solvent resistant polyphenylsulfone/polyimide nanofiltration membranes,J.Membr.Sci.447(2013)107-118.
[26]C.Guizard,A.Ayral,A.Julbe,Potentiality of organic solvents filtration with ceramic membranes.A comparison with polymer membranes,Desalination147(2002)275-280.
[27]T.Tsuru,M.Miyawaki,H.Kondo,T.Yoshioka,M.Asaeda,Inorganic porous membranes for nanofiltration of nonaqueous solutions,Sep.Purif.Technol.32(2003)105-109.
[28]T.Tsuru,M.Narita,R.Shinagawa,T.Yoshioka,Nanoporous titania membranes for permeation and filtration of organic solutions,Desalination233(2008)1-9.
[29]A.Dobrak,B.Verrecht,H.Van den Dungen,A.Buekenhoudt,I.F.J.Vankelecom,B.Van der Bruggen,Solvent flux behavior and rejection characteristics of hydrophilic and hydrophobic mesoporous and microporous TiO2and ZrO2membranes,J.Membr.Sci.346(2010)344-352.
[30]A.Buekenhoudt,F.Bisignano,G.De Luca,P.Vandezande,M.Wouters,K.Verhulst,Unravelling the solvent flux behaviour of ceramic nanofiltration and ultra filtration membranes,J.Membr.Sci.439(2013)36-47.
[31]K.Goh,H.E.Karahan,L.Wei,T.H.Bae,A.G.Fane,R.Wang,Y.Chen,Carbon nanomaterials for advancing separation membranes:A strategic perspective,Carbon109(2016)694-710.
[32]S.Hermans,H.Marien,C.Van Goethem,I.F.J.Vankelecom,Recent developments in thin film(nano)composite membranes for solvent resistant nanofiltration,Curr.Opin.Chem.Eng.8(2015)45-54.
[33]L.G.Peeva,M.Sairam,A.G.Livingston,2.05—Nano filtration operations in nonaqueous systems,in:E.Drioli,L.Giorno(Eds.),Comprehensive Membrane Science and Engineering,Elsevier,Oxford 2010,pp.91-113.
[34]J.Mulder,Basic Principles of Membrane Technology,2nd ed Kluwer Academic,Dordrecht,Boston,1996.
[35]K.Vanherck,A.Cano-Odena,G.Koeckelberghs,T.Dedroog,I.Vankelecom,A simplified diamine crosslinking method for PI nanofiltration membranes,J.Membr.Sci.353(2010)135-143.
[36]K.Vanherck,P.Vandezande,S.O.Aldea,I.F.J.Vankelecom,Cross-linked polyimide membranes for solvent resistant nanofiltration in aprotic solvents,J.Membr.Sci.320(2008)468-476.
[37]K.Vanherck,G.Koeckelberghs,I.F.J.Vankelecom,Crosslinking polyimides for membrane applications:A review,Prog.Polym.Sci.38(2013)874-896.
[38]P.S.Tin,T.S.Chung,Y.Liu,R.Wang,S.L.Liu,K.P.Pramoda,Effects of cross-linking modification on gas separation performance of Matrimid membranes,J.Membr.Sci.225(2003)77-90.
[39]Y.Liu,R.Wang,T.S.Chung,Chemical cross-linking modification of polyimide membranes for gas separation,J.Membr.Sci.189(2001)231-239.
[40]K.Hendrix,K.Vanherck,I.F.J.Vankelecom,Optimization of solvent resistant nanofiltration membranes prepared by the in-situ diamine crosslinking method,J.Membr.Sci.421(2012)15-24.
[41]I.Soroko,M.P.Lopes,A.Livingston,The effect of membrane formation parameters on performance of polyimide membranes for organic solventnano filtration(OSN):Part A.Effect of polymer/solvent/non-solvent system choice,J.Membr.Sci.381(2011)152-162.
[42]I.Soroko,M.Makowski,F.Spill,A.Livingston,The effect of membrane formation parameters on performance of polyimide membranes for organic solvent nanofiltration(OSN).Part B:Analysis of evaporation step and the role of a cosolvent,J.Membr.Sci.381(2011)163-171.
[43]I.Soroko,M.Sairam,A.G.Livingston,The effect of membrane formation parameters on performance of polyimide membranes for organic solvent nanofiltration(OSN).Part C.Effect of polyimide characteristics,J.Membr.Sci.381(2011)172-182.
[44]S.M.Dutczak,F.P.Cuperus,M.Wessling,D.F.Stamatialis,New crosslinking method of polyamide-imide membranes for potential application in harsh polar aprotic solvents,Sep.Purif.Technol.102(2013)142-146.
[45]K.Hendrix,M.Van Eynde,G.Koeckelberghs,I.F.J.Vankelecom,Crosslinking of modified poly(ether ether ketone)membranes for use in solvent resistant nanofiltration,J.Membr.Sci.447(2013)212-221.
[46]X.X.Loh,M.Sairam,J.H.Steinke,A.G.Livingston,A.Bismarck,K.Li,Polyaniline hollow fibres for organic solvent nanofiltration,Chem.Commun.(2008)6324-6326.
[47]X.X.Loh,M.Sairam,A.Bismarck,J.H.G.Steinke,A.G.Livingston,K.Li,Crosslinked integrally skinned asymmetric polyaniline membranes for use in organic solvents,J.Membr.Sci.326(2009)635-642.
[48]M.Sairam,X.X.Loh,K.Li,A.Bismarck,J.H.G.Steinke,A.G.Livingston,Nanoporous asymmetric polyaniline films for filtration of organic solvents,J.Membr.Sci.330(2009)166-174.
[49]M.Sairam,X.X.Loh,Y.Bhole,I.Sereewatthanawut,K.Li,A.Bismarck,J.H.G.Steinke,A.G.Livingston,Spiral-wound polyaniline membrane modules for organic solvent nanofiltration(OSN),J.Membr.Sci.349(2010)123-129.
