Recent developments in nanofiltration membranes based on nanomaterials☆
2017-05-30YanliJiWeijieQianYaweiYuQuanfuAnLifenLiuYongZhouCongjieGao
Yanli Ji,Weijie Qian ,Yawei Yu ,Quanfu An ,Lifen Liu ,Yong Zhou ,*,Congjie Gao
1 Center for Membrane and Water Science&Technology,Ocean College,Zhejiang University of Technology,Hangzhou 310014,China
2 Beijing Key Laboratory for Green Catalysis and Separation,College of Environmental and Energy Engineering,Beijing University of Technology,Beijing 100124,China
1.Introduction
Wateris an essential resource for our lives,however,3.9 billion people would live in water-scarcity regions on the earth by 2030(as estimated by World Water Council).In addition to the shortage of water resources,serious environmental pollution is another important issue for us[1,2].In the face of the above serious problems,membrane separation techniques have been quickly developed.Membrane separation as an environmental friendly,low energy consumption,and high separation efficiency technology,has been applied in many fields,such as the water and waste water treatment and the separation and purification of substances in industrial sectors like food,chemicals,medicine,and biotechnology[3,4].Pressure-driven membrane separation processes,i.e.,micro filtration,ultra filtration,nano filtration and reverse osmosis/forward osmosis are the most widely used membrane technologies for above separation applications[5].Nano filtration with a membrane pore size(0.5-2.0 nm)and operation pressure between reverse osmosis and ultra filtration,combines their advantages of high-solute rejection and low-energy consumption[6].Based on the electrostatic repulsive effect and the steric hindrance effect,nano filtration membranes can separate mono/multi-valent salts,and different size organic molecules.With the rapid development of nanofiltration technologies,nanofiltration application market is currently growing in a fast rate and the global sales is expected to reach ca.$450 million by 2019[7].
Since the pasttwo decades,polymeric membranes are being the main research object due to their good film-forming property,suitable flexibility and mechanical strength.Nowadays,commercially available nanofiltration membranes are mainly prepared with polymers,e.g.polyamides(PA)and cellulose acetate(CA)[8,9].Some other polymers like polyether sulfone(PES),sulfonated polysulfone(SPSF),polyimide(PI),polyvinyl alcohol(PVA),and chitosan(CS)are also being studied and used for preparing nanofiltration membranes[10-12].Although there is a great success for the development of nanofiltration technologies,the nanofiltration membranes with superior separation performance and anti-fouling property are required for the more complicated applications.The polymer selective layer,which rejects solutes while allowing water/solvent molecules to pass,is the crucial part which is expected to render high productivity/selectivity coupled with anti-fouling properties.However,the membranes constructed with the highly cross-linked and restrained polymer chains resist further enhancing membrane performance,and there is much room for improvement.
Recently,membrane scientists have come up with the research of modifying conventional polymeric membranes with nanoparticles,wherein nanoparticles were incorporated into/onto membranes.Introducing nanoparticles into monomer/polymer solution before membrane formation,the resultant nanoparticle-blended membranes or nanoparticle-entrapped membranes are often called mixed matrix membranes(MMMs)[13].While,in another type,nanoparticles are self-assembled onto membrane surface by dip-coating or pressurized deposition on the prepared membrane.Most of studies embedded nanofillers into polyamide thin film layer to form MMMs,which are usually referred to as thin film nanocomposite membranes(TFNMs)[14].In the last few years,remarkable progress has been made in the fabrication of nanomaterial based nanofiltration membranes,which not only demonstrates promising potential in overriding the “trade-off”between solvent permeability and solute selectivity,but also showed excellent mechanical/thermal stability and antifouling properties[15-17].The fabrication of advanced nano-based nanofiltration membranes should meet the following requirements:the well-dispersed nanomaterials,good compatibility of nanomaterials with polymers,and strong and stable interaction between nanomaterials with the polymeric matrix.However,the traditional nanoparticle-introduction strategy(physical blending and dip-coating method)using commercial or as-prepared nanoparticles can hardly fulfill the aforementioned requirements.Therefore,the innovation of advanced nanomaterials and development of appropriate membrane fabrication methodsis essentialto achieve nanofiltration membranes with superior comprehensive properties.
In the present context,the recent progress in nano-based nanofiltration membranes has been overviewed,as shown in Fig.1,a wide range ofnanomaterials,such as the metaland metaloxide nanoparticles,carbon-based nanomaterials,metal-organic frameworks,water channel proteins,and organic micro/nanoparticles have been adopted to prepare nano-based nanofiltration membranes.This review intends to summarize the recent studies on nanomaterial prepared membranes used for nanofiltration,including mixed matrix membranes,thin film nanocomposite membranes,and nanomaterial self-assembly membrane.In particular,the superiority of nanomaterial based membranes,including high perm-selectivity,good stability and antifouling properties is emphasized.
