Mass transfer model,preparation and applications of zeolite membranes for pervaporation dehydration:A review☆
2017-05-30ChunZhangLiPengJiJiangXuehongGu
Chun Zhang,Li Peng,Ji Jiang,Xuehong Gu*
State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemical Engineering,Synergetic Innovation Center for Advanced Materials,Nanjing Tech University,Nanjing 210009,China
1.Introduction
With the rapid development of industry,the demands for organic solvent are growing worldwide.In the processes of the organic solvent production and usage,it is very important to dehydrate organic solvents with high efficiency.Unfortunately,most of organic solvent/water mixtures are easy to form azeotropic or close azeotropic mixtures,which cause high energy consumption and waste emission using traditional technologies such as distillation and adsorption for separation.Therefore,the development of efficient organic solvent separation technology is the key to implement clean production and promote recycling efficiency.Membrane separation is a new dehydration technology with high efficiency and energy saving.Moreover,the process is easy to control and space saving.The separation processes are related with the sorption-diffusion rate of the components but not limited by vapor liquid equilibrium system,which is especially suitable for the separation of azeotropic or close azeotropic mixtures[1-5].
Zeolite membranes have been a new generation of membranes for pervaporation.The membranes belong to inorganic membranes and possess a well-defined microporous structure,which show high permeation flux,selectivity and thermochemical stability as compared with polymeric membranes.Hydrophilic zeolite membranes have strong adsorption to water,showing very high water separation selectivity.The separation processes are mainly operated by pervaporation(including vapor permeation).Fig.1 shows the schematic diagram for pervaporation of hydrophilic zeolite membranes.The feed(organic solvent/water mixture)is introduced through one side of a zeolite membrane tube at atmospheric pressure or higher pressure.The other side of the membrane was evacuated with a vacuum pump through a vacuum line,which maintained a vacuum pressure throughout the operation.Water selectively permeates through the zeolite membrane and purified organic solvent could be obtained in the retentate.In the pervaporation dehydration process,the micro pore structure(pore diameter<1 nm)confined effect of zeolite membranes is used to realize the components separation.
Obviously membranes are the most important aspects for the separation process.In the past few decades,many efforts have been devoted to the preparation of high-quality zeolite membranes for solvent dehydration.In particular,NaA-type zeolite membranes show excellent dehydration performance for the mixtures of water and alcohols[6,7].However,the poor acid stability of NaA zeolite membrane which originated from the high aluminum content in the framework(Si/Al=1)limited its applications only in the neutral systems[8-10].Therefore,in the recent few years,several types of zeolite membranes with higher Si/Al ratio,such as FAU[11],T-type[12,13],MOR[14,15],ZSM-5[16],and CHA[17-20],have been prepared to improve the acid resistance for pervaporation dehydration process.These membranes can be applied in alcohols,esters,ethers,ketone,aromatic hydrocarbons and some other organic solvents for pervaporation dehydration.
Nowadays,NaAzeolite membrane has been industrialized by several commercial companies,such as Mitsui Engineering and Shipbuilding(Japan),Jiangsu Nine Heaven Co.Ltd.(China)and Inocermic GmbH(Germany)[5,21,22].Over 200 membrane plants for dehydration of industrial solvents were constructed in chemical and pharmaceutical industry.However,it is still a challenge for the rapid development of application market.There are three issues limiting the wide application of zeolite membranes:(i)quality control of large scale preparation;(ii)high fabrication cost of membrane equipment;and(iii)stability of membrane structure in complex application environment.In this review,we intend to give an introduction on mass transfer model,preparation method and applications of the prevalent zeolite membranes for pervaporation dehydration.It is expected to find out ways to address the key issues mentioned above.Finally,a brief perspective on the possible future direction of pervaporation dehydration zeolite membrane is presented.

Fig.1.Schematic diagram for pervaporation of hydrophilic zeolite membranes.
2.Mass Transfer Model
The mass transfer model of pervaporation membrane can be divided into empirical model,semi-empirical model and theoretical model.The solution-diffusion model proposed by Binning and Lee[23]and the pore flow model by Okadaet al.[24]for pervaporation process belong to semi-empirical models.The virtual phase transformation solution diffusion model proposed by Shieh and Huang[25]and the irreversible thermodynamic model put forward by Kedem[26]for characterization of the “coupling”effect in the pervaporation process are theoretical models.For now,transport mechanism applied in modeling permeation through pervaporation zeolite membranes is mainly described with the adsorption-diffusion model which is also a kind of theoretical model.The transport of the solvent species through the zeolite matrix comprises three steps:(i)selective adsorption of the species on the surface of zeolite membrane,(ii)diffusion inside the membrane of species dissolved on the surface driven by the gradient of chemical potential and(iii)vaporization of species on permeate side.Molecular simulation technology was used to study the adsorption and diffusion behaviors of the components through zeolite membranes.With the assistance of molecular simulation,the multicomponent separation can be better understood on a molecular level.The generalized Maxwell-Stefan(GMS)theory,traditionally applied for the description of gas permeation through MFI zeolite membranes,has been extended to provide a fundamental description for pervaporation dehydration through hydrophilic zeolite membranes.Krishna and co-workers[27-33]have extended this theory to describe multicomponent diffusion through zeolite membranes.
2.1.Adsorption simulation
Monte Carlo(MC)calculation refers to a calculation method of citing random numbers in every calculation which is one type of common simulation method used for simplifying the force field.Under a certain ensemble,the particles can randomly rotate,displace in the system or transfer between two positions.Combined with the given molecular potential energy function,the energy between particles could be superimposed.The Metropolis sampling method was used to generate a random con figuration of a series of microscopic particles in the system.Microscopic state of the particles is randomly selected from all possible particles,thus gradually approaching the equilibrium of the Boltzmann distribution.It is unable to get the real movement of particles because the particle displacement in MC simulation is virtual.Simulating the transmission properties of the system is difficult.In addition,it is necessary to selecta large number of samples in the process ofMC simulation.Otherwise it does not have statistical sense.Also the selected samples must be representative,which means a uniform distribution of the random number sequence is required.The Monte Carlo method is introduced into the probability model by mathematical method.Combined with the computer technology,the numerical simulation experiment is carried out.The Monte Carlo method can solve the problem with complicated factors simply compared with the general calculation method.
At present,the MC method has been widely applied into simulating the adsorption of guest molecules in zeolites(e.g.zeolite MFI,LTA,FAU,MTW),MOFs(e.g.IRMOF-1),COFs(e.g.COF-108),etc.These guest molecules include CH4,CO2,N2,H2,CO,SO2,O2,He,Ar and BTEX[31,34-35].The reliability of adsorption results has been widely recognized.For now,the grand canonical ensemble Monte Carlo(GCMC)method has received extensive attention from many researchers.This method,as an important MC simulation method,can be used to simulate the adsorption process.Because the temperatureT,volumeV,and chemical potential energy μ of the ensemble are a large portfolio of system for the set value,the simulation environment is close to the experimental conditions which could compare the simulation results with the experimental results easily.By optimizing the potential parameters with a quantitative method,Rutkai and Csanyiet al.[36,37]developed new models and investigated the adsorption behavior of alcohol/water vapor through NaA zeolite membranes using the GCMC method.Yang and Wuet al.[38,39]adopted the GCMC method to study the adsorption behavior of ethanol/water mixture in MFI and NaA zeolite.Other researchers[40,41]used the GCMC method to simulate the adsorption behavior of water in silicalite,DDR,NaY,NaX and ZSM-5 zeolites,which were consistent with the experimental values in equilibrium data of heats of adsorption,the adsorption capacity and adsorption isotherm.Competitive adsorption was found for the water/ethanol mixture adsorbed in NaA zeolite as shown in Fig.2[42].
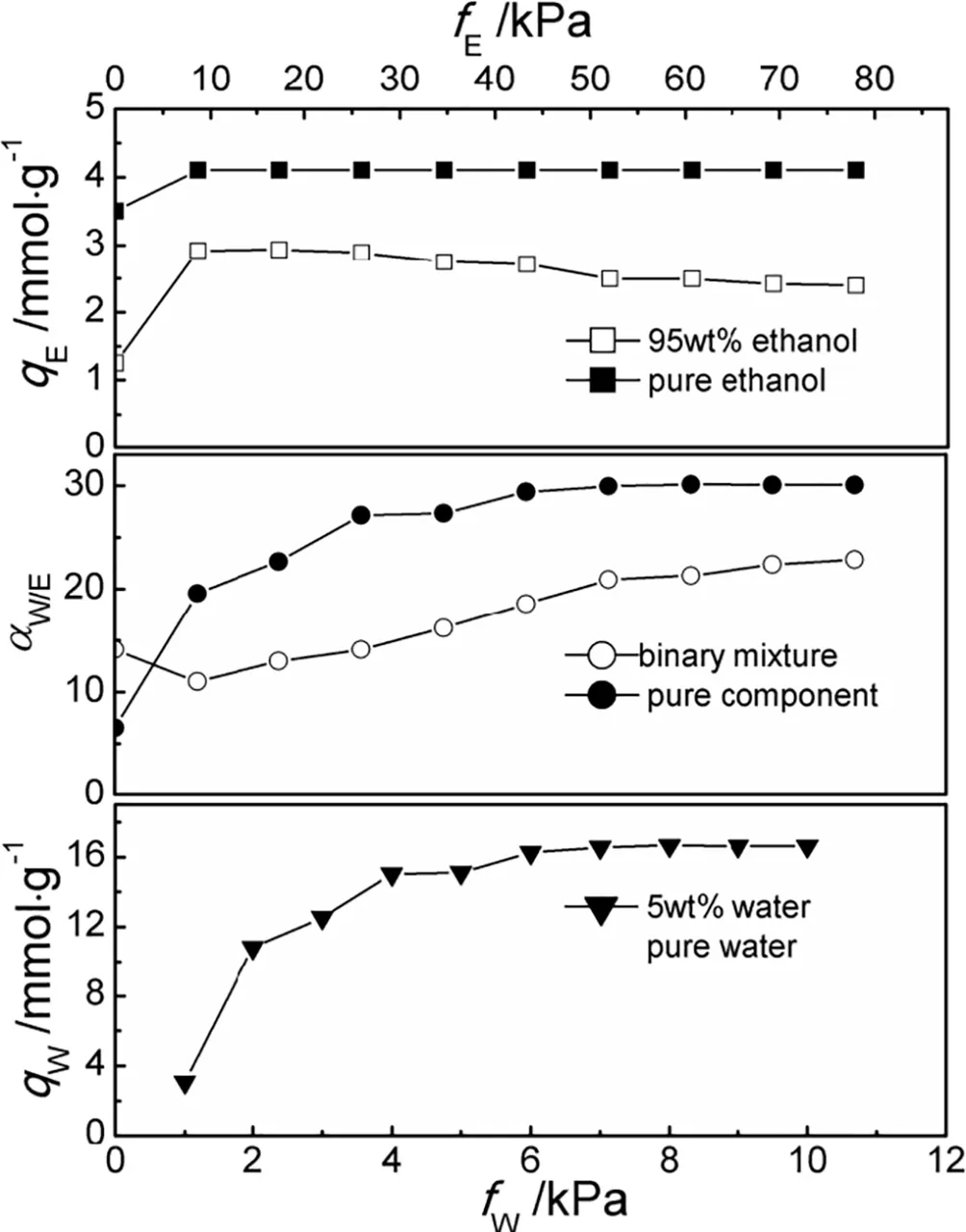
Fig.2.Comparison of the adsorption isotherms and selectivities of the ethanol/water binary mixture at a feed ethanol concentration of 95 wt%and their pure components in NaA zeolite at 348 K[42].