[50]I.Struzynska-Piron,J.Loccu fier,L.Vanmaele,I.F.Vankelecom,Synthesis of solvent stable polymeric membranesviaUV depth-curing,Chem.Commun.49(2013)11494-11496.
[51]I.Struzynska-Piron,M.R.Bilad,J.Loccufier,L.Vanmaele,I.F.J.Vankelecom,Influence of UV curing on morphology and performance of polysulfone membranes containing acrylates,J.Membr.Sci.462(2014)17-27.
[52]A.K.Hołda,B.Aernouts,W.Saeys,I.F.J.Vankelecom,Study of polymer concentration and evaporation time as phase inversion parameters for polysulfone-based SRNF membranes,J.Membr.Sci.442(2013)196-205.
[53]A.K.Hołda,I.F.J.Vankelecom,Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part A:Influence of high molecular weight additives,J.Membr.Sci.450(2014)512-521.
[54]A.K.Hołda,I.F.J.Vankelecom,Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part B:Influence of low molecular weight additives,J.Membr.Sci.450(2014)499-511.
[55]I.B.Valtcheva,S.C.Kumbharkar,J.F.Kim,Y.Bhole,A.G.Livingston,Beyond polyimide:Crosslinked polybenzimidazole membranes for organic solvent nanofiltration(OSN)in harsh environments,J.Membr.Sci.457(2014)62-72.
[56]I.B.Valtcheva,P.Marchetti,A.G.Livingston,Cross linked polybenzimidazole membranes for organic solvent nanofiltration(OSN):Analysis of crosslinking reaction mechanism and effects of reaction parameters,J.Membr.Sci.493(2015)568-579.
[57]X.F.Li,S.De Feyter,I.F.J.Vankelecom,Poly(sulfone)/sulfonated poly(ether ether ketone)blend membranes:Morphology study and application in the filtration of alcohol based feeds,J.Membr.Sci.324(2008)67-75.
[58]S.Darvishmanesh,J.C.Jansen,F.Tasselli,E.Tocci,P.Luis,J.Degreve,E.Drioli,B.van der Bruggen,Novel polyphenylsulfone membrane for potential use in solvent nanofiltration,J.Membr.Sci.379(2011)60-68.
[59]S.Darvishmanesh,F.Tasselli,J.C.Jansen,E.Tocci,F.Bazzarelli,P.Bernardo,P.Luis,J.Degreve,E.Drioli,B.Van der Bruggen,Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes,J.Membr.Sci.384(2011)89-96.
[60]Solvay website,Markets&products,Featured products(Radel®,Veradel®and Acudel®),http://www.solvay.com/en/markets-and-products/featured-products/index.html(Last accessed:August 8,2017).
[61]Y.C.Xu,X.Q.Cheng,J.Long,L.Shao,A novel monoamine modification strategy toward high-performance organic solvent nanofiltration(OSN)membrane for sustainable molecular separations,J.Membr.Sci.497(2016)77-89.
[62]D.Y.Koh,B.A.McCool,H.W.Deckman,R.P.Lively,Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes,Science353(2016)804-807.
[63]A.G.Fane,R.Wang,M.X.Hu,Synthetic membranes for water purification:Status and future,Angew.Chem.Int.Ed.54(2015)3368-3386.
[64]M.F.Jimenez Solomon,Y.Bhole,A.G.Livingston,High flux membranes for organic solvent nanofiltration(OSN)—Interfacial polymerization with solvent activation,J.Membr.Sci.423-424(2012)371-382.
[65]S.-P.Sun,T.-S.Chung,K.-J.Lu,S.-Y.Chan,Enhancement of flux and solvent stability of Matrimid®thin- film composite membranes for organic solvent nanofiltration,AIChE J.60(2014)3623-3633.
[66]M.F.Jimenez Solomon,Y.Bhole,A.G.Livingston,High flux hydrophobic membranes for organic solvent nanofiltration(OSN)—Interfacial polymerization,surface modification and solvent activation,J.Membr.Sci.434(2013)193-203.
[67]H.Q.Zhang,Y.J.Zhang,L.B.Li,S.Zhao,H.O.Ni,S.K.Cao,J.T.Wang,Cross-linked polyacrylonitrile/polyethyleneimine-polydimethylsiloxane composite membrane for solvent resistant nanofiltration,Chem.Eng.Sci.106(2014)157-166.
[68]S.Karan,Z.Jiang,A.G.Livingston,Sub-10 nm polyamide nano films with ultrafast solvent transport for molecular separation,Science348(2015)1347-1351.
[69]M.F.Jimenez-Solomon,Q.Song,K.E.Jelfs,M.Munoz-Ibanez,A.G.Livingston,Polymer nanofilms with enhanced microporosity by interfacial polymerization,Nat.Mater.15(2016)760-767.
[70]L.E.M.Gevers,S.Aldea,I.F.J.Vankelecom,P.A.Jacobs,Optimisation of a lab-scale method for preparation of composite membranes with a filled dense top-layer,J.Membr.Sci.281(2006)741-746.
[71]L.E.M.Gevers,I.F.J.Vankelecom,P.A.Jacobs,Solvent-resistant nanofiltration with filled polydimethylsiloxane(PDMS)membranes,J.Membr.Sci.278(2006)199-204.
[72]N.Sta fie,D.F.Stamatialis,M.Wessling,Effect of PDMS cross-linking degree on the permeation performance of PAN/PDMS composite nanofiltration membranes,Sep.Purif.Technol.45(2005)220-231.
[73]S.Aerts,A.Vanhulsel,A.Buekenhoudt,H.Weyten,S.Kuypers,H.Chen,M.Bryjak,L.E.M.Gevers,I.F.J.Vankelecom,P.A.Jacobs,Plasma-treated PDMS-membranes in solvent resistant nanofiltration:Characterization and study of transport mechanism,J.Membr.Sci.275(2006)212-219.