2.Nano filtration Membranes Based on Nanomaterials
2.1.Nanofiltration membranes prepared with metal and metal oxide nanoparticles
A number of metal and metal oxide nanoparticles,i.e.,zeolite,silica(SiO2),titanium dioxide(TiO2),and silver nanoparticle(Ag NPs),have been incorporated into polymeric matrix for preparing nanofiltration membranes,and improved the membrane performances,including the flux and rejection,mechanical and thermal stability,and antifouling and-bacteria properties(Table 1).Jeonget al.[18] firstly developed a kind of zeolite/polyamide TFNMs by interfacial polymerization.The water permeability of the membrane was improved nearly double that of pristine polyamide membranes with comparable solute rejections.The nanocomposite membranes become much smoother,more hydrophilic and negatively charged.It was worth mentioning that,the opened pores within zeolites could provide extra pathway for water molecules transporting through membranes,as a result the membrane separation performance was enhanced.Lindet al.[19,20]similarly prepared polyamide TFNMs with incorporating LTA zeolites by interfacial polymerization.The particle size has a greatinfluence on the membrane physical characteristics and separation performances.Larger nanoparticles induce the formation of much negatively charged,thicker and rougher membrane surface,while smaller nanoparticles increased the free volume and permeability of the membrane.Huanget al.[21]systematically investigated the behavior and role of NaA zeolites in the interfacial polymerization for preparing polyamide TFNMs.The nanoparticles were added into aqueous/organic phase,as shown in Fig.2,the membrane structures and separation performances are different.There is a dense polyamide layer with low concentration of zeolites by adding zeolites into aqueous phase,while more zeolites were embedded throughout the whole polyamide layer with incorporation in organic phase.The nanocomposite membrane with the addition of zeolites into the organic phase had a better separation performance,since the porous zeolite appeared in the polyamide selective layer acted as a water channel carrier.
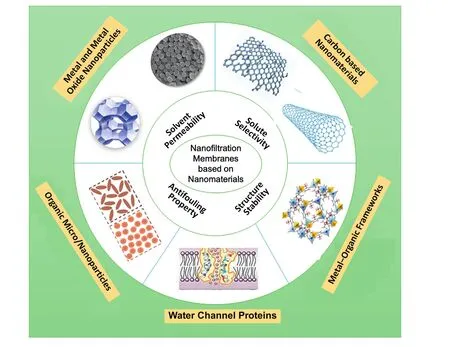
Fig.1.Overview of nanofiltration membranes based on various nanomaterials with improved permeability,selectivity,stability and antifouling properties.
In generally,there is a weak interaction between inorganic nanoparticles and the polymeric matrix,and more problematically,the agglomeration of nanoparticles in the selective layer,leading to the formation of“defects”and a poor separation performance.Developing new membrane fabrication method or modifying the nanoparticles might improve the membrane structure and separation performance.Donget al.[22]reported a new approach for fabricating TFNMs,involving preparation of a polysulfone support in-situ embedded with zeolite nanoparticles followed by interfacial polymerization to form the polyamide layer.The new nanocomposite membrane with higher zeoliteloading content and more uniform particle dispersion in the polyamide layer showed a stable nanofiltration performance.Wuet al.[23]synthesized a new kind of mesoporous SiO2nanoparticles with amino groups,and added them into piperazine(PIP)aqueous solution,the nanoparticles would take part in the interfacial polymerization between PIP and trimesoyl chloride(TMC).Thus,there is a covalent bonding between the SiO2nanoparticles and polyamide selective layer.The resultant membranes with appropriate concentration of SiO2nanoparticles in aqueous phase(0.03 wt%),exhibited a maximum water flux of 32.4 L·m-1·h-1(about 1.5 times as high as that of pristine polyamide membrane),while kept a relative high Na2SO4rejection(>80%).Liet al.[24]synthesized three kinds of SiO2nanospheres with the functional groups of phenyl,sulfonic,and pyridyl,then introduced them into polyethyleneimine(PEI)matrix for preparing nanofiltration membranes.These SiO2nanospheres were uniformly dispersed in PEImatrix,offering an excellent thermal stability and good solvent resistance in nanofiltration.The microstructures of nanocomposite membranes were tuned by varying the fractional free volume and surface hydrophilic/hydrophobic properties.The ethanol permeation of the membrane was increased from 21.2 to 30.8 L·m-1·h-1with the increase of fractional free volume from 0.452%to 0.473%by incorporating hydrophilic SiO2nanospheres.While,the nanocomposite membrane with hydrophobic SiO2nanospheres showed a significant increasing flux for nheptane from 0.1 to 21.7 L·m-1·h-1,due to the enhanced affinity of membranes to the non-polar organic solvent.