The adsorption isotherm is the most important adsorption curve for describing the characteristics of the adsorption process.The types of adsorption isotherms vary with different kinds of adsorption systems.Different adsorption theory and adsorption physical models were used to describe and explain the molecular adsorption behavior in the system.As the researchers continue to get insight into the adsorption process,many more accurate and complex adsorption models are derived.Gorbach and others[43]researched the adsorption process of water vapor through NaA molecular sieve and reviewed eighteen kinds of commonly used adsorption models.The Langmuir model is a monolayer adsorption theory of the molecular adsorption of gas molecules on the surface of solid derived from the kinetics.Loughlin[44]deduced several other adsorption theory and model according to the adsorption properties of water through NaA zeolite.They used dual-site Langmuir adsorption model to fit adsorption isotherms of water and obtained good results.If the adsorbing species have the same molarsaturation loadingqM,i,the multicomponent adsorption behavior can be described by the extended Langmuir equation:

wherePiis the fugacity of componentiandKiis the adsorption constant of componenti.
2.2.Diffusion simulation
Molecular dynamics(MD)simulation is used to calculate the real trajectory of particle motion,which can simulate transmission properties.The equilibrium properties and various dynamical properties of different system could be calculated,in which it is closely related to the applicability of the force field,the calculation speed,the correctness of the calculation method and the rationality of the initial structure.There are four kinds of common methods mainly used to solve Newtonian motion equations in the process of MD calculation including Verlet algorithm,leap frog method,Beeman method and Gear predicted-correction method.Small molecular diffusion in porous materials mainly adopts the Equilibrium Molecular Dynamics(EMD)method which can accurately estimate the transport properties of single molecules.It can also roughly estimate the mixture properties with low estimation accuracy of the mixture diffusion coefficient.While the Non-Equilibrium Molecular Dynamics(NEMD)method can make up for the deficiencies of the EMD method in the aspect of estimating external force field so as to obtain the non-equilibrium statistical characteristics and the transfer characteristics of components.The diffusion process is more accurate,but the calculation is relatively complex which need further improvement.
The MD method is mainly used to simulate the diffusion behavior and it is also the common method for studying the diffusion of organic solvents/water in zeolites.Krishnaet al.[45-47]researched the diffusion behavior of gas molecules through MFI,FVU,DDR and LTA zeolite using MD and KMC simulation method to investigate the effect of number of crystal molecules,temperature and molecular entropy on the activation energy and the diffusion coefficient.Their research laid the foundation for the study of molecular diffusion process through zeolites and diffusion mechanism which also provided theoretical basis for the diffusion of organic solvent/water mixtures through zeolites.
At present,most of researchers are focused on the investigation of the adsorption and diffusion of molecules in zeolites using molecular simulation techniques.However,it is also very important to study the permeability of zeolite membranes based on the molecular simulation technique,which is helpful for understanding the membrane performance and optimizing the operation parameters.Yanget al.[38]combined the adsorption and diffusion simulation with the permeation theory to probe the permeation performance of pure water and ethanol through silicalite membranes at room temperature.The permeation performance on all-silica DD3R membranes[40]was also investigated by the use of molecular simulations and the Maxwell-Stefan theory.Guoet al.[42]predicted permeation fluxes of pure water and ethanol through a NaA zeolite membrane based on the simulated data of adsorption and diffusion using the GCMC and Equilibrium Molecular Dynamics(EMD)methods.Yanget al.[48]simulated diffusion behavior of ethanol/water and methanol/water in MFI zeolite using NVY-EMD to investigate the influence of molecular interaction on diffusion coefficient and revealed the factors that affect the component diffusion coefficient in zeolites.Csanyiet al.[37]studied diffusion behavior of ethanol/methanol mixture in NaA zeolite with molecular simulation method.The effects of atomic charges in zeolite model on molecular diffusion were further investigated and the simulated diffusion coefficients were compared with the obtained experimental values.The position of Na+cation in the zeolitic framework has a significant influence on the diffusion process.The self-diffusivities of the molecules can be calculated by the Einstein equation:
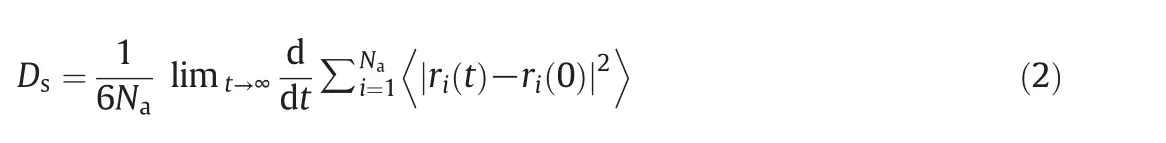
where Nais the number of diffusing molecules,and 〈|ri(t)-ri(0)|2〉is the average of the mean squared displacement(MSD)of the diffusing molecules.Jia and Murad[49]compared the pervaporation performances of silicalite,NaA and chabazite zeolite membranes with a molecular dynamics(MD)simulation.Important factors were studied,including the molecular density distribution and idealseparation factor.Wuet al.[39]used the GCMC and MD methods to investigate the adsorption and diffusion properties of water/methanol and water/ethanol mixtures in NaA zeolite at 298 K.Special attention was paid to the effects of electrostatic interactions and a vibrating framework on the diffusion.
2.3.Mass transfer simulation of pervaporation
For now,the high pervaporation flux is mainly attributed to the zeolite membrane layer.Several literatures[50-52]have been reported about the influence of zeolite membrane layer thickness on the permeability of the membrane without considering the effectofsupport layer.The lack of overall research on the pervaporation zeolite membrane lies in focusing on the influence of the confinement structure of single zeolite layer.The asymmetric structure and morphology of the ceramic porous hollow fiber had a great influence on the overall permeability and separation selectivity of zeolite membranes in the actual process[53-56].Farooq and Karimi[53]proposed a simple pseudo-binary diffusion model for the gas transport in the support.Proposed models and procedures successfully predicted the published experimental results for a binary system.Simulation results suggested that the permeate flux would increase significantly at low support resistances.de Bruijnet al.[55]established a simple dual-layer(membrane/substrate)pressure drop modelto evaluate the effect of the support on transport resistance.Based on a variety of experimental data from published reports,they calculated the interfacial pressure between the membrane and the support layer to reveal the resistance contribution of the support layer to the overall resistance.Shaoet al.[56]reported the effect of the porosity of hollow fiber support on the pervaporation performance of NaA zeolite membrane.Although it is recognized that the support layer has effect on the permeability,the theoretical research on the mechanism and the separation process of the support layer is still scary.
The mass transfer mechanism of mixed gas through porous materials is mainly described by the Dusty Gas Model(DGM)[57-58].The parameters of the model are related to the porous structure and the interaction between the gas and the support,and do not vary with the change of temperature and pressure.In the DGM,the porous structure of support is considered to be a fixed array of dust particles in space.The DGM consists of mainly three kinds of diffusion mechanism:Knudsen diffusion,viscous flow diffusion and mixed diffusion.The study of mass transfer mechanism of supports and establishment of mass transfer model are mostly based on the DGM model.Beuscher and Gooding[58]investigated the mass transfer mechanism of trichloroethylene through flat sheet and hollow fiber membrane supports with DGM.The components were mainly controlled by Knudsen diffusion and mixed diffusion.The DGM shows an excellent fit to experimental data when the asymmetric structure of the membrane supports is taken into account.Zah and Sato[59-60]revealed that the pervaporation process through tubular ceramic support is described by the Knudsen diffusion.Wu and Garcia[61-62]calculated the mean free path of gas through sponge-like layer and finger-like layer of ceramic porous hollow fiber support and concluded that the Knudsen diffusion occurs mainly in the sponge-like layer,and the mass transfer resistance of the finger-like layer can be ignored.
Towards the mass transfer through the hollow fiber NaA zeolite membrane,Yeet al.[63]developed a multi-layer series-resistance(MLSR)model to describe pervaporation dehydration of ethanol through hollow fiber NaA zeolite membranes.The membrane was divided into three transfer-resistance regions in series as shown in Fig.3:(i)NaA zeolite layer;(ii)sponge-like layer of the support;and(iii) finger-like layer of the support.The sponge-like region of hollow fiber support made a significant contribution to overall membrane transfer resistance while the finger-like layer had less effect.
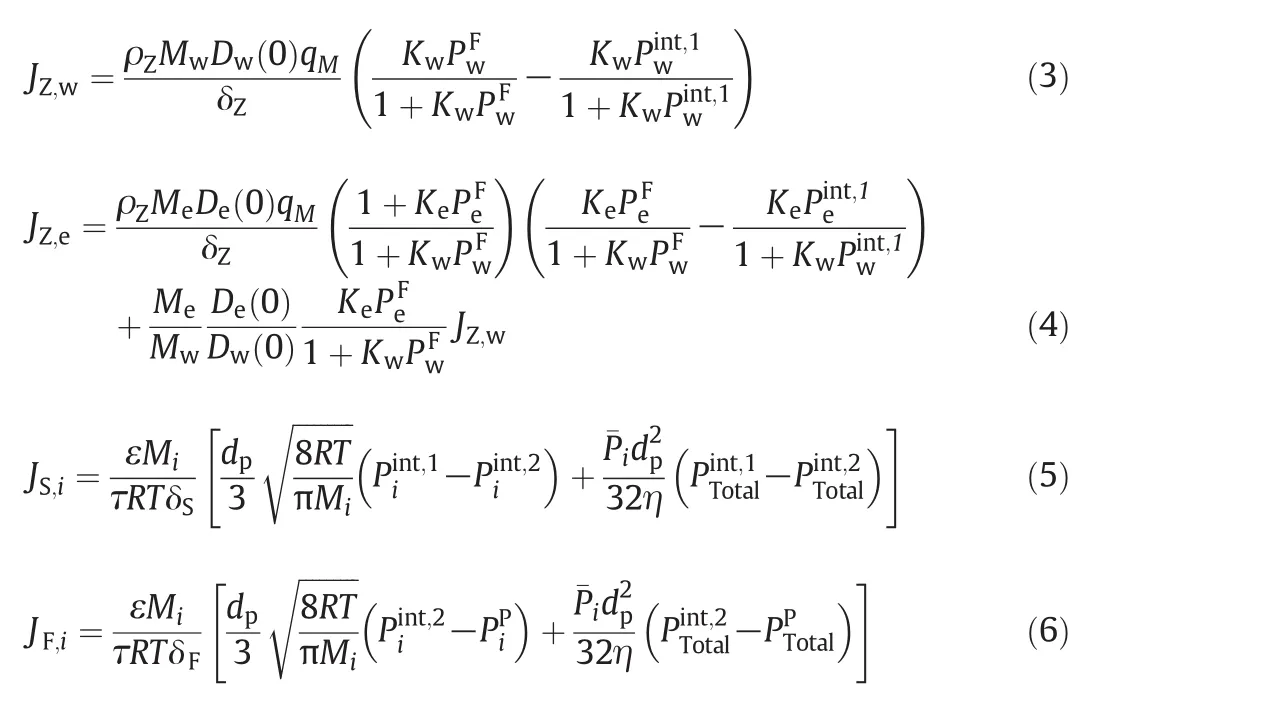
The mass transfer through zeolite layer was described by the Maxwell-Stefan mechanism based on adsorption and diffusion parameters obtained from molecular simulation.Eqs.(3)and(4)were used to predict permeation flux of component water and ethanol(JZ,wandJZ,e)through the zeolite membrane respectively.Based on both Knudsen diffusion and viscous flow,the permeation fluxes of componentithrough the sponge-like region and the finger-like region were described by Eqs.(5)and(6),respectively.The steady-state mass flow rate through the different regions should be equal,which follows the formula:


Fig.3.Schematic diagram of MLSR model through the hollow fiber NaAzeolite membrane[63].
The water permeation flux could be calculated well by the MLSR model.The sponge-like layer of support made an important transfer resistance contribution to the overall membrane resistance except for zeolite membrane layer while the finger-like layer showed low transfer resistance.The thickness of sponge-like layer could be reduced to improve the water flux while the thickness of finger-like layer could be increased modestly to improve the mechanical strength for performance optimization of hollow fiber NaA zeolite membrane.
3.Preparation of Hydrophilic Zeolite Membranes
Zeolite membranes for pervaporation can be prepared by several methods,but the most common ones are the in situ crystallization and secondary growth methods[64],although dry gel methods[5]have also been used.Hydrothermal synthesis involves crystallization of a zeolite layer onto a porous support from a gel that is usually composed of water,amorphous silica,a source for tetrahedral framework atoms other than Si,a structure directing organic template,and sometimes a mineralizing agent,such as NaOH[22].