[74]E.Florian,M.Modesti,M.Ulbricht,Preparation and characterization of novel solvent-resistant nanofiltration composite membranes based on crosslinked polyurethanes,Ind.Eng.Chem.Res.46(2007)4891-4899.
[75]X.F.Li,P.Vandezande,I.F.J.Vankelecom,Polypyrrole modified solvent resistant nanofiltration membranes,J.Membr.Sci.320(2008)143-150.
[76]L.Shao,X.Q.Cheng,Z.X.Wang,J.Ma,Z.H.Guo,Tuning the performance of polypyrrole-based solvent-resistant composite nanofiltration membranes by optimizing polymerization conditions and incorporating graphene oxide,J.Membr.Sci.452(2014)82-89.
[77]A.V.Volkov,V.V.Parashchuk,D.F.Stamatialis,V.S.Khotimsky,V.V.Volkov,M.Wessling,High permeable PTMSP/PAN composite membranes for solvent nanofiltration,J.Membr.Sci.333(2009)88-93.
[78]P.M.Budd,B.S.Ghanem,S.Makhseed,N.B.McKeown,K.J.Msayib,C.E.Tattershall,Polymers of intrinsic microporosity(PIMs):Robust,solution-processable,organic nanoporous materials,Chem.Commun.(2004)230-231.
[79]N.B.McKeown,Polymers of intrinsic microporosity,ISRN Materials Science2012(2012)1-16.
[80]D.Fritsch,P.Merten,K.Heinrich,M.Lazar,M.Priske,High performance organic solvent nanofiltration membranes:Development and thorough testing of thin if lm composite membranes made of polymers of intrinsic microporosity(PIMs),J.Membr.Sci.401(2012)222-231.
[81]P.Gorgojo,S.Karan,H.C.Wong,M.F.Jimenez-Solomon,J.T.Cabral,A.G.Livingston,Ultrathin polymer films with intrinsic microporosity:Anomalous solvent permeation and high flux membranes,Adv.Funct.Mater.24(2014)4729-4737.
[82]J.K.Kim,S.Y.Yang,Y.Lee,Y.Kim,Functional nanomaterials based on block copolymer self-assembly,Prog.Polym.Sci.35(2010)1325-1349.
[83]X.F.Li,C.A.Fustin,N.Lefevre,J.F.Gohy,S.De Feyter,J.De Baerdemaeker,W.Egger,I.F.J.Vankelecom,Ordered nanoporous membranes based on diblock copolymers with high chemical stability and tunable separation properties,J.Mater.Chem.20(2010)4333-4339.
[84]E.Fontananova,G.Di Profio,F.Artusa,E.Drioli,Polymeric homogeneous composite membranes for separations in organic solvents,J.Appl.Polym.Sci.129(2013)1653-1659.
[85]N.Joseph,P.Ahmadiannamini,R.Hoogenboom,I.F.J.Vankelecom,Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation,Polm.Chem.5(2014)1817-1831.
[86]Q.Zhao,Q.F.F.An,Y.L.Ji,J.W.Qian,C.J.Gao,Polyelectrolyte complex membranes for pervaporation,nano filtration and fuel cell applications,J.Membr.Sci.379(2011)19-45.
[87]X.F.Li,S.De Feyter,D.J.Chen,S.Aldea,P.Vandezande,F.Du Prez,I.F.J.Vankelecom,Solvent-resistant nanofiltration membranes based on multilayered polyelectrolyte complexes,Chem.Mater.20(2008)3876-3883.
[88]X.F.Li,W.Goyens,P.Ahmadiannamini,W.Vanderlinden,S.De Feyter,I.Vankelecom,Morphology and performance of solvent-resistant nanofiltration membranes based on multilayered polyelectrolytes:Study of preparation conditions,J.Membr.Sci.358(2010)150-157.
[89]P.Ahmadiannamini,X.F.Li,W.Goyens,N.Joseph,B.Meesschaert,I.F.J.Vankelecom,Multilayered polyelectrolyte complex based solvent resistant nanofiltration membranes prepared from weak polyacids,J.Membr.Sci.394(2012)98-106.
[90]P.Ahmadiannamini,X.F.Li,W.Goyens,B.Meesschaert,W.Vanderlinden,S.De Feyter,I.F.J.Vankelecom,Influence of polyanion type and cationic counter ion on the SRNF performance of polyelectrolyte membranes,J.Membr.Sci.403(2012)216-226.
[91]D.J.Chen,Solvent-resistant nanofiltration membranes based on multilayered polyelectrolytes deposited on silicon composite,J.Appl.Polym.Sci.129(2013)3156-3161.
[92]S.Ilyas,N.Joseph,A.Szymczyk,A.Volodin,K.Nijmeijer,W.M.de Vos,I.F.J.Vankelecom,Weak polyelectrolyte multilayers as tunable membranes for solvent resistant nanofiltration,J.Membr.Sci.514(2016)322-331.
[93]J.da Silva Burgal,L.G.Peeva,S.Kumbharkar,A.Livingston,Organic solvent resistant poly(ether-ether-ketone)nanofiltration membranes,J.Membr.Sci.479(2015)105-116.
[94]M.G.Buonomenna,G.Golemme,J.C.Jansen,S.H.Choi,Asymmetric PEEKWC membranes for treatment of organic solvent solutions,J.Membr.Sci.368(2011)144-149.
[95]C.Ursino,S.Simone,L.Donato,S.Santoro,M.P.De Santo,E.Drioli,E.Di Nicolò,A.Figoli,ECTFE membranes produced by non-toxic diluents for organic solvent filtration separation,RSC Adv.6(2016)81001-81012.
[96]D.J.Chen,X.Liu,D.D.Li,X.F.Li,Highly stable polysulfone solvent resistant nanofiltration membranes with internal cross-linking networks,RSC Adv.6(2016)29570-29575.
[97]D.J.Chen,S.S.Yu,M.Yang,D.D.Li,X.F.Li,Solvent resistant nanofiltration membranes based on crosslinked polybenzimidazole,RSC Adv.6(2016)16925-16932.