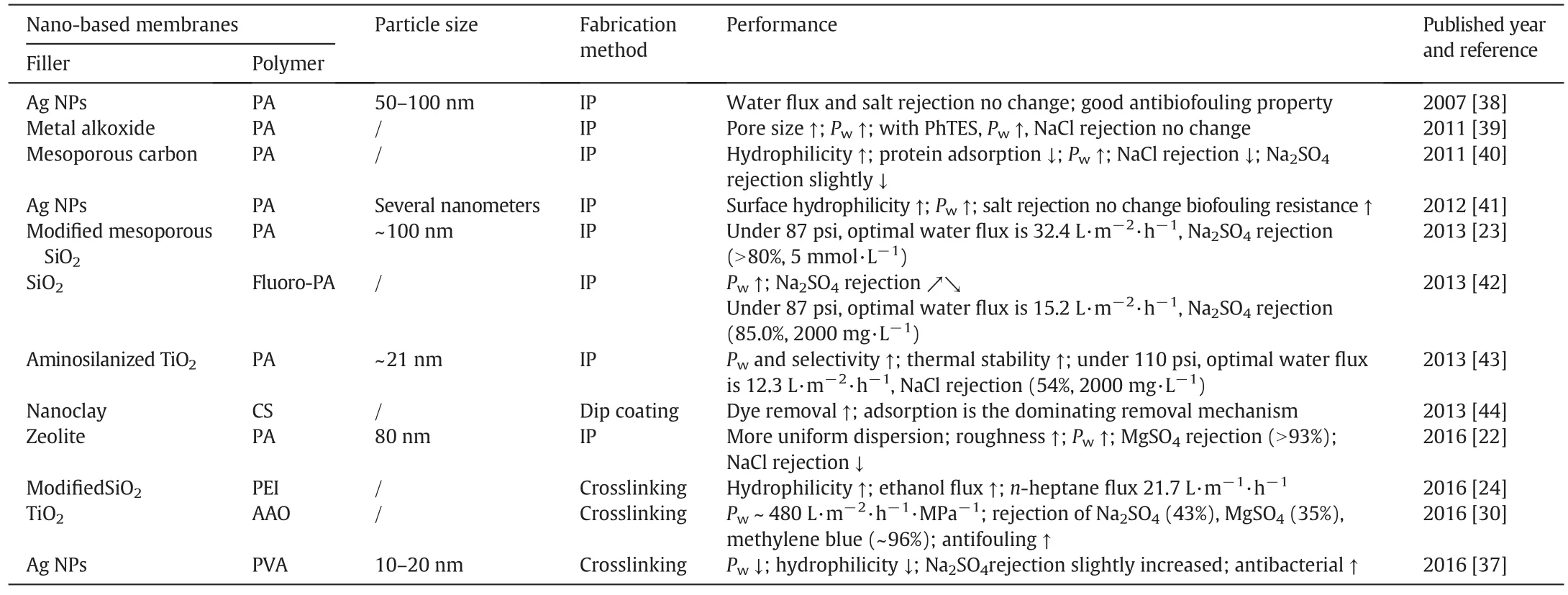
Table 1Summary of nanofiltration membranes prepared with metal and metal oxide nanoparticles
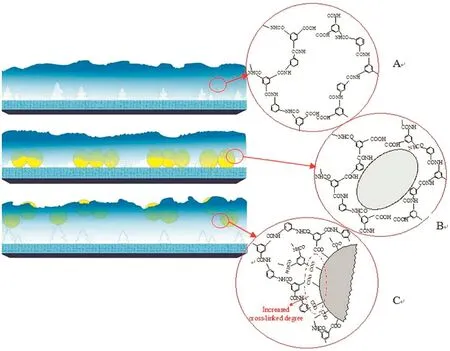
Fig.2.Schematic of the membrane formation mechanism for the(A)TFC membrane;(B)a-TFN membrane prepared with addition of zeolites into aqueous phase;(C)o-TFN membrane prepared with addition of zeolites into organic phase.Reprinted with permission from Ref.[21].Copy right(2013)Royal Society of Chemistry.
Titanium oxide(TiO2)as a photocatalytic material,can disintegrate/degrade organic compounds in water,which has been widely used for preparing antifouling separation membranes[25,26].Ahybrid TiO2/polyamide membrane was prepared by self-assembly of TiO2nanoparticles on the surface of polyamide layer[27].There is a coordination and H-bonding interaction between TiO2and carboxyl groups of polyamide layer.In the antifouling test,the hybrid membrane has less loss of flux and salt rejection in filtrating water solution containingEscherichia coliunder UVlight illumination.There are two main reasons for the excellent antifouling performance,one is photo-catalysis decomposes organic foulants and microbes,and the other is the enhanced hydrophilicity,the hydration layer of water on the membrane surface repels hydrophobic foulants[28,29],resulting in an improved anti-fouling performance.Recently,Songet al.[30]prepared TiO2nanofiltration membranes with precursors(TiCl4and ethylene glycol)by molecular layer deposition method,with the optimum preparation condition the membrane pore size was tuned to be approximately 1 nm.There is a high pure water permeability~480 L·m-2·h-1·MPa-1,and moderate rejection of Na2SO4(43%)and MgSO4(35%)and high rejection of methylene blue(~96%)and natural organic matter(~99%),respectively.Other than metal oxide TiO2,Ag nanoparticle(Ag NP)is another photocatalytic,hydrophilic and antibacterial particle,has been used for preparing antifouling membranes[31-33].The most common method is to introduce Ag NPs inside the membrane matrix,which limits their antifouling efficiency in applications.Liuet al.[34]synthesized antibacterial silver nanocomposite nanofiltration by layer-by-layer assembly method.Some other workers attached Ag+or Ag NPs onto the membrane surface through covalent bond[35,36].Zhanget al.[37]modified a commercial nanofiltration membrane by in-situ formation of Ag NPs on the membrane surface.PVA was first coated onto the nanofiltration membrane,then silver salt was deposited on PVA layer.Ag NPs with 10-20 nm in diameter were in-situ formed and immobilized onto the membrane surface.These methods not only improved the anti-biofouling performance,but also avoid Ag NPs leaching out from the membranes.