For the in situ crystallization method,the support surface is brought in contact with a zeolite synthesis solution containing zeolite precursors and,in most cases,an organic structure directing agent(template)at controlled pH.The process should be controlled so that zeolite nuclei are formed on the support surface,rather than in the synthesis solution.This requires creation of local supersaturation on the support surface by various physical means.Support surface functionalization using an organic linker was also reported to facilitate nucleation on the surface[65].The nuclei formed on the support surface are then grown into a continuous zeolite film.The advantage of the in situ method is that the zeolite film growth is completed in one step,but it is difficult to identify conditions of local supersaturation to ensure high reproducibility of zeolite membrane synthesis by this method.Furthermore,it is very difficult to form a dense and continuous zeolite layer in one synthesis time.Therefore,in most cases,more than 2 times of crystallization is necessary,which might lead to the increase of membrane thickness and total crystallization time and thus inhibit the formation of high flux zeolite membranes[22].
In the secondary growth method,a zeolite seed layer is coated from a suspension containing zeolite crystals onto the support.The support with a zeolite seed layeris then broughtin contactwith zeolite synthesis solution and grows into a continuous zeolite film.The secondary growth method exhibits advantages such as better control over membrane microstructure(e.g.thickness,orientation)and higher reproducibility.Therefore,the preparation of zeolite films using secondary growth of precursor particle layers has attracted considerable interestas a possible route to zeolite membrane synthesis[66].
The majority of the zeolite membranes prepared are supported,due to their greater structural stability.The most frequently used supports are alumina and stainless steel tubes or discs.Since the average pore sizes of these supports are quite different(mainly from 5 nm to 10 μm),the seeding process is very important.For instance,Gu and his coworkers[67]found it difficult to cover seeds on mullite tubes with dents and pinholes by the dip-coating method,while rubbing treatment with seed paste resulted in non-uniform coverage on flat area.Therefore,a combined method was used so as to produce highquality membranes with high reproducibility.For better coverage of supports,a ball-milled seeding process was developed[20,68].Not only the pervaporation performance of the membranes was improved,but the crystallization time was shortened as well.Furthermore,it is very useful in controlling the formation of impurity phase and thus improved the stability of zeolite membranes[20].To further improve the reproducibility of zeolite membrane synthesis,some colloidal SiO2was added into seed suspension before coating the supports.In this way,zeolite T membranes with high flux and reproducibility could be achieved[69].
For preparation of high-quality zeolite membranes on coarse macroporous α-Al2O3supports,a varying temperature hot dip coating method was developed by Wang's group[70].This method could effectively avoid the penetration of small zeolite seeds from entering into the pores of support and thus high flux zeolite membranes were available.This seeding method is reported to allow for reproducible membrane manufacturing of relatively thick films on coarse supports.Based on the varying temperature hot dip coating method,several types of zeolite membranes,such as NaA[71],T[72],MOR[73],and ZSM-5[70],have been prepared.
In recent years,hollow fiber zeolite membranes have drawn many attentions due to high permeation flux as well as high packing density.Hollow fiber(HF)supports are too slender and brittle for rubbing powdered seed crystals directly onto them by hand.Furthermore,it is very difficult to seeding the support due to low capillary force.Therefore,a composited dipcoating-wiping seed method was developed by Wanget al.[7],and a very high flux of 9.0 kg·m-2·h-1with a separation factor of>10000 was achieved with NaA zeolite membranes.Chenet al.[14]also developed a rubbing method for preparation of T-type zeolite membranes on yttria-stabilized zirconia(YSZ)hollow fibers.Although high performance zeolite T-type zeolite membranes could be achieved by this method,it might be not be suitable for industrial production of zeolite membranes.Therefore,a vacuum seeding method was used for preparation of high performance T-type zeolite membranes.Finally,a water permeation flux of 7.36 kg·m-2·h-1with a separation factor of>10000 was obtained for dehydration of 90 wt%isopropanol solution at 75°C[14].The membrane synthesisviathe seeding approach of vacuum-coating could produce hollow fiber T-type zeolite membranes in batch scale.Moreover,hollow fiber membrane modules withca.0.1 m2membrane area were fabricated and assembled for propanal dehydration to evaluate the technical feasibility.Based on this method,high performance NaA zeolite membranes have been prepared in large-scale and zeolite membrane module with different membrane areas has been fabricated for solvent dehydration[74].Although single-channel hollow fiber supported zeolite membranes showed higher packing density and permeation flux,too low mechanical strength of the supports significantly hindered its wide research and industrial applications.Recently,multi-channel(3,4 or 7 channels)alumina hollow fibers(Fig.4(a))were fabricated in Gu's lab[75,76]and Li's lab[77],which not only showed high permeability but also high mechanical strength.The successful preparation of the high-performance multi-channel hollow fibers made it possible for wide research and application of hollow fiber zeolite membranes.Due to the high capillary force of four-channel supports,very high flux NaA zeolite membranes(the flux was 12.8 kg·m-2·h-1and the separation factor >10000)could be prepared only by the dip-coating method[6],which has been demonstrated to be the most efficient approach for zeolite membrane synthesis in large-scale.In addition,high-quality SAPO-34 membranes supported on four-channel hollow fibers were also prepared by a similar seeding approach by Chenet al.[78].
Although the above seeding methods are very effective in the preparation of zeolite membranes with high separation performances,most of those as-synthesized membranes are randomly-orientated and have thicker membrane layers.Therefore,those membranes usually showed relatively lower permeation fluxes.Tsapatsis[79]suggested that only a 10-fold increase in flux compared to the current state of the art might put forward zeolite membranes for wide applications.Therefore,a self-assembly of orientated zeolite layer is often used to prepare orientated zeolite membranes.Manual and self-assembly of seed monolayers was studied extensively by Yoon and co-workers and it has been reviewed in 2007[80].According to one version of this technique,zeolite seeds are manually rubbed on a substrate.Either ionic bonding or hydrogen bonding was found to be responsible for the assembly.A direct covalent bonding between the support and the seed crystals can be established by using water-soluble bi-dentate additives like diisocyanates as proposed by Yoon[81].Although orientated zeolite membranes with thinner thickness could be prepared in this approach,an organic structure directing agent is often necessary.Therefore,the fabrication cost of zeolite membranes is much higher.So far,very few pervaporation membranes manufactured in this approach were reported until last year[82].
Except for the seeding method affected the preparation of high performance zeolite membranes,the heating approach or synthesis conditions also had important effects on membrane crystallization.In recent years,microwave synthesis was adopted to the synthesis of zeolite membranes.Conventional heating has a heat source on the outside and relies on transferring the heat to the surface of the material and then conducting the heat to the middle of the material.Compared with conventional heating,microwave dielectric heating has the following advantages for chemical synthesis:(i)the introduction of microwave energy into a chemical reaction can lead to much higher heating rates than those which are achieved conventionally;(ii)the microwave energy is introduced into the chemical reactor remotely without direct contact between the energy source and the reacting chemicals;(iii)it is volumetric and instantaneous(or rapid)heating with no wall or heat diffusion effects;(iv)it can realize selective heating because chemicals and the containment materials for chemical reactions do not interact equally with microwaves;and(v)“hot spots”yielded on local boundaries by reflections and refractions may result in a “super-heating”effect,which can be described best as local overheating and is comparable to the delayed boiling of overheated liquids under conventional conditions[83].Therefore,the crystallization duration is often much shorter and the thickness of zeolite membranes is much thinner.Till now,LTA[84],MFI[85],AFI[86],FAU[87],SOD[88],CHA[89]and ETS-4[90]types of zeolite membranes have been successfully synthesized by microwave heating.Forinstance,Huet al.successfully synthesized CHA-type zeolite membranes on stainless steel supports by microwave synthesis,which exhibited water fluxes of 7.3 and 9.1 kg·m-2·h-1and separation factors of 2000 and 2500 for 90 wt%ethanoland isopropanol aqueous solutions at348 K,respectively[89].These fluxes were twice as high as those of the chabazite membranes prepared by conventional heating due to the thinner zeolite layers and lower resistance of the support layer in the microwave heating system.
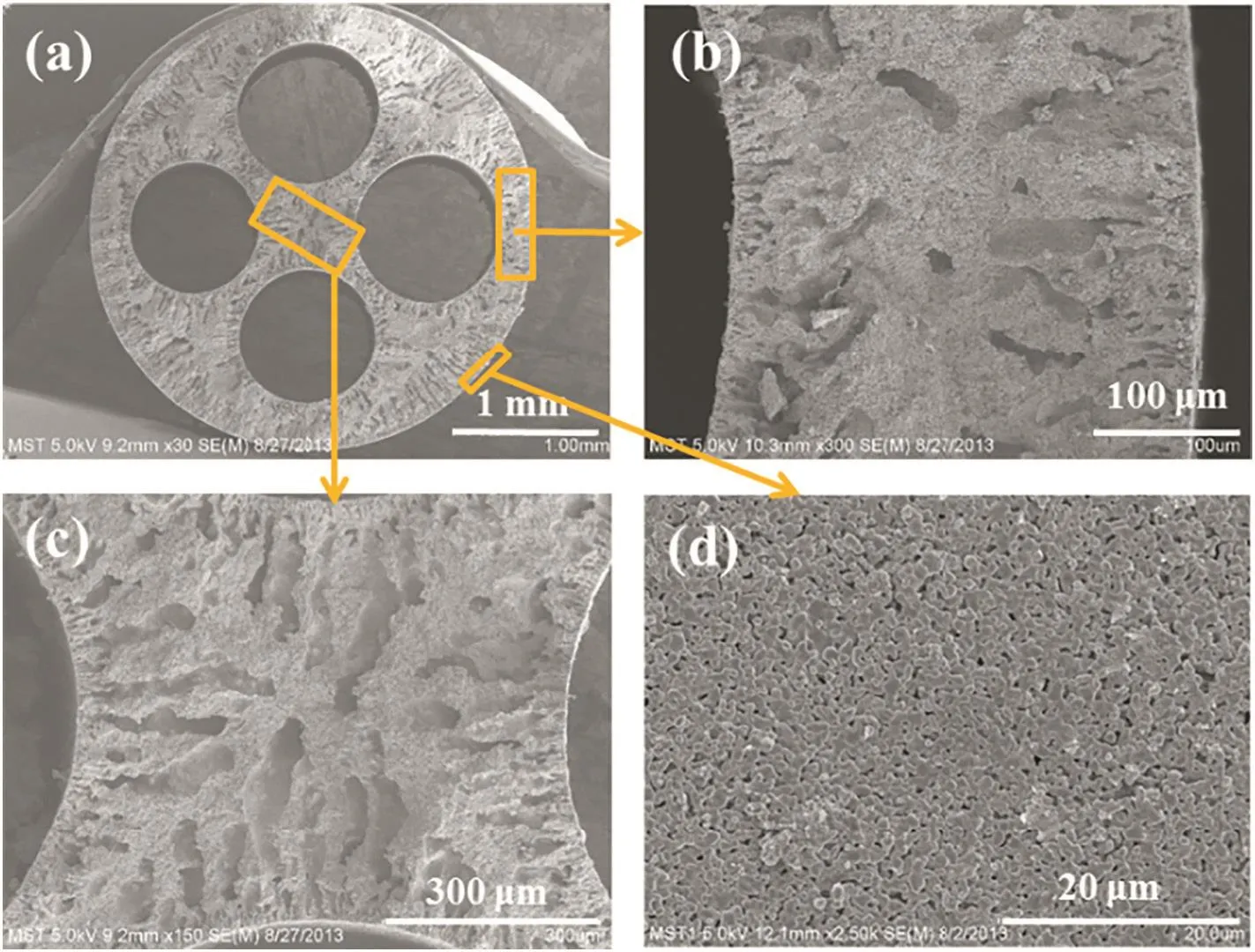
Fig.4.SEM images of the cross-sections(a-c)and the outer surface(d)of four-channel α-Al2O3 hollow fiber for pervaporation[6].