[98]S.M.Dutczak,C.R.Tanardi,K.K.Kopec,M.Wessling,D.Stamatialis,"Chemistry in a spinneret"to fabricate hollow fibers for organic solvent filtration,Sep.Purif.Technol.86(2012)183-189.
[99]S.K.Lim,L.Setiawan,T.H.Bae,R.Wang,Polyamide-imide hollow fiber membranes crosslinked with amine-appended inorganic networks for application in solvent resistant nanofiltration under low operating pressure,J.Membr.Sci.501(2016)152-160.
[100]S.P.Sun,S.Y.Chan,T.S.Chung,A slow-fast phase separation(SFPS)process to fabricate dual-layer hollow fiber substrates for thin- film composite(TFC)organic solvent nanofiltration(OSN)membranes,Chem.Eng.Sci.129(2015)232-242.
[101]S.P.Sun,S.Y.Chan,W.H.Xing,Y.Wang,T.S.Chung,Facile synthesis of dual-layer organic solvent nanofiltration(OSN)hollow fiber membranes,ACS Sustain.Chem.Eng.3(2015)3019-3023.
[102]H.Siddique,E.Rundquist,Y.Bhole,L.G.Peeva,A.G.Livingston,Mixed matrix membranes for organic solvent nanofiltration,J.Membr.Sci.452(2014)354-366.
[103]M.F.Jimenez-Solomon,P.Gorgojo,M.Munoz-Ibanez,A.G.Livingston,Beneath the surface:Influence of supports on thin film composite membranes by interfacial polymerization for organic solvent nanofiltration,J.Membr.Sci.448(2013)102-113.
[104]S.Hermans,E.Dom,H.Marien,G.Koeckelberghs,I.F.J.Vankelecom,Efficient synthesis of interfacially polymerized membranes for solvent resistant nanofiltration,J.Membr.Sci.476(2015)356-363.
[105]L.Pérez-Manríquez,A.R.Behzad,K.-V.Peinemann,Sub-6 nm thin cross-linked dopamine films with high pressure stability for organic solvent nanofiltration,Macromol.Mater.Eng301(2016)1437-1442.
[106]Y.J.Zhang,H.Q.Zhang,Y.F.Li,H.Mao,G.H.Yang,J.T.Wang,Tuning the performance of composite membranes by optimizing PDMS content and cross-linking time for solvent resistant nanofiltration,Ind.Eng.Chem.Res.54(2015)6175-6186.
[107]C.Liu,S.Kulprathipanja,A.M.W.Hillock,S.Husain,W.J.Koros,Recent progress in mixed-matrix membranes,In:Advanced Membrane Technology and Applications,Hoboken,New Jersey,2008,pp.787-819.
[108]R.Mahajan,W.J.Koros,Factors controlling successful formation of mixed-matrix gas separation materials,Ind.Eng.Chem.Res.39(2000)2692-2696.
[109]M.Y.Kariduraganavar,J.G.Varghese,S.K.Choudhari,R.H.Olley,Organic-inorganic hybrid membranes:Solving the trade-off phenomenon between permeation flux and selectivity in pervaporation,Ind.Eng.Chem.Res.48(2009)4002-4013.
[110]H.Dong,X.Y.Qu,L.Zhang,L.H.Cheng,H.L.Chen,C.J.Gao,Preparation and characterization of surface-modified zeolite-polyamide thin film nanocomposite membranes for desalination,Desalin.Water Treat.34(2011)6-12.
[111]K.Goh,Y.Chen,Controlling water transport in carbon nanotubes,Nano Today14(2017)13-15.
[112]J.Kim,B.Van der Bruggen,The use of nanoparticles in polymeric and ceramic membrane structures:review of manufacturing procedures and performance improvement for water treatment,Environ.Pollut.158(2010)2335-2349.
[113]T.T.Moore,W.J.Koros,Non-ideal effects in organic-inorganic materials for gas separation membranes,J.Mol.Struct.739(2005)87-98.
[114]K.Goh,L.Setiawan,L.Wei,W.Jiang,R.Wang,Y.Chen,Fabrication of novel functionalized multi-walled carbon nanotube immobilized hollow fiber membranes for enhanced performance in forward osmosis process,J.Membr.Sci.446(2013)244-254.
[115]E.S.Tarleton,J.P.Robinson,C.R.Millington,A.Nijmeijer,Non-aqueous nanofiltration:Solute rejection in low-polarity binary systems,J.Membr.Sci.252(2005)123-131.
[116]L.E.Gevers,I.F.Vankelecom,P.A.Jacobs,Zeolite filled polydimethylsiloxane(PDMS)as an improved membrane for solvent-resistant nanofiltration(SRNF),Chem.Commun.(2005)2500-2502.
[117]K.Vanherck,A.Aerts,J.Martens,I.Vankelecom,Hollow filler based mixed matrix membranes,Chem.Commun.46(2010)2492-2494.
[118]I.Soroko,A.Livingston,Impact of TiO2nanoparticles on morphology and performance of crosslinked polyimide organic solvent nanofiltration(OSN)membranes,J.Membr.Sci.343(2009)189-198.
[119]M.Namvar-Mahboub,M.Pakizeh,Development of a novel thin film composite membrane by interfacial polymerization on polyetherimide/modified SiO2support for organic solvent nanofiltration,Sep.Purif.Technol.119(2013)35-45.
[120]J.L.C.Rowsell,O.M.Yaghi,Metal-organic frameworks:a new class of porous materials,Micropor.Mesopor.Mat.73(2004)3-14.
[121]B.Zornoza,C.Tellez,J.Coronas,J.Gascon,F.Kapteijn,Metal organic framework based mixed matrix membranes:An increasingly important field of research with a large application potential,Micropor.Mesopor.Mat.166(2013)67-78.