2.2.Nanofiltration membranes prepared with carbon-based nanomaterials
Carbon-based nanomaterials,such as carbon nanotubes(CNTs)and graphene have been thought to be alternative candidates for preparing nanofiltration membranes(Table 2).Carbon nanotube(CNT)as a onedimensional nanomaterial with the hydrophobic,smooth inner walls displays a unique water transport capability.Molecular simulation has proved that the multiwalled CNTs(MWCNTs)with a pore diameter of 7 nm has four to five orders of magnitude faster water flux than the theoretical value obtained from conventional fluid calculations[45].Mixed matrix membranes(MMMs)have been prepared with the addition of CNTs,which exhibit improved separation and antifouling performances.For example,Wuet al.[46]fabricated a TFNM with TMC,triethanolamine(TEOA)and MWCNTviaa reverse interfacialpolymerization.The resultant nanocomposite membrane with a looser cross-linked structure,hydrophilic and negatively charged surface,resulted in a high water flux but low salt rejection.Tiraferriet al.[47]modified the single-walled carbon nanotubes(SWNTs)with ethylenediamine and deposited them onto the polyamide membrane surface.TheE.colicell adsorption experiments showed that an enhanced bacterial cytotoxicity for the SWNT-coated membranes.The incorporation of CNTs into polymeric matrix is an effective way to obtain nanofiltration membranes with high separation performance.However,there are still some issues which should be solved to obtain perfect CNT MMMs: first,it is the dispersion of CNTs in the polymeric matrix,aggregations will make defects and voids,leading to a poor substance rejection.In addition,the end-cap opened CNTs are required to efficiently play their role in the water transport and solute selectivity.Moreover,the length and tortuosity of CNTs should be decreased to fit the thin film selective layer.Leeet al.[48]prepared a TFNM with the incorporation of hydrophilic,cap-opened MWCNTs into the polyamide selective layer.The MWCNTs were firstly treated with a thermo-oxidative process and surface modified with dopamine,then they were in-situ interfacial polymerization with the metaphenylene diamine(MPD)and TMC.Open ended MWCNTs/PA-TFC membranes showed better water flux than pristine polyamide membrane.Zhaoet al.[49]embedded poly(dopamine)modified multiwall carbon nanotubes(MWCNTs@PDA)into polyethyleneimine(PEI)/TMC polyamide thin film composite membranes(Fig.3).The compatibility and interfacial interaction between modified MWCNTs and polyamide matrix were improved,as a result the water permeability of PEI/MWCNTs@PDA/TMC nanocomposite membrane(153.2 L·m-2·h-1·MPa-1)is about 1.6 times increased compared with that of pristine PEI/TMC membranes.In addition,they prepared a new type of polyelectrolyte complex(PEC)/MWCNT nanocomposite membranesviain-situ ionic cross-linking with sodium alginate(SA)and polyethyleneimine(PEI)[50].Both the waterpermeability and stability of nanocomposite membranes were enhancedviaintroducing MWCNTs into membranes.This is because the MWCNTs have taken part in the ionic cross-linking reaction with the protonation of--NH3groups of PEI,thus there is a strong interfacial force between MWCNTs and the PEC matrix,resulting in a good nanofiltration performance.

Table 2Summary of nanofiltration membranes prepared with carbon-based nanomaterials
Vertically aligned CNT nanocomposite membranes with a high porosity and straightforward porous structure would be most preferable to achieve a high separation performance.CNT is easier to be introduced into thin film membranes but more difficult to be oriented because it is more soft and sensitive to random thermal motion.Moreover,most of the existing alignment methods need a special substrate and manufacturing equipment,which limited the application of aligned CNTs in polymeric matrix[51-53].Recently,Chanet al.[54]used high-vacuum filtration to disperse the zwitterion functionalized CNTs on the PES support layer,then the support with semialigned CNTs was wetted in MPD and TMC solutions for interfacial polymerization,and the CNT/PA nanocomposite membrane was obtained.The zwitterionic functionalized CNTs within polyamide layer were partially aligned through a high-vacuum filtration step(Fig.4).The membrane water flux was increased by more than a factor of 4,from 6.8 to 28.7 GFD(gallons per square foot per day)with increasing the CNT content from 0 to 20 wt%.Zhaoet al.[55]proposed a new method,hot-press combined with peel-off,wherein the CNT can be vertically aligned on the polymeric substrate.Shear force and mechanical stretch are the main forces to align the tubes perpendicular to the substrate surface during the peel-off process.The aligned CNTs could be maintained in a thin hybrid membrane with dip-coating cellulose acetate dope solution,showing a potential for constructing aligned CNTs TFNMs.
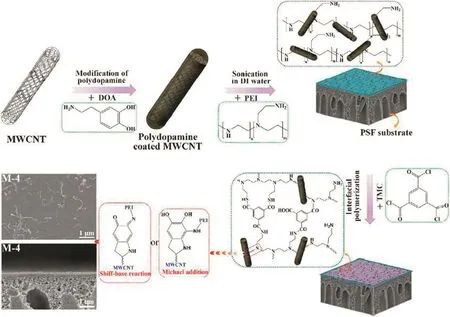
Fig.3.Schematic preparation of PEI/MWCNTs@PDA/TMC NF membranes.Reprinted with permission from Ref.[49].Copy right(2016)American Chemical Society.