4.Stabilities of Hydrophilic Zeolite Membranes
With a well-de fined pore size of~0.42 nmand strong hydrophilicity,NaA zeolite membrane exhibits high permeation flux and excellent separation selectivity in pervaporation dehydration of organic solvents.The stability of NaA zeolite membrane is a big issue concerning the practical pervaporation dehydration application.pH values,inorganic salts,as well as water content in the feed were found to have a significant effect on the stability and separation performance of NaA zeolite membrane.
The industrial reactants or solvents needed for separation usually contain acetic acid,sulfuric and/or formic acid which are frequently used in many industrial processes.It has been comprehensively documented that the acid stability of zeolites depends strongly on the Si/Al ratio of the zeolite framework.In general,with an increase in Al content in the framework,the hydrophilicity of a zeolite increases,whereas the acid resistance simultaneously decreases as a strong acid would leach Al from the zeolite to decompose its framework structure[9].NaA zeolite membrane exhibits low stability in acid solution due to the low Si/Al ratio of 1 for the framework.
Hasegawaet al.[9]adopted a mass spectrometer to monitor the influence of acid on the permeation performance of NaA zeolite membranes in real-time,and the water permeation flux was observed first declined and then increased dramatically.The separation selectivity is finally lost due to the damage of the membrane structure caused by the dealumination in acid environment.Yuet al.[91]also found similar results when they studied the separation performance of NaA zeolite membranes after long-term(100 day)immersion in organic solvents with different pH values at room temperature.The PV performance of the membrane was significantly damaged after the treatment in the low pH solution(acidic condition).Both water flux and permeate water content were unchanged for the membranes immersed in pH~7-9.Meanwhile zeolite crystals were well-preserved without any defects found on the membrane surfaces.
Inorganic salts,which are usually contained in these organic solvents from fine chemical and pharmaceutical chemical production,were found to have influence on the separation performance of NaA zeolite membranes.Yanget al.[92]investigate the effects of salt concentration,cations,and anions on the NaA membrane pervaporation performance with a model system of ethanol/H2O/salt mixture by choosing five kinds of salts NaCl,NaNO3,KCl,MgCl2,and CaCl2.Their work demonstrates that inorganic salts in the alcohol/H2O mixture decreased the flux or separation selectivity.The water flux decreased greatly with increasing salt concentrations due to the reduced water driving force/activity and negative salt physical blocking on the outer membrane surface.Potassium ion led to the largest flux drop among all these abovementioned cations and the water flux can be recovered to different percentages of the original level with different salts system after flushing regeneration.These complex flux behaviors could be mainly attributed to the ion exchange and ion blocking effects.
Water content in the feed was also found to have a significant effect on hydrothermal stability of NaA zeolite membrane,which mightbe related to the hydrolysis of molecular sieve in higher water content.Liet al.[93]investigated the effects of water content and operation temperature on NaA zeolite membrane for pervaporation dehydration.They noticed that the separation factor decreased quickly while the water flux increased rapidly with the increase of water content.It was indicated that the hydrolysis of amorphous substance in intergranular could be accelerated in high water content system.Thus,some cracks and pinhole defects occurred and led to the decrease of separation performance.Yuet al.[94]also suggested that a relatively low operation temperature and water content(<20 wt%)were beneficial to industrial applications of NaA zeolite membranes.
In order to further understand the membrane stability in harsh conditions,electrochemical impedance spectroscopy(EIS)characterization in combination with pervaporation dehydration performance was adopted to monitor the micro-structure evolution of NaA zeolite membranes in acidic water/ethanol solution by Caiet al.[95]recently.Diskshaped NaA zeolite membranes were immersed in 10 wt%water/ethanol solution with pH=3 for different durations between 0 and 107 h,and the Nyquist plot and impedance modulus curve of the immersed samples were collected in Fig.5.The results showed that the electrochemical impedance of the membranes decreased at the initial contact between the membranes and acid solution,and then a rapidly increasing trend occurred before a monotonically decreasing in the end.The fitted results were in good agreement with the PV results,which made EIS characterization a promising technique to monitor and analyze the microstructure evolution of zeolite membranes in practical applications.
In the last few decades,many efforts have been made to develop novel zeolite membranes with higher Si/Al ratio(e.g.CHA,FAU,T,MER,MOR,ZSM-5)to improve the acid stability.Among those high acid-resistant membranes,zeolite T,CHA,MOR and ZSM membranes were widely studied,which should be related to the high water permeation flux orits superiority in organic acid dehydration.Tanakaet al.[97]reported a high acid-resistance T-type zeolite membrane,which showed relatively stable performance after immersing in 50 wt%acetic acid solution at room temperature for 36 days.Besides,the water permeation flux and separation factor of the T-type membrane were still as high as 0.42 kg·m-2·h-1and 320 after long term dehydration of acetic acid solution for7 days.Recently,a hollow fiber zeolite T-type membrane module with a membrane area of0.1 m2and packing density of360 m2·m-3has been fabricated by Wanget al.[12],which showed stable dehydration performance in industrial propanal/water mixture for over 60 h.CHA zeolite membrane with Si/Al ratios of 2-∞and a pore diameter of 0.38 nm has been proven to exhibit not only high permeation flux but also high acid resistance in acidic solvent dehydration[98].Jianget al.[99]reported a CHA zeolite membrane prepared in clear solution,which showed a high water permeation flux of 13.3 kg·m-2·h-1with a separation factor ofca.6000.Besides,the as-synthesized membrane was stable in pH~3 for over 550 h.Satoet al.[100]also reported the synthesis of high-silica CHA zeolite membranes and membrane module,which has been proven to be of high hydrothermal stability in 50 wt%N-Methyl pyrrolidone(NMP)solution dehydration for over 6 months in practical application.Yamanakaet al.[101]also reported the synthesis of high-silica CHA zeolite membranes,which showed a high water permeation flux of 7.9 kg·m-2·h-1with a separation factor ofca.2500 in 50 wt%acetic acid solution for 7 days.Chenet al.[102]reported a high acid-resistance MOR zeolite membrane,which showed long-term stable PV performance in 83 wt%H2O/AcOH mixture dehydration at 80°C,although water permeation flux of the membrane was onlyca.0.27 kg·m-2·h-1.Zhuet al.[103]reported a high-silica ZSM-5 membrane,which has been proven to be stable in 90 wt%HAc/H2O mixture dehydration at 75°C for 60 days.All these results indicated that improving the Si/Al ratio in the framework would be helpful for improving the stability of zeolite membranes.
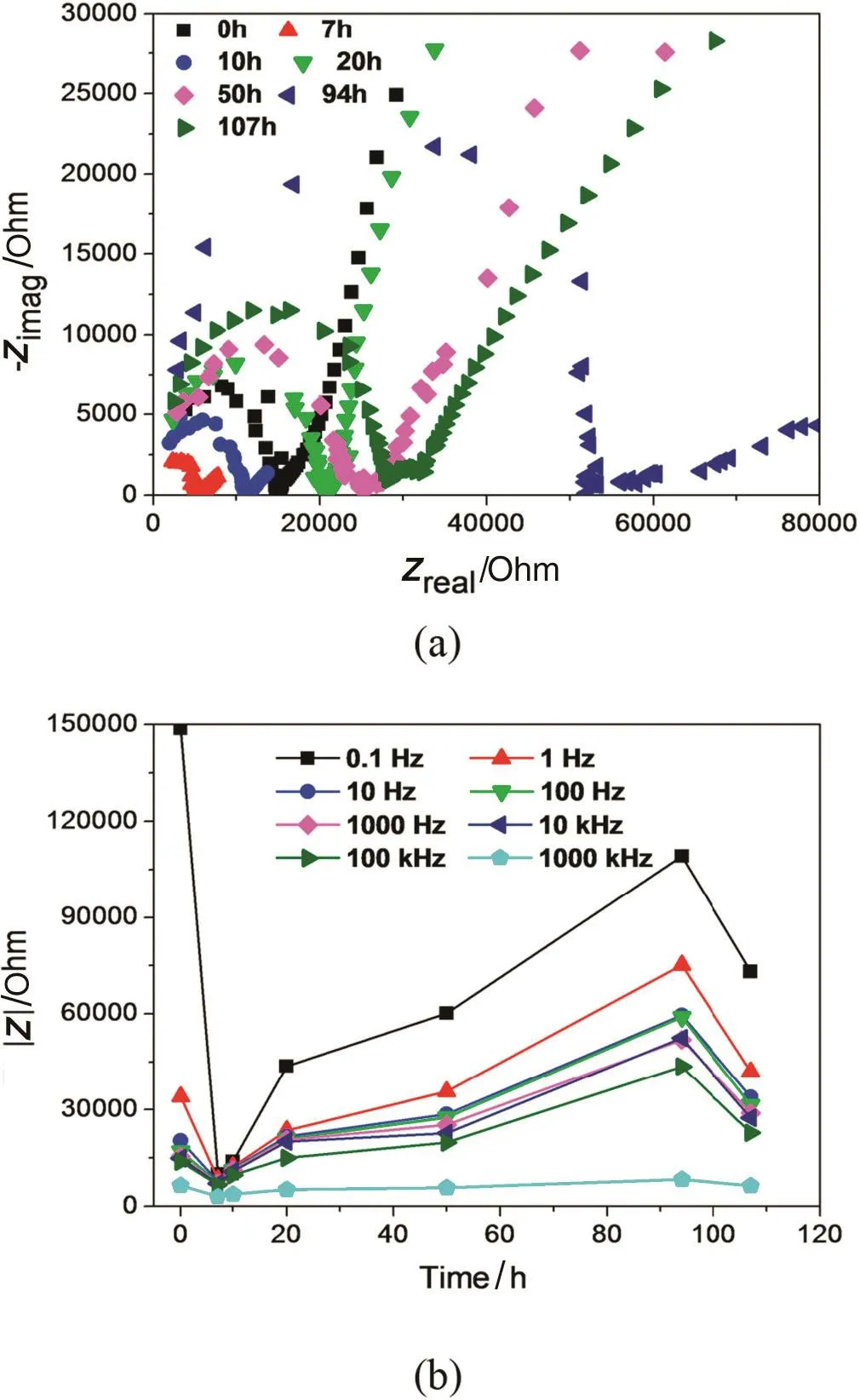
Fig.5.Time dependence of impedance for NaA zeolite membranes in 10 wt%water/ethanol solution at pH=3.(a)Nyquist plot,(b)impedance modulus curve[95].
Besides the influence of the solvent properties,contraction/expansion of the zeolite crystals is another important effect on the membrane separation performance due to the intercrystalline defects.Falconeret al.[104]found that NaA zeolite crystal unit cell expanded or contracted after the adsorption of water,methanol,etc.Quet al.[105]investigated the effect of adsorption-induced changes in zeolite crystal size on the separation performance of NaA zeolite membrane for dehydration of alcohols using both experimental measurements and molecular simulations.Their study shows that the alcohol fluxes were almost independent of the feed water concentration in the case of water/methanol and water/ethanol mixtures,nevertheless water/IPA showed a dramatic decrease in selectivity due to an increase of IPA flux as the feed water concentration decreased.It was suggested that the presence of methanol or ethanol in the water/isopropanol mixtures with low water contentcan improve the dehydration performance of NaA zeolite membrane because both methanol and ethanol can enter the zeolite crystal to reduce the crystal contraction effects caused by low water loading and block the defects by adsorbing at the defect sites.
5.Applications of Zeolite Membranes for Pervaporation Dehydration
The applications of pervaporation membranes include the dehydration of organic solvents,recycle of organics in water and separation of organic compounds.Early pervaporation technology research and application is dehydration of organic solvents,and it is still a hot topic now.Suitable pervaporation solvent systems include organic/water azeotropic or near-boiling mixtures,such as alcohols,ketones,esters,and amines.Generally speaking,pervaporation separation is an easy and cost-effective route for the dehydration of organic solvents with a water content between 0.1wt%and 20 wt%.Another advantage of pervaporation dehydration technology is the universality of its equipment,because the same pervaporation system can be used for the dehydration of various organic solvents.For example,the GFT Company designed a pervaporation dehydration plant with the total membrane area being 120 m2,and it can be used for ethanol and tetrahydrofuran dehydration[106].