[122]H.B.Tanh Jeazet,C.Staudt,C.Janiak,Metal-organic frameworks in mixed-matrix membranes for gas separation,Dalton Trans.41(2012)14003-14027.
[123]S.Basu,M.Maes,A.Cano-Odena,L.Alaerts,D.E.De Vos,I.F.J.Vankelecom,Solvent resistant nanofiltration(SRNF)membranes based on metal-organic frameworks,J.Membr.Sci.344(2009)190-198.
[124]B.H.Jeong,E.M.V.Hoek,Y.S.Yan,A.Subramani,X.F.Huang,G.Hurwitz,A.K.Ghosh,A.Jawor,Interfacial polymerization of thin film nanocomposites:A new concept for reverse osmosis membranes,J.Membr.Sci.294(2007)1-7.
[125]S.Sorribas,P.Gorgojo,C.Tellez,J.Coronas,A.G.Livingston,High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration,J.Am.Chem.Soc.135(2013)15201-15208.
[126]M.Peyravi,M.Jahanshahi,A.Rahimpour,A.Javadi,S.Hajavi,Novel thin film nanocomposite membranes incorporated with functionalized TiO2nanoparticles for organic solvent nanofiltration,Chem.Eng.J.241(2014)155-166.
[127]C.Van Goethem,R.Verbeke,S.Hermans,R.Bernstein,I.F.J.Vankelecom,Controlled positioning of MOFs in interfacially polymerized thin- film nanocomposites,J.Mater.Chem.A4(2016)16368-16376.
[128]K.Goh,L.Setiawan,L.Wei,R.Si,A.G.Fane,R.Wang,Y.Chen,Graphene oxide as effective selective barriers on a hollow fiber membrane for water treatment process,J.Membr.Sci.474(2015)244-253.
[129]K.Goh,J.K.Heising,Y.Yuan,H.E.Karahan,L.Wei,S.Zhai,J.X.Koh,N.M.Htin,F.Zhang,R.Wang,A.G.Fane,M.Dekker,F.Dehghani,Y.Chen,Sandwicharchitectured poly(lactic acid)-graphene composite food packaging films,ACS Appl.Mater.Interfaces8(2016)9994-10004.
[130]L.Huang,Y.Li,Q.Zhou,W.Yuan,G.Shi,Graphene oxide membranes with tunable semipermeability in organic solvents,Adv.Mater.27(2015)3797-3802.
[131]K.Goh,W.C.Jiang,H.E.Karahan,S.L.Zhai,L.Wei,D.S.Yu,A.G.Fane,R.Wang,Y.Chen,All-carbon nanoarchitectures as high-performance separation membranes with superior stability,Adv.Funct.Mater.25(2015)7348-7359.
[132]L.Huang,J.Chen,T.Gao,M.Zhang,Y.Li,L.Dai,L.Qu,G.Shi,Reduced graphene oxide membranes for ultrafast organic solvent nanofiltration,Adv.Mater.28(2016)8669-8674.
[133]R.Ding,H.Q.Zhang,Y.F.Li,J.T.Wang,B.B.Shi,H.Mao,J.C.Dang,J.D.Liu,Graphene oxide-embedded nanocomposite membrane for solvent resistant nanofiltration with enhanced rejection ability,Chem.Eng.Sci.138(2015)227-238.
[134]X.L.Wu,L.Hao,J.K.Zhang,X.Zhang,J.T.Wang,J.D.Liu,Polymer-Ti3C2Txcomposite membranes to overcome the trade-off in solvent resistant nanofiltration for alcohol-based system,J.Membr.Sci.515(2016)175-188.
[135]H.Mao,H.Zhang,Y.Li,Y.Xue,F.Pei,J.Wang,J.Liu,Tunable solvent permeation properties of thin film nanocomposite membrane by constructing dual-pathways using cyclodextrins for organic solvent nanofiltration,ACS Sustain.Chem.Eng.3(2015)1925-1933.
[136]H.Q.Zhang,H.Mao,J.T.Wang,R.Ding,Z.Du,J.D.Liu,S.K.Cao,Mineralization inspired preparation of composite membranes with polyethyleneiminenanoparticle hybrid active layer for solvent resistant nanofiltration,J.Membr.Sci.470(2014)70-79.
[137]Y.F.Li,H.Mao,H.Q.Zhang,G.H.Yang,R.Ding,J.T.Wang,Tuning the microstructure and permeation property of thin film nanocomposite membrane by functionalized inorganic nanospheres for solvent resistant nanofiltration,Sep.Purif.Technol.165(2016)60-70.
[138]M.Namvar-Mahboub,M.Pakizeh,S.Davari,Preparation and characterization of UZM-5/polyamide thin film nanocomposite membrane for dewaxing solvent recovery,J.Membr.Sci.459(2014)22-32.
[139]A.Dobrak-Van Berlo,I.F.J.Vankelecom,B.Van der Bruggen,Parameters determining transport mechanisms through un filled and silicalite filled PDMS-based membranes and dense PI membranes in solvent resistant nanofiltration:Comparison with pervaporation,J.Membr.Sci.374(2011)138-149.
[140]Y.Li,T.Verbiest,I.Vankelecom,Improving the flux of PDMS membranes via localized heating through incorporation of gold nanoparticles,J.Membr.Sci.428(2013)63-69.
[141]K.Vanherck,I.Vankelecom,T.Verbiest,Improving fluxes of polyimide membranes containing gold nanoparticles by photothermal heating,J.Membr.Sci.373(2011)5-13.
[142]K.Vanherck,T.Verbiest,I.Vankelecom,Comparison of two synthesis routes to obtain gold nanoparticles in polyimide,J.Phys.Chem.C116(2012)115-125.
[143]K.Vanherck,S.Hermans,T.Verbiest,I.Vankelecom,Using the photothermal effect to improve membrane separations via localized heating,J.Mater.Chem.21(2011)6079-6087.