Two-dimensional(2D)graphene-based nanosheets including graphene,graphene oxide(GO)and chemically-converted graphene have become a new generation of carbon nanomaterials for preparing nanofiltration membranes.Recently,Liuet al.[56]have given a comprehensive review about the graphene-based separation membranes.Here we just summarize the novel type of graphene-based nanofiltration membranes.Wanget al.[57]incorporated GO into polyelectrolyte complexes(PECs).The PEI-modified GO and PAA solutions were alternately poured onto a hydrolyzed polyacrylonitrile ultra filtration supporting membrane,then the(PEI-modified GO)/PAA multilayer membranes were immersed in PVA solution and cross-linked by glutaraldehyde.Both the mechanical and thermal properties of the GO/PEC nano-hybrid membranes were improved a lot.The membrane showed good separation performance,i.e.,the retention of Congo red,Mg2+and Na+were 99.5%,92.6%and 43.2%,respectively,however,water flux of the membrane is relatively low(8.4 kg·m-2·h-1·MPa-1).Banoet al.[58]prepared polyamide nanocomposite membranes embedded with GO,and the prepared polyamide/GO membrane showed a higher water flux and good saltrejection with the optimum GO loading content.The incorporated GO would provide an additional pathway for water molecules transporting through the membrane.Meanwhile,the introduction of GO nanosheets might disrupt polyamide chains packing in matrix,leading to an increased free volume and high water permeability.However,when the GO loading content is too much higher,they would aggregate and increase the transmission resistance,the water flux was decreased.Hu and Mi[59]reported a new type of nanofiltration membranes with stacked GO nanosheets,the GO nanosheets were depositedvialayerby-layer(LbL)on a polydopamine-coated polysulfone support and then cross-linked with TMC(Fig.5).It is found that the GO membrane flux is in the range of 80 and 276 L·m-2·h-1·MPa-1much higher than that of most commercial nanofiltration membranes,while the salt rejection is relatively low(6%-46%for monovalent and divalent salts).These results demonstrate that the LbL GO films with thin thickness have higher water permeability,but their cross-linked density should be further enhanced for satisfying the substance separation.
Molecular simulation has predicted that two-dimensional channels could be formed between stacked GO nanosheets which might allow water molecules passing through while rejecting solutes,leading to an extremely high perm-selectivity in nanofiltration process[60,61].Qiuet al.[62] first prepared graphene membrane using chemically converted graphene by vacuum filtration and explored its potential application in nanofiltration,the pure water flux of the membrane is 400 L·m-2·h-1·MPa-1and the rejection of Direct Yellow is 67%.Hanet al.