5.1.NaA zeolite membrane
NaA zeolite membranes have attracted much attention because of its excellent performance for pervaporation.Since the early 1990s,researchers have devoted to the preparation and applications of NaA zeolite membranes.Currently,there are many industrial applications in pervaporation dehydration of NaA zeolite membranes.In 1999,Mitsui Shipbuilding Company in Japan first developed NaA zeolite membrane products in collaboration with professor Kita[21].The membranes were successfully constructed to the first industrial plant for dehydration of ethanol.Subsequently,Mitsui has established more than 60 plants of NaA zeolite membranes,widely used in chemical,pharmaceutical,food,microelectronics and other fields[3,107-109].From 2002,Inocermic Company in Germany has also successfully developed a high-performance four-channel NaA zeolite membrane product.Using the membranes,GFT Company developed the process of ethanol dehydration and established a couple of industrial plants.Gu and his co-workers in Nanjing Tech University made a lot of work for the zeolite membrane industrialization in China.Their first industrial apparatus was built up in 2009[91],which was used for dehydration of isopropanol from a pharmaceutical company.Since 2011,Jiangsu Nine Heaven High-Tech Co.,Ltd.has built a 20000 m2·a-1NaA zeolite membrane scale production line,specializing in the production and application of zeolite membrane.Until now,more than 100 sets of pervaporation plants have been successfully designed and put into use for dehydration of industrial solvents from chemical and pharmaceutical industry in China.The separation systems concern methanol,ethanol,isopropanol,acetonitrile,tetrahydrofuran,MTBE,etc.
Hoofet al.[110]compared the dehydration performance of four commercially available organic membranes of Mitsui NaA zeolite membrane with those supplied by Sulzer and Celfa of Sweden,and the dehydration experiments of isopropanol,acetonitrile and methyl ethyl ketone were carried out at 70°C.The results showed that when the water content was low,NaA zeolite membrane had better separation performance than other organic membranes,indicating that NaA zeolite membrane had more advantages in dehydration and purification of high purity organic solvents.Sommer and Melin[111]studied the effect of operating parameters on the separation performance of NaA zeolite membranes.It was found that the permeate flux of membrane increased linearly with the increase of water content in the feed and the increase of vacuum in the permeate side,and it increases exponentially with temperature.Because the NaA zeolite membrane has good thermal stability,it can be used in the dehydration process at higher temperature and obtain higher permeation flux.The permeation flux of the 90 wt%ethanol/water mixture was as high as 37 kg·m-2·h-1at 145 °C using NaA zeolite membrane,while the separation factor remained at a high level[112].Satoet al.[113]used distillation coupled with pervaporation to treat sugarcane fermentation liquor to produce absolute ethanol.The top product of the distillation column was 88 wt%ethanol/water mixed steam with temperature and vapor pressure of 130°C and 550 kPa respectively.The product with ethanol content greater than 99.6 wt%can be obtained by steam permeation dehydration of the two-stage NaA zeolite membrane module.For most organic membranes,due to their poor thermal stability and mechanical strength,the operating conditions are generally at atmospheric pressure and below 100°C,limiting their permeation flux enhancement.Sommer and Melin[114]used a commercial NaA zeolite membrane to dehydrate various organic solvents.The separation results are shown in Table 1.The NaA zeolite membrane exhibited high separation selectivity and high permeation flux.
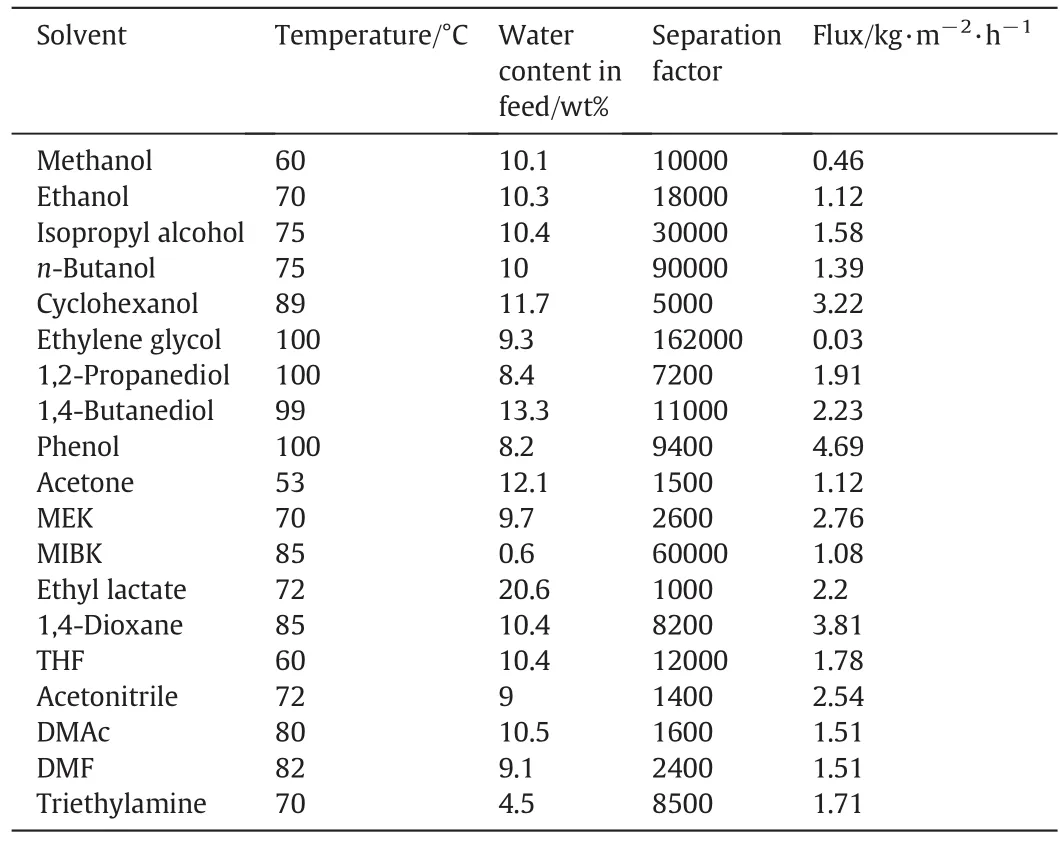
Table 1Separation performance of NaA zeolite membrane from Mitsui for dehydration of various organic solvents[114]
In order to fulfill the pervaporation demand in organic solvent dehydration,the pretreatment steps such as pH adjustment and rectification could be employed to effectively adjust properties of raw liquids,and the stability of NaA membrane was significantly improved,which paved a way for large-scale industrial applications of the dehydration technology[112].For instance,in the production of cephalosporin,the isopropyl alcohol needs to recycle after dehydration.In conventional process,the caustic alkali dehydration was employed,which requires several steps including initial distillation,rectification,alkali dehydration,second distillation and pH adjustment.The membrane dehydration technique is much more convenient as compared with alkali dehydration.Both separation processes are presented in Fig.6.A 5000 tons·year-1membrane separation facility was successfully built up by Jiangsu Nine Heaven High-Tech Co.Ltd.for dehydration of isopropanol.The water content in isopropanol solution was dehydrated from 17 wt%to less 2 wt%,as required by the customer.The solvent dehydration industrial plant achieved a long-term stable operation for more than 3 years.
Fig.7 shows the high-throughput industrial apparatus from Jiangsu Nine Heaven High-Tech Co.,Ltd.for dehydration of alcohol mixture(34400 t·a-1)from 97.9 wt%to 99.7 wt%.The apparatus has 12 membrane modules connected in series.Each membrane module has a membrane area of 20 m2.The apparatus is operated under vapor permeation mode.A significant reduction(over 50%)in the separation cost was achieved for the membrane technique compared with distillation.The apparatus has been stably running for more than one year.Fig.8 is another apparatus for dehydration of acetonitrile(15000 t·a-1)from 80 wt%to 99 wt%.The apparatus has 20 membrane modules(10 m2per module)with total membrane area of 200 m2.The membrane dehydration technique can save more than 50%steam consumption for production of acetonitrile compared with rectification under vacuum.Fig.9 shows the membrane modules(the membrane areas:1 m2,3 m2and 10 m2)for pervaporation dehydration from Jiangsu Nine Heaven High-Tech Co.Ltd.
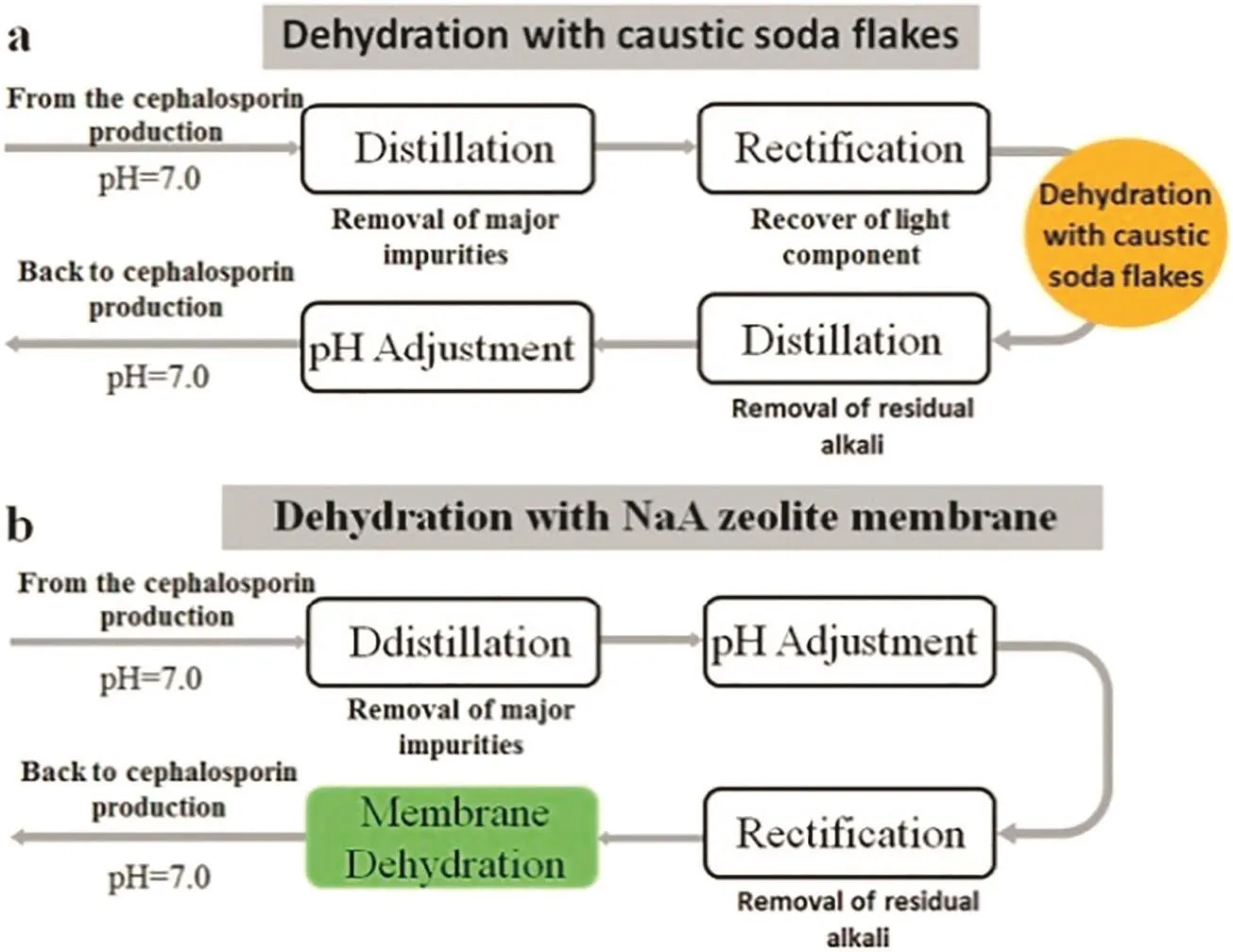
Fig.6.Comparison of different feed pretreatment processes for isopropanol dehydration:(a)dehydration with caustic soda flakes,(b)dehydration with NaA zeolite membrane.