[144]H.Siddique,L.G.Peeva,K.Stoikos,G.Pasparakis,M.Vamvakaki,A.G.Livingston,Membranes for organic solvent nanofiltration based on preassembled nanoparticles,Ind.Eng.Chem.Res.52(2013)1109-1121.
[145]N.A.A.Sani,W.J.Lau,A.F.Ismail,Polyphenylsulfone-based solvent resistant nanofiltration (SRNF) membrane incorporated with copper-1,3,5-benzenetricarboxylate(Cu-BTC)nanoparticles for methanol separation,RSC Adv.5(2015)13000-13010.
[146]J.Campbell,G.Szekely,R.P.Davies,D.C.Braddock,A.G.Livingston,Fabrication of hybrid polymer/metal organic framework membranes:Mixed matrix membranes versus in situ growth,J.Mater.Chem.A2(2014)9260-9271.
[147]J.Campbell,J.D.Burgal,G.Szekely,R.P.Davies,D.C.Braddock,A.G.Livingston,Hybrid polymer/MOF membranes for Organic Solvent Nano filtration(OSN):Chemical modification and the quest for perfection,J.Membr.Sci.503(2016)166-176.
[148]Y.Li,L.H.Wee,A.Volodin,J.A.Martens,I.F.Vankelecom,Polymer supported ZIF-8 membranes prepared via an interfacial synthesis method,Chem.Commun.51(2015)918-920.
[149]S.Roy,S.A.Ntim,S.Mitra,K.K.Sirkar,Facile fabrication of superior nanofiltration membranes from interfacially polymerized CNT-polymer composites,J.Membr.Sci.375(2011)81-87.
[150]B.C.Shi,P.Marchetti,D.Peshev,S.F.Zhang,A.G.Livingston,Performance of spiralwound membrane modules in organic solventnano filtration—Fluid dynamics and mass transfer characteristics,J.Membr.Sci.494(2015)8-24.
[151]A.R.Korikov,R.Kosaraju,K.K.Sirkar,Interfacially polymerized hydrophilic microporous thin film composite membranes on porous polypropylene hollow fibers and flat films,J.Membr.Sci.279(2006)588-600.
[152]P.B.Kosaraju,K.K.Sirkar,Interfacially polymerized thin film composite membranes on microporous polypropylene supports for solvent-resistant nanofiltration,J.Membr.Sci.321(2008)155-161.
[153]K.K.Kopec,S.M.Dutczak,M.Wessling,D.F.Stamatialis,Chemistry in a spinneret—On the interplay of crosslinking and phase inversion during spinning of novel hollow fiber membranes,J.Membr.Sci.369(2011)308-318.
[154]S.M.Dutczak,M.W.J.Luiten-Olieman,H.J.Zwijnenberg,L.A.M.Bolhuis-Versteeg,L.Winnubst,M.A.Hempenius,N.E.Benes,M.Wessling,D.Stamatialis,Composite capillary membrane for solvent resistant nanofiltration,J.Membr.Sci.372(2011)182-190.
[155]Inopor®website,Products,Membranes,Membrane materials and pore sizes,http://inopor.com/en/products/membranes.html(Last accessed:August 8,2017).
[156]I.C.Kim,J.H.Kim,K.H.Lee,T.M.Tak,Phospholipids separation(degumming)from crude vegetable oil by polyimide ultra filtration membrane,J.Membr.Sci.205(2002)113-123.
[157]C.Pagliero,M.Mattea,N.Ochoa,J.Marchese,Fouling of polymeric membranes during degumming of crude sun flower and soybean oil,J.Food Eng.78(2007)194-197.
[158]N.Sta fie,D.F.Stamatialis,A.Wessling,Insight into the transport of hexane-solute systems through tailor-made composite membranes,J.Membr.Sci.228(2004)103-116.
[159]B.M.Bhosle,R.Subramanian,New approaches in deacidification of edible oils—a review,J.Food Eng.69(2005)481-494.
[160]M.Cheryan,Membrane technology in the vegetable oil industry,Membr.Technol.2005(2005)5-7.
[161]W.B.Cai,Y.Z.Sun,X.L.Piao,J.D.Li,S.L.Zhu,Solvent recovery from soybean oil/hexane miscella by PDMS composite membrane,Chinese J.Chem.Eng.19(2011)575-580.
[162]J.R.Kwiatkowski,M.Cheryan,Recovery of corn oil from ethanol extracts of ground corn using membrane technology,J.Am.Oil Chem.Soc.82(2005)221-227.
[163]M.S.Kuk,R.Tetlow,M.K.Dowd,Cottonseed extraction with mixtures of acetone and hexane,J.Am.Oil Chem.Soc.82(2005)609-612.
[164]S.Darvishmanesh,T.Robberecht,P.Luis,J.Degreve,B.Van der Bruggen,Performance of nanofiltration membranes for solvent purification in the oil industry,J.Am.Oil Chem.Soc.88(2011)1255-1261.
[165]A.V.Volkov,G.A.Korneeva,G.F.Tereshchenko,Organic solvent nanofiltration:Prospects and application,Russ.Chem.Rev.77(2008)983-993.
[166]L.R.Firman,N.A.Ochoa,J.Marchese,C.L.Pagliero,Deacidification and solvent recovery of soybean oil by nanofiltration membranes,J.Membr.Sci.431(2013)187-196.
[167]E.M.Tsui,M.Cheryan,Membrane processing of xanthophylls in ethanol extracts of corn,J.Food Eng.83(2007)590-595.
[168]B.Tylkowski,B.Trusheva,V.Bankova,M.Giamberini,G.Peev,A.Nikolova,Extraction of biologically active compounds from propolis and concentration of extract by nanofiltration,J.Membr.Sci.348(2010)124-130.
[169]I.Sereewatthanawut,I.I.Baptista,A.T.Boam,A.Hodgson,A.G.Livingston,Nano filtration process for the nutritional enrichment and re fining of rice bran oil,J.Food Eng.102(2011)16-24.