[63]fabricated ultrathin(22-53 nm thick)chemically converted graphene membranes by pressure filtration(Fig.6).Separation performance was examined on a dead end filtration device,and the pure water flux is 218 L·m-2·h-1·MPa-1,there is a higher organic dye rejection(>99%)and lower salt rejection(≈20%-60%).The authors thought that the retention of dye molecules is attributed to three reasons:one is the direct absorption of dye molecules by reduced GO,the second is the size sieving by 2D nanochannels in the membrane,and the third is the electrostatic repulsive interaction between oppositely charged dye molecules and the negatively charged groups in reduced GO.However,the lower salt rejection is mainly due to some larger pores(1-5 nm)produced by the unzipped slits within GO membranes in water environment.Thus,the structural integrity and stability of the laminar GO membranes should be improved.Recently,they fabricated a new type of nanofiltration membranes by synergistic assembling of graphene and MWCNTs[64].The resultant membrane(GCNTm)showed a water flux up to 113 L·m-2·h-1·MPa-1,while keeping high dye rejection(>99%)and good salt rejection(i.e.,83.5%for Na2SO4,51.4%for NaCl).Herein,the MWCNTs act as space holders to avoid shrinkage of nanochannels within graphene layers,endowing GCNT mmembrane better structure stability and nanofiltration performance.Hunget al.[65]have used different kinds of diamine monomers cross-linking GO nanosheets to prepare composite graphene oxide-framework membranes.The size of nanochannels could be easily tuned by the diamine monomers,and the stability of the membrane was improved.
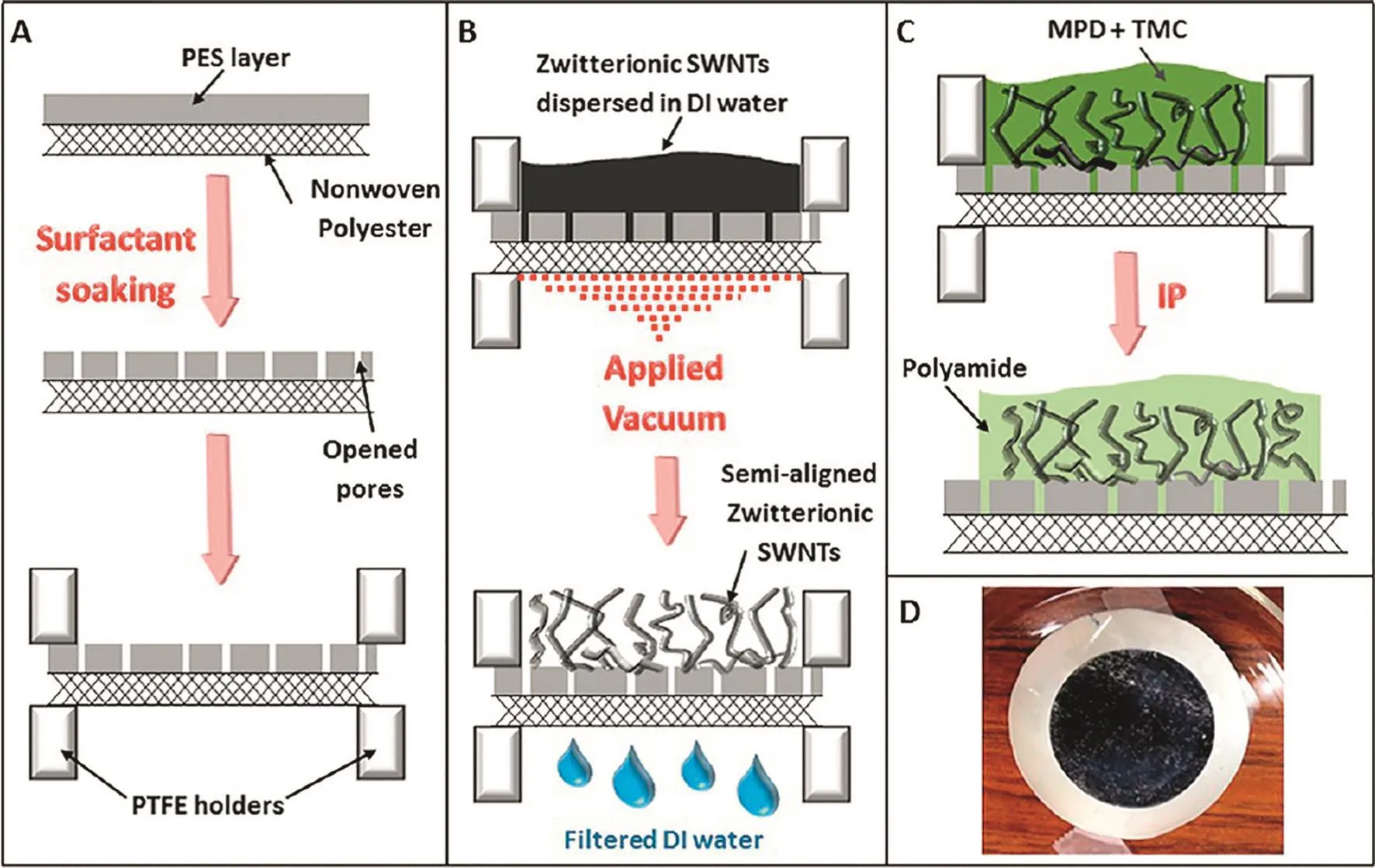
Fig.4.Cross-sectional schematics of the fabrication procedure for CNT nanocomposite membrane.(A)PES ultra filtration membrane,composed of a thin PES layer covered on a nonwoven polyester web,soaked in surfactant solution to clean the pores and increase hydrophilicity.The membrane was then sandwiched by two PTFE holders.(B)Zwitterion functionalized CNTs,depositing onto the pretreated PES membrane,through vacuum filtration.(C)Interfacial polymerization of polyamide carried out between semialigned functionalized CNTs at which aqueous solution of MPD comes in contact with nonaqueous solution of TMC.(D)Photograph of the top of CNT nanocomposite membrane that is exposed to the feed.Reprinted with permission from Ref.[54].Copy right(2013)American Chemical Society.
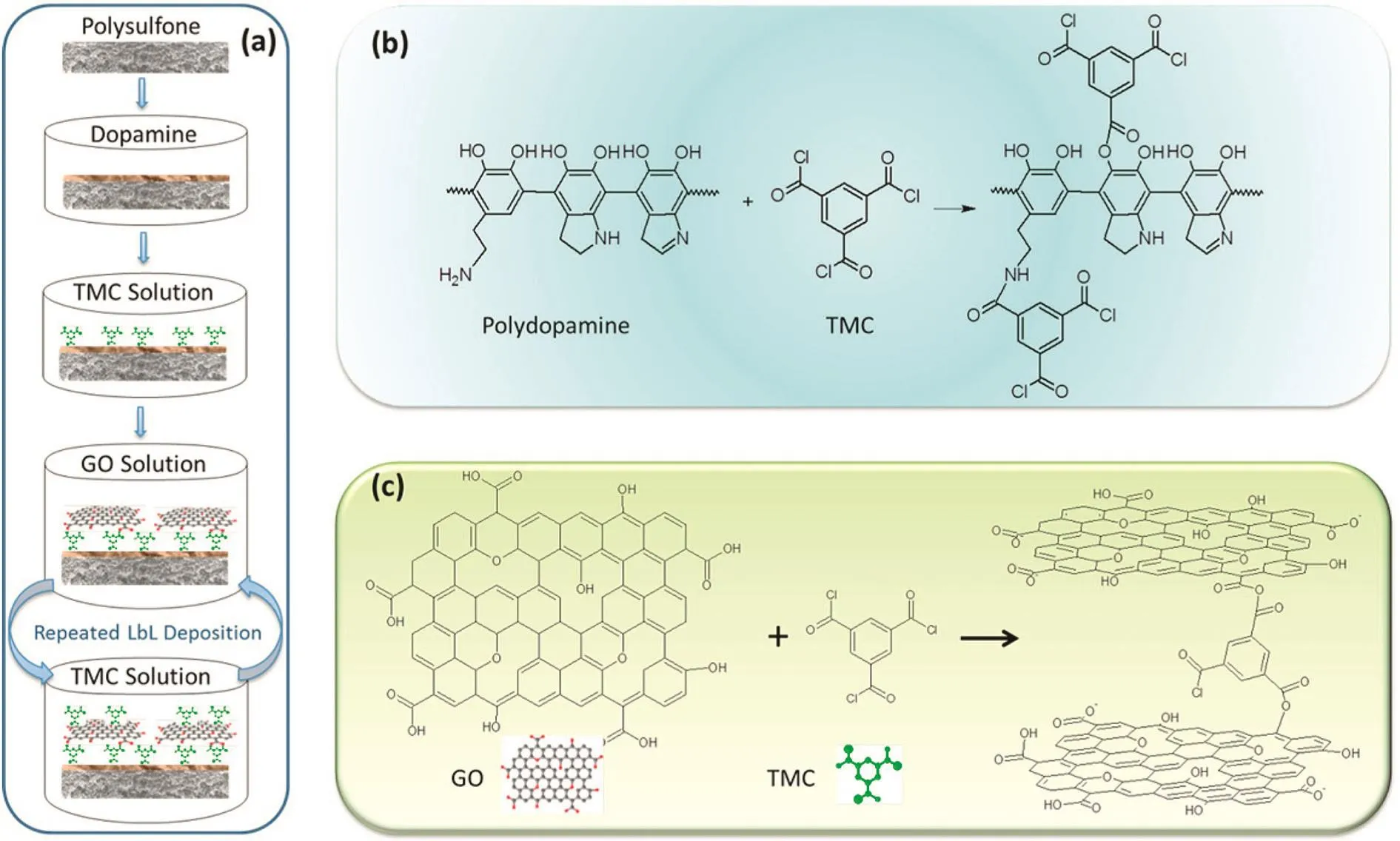
Fig.5.Schematic illustration of(a)a step-by-step procedure to synthesize the GO membrane,(b)the mechanism of reactions between polydopamine and TMC,and(c)the mechanism of reactions between GO and TMC.Reprinted with permission from Ref.[59].Copy right(2013)American Chemical Society.