Fig.7.The apparatus for dehydration of alcohol mixture(34400 t·a-1).

Fig.8.The apparatus for dehydration of acetonitrile(15000 t·a-1).

Fig.9.Zeolite membrane modules for pervaporation dehydration.
5.2.T-type and CHA zeolite membranes
Sommer and Melin[114]also used the commercial T-type zeolite membrane to dehydrate variousorganic solvents.The separation results are shown in Table 2.The T-type zeolite membrane exhibited similar separation selectivity and permeation flux with NaA zeolite membrane.The NaAzeolite membrane exhibits excellent separation factors,but has a very limited resistance in an acidic environment.The T-type membrane combines reasonable methanol/water selectivity with a higher acid stability.Zhouet al.[115]investigated the hydrothermal stability and acid stability of T-type zeolite membrane by on-line pervaporation.They performed the separation of 70 wt%ethanol/water mixture at 75 °C for 160 h.The permeate flux was stable at 4.5 kg·m-2·h-1,and the water content in the permeate side maintained above 99.9%.The membrane was stable for more than 30 days in ethanol/water mixture(containing acetic acid)with pH=3 at 75°C.
In view of the good stability of T-type zeolite membrane in acidic environment,it has a wide application prospect in the aspects of coupling the pervaporation dehydration technology with the esterification reaction to break the chemical equilibrium limit and enhance the reaction conversion rate.In the literature,some researchers coupled the pervaporation dehydration technology with ethyl acetate[96],ethyl lactate[97]and n-butyl acetate[116]esterification process,conversion rate of acetic acid and lactic acid up to 100%.Recently,Truonget al.[117]investigated the pervaporation performance of the T-type zeolite membrane of Mitsui Shipbuilding Company and Pervap#1201 membrane of Sulzer Company in the ethyl acrylate reaction system,the permeation flux of the T-type zeolite membrane at 65°C was 20%higher than that of Pervap#1201 membrane,which shows a good industrial application prospect.
Satoet al.[100]reported the first practically available high-silica CHA-type zeolite membranes in an industrial scale synthesized using an organic structure directing agent for an application of dehydration of N-Methylpyrrolidone solution by pervaporation.It was also reported that separating membranes of high-silica CHA-type of SSZ-13 were synthesized to dehydrate HNO3/H2O solution in pervaporation[118].Yamanakaet al.[101]synthesized the CHA-type zeolite membrane by the interzeolite conversion method,and their high acid stability towards several common mineral acids,such as HCl,H2SO4,and HNO3,was also con firmed.The CHA-type zeolite membrane could be used for the esterification of adipic acid with isopropyl alcohol using sulfuric acid as the catalyst.The yield of diisopropyl adipate increased from 56%to 98%by membrane assistance[20].
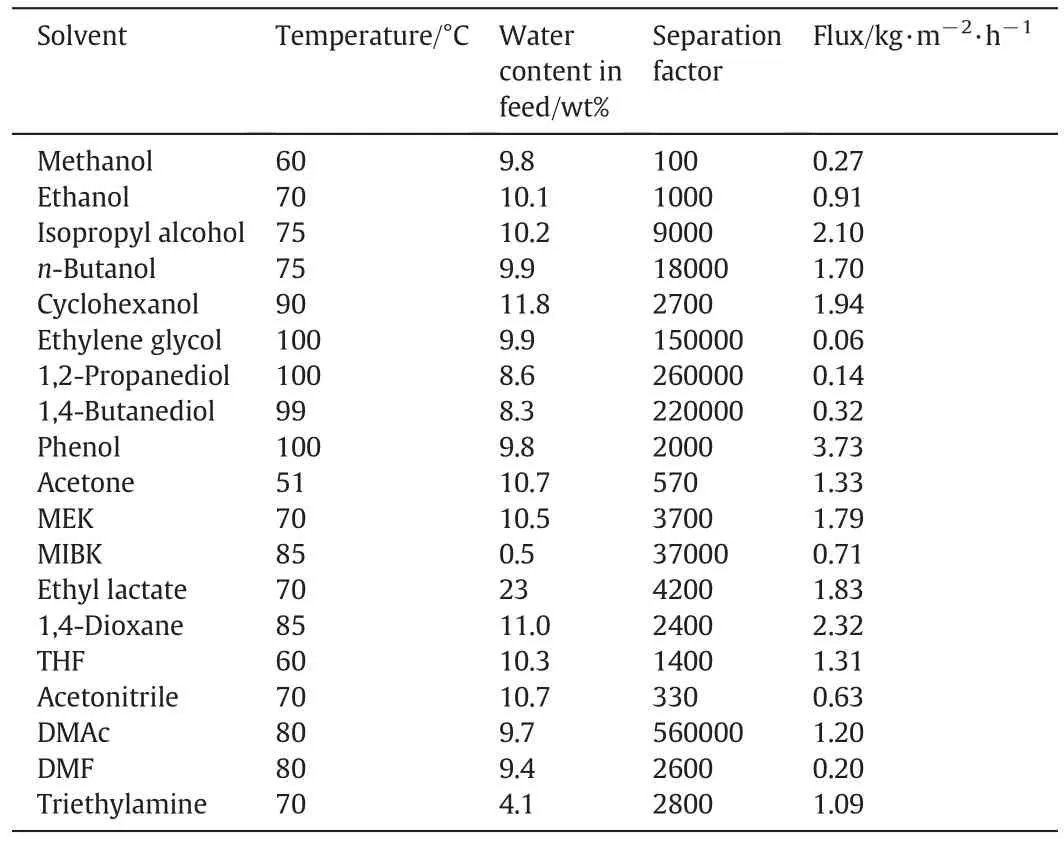
Table 2Separation performance of T-type zeolite membrane from Mitsui for dehydration of various organic solvents[114]
6.Conclusions and Perspectives
This review gives a brief introduction on mass transfer model,membrane preparation and application of zeolite membranes for pervaporation dehydration.The technology shows promising applications in organic solvent purification and separation due to high energy saving and better operation stability.Despite great developments having been achieved,there are still some challenges which should be focused on for the future work.(i)The fabrication cost of NaA zeolite membranes is still an issue for bursting applications and the potential applications of the membranes also need to be developed.(ii)Hollow fiber supported zeolite membranes showed both high permeation flux and packing density,which could be the next-generation products.However,the mechanical strength and batch scale production should be solved for practical applications.(iii)The stability of zeolite membrane in industrial environments is still a challenging issue for largescale applications.Some acid-resistance zeolite membranes such as T-type,MOR and CHA have better prospects in complex separation systems but need to improve their permeation flux further.High-silica membrane materials with thinner separation layer would be necessary for the harsh environments.
Nomenclature
Dsself-diffusivity,m2·s-1
D(0) the MS surface diffusivity at zero coverage
dppore diameter,m
Jpermeation flux,kg·m-2·h-1
Kadsorption constant,Pa-1
Mmolecular weight,kg·mol-1
Nanumber of diffusing molecules
Paverage partial pressure,kPa
PFpartial pressure in feed side,kPa
PPpartial pressure in permeate side,kPa
qadsorption loading,mol·kg-1
qMsaturated loading,mol·kg-1
Rgas constant(=8.314 Pa·m3·mol-1·K-1)
Ttemperature,K
δ thickness
ε support porosity
η viscosity,Pa·s
θ adsorption coverage
ρZdensity of zeolite layer,kg·m-3
τ support tortuosity
Subscripts
e ethanol
F finger-like layer
w water
Z NaA zeolite layer
Superscripts
int,1 zeolite/support interface
int,2 sponge-like/ finger-like interface
F feed side
P permeate side
Acknowledgments
We would like to thank Jiangsu Nine Heaven High-Tech Co.Ltd.for the information on the technical application of NaA zeolite membrane for pervaporation dehydration.
[1]X.Feng,R.Y.M.Huang,Liquid separation by membrane pervaporation:a review,Ind.Eng.Chem.Res.36(1997)1048-1066.
[2]W.H.Yuan,Y.S.Lin,W.S.Yang,Molecular sieving MFI-type zeolite membranes for pervaporation separation ofxylene isomers,J.Am.Chem.Soc.126(2004)4776-4777.
[3]T.C.Bowen,R.D.Noble,J.L.Falconer,Fundamentals and applications of pervaporation through zeolite membranes,J.Membr.Sci.245(2004)1-33.
[4]P.D.Chapman,T.Oliveira,A.G.Livingston,K.Li,Membranes for the dehydration of solvents by pervaporation,J.Membr.Sci.318(2008)5-37.
[5]J.Gascon,F.Kapteijn,B.Zornoza,V.Sebastián,C.Casado,J.Coronas,Practical approach to zeolitic membranes and coatings:state of the art,opportunities,barriers,and future perspectives,Chem.Mater.24(2012)2829-2844.
[6]D.Z.Liu,Y.T.Zhang,J.Jiang,X.R.Wang,C.Zhang,X.H.Gu,High-performance NaA zeolite membranes supported on four-channel ceramic hollow fibers for ethanol dehydration,RSC Adv.5(2015)95866-95871.
[7]Z.B.Wang,Q.Q.Ge,J.Shao,Y.S.Yan,High performance zeolite LTA pervaporation membranes on ceramic hollow fibers by dipcoating-wiping seed deposition,J.Am.Chem.Soc.131(2009)6910-6911.
[8]J.Caro,D.Albrecht,M.Noack,Why is it so extremely difficult to prepare shapeselective Al-rich zeolite membranes like LTA and FAU for gas separation?Sep.Purif.Technol.66(2009)143-147.
[9]Y.Hasegawa,T.Nagase,Y.Kiyozumi,T.Hanaoka,F.Mizukami,Influence of acid on the permeation properties of NaA-type zeolite membranes,J.Membr.Sci.349(2010)189-194.
[10]R.A.Rakoczy,Y.Traa,Nanocrystalline zeolite A:synthesis,ion exchange and dealumination,Microporous Mesoporous Mater.60(2003)69-78.
[11]G.Q.Zhu,Y.S.Li,H.Zhou,J.Liu,W.S.Yang,Microwave synthesis of high performance FAU-type zeolite membranes:optimization,characterization and pervaporation dehydration of alcohols,J.Membr.Sci.337(2009)47-54.
[12]X.R.Wang,Y.Chen,C.Zhang,X.H.Gu,N.P.Xu,Preparation and characterization of high- flux T-type zeolite membranes supported on YSZ hollow fibers,J.Membr.Sci.455(2014)294-304.
[13]Y.Cui,H.Kita,K.-I.Okamoto,Zeolite T membrane:preparation,characterization,pervaporation of water/organic liquid mixtures and acid stability,J.Membr.Sci.236(2004)17-27.
[14]Z.Chen,J.H.Yang,D.H.Yin,Y.H.Li,S.F.Wu,J.M.Lu,J.Q.Wang,Fabrication of poly(1-vinylimidazole)/mordenite grafting membrane with high pervaporation performance for the dehydration of acetic acid,J.Membr.Sci.349(2010)175-182.
[15]Z.Chen,D.H.Yin,Y.H.Li,J.H.Yang,J.M.Lu,Y.Zhang,J.Q.Wang,Functional defect patching of a zeolite membrane for the dehydration of acetic acid by pervaporation,J.Membr.Sci.369(2011)506-513.
[16]X.S.Li,H.Kita,H.Zhu,Z.J.Zhang,K.Tanaka,Synthesis of long-term acid-stable zeolite membranes and their potential application to esterification reactions,J.Membr.Sci.339(2009)224-232.
[17]Y.Hasegawa,H.Hotta,K.Sato,T.Nagase,F.Mizukami,Preparation of novel chabazite(CHA)-type zeolite layer on porous α-Al2O3tube using template-free solution,J.Membr.Sci.347(2010)193-196.