[170]C.M.Gilmer,N.B.Bowden,Highly cross-linked epoxy nanofiltration membranes for the separation of organic chemicals and fish oil ethyl esters,ACS Appl.Mater,Interfaces8(2016)24104-24111.
[171]I.Tsibranska,I.Saykova,Combining nanofiltration and other separation methods(review),J.Univ.Chem.Technol.Metall.48(2013)333-340.
[172]D.Peshev,L.G Peeva,G.Peev,I.I.R Baptista,A.T Boam,Application of organic solvent nanofiltration for concentration of antioxidant extracts of rosemary(Rosmarinus officiallis L.),Chem.Eng.Res.Des.89(2011)318-327.
[173]C.Abels,F.Carstensen,M.Wessling,Membrane processes in biore finery applications,J.Membr.Sci.444(2013)285-317.
[174]R.Othman,A.W.Mohammad,M.Ismail,J.Salimon,Application of polymeric solvent resistant nanofiltration membranes for biodiesel production,J.Membr.Sci.348(2010)287-297.
[175]H.Werhan,A.Farshori,P.R.von Rohr,Separation of lignin oxidation products by organic solvent nanofiltration,J.Membr.Sci.423(2012)404-412.
[176]R.M.Gould,L.S.White,C.R.Wildemuth,Membrane separation in solvent lube dewaxing,Environ.Prog.20(2001)12-16.
[177]L.S.White,Transport properties of a polyimide solvent resistant nanofiltration membrane,J.Membr.Sci.205(2002)191-202.
[178]L.S.White,C.R.Wildemuth,Aromatics enrichment in re finery streams using hyper filtration,Ind.Eng.Chem.Res.45(2006)9136-9143.
[179]G.Szekely,J.Bandarra,W.Heggie,B.Sellergren,F.C.Ferreira,Organic solvent nanofiltration:A platform for removal of genotoxins from active pharmaceutical ingredients,J.Membr.Sci.381(2011)21-33.
[180]G.Szekely,M.Gil,B.Sellergren,W.Heggie,F.C.Ferreira,Environmental and economic analysis for selection and engineering sustainable API degenotoxification processes,Green Chem.15(2013)210-225.
[181]W.E.Siew,A.G.Livingston,C.Ates,A.Merschaert,Continuous solute fractionation with membrane cascades—A high productivity alternative to dia filtration,Sep.Purif.Technol.102(2013)1-14.
[182]J.F.Kim,A.M.Freitas da Silva,I.B.Valtcheva,A.G.Livingston,When the membrane is not enough:A simpli fied membrane cascade using Organic Solvent Nano filtration(OSN),Sep.Purif.Technol.116(2013)277-286.
[183]J.F.Kim,G.Szekely,I.B.Valtcheva,A.G.Livingston,Increasing the sustainability of membrane processes through cascade approach and solvent recoverypharmaceutical purification case study,Green Chem.16(2014)133-145.
[184]E.Rundquist,C.Pink,E.Vilminot,A.Livingston,Facilitating the use of counter current chromatography in pharmaceutical purification through use oforganic solvent nanofiltration,J.Chromatogr.A1229(2012)156-163.
[185]I.Sereewatthanawut,F.C.Ferreira,N.F.Ghazali,A.G.Livingston,Enantioseparation via EIC-OSN:Process design and improvement of enantiomers resolvability and separation performance,AIChE J56(2010)893-904.
[186]N.F.Ghazali,D.A.Patterson,A.G.Livingston,Elucidation of the mechanism of chiral selectivity in diastereomeric salt formation using organic solvent nanofiltration,Chem.Commun.(2004)962-963.
[187]C.Roengpithya,D.A.Patterson,P.C.Taylor,A.G.Livingston,Development of stable organic solvent nanofiltration membranes for membrane enhanced dynamic kinetic resolution,Desalination199(2006)195-197.
[188]N.F.Ghazali,F.C.Ferreira,A.J.P.White,A.G.Livingston,Enantiomer separation by enantioselective inclusion complexation-organic solvent nanofiltration,Tetrahedron Asymmetry17(2006)1846-1852.
[189]H.T.Wong,Y.H.See-Toh,F.C.Ferreira,R.Crook,A.G.Livingston,Organic solvent nanofiltration in asymmetric hydrogenation:enhancement of enantioselectivity and catalyst stability by ionic liquids,Chem.Commun.(2006)2063-2065.
[190]W.E.Siew,C.Ates,A.Merschaert,A.G.Livingston,Efficient and productive asymmetric Michael addition:Development of a highly enantioselective quinidinebased organocatalyst for homogeneous recycling via nanofiltration,Green Chem.15(2013)663-674.
[191]M.Janssen,C.Mueller,D.Vogt,Recent advances in the recycling of homogeneous catalysts using membrane separation,Green Chem.13(2011)2247-2257.
[192]G.Székely,P.Marchetti,M.F.Jimenez-Solomon,A.G.Livingston,E.M.V.Hoek,V.V.Tarabara,Organic solvent nanofiltration,In:Encyclopedia of Membrane Science and Technology,John Wiley&Sons,Inc,Hoboken,New Jersey,2013.
[193]D.Nair,J.T.Scarpello,I.F.J.Vankelecom,L.M.F.Dos Santos,L.S.White,R.J.Kloetzing,T.Welton,A.G.Livingston,Increased catalytic productivity for nanofiltration coupled Heck reactions using highly stable catalyst systems,Green Chem.4(2002)319-324.
[194]A.Datta,K.Ebert,H.Plenio,Nano filtration for homogeneous catalysis separation:Soluble polymer-supported palladium catalysts for Heck,Sonogashira,and Suzuki coupling of aryl halides,Organometallics22(2003)4685-4691.
[195]H.T.Wong,C.J.Pink,F.C.Ferreira,A.G.Livingston,Recovery and reuse of ionic liquids and palladium catalyst for Suzuki reactions using organic solvent nanofiltration,Green Chem.8(2006)373-379.