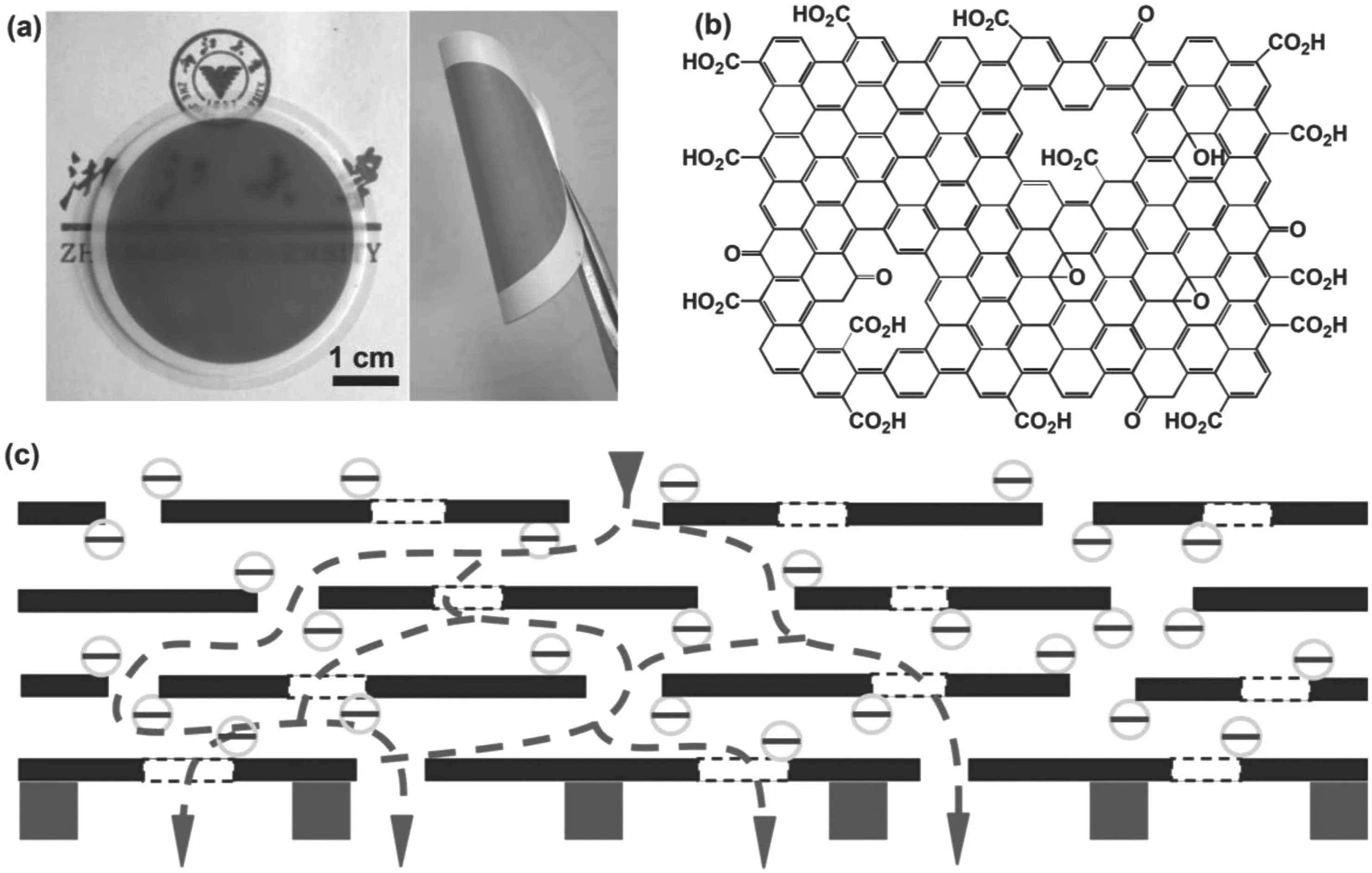
Fig.6.(a)Digital photo of an uGNM coated on an AAO disk(left)and a twisted uGNM coated on a PVDF membrane(right).(b)Schematic representation of a brGO:graphene sheet with a certain amount of holes and most of the oxidized groups are located on the edges and the periphery of the holes on it.Note that the real graphene sheets extend further than depicted.(c)Schematic view for possible permeation route:water molecules go through the nanochannels of the uGNMs and the holes on the graphene sheets and at last reach the pores of supporting membranes.The blank squares present the holes on the graphene sheets(black line).The edges of the brGO and the periphery of the holes are negatively charged.Reprinted with permission from Ref.[63].Copy right(2013)Wiley-VCH.