[18]J.Jiang,X.R.Wang,Y.T.Zhang,D.Z.Liu,X.H.Gu,Fabrication of pure-phase CHA zeolite membranes with ball-milled seeds at low K+concentration,Microporous Mesoporous Mater.215(2015)98-108.
[19]R.F.Zhou,Y.Q.Li,B.Liu,N.Hu,X.S.Chen,H.Kita,Preparation of chabazite membranes by secondary growth using zeolite-T-directed chabazite seeds,Microporous Mesoporous Mater.179(2013)128-135.
[20]Y.Hasegawa,C.Abe,F.Mizukami,Y.Kowata,T.Hanaoka,Application ofa CHA-type zeolite membrane to the esterification of adipic acid with isopropyl alcohol using sulfuric acid catalyst,J.Membr.Sci.415(2012)368-374.
[21]Y.Morigami,M.Kondo,J.Abe,H.Kita,K.Okamoto,The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane,Sep.Purif.Technol.25(1-3)(2001)251-260.
[22]Y.S.Lin,M.C.Duke,Recent progress in polycrystalline zeolite membrane research,Curr.Opin.Chem.Eng.2(2013)209-216.
[23]R.C.Binning,R.J.Lee,Separation of liquid mixtures by pervaporation,Ind.Eng.Chem.53(1961)45-54.
[24]T.Okada,M.Yoshikawa,T.Matsuura,A study on the pervaporation of ethanol/water mixtures on the basis of pore flow mode,J.Membr.Sci.107(1995)1-21.
[25]J.J.Shieh,R.Y.M.Huang,A pseudophase-change solution-diffusion model for pervaporation.II.Binary mixture permeation,Sep.Sci.Technol.33(1998)933-957.
[26]O.Kedem,The role of coupling in pervaporation,J.Membr.Sci.47(1989)277-285.
[27]R.Krishna,L.J.P.Van den Broeke,The Maxwell-Stefan description of mass transport across zeolite membranes,Chem.Eng.J.57(1995)155-162.
[28]R.Krishna,J.A.Wesselingh,The Maxwell-Stefan approach to mass transfer,Chem.Eng.Sci.52(1997)861-911.
[29]F.Kapteijn,J.A.Moulijn,R.Krishna,The generalized Maxwell-Stefan model for diffusion in zeolites:sorbate molecules with different saturation loadings,Chem.Eng.Sci.55(15)(2000)2923-2930.
[30]R.Krishna,R.Baur,Modelling issues in zeolite based separation processes,Sep.Purif.Technol.33(2003)213-254.
[31]R.Krishna,Describing the diffusion of guest molecules inside porous structures,J.Phys.Chem.C113(2009)19756-19781.
[32]R.Krishna,J.M.van Baten,A molecular dynamics investigation of the diffusion characteristics of cavity-type zeolites with 8-ring windows,Microporous Mesoporous Mater.137(2011)83-91.
[33]R.Krishna,The Maxwell-Stefan description of mixture diffusion in nanoporous crystalline materials,Microporous Mesoporous Mater.185(2014)30-50.
[34]A.H.Fuchs,A.K.Cheetham,Adsorption of guest molecules in zeolite materials:computational aspects,J.Phys.Chem.B105(2001)7375-7383.
[35]R.Nagumo,H.Takaba,S.Suzuki,Estimation of inorganic gas permeability through an MFI-type silicalite membrane by a molecular simulation technique combined with permeation theory,Microporous Mesoporous Mater.48(2001)247-254.
[36]G.Rutkai,E.Csanyi,T.Kristof,Prediction of adsorption and separation of wateralcohol mixtures with zeolite NaA,Microporous Mesoporous Mater.114(2008)455-464.
[37]E.Csanyi,T.Kristof,G.Lendva,Potential model development using quantum chemical information for molecular simulation ofadsorption equilibria of water-methanol(ethanol)mixtures in zeolite NaA-4,J.Phys.Chem.C113(2009)12225-12235.
[38]J.Z.Yang,Q.L.Liu,H.T.Wang,Analyzing adsorption and diffusion behaviors of ethanol/water through silicalite membranes by molecular simulation,J.Membr.Sci.291(2007)1-9.
[39]J.Y.Wu,Q.L.Liu,Y.Xiong,Molecular simulation of water/alcohol mixtures adsorption and diffusion in zeolite 4A membranes,J.Phys.Chem.B113(2009)4267-4274.
[40]J.Kuhn,J.M.Castillo-Sanchez,J.Gascon,S.Calero,D.Dubbeldam,T.J.H.Vlugt,F.Kapteijn,J.Gross,Adsorption and diffusion of water,methanol,and ethanol in allsilica DD3R:experiments and simulation,J.Phys.Chem.C113(2009)14290-14301.
[41]D.A.Lella,N.Desbiens,A.Boutin,I.Demachy,P.Ungerer,J.-P.Bellat,A.H.Fuchs,Molecular simulation studies of water physisorption in zeolite,Phys.Chem.Chem.Phys.8(2006)5396-5406.
[42]S.Y.Guo,C.L.Yu,X.H.Gu,W.Q.Jin,J.Zhong,C.L.Chen,Simulation of adsorption,diffusion,and permeability of water and ethanol in NaA zeolite membranes,J.Membr.Sci.376(2011)40-49.
[43]A.Gorbach,M.Stegmaier,G.Eigenberger,Measurement and modeling of water vapor adsorption on zeolite 4A-equilibria and kinetics,Adsorption10(2004)29-46.
[44]K.F.Loughlin,Water isotherm models for 4A(NaA)zeolite,Adsorption15(2009)337-353.
[45]R.Krishna,D.Paschek,Self-diffusivities in multicomponent mixtures in zeolites,Phys.Chem.Chem.Phys.4(10)(2002)1891-1898.
[46]R.Krishna,J.M.van Baten,Diffusion of alkane mixtures in zeolites:validating the Maxwell-Stefan formulation using MD simulations,J.Phys.Chem.B109(2005)6386-6396.
[47]R.Krishna,J.M.van Baten,Diffusion of hydrocarbon mixtures in MFI zeolite:influence of intersection blocking,Chem.Eng.J.140(2008)614-620.
[48]J.Z.Yang,Y.Chen,A.M.Zhu,Q.L.Liu,J.Y.Wu,Analyzing diffusion behaviors of methanol/water through MFI membranes by molecular simulation,J.Membr.Sci.318(2008)327-333.
[49]W.Jia,S.Murad,Molecular dynamics simulation of pervaporation in zeolite membranes,Mol.Phys.104(2006)3033-3043.
[50]D.Shen,W.Xiao,J.H.Yang,N.B.Chu,J.M.Lu,D.H.Yin,J.Q.Wang,Synthesis of silicalite-1 membrane with two silicon source by secondary growth method and its pervaporation performance,Sep.Purif.Technol.26(2011)309-315.
[51]D.Kunnakorn,T.Rirksomboon,P.Aungkavattana,N.Kuanchertchoo,D.Atong,K.Hemra,S.Kulprathipanja,S.Wongkasemjit,Optimization of synthesis time for high performance of NaA zeolite membranes synthesized via autoclave for water-ethanol separation,Desalination280(2011)259-265.
[52]V.Nikalakis,G.Xomeritakis,A.Abibi,M.Dickson,M.Tsapatsis,D.G.Vlachos,Growth of a faujasite-type zeolite membrane and its application in the separation of saturated/unsaturated hydrocarbon mixtures,J.Membr.Sci.184(2001)209-219.
[53]S.Farooq,I.A.Karimi,Modeling support resistance in zeolite membranes,J.Membr.Sci.186(2001)109-121.
[54]S.S.Madaeni,R.Pourghorbani,V.Vatanpour,Investigation of parameters affecting the flux of micro filtration poly(vinylidene fluoride)membranes for particulate removal,Adv.Polym.Technol.31(2012)29-40.
[55]F.T.de Bruijn,L.Sun,Z.Olujic,P.J.Jansens,F.Kapteijn,Influence of the supportlayer on the flux limitation in pervaporation,J.Membr.Sci.223(2003)141-156.
[56]J.Shao,Z.Y.Zhan,J.G.Li,Z.B.Wang,K.Li,Y.S.Yan,Zeolite NaAmembranes supported on alumina hollow fibers:effect of support resistances on pervaporation performance,J.Membr.Sci.451(2014)10-17.
[57]U.Beuscher,C.H.Gooding,The permeation of binary gas mixtures through support structures of composite membranes,J.Membr.Sci.150(1998)57-73.
[58]U.Beuscher,C.H.Gooding,The influence of the porous support layer of composite membranes on the separation of binary gas mixtures,J.Membr.Sci.152(1999)99-116.
[59]J.Zah,H.M.Krieg,J.C.Breytenbach,Pervaporation and related properties of time dependent growth layers of zeolite NaA on structured ceramic supports,J.Membr.Sci.284(2006)276-290.
[60]K.Okamoto,H.Kita,K.Horii,K.Tanaka,M.Kondo,Zeolite NaA membrane:Preparation,single-gas permeation,and pervaporation and vapor permeation of water/organic liquid mixtures,Ind.Eng.Chem.Res.40(2001)163-175.
[61]Z.T.Wu,I.M.D.Hatim,B.F.K.Kingsbury,E.Gbenedio,K.Li,A novel inorganic hollow fiber membrane reactor for catalytic dehydrogenation of propane,AIChE J.55(2009)2389-2398.
[62]F.R.Garcia-Garcia,K.Li,New catalytic reactors prepared from symmetric and asymmetric ceramic hollow fibers,Appl.Catal.A Gen.456(2013)1-10.
[63]P.Ye,Y.T.Zhang,H.F.Wu,X.H.Gu,Mass transfer simulation on pervaporation dehydration of ethanol through hollow fiber NaA zeolite membranes,AIChE J.62(7)(2016)2468-2478.
[64]N.Rangnekar,N.Mittal,B.Elyassi,J.Caro,M.Tsapatsis,Zeolite membranes-a review and comparison with MOFs,Chem.Soc.Rev.44(2015)7128-7154.
[65]A.S.Huang,J.Caro,Facile synthesis of LTA molecular sieve membranes on covalently functionalized supports by using diisocyanates as molecular linkers,J.Mater.Chem.21(2011)11424-11429.
[66]S.L.Wee,C.T.Tye,S.Bhatia,Membrane separation process—pervaporation through zeolite membrane,Sep.Purif.Technol.63(3)(2008)500-516.
[67]Y.M.Liu,Z.Z.Yang,C.L.Yu,X.H.Gu,N.P.Xu,Effect of seeding methods on growth of NaA zeolite membranes,Microporous Mesoporous Mater.143(2)(2011)348-356.
[68]Z.Z.Yang,Y.M.Liu,C.L.Yu,X.H.Gu,N.P.Xu,Ball-milled NaA zeolite seeds with submicron size for growth of NaA zeolite membranes,J.Membr.Sci.392(2012)18-28.
[69]X.R.Wang,Z.Z.Yang,C.L.Yu,L.W.Yin,C.Zhang,X.H.Gu,Preparation of T-type zeolite membranes using a dip-coating seeding suspension containing colloidal SiO2,Microporous Mesoporous Mater.197(2014)17-25.
[70]L.Q.Li,J.H.Yang,J.J.Li,J.Q.Wang,J.M.Lu,D.H.Yin,Y.Zhang,High performance ZSM-5 membranes on coarse macroporous α-Al2O3supports for dehydration of alcohols,AIChE J.(2016).
[71]H.Z.Li,J.Q.Wang,J.Xu,X.D.Meng,B.Xu,J.H.Yang,S.Y.Li,J.M.Lu,Y.Zhang,X.L.He,Synthesis of zeolite NaA membranes with high performance and high reproducibility on coarse macroporous supports,J.Membr.Sci.444(2013)513-522.
[72]X.X.Chen,J.Q.Wang,D.H.Yin,J.H.Yang,J.M.Lu,Y.Zhang,Z.Chen,High performance zeolite T membrane for dehydration of organics by a new varying temperature hot-dip coating method,AIChE J.59(3)(2013)936-947.