[196]A.Tsoukala,L.Peeva,A.G.Livingston,H.R.Bjorsvik,Separation of reaction product and palladium catalyst after a Heck coupling reaction by means of organic solvent nanofiltration,ChemSusChem5(2012)188-193.
[197]P.G.N.Mertens,M.Bulut,L.E.M.Gevers,I.F.J.Vankelecom,P.A.Jacobs,D.E.De Vos,Catalytic oxidation of 1,2-diols to alpha-hydroxy-carboxylates with stabilized gold nanocolloids combined with a membrane-based catalyst separation,Catal.Lett.102(2005)57-61.
[198]S.Aerts,A.Buekenhoudt,H.Weyten,L.E.M.Gevers,I.F.G.Vankelecom,P.A.Jacobs,The use of solvent resistant nanofiltration in the recycling of the Co-Jacobsen catalyst in the hydrolytic kinetic resolution(HKR)of epoxides,J.Membr.Sci.280(2006)245-252.
[199]P.T.Witte,S.R.Chowdhury,J.E.ten Elshof,D.Sloboda-Rozner,R.Neumann,P.L.Alsters,Highly efficient recycling of a"sandwich"type polyoxometalate oxidation catalyst using solvent resistant nanofiltration,Chem.Commun.(2005)1206-1208.
[200]S.Roy Chowdhury,P.T.Witte,D.H.Blank,P.L.Alsters,J.E.Ten Elshof,Recovery of homogeneous polyoxometallate catalysts from aqueous and organic media by a mesoporous ceramic membrane without loss of catalytic activity,Chemistry12(2006)3061-3066.
[201]A.Keraani,T.Renouard,C.Fischmeister,C.Bruneau,M.Rabiller-Baudry,Recovery of enlarged ole fin metathesis catalysts by nanofiltration in an eco-friendly solvent,ChemSusChem1(2008)927-933.
[202]D.Schoeps,K.Buhr,M.Dijkstra,K.Ebert,H.Plenio,Batchwise and continuous organophilic nanofiltration of Grubbs-type ole fin metathesis catalysts,Chemistry15(2009)2960-2965.
[203]A.Kajetanowicz,J.Czaban,G.R.Krishnan,M.Malinska,K.Wozniak,H.Siddique,L.G.Peeva,A.G.Livingston,K.Grela,Batchwise and continuous nanofiltration of POSS-tagged Grubbs-Hoveyda-type ole fin metathesis catalysts,ChemSusChem6(2013)182-192.
[204]M.Rabiller-Baudry,G.Nasser,T.Renouard,D.Delaunay,M.Camus,Comparison of two nanofiltration membrane reactors for a model reaction of ole fin metathesis achieved in toluene,Sep.Purif.Technol.116(2013)46-60.
[205]M.Priske,K.D.Wiese,A.Drews,M.Kraume,G.Baumgarten,Reaction integrated separation of homogenous catalysts in the hydroformylation of higher ole fins by means of organophilic nanofiltration,J.Membr.Sci.360(2010)77-83.
[206]P.I.Dalko,L.Moisan,In the golden age of organocatalysis,Angew.Chem.Int.Ed.43(2004)5138-5175.
[207]T.Fahrenwaldt,J.Grosseheilmann,F.Erben,U.Kragl,Organic solvent nanofiltration as a tool for separation of quinine-based organocatalysts,Org.Process.Res.Dev.17(2013)1131-1136.
[208]J.Großeheilmann,H.Büttner,C.Kohrt,U.Kragl,T.Werner,Recycling of phosphorus-based organocatalysts by organic solvent nanofiltration,ACS Sustain.Chem.Eng.3(2015)2817-2822.
[209]J.F.Brennecke,E.J.Maginn,Ionic liquids:Innovative fluids for chemical processing,AIChE J.47(2001)2384-2389.
[210]S.Han,H.T.Wong,A.G.Livingston,Application of organic solvent nanofiltration to separation of ionic liquids and products from ionic liquid mediated reactions,Chem.Eng.Res.Des.83(2005)309-316.
[211]A.Buekenhoudt,H.Beckers,D.Ormerod,M.Bulut,P.Vandezande,R.Vleeschouwers,Solvent based membrane nanofiltration for process intensification,Chem.Ing.Tech.85(2013)1243-1247.
[212]I.Soroko,Y.Bhole,A.G.Livingston,Environmentally friendly route for the preparation of solvent resistant polyimide nanofiltration membranes,Green Chem.13(2011)162-168.
[213]D.Y.Xing,S.Y.Chan,T.S.Chung,The ionic liquid[EMIM]OAc as a solvent to fabricate stable polybenzimidazole membranes for organic solvent nanofiltration,Green Chem.16(2014)1383-1392.
[214]H.Mariën,L.Bellings,S.Hermans,I.F.J.Vankelecom,Sustainable process for the preparation of high-performance thin- film composite membranes using ionic liquids as the reaction medium,ChemSusChem9(2016)1101-1111.
[215]M.Amirilargani,M.Sadrzadeh,E.J.R.Sudholter,L.C.P.M.de Smet,Surface modification methods of organic solvent nanofiltration membranes,Chem.Eng.J.289(2016)562-582.
[216]S.Karan,S.Samitsu,X.Peng,K.Kurashima,I.Ichinose,Ultrafast viscous permeation of organic solvents through diamond-like carbon nanosheets,Science335(2012)444-447.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Positively charged nanofiltration membrane fabricated by poly(acid-base)complexing effect induced phase inversion method for heavy metal removal☆
- Substrate matters:The influences of substrate layers on the performances of thin- film composite reverse osmosis membranes☆
- Recent developments in nanofiltration membranes based on nanomaterials☆
- Mass transfer model,preparation and applications of zeolite membranes for pervaporation dehydration:A review☆
- Manipulation of confined structure in alcohol-permselective pervaporation membranes☆
- Monovalent cation perm-selective membranes(MCPMs):New developments and perspectives☆