[73]L.Q.Li,J.H.Yang,J.J.Li,P.Han,J.X.Wang,Y.Zhao,J.Q.Wang,J.M.Lu,D.H.Yin,Y.Zhang,Synthesis of high performance mordenite membranes from fluoride-containing dilute solution under microwave-assisted heating,J.Membr.Sci.512(2016)83-92.
[74]Y.M.Liu,X.R.Wang,Y.T.Zhang,Y.He,X.H.Gu,Scale-up of NaA zeolite membranes on α-Al2O3hollow fibers by a secondary growth method with vacuum seeding,Chin.J.Chem.Eng.23(7)(2015)1114-1122.
[75]C.Cai,Y.T.Zhang,C.Zhang,X.H.Gu,Microstructure modulation of α-Al2O3hollow fiber membranes with four-channel geometric con figuration,Asia Pac.J.Chem.Eng.11(2016)949-957.
[76]Z.Z.Shi,Y.T.Zhang,C.Cai,C.Zhang,X.H.Gu,Preparation and characterization of α-Al2O3hollow fiber membranes with four-channel con figuration,Ceram.Int.41(2015)1333-1339.
[77]M.Lee,Z.T.Wu,B.Wang,K.Li,Micro-structured alumina multi-channel capillary tubes and monoliths,J.Membr.Sci.489(2015)64-72.
[78]Y.Chen,Y.T.Zhang,C.Zhang,J.Jiang,X.H.Gu,Fabrication of high- flux SAPO-34 membrane on α-Al2O3four-channel hollow fibers for CO2capture from CH4,J.CO2 Util.18(2017)30-40.
[79]M.Tsapatsis,Toward high-throughput zeolite membranes,Science334(6057)(2011)767-768.
[80]K.B.Yoon,Organization of zeolite microcrystals for production of functional materials,Acc.Chem.Res.40(1)(2007)29-40.
[81]Y.S.Chun,K.Ha,Y.J.Lee,J.S.Lee,H.S.Kim,Y.S.Park,K.B.Yoon,Diisocyanates as novel molecular binders for monolayer assembly of zeolite crystals on glass,Chem.Commun.17(2002)1846-1847.
[82]B.Elyassi,M.Y.Jeon,M.Tsapatsi,K.Narasimharao,S.N.Basahel,S.Al-Thabiaiti,Ethanol/water mixture pervaporation performance of b-oriented silicalite-1 membranes made by gel-free secondary growth,AIChE J.62(2)(2016)556-563.
[83]Y.S.Li,W.S.Yang,Microwave synthesis of zeolite membranes:a review,J.Membr.Sci.316(1)(2008)3-17.
[84]Y.S.Li,J.Liu,W.S.Yang,Formation mechanism of microwave synthesized LTA zeolite membranes,J.Membr.Sci.281(1)(2006)646-657.
[85]J.Motuzas,S.Heng,P.P.S.Z.Lau,K.L.Yeung,Z.J.Beresnevicius,A.Julbe,Ultra-rapid production of MFI membranes by coupling microwave-assisted synthesis with either ozone or calcination treatment,Microporous Mesoporous Mater.99(1)(2007)197-205.
[86]S.Mintova,S.Mo,T.Bein,Nanosized AlPO4-5 molecular sieves and ultrathin films prepared by microwave synthesis,Chem.Mater.10(12)(1998)4030-4036.
[87]K.Weh,M.Noack,I.Sieber,J.Caro,Permeation of single gases and gas mixtures through faujasite-type molecular sieve membranes,Microporous Mesoporous Mater.54(1)(2002)27-36.
[88]X.C.Xu,Y.Bao,C.S.Song,W.S.Yang,J.Liu,L.W.Lin,Microwave-assisted hydrothermal synthesis of hydroxy-sodalite zeolite membrane,Microporous Mesoporous Mater.75(3)(2004)173-181.
[89]N.Hu,Y.Q.Li,S.L.Zhong,B.Wang,F.Zhang,T.Wu,Z.Yang,R.F.Zhou,X.S.Chen,Microwave synthesis of zeolite CHA(chabazite)membranes with high pervaporation performance in absence oforganic structure directing agents,Microporous Mesoporous Mater.228(2016)22-29.
[90]D.Coutinho,J.A.Losilla,K.J.Balkus,Microwave synthesis of ETS-4 and ETS-4 thin films,Microporous Mesoporous Mater.90(1)(2006)229-236.
[91]C.L.Yu,Y.M.Liu,G.L.Chen,X.H.Gu,W.H.Xing,Pretreatment of isopropanol solution from pharmaceutical industry and pervaporation dehydration by NaA zeolite membranes,Chin.J.Chem.Eng.19(6)(2011)904-910.
[92]J.H.Yang,H.Z.Li,J.Xu,J.Q.Wang,X.D.Meng,K.Bai,J.M.Lu,Y.Zhang,D.H.Yin,Influences of inorganic salts on the pervaporation properties of zeolite NaA membranes on macroporous supports,Microporous Mesoporous Mater.192(2014)60-68.
[93]Y.S.Li,H.Zhou,G.Q.Zhu,J.Liu,W.S.Yang,Hydrothermal stability of LTA zeolite membranes in pervaporation,J.Membr.Sci.297(1)(2007)10-15.
[94]C.L.Yu,C.Zhong,Y.M.Liu,X.H.Gu,W.H.Xing,N.P.Xu,Pervaporation dehydration of ethylene glycol by NaA zeolite membranes,Chem.Eng.Res.Des.90(2012)1372.
[95]X.S.Cai,Y.T.Zhang,L.W.Yin,D.D.Ding,W.H.Jing,X.H.Gu,Electrochemical impedance spectroscopy for analyzing microstructure evolution of NaA zeolite membrane in acid water/ethanol solution,Chem.Eng.Sci.153(2016)1-9.
[96]K.Tanaka,R.Yoshikawa,C.Ying,H.Kita,K.-I.Okamoto,Application of zeolite membranes to esterification reactions,Catal.Today67(1-3)(2001)121-125.
[97]K.Tanaka,R.Yoshikawa,C.Ying,H.Kita,K.-I.Okamoto,Application of zeolite T membrane to vapor-permeation-aided esterification of lactic acid with ethanol,Chem.Eng.Sci.57(9)(2002)1577-1584.
[98]Y.Hasegawa,C.Abe,M.Nishioka,K.Sato,T.Nagase,T.Hanaoka,Formation of high flux CHA-type zeolite membranes and their application to the dehydration of alcohol solutions,J.Membr.Sci.364(1)(2010)318-324.
[99]J.Jiang,L.Wang,L.Peng,C.Cai,C.Zhang,X.R.Wang,X.H.Gu,Preparation and characterization of high performance CHA zeolite membranes from clear solution,J.Membr.Sci.527(2017)51-59.
[100]K.Sato,K.Sugimoto,N.Shimotsuma,T.Kikuchi,T.Kyotani,T.Kurata,Development of practically available up-scaled high-silica CHA-type zeolite membranes for industrial purpose in dehydration ofN-methyl pyrrolidone solution,J.Membr.Sci.409(2012)82-95.
[101]N.Yamanaka,M.Itakura,Y.Kiyozumi,Y.Ide,M.Sadakane,T.Sano,Acid stability evaluation of CHA-type zeolites synthesized by interzeolite conversion of FAU-type zeolite and their membrane application for dehydration of acetic acid aqueous solution,Microporous Mesoporous Mater.158(2012)141-147.
[102]Z.Chen,Y.H.Li,D.H.Yin,Y.M.Song,X.X.Ren,J.M.Lu,J.H.Yang,J.Q.Wang,Microstructural optimization of mordenite membrane for pervaporation dehydration of acetic acid,J.Membr.Sci.411-412(2012)182-192.
[103]M.H.Zhu,S.L.Xia,X.M.Hua,Z.J.Feng,N.Hu,F.Zhang,I.Kumakiri,Z.H.Lu,X.S.Chen,H.Kita,Rapid preparation of acid-stable and high dehydration performance mordenite membranes,Ind.Eng.Chem.Res.53(2014)19168-19174.
[104]S.G.Sorenson,E.A.Payzant,W.T.Gibbons,B.Soydas,H.Kita,R.D.Noble,J.L.Falconer,Influence of zeolite crystal expansion/contraction on NaA zeolite membrane separations,J.Membr.Sci.366(2011)413-420.
[105]F.Y.Qu,R.Shi,L.Peng,Y.T.Zhang,X.H.Gu,X.Y.Wang,S.Murad,Understanding the effect of zeolite crystal expansion/contraction on separation performance of NaA zeolite membrane:a combined experimental and molecular simulation study,J.Membr.Sci.539(2017)14-23.
[106]A.Jonquières,R.Clément,P.Lochon,J.Néel,M.Dresch,B.Chrétien,Industrial state of-the-art of pervaporation and vapour permeation in the western countries,J.Membr.Sci.206(2002)87-117.
[107]J.Caro,M.Noack,Zeolite membranes—recent developments and progress,Microporous Mesoporous Mater.115(2008)215-233.
[108]M.P.Pina,R.Mallada,M.Arruebo,M.Urbiztondo,N.Navascués,O.de la Iglesia,J.Santamaria,Zeolite films and membranes.Emerging applications,Microporous Mesoporous Mater.144(2011)19-27.
[109]B.Bolto,M.Hoang,Z.L.Xie,A review of water recovery by vapour permeation through membranes,Water Res.46(2012)259-266.
[110]V.Hoof,C.Dotremont,A.Buekenhoudt,Performance of Mitsui NaA type zeolite membranes for the dehydration of organic solvents in comparison with commercial polymeric pervaporation membranes,Sep.Purif.Technol.48(2006)304-309.
[111]S.Sommer,T.Melin,Influence of operation parameters on the separation of mixtures by pervaporation and vapor permeation with inorganic membranes.Part 1:dehydration of solvents,Chem.Eng.Sci.60(2005)4509-4523.
[112]K.Sato,K.Sugimoto,T.Nakane,Preparation of higher flux NaA zeolite membrane on asymmetric porous support and permeation behavior at higher temperatures up to 145°C in vapor permeation,J.Membr.Sci.307(2008)181-195.
[113]K.Sato,K.Aoki,K.Suimoto,K.Izumi,S.Inoue,J.Saito,S.Ikeda,T.Nakane,Dehydrating performance of commercial LTA zeolite membranes and application to fuel grade bio-ethanol production by hybrid distillation/vaporpermeation process,Microporous Mesoporous Mater.115(2008)184-188.
[114]S.Sommer,T.Melin,Performance evaluation of microporous inorganic membranes in the dehydration of industrial solvents,Chem.Eng.Process.44(2005)1138-1156.
[115]R.F.Zhou,L.L.Hu,Y.J.Zhang,N.Hu,X.S.Chen,X.Lin,H.Kita,Synthesis of oriented zeolite T membranes from clear solutions and their pervaporation properties,Microporous Mesoporous Mater.174(2013)81-89.
[116]H.Zhou,Y.S.Li,G.Q.Zhu,J.Liu,L.W.Lin,W.S.Yan,Microwave synthesis of a&boriented zeolite t membranes and their application in pervaporation-assisted esterification,Chin.J.Catal.29(7)(2008)592-594.
[117]H.T.Truong,S.Rode,D.Roizard,S.Mouzon-Pelletier,S.Tretjak,Dehydration of reactive industrial mixtures by pervaporation:an innovative approach in acrylic esters processes,Sep.Purif.Technol.120(2013)24-34.
[118]H.Kalipcilar,T.C.Bowen,R.D.Noble,L.Falconer,Synthesis and separation performance of SSZ-13 zeolite membranes on tubular supports,Chem.Mater.14(8)(2002)3458-3464.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Positively charged nanofiltration membrane fabricated by poly(acid-base)complexing effect induced phase inversion method for heavy metal removal☆
- Substrate matters:The influences of substrate layers on the performances of thin- film composite reverse osmosis membranes☆
- Polymer-based membranes for solvent-resistant nanofiltration:A review
- Recent developments in nanofiltration membranes based on nanomaterials☆
- Manipulation of confined structure in alcohol-permselective pervaporation membranes☆
- Monovalent cation perm-selective membranes(MCPMs):New developments and perspectives☆