Perreaultet al.[66]have reported that the polyamide membrane modified with GO exhibited an enhanced antimicrobial property,the number of viableE.colicells absorbed on the membrane surface was reduced by 64.5%after contact with the membrane for 1 h.SEM results showed that the cells adsorbed on the GO-TFNMs are to be flattened or shrunk compared to those on the polyamide membrane.Antibacterial mechanism of graphene-based nanomaterials has been investigated by Liuet al.[67]They found that there is a three step antibacterial mechanism.First graphene nanomaterials with amounts of functional groups have more chances to interact with bacterial cells,the cell deposits on it and directly contacts,then the graphene nanosheets induce membrane stress by disrupting and damaging cell membranes,as a result the cell becomes dying.Wanget al.[68]preparedO-(carboxymethyl)-chitosan(OCMC)nano filtration membranes with surface functionalization by GO nanosheets.The GO nanosheets were bounded on the membrane surfaceviaring-opening polymerization between epoxy groups of GO nanosheets and amino groups of OCMC.The membrane surface became much hydrophilic,negatively charged and smooth with deposition ofGO nanosheets on membrane surface,thus the nanofiltration performance of the membrane was enhanced.
2.3.Nanofiltration membranes prepared with metal-organic frameworks(MOFs)
MOF nanocomposite membranes have shown an excellent gas separation performance,and have been comprehensively summarized in previous review[74].Recently,MOFs are emerging as great potential porous nanomaterials for preparing nanofiltration membranes[75-78].In addition to a high surface area and pore volume,their chemicalstructure,surface properties and particle size can be readily tuned to meet the requirements of membrane fabrication and nanofiltration applications(Table 3).Early Sorribaset al.[75]have fabricated series of TFNMs containing a range of 50-150 nm MOFs[ZIF-8,MIL-53(Al),NH2-MIL-53(Al)and MIL-101(Cr)]viain-situ interfacial polymerization on top of crosslinked polyimide porous supports.These TFN membranes showed dramatically increased permeance of solvent compared to the pristine polyamide membrane,without sacrificing rejection.It is found that the porous MOFs provided preferential flow paths for the solvents,while the polyamide layer surrounding MOFs maintained a high solute rejection.Later,various PA/MOF TFN membranes were prepared by interfacial polymerization with the addition of different MOFs in aqueous/organic phases[76-78].Although there is an excellent compatibility between MOF particles and polymeric matrix,the MOF aggregates might form with a high MOF loading content during interfacial polymerization and/or solvent evaporation.It is desired to develop novel fabrication methodology for improving membrane performance ofPA/MOF TFN membranes at high MOF loading content.
MOF particle embedded nanocomposite membranes have shown an increasing solvent permeability in nanofiltration process,while their structural stability and substance separation performance should be further improved in the consideration of applications.Zhanget al.[79]propose a simple and powerful strategy,coordination driven in-situ self-assemble for preparing MOF hybrid membranes.The coordination interactions between metal ions and ligands and/or the functional groups of polymer,improve the MOF-particle dispersion in and compatibility with the polymeric matrix.Similarly,they prepared the ZIF-8/PSS hybrid membrane on a tubular alumina substrate by LbL self-assembly method[80](Fig.7).Effects of layer numbers and Zn(NO3)2concentration on the nanofiltration performance were systematically studied.The membrane prepared with optimized conditions showed outstanding nanofiltration performance with 210 L·m-2·h-1·MPa-1flux and 98.6%methyl blue rejection.This work illustrates a simple approach for fabricating MOF hybrid membranes on porous substrates,having great potential for industrial applications.In addition,Campbellet al.[81]prepared a MOF hybrid membrane by in-situ growth(ISG)of HKUST-1 within the aryl carboxylic acid modified pores of polyimide(PI)membranes.The resultant hybrid membrane had a narrowest pore size distribution,and displayed high rejection with a molecular weight cut-off of 794 g·mol-1,and high solvent permeation.
The nanocomposite membranes fabricated with hybrid nanomaterials have shown a synergistic advantage over single type of nanoparticle based membranes[82,83].Wanget al.[84]reported a new type of polyamide TFNMs incorporated with zeolitic imidazolate framework-8(ZIF-8)and GO.The resultant hybrid nanocomposite membranes exhibited the merits of both ZIF-8 and GO,simultaneously,there was a uniform dispersion of ZIF-8 onto GO nanosheets.The ZIF-8/GO TFN membrane had water permeability(40.63 L·m-2·h-1·MPa-1)and allowed for efficient bivalent salt removal.Especially,there was a betterantimicrobial activity of the hybrid nanocomposite membrane than those membranes just prepared with ZIF-8 and GO separately.In order to control the size and dispersion of MOF particles in the polymeric matrix,a new concept of nanoconfined composite(NCC)membranes was invented by Wanget al.[85]The nanopores in the substrate membrane were used as template to confine the formation of MOF particles.Consequently,there was a nearly zero thickness of MOF layer on the substrate,which greatly reduced the mass transfer resistance.The water flux(464 L·m-2·h-1·MPa-1)of ZIF-11/PAN NCC membrane is much higher than most of other nanofiltration membranes,and simultaneously the membrane had an excellent rejection(98.4%)to methyl blue dye molecules.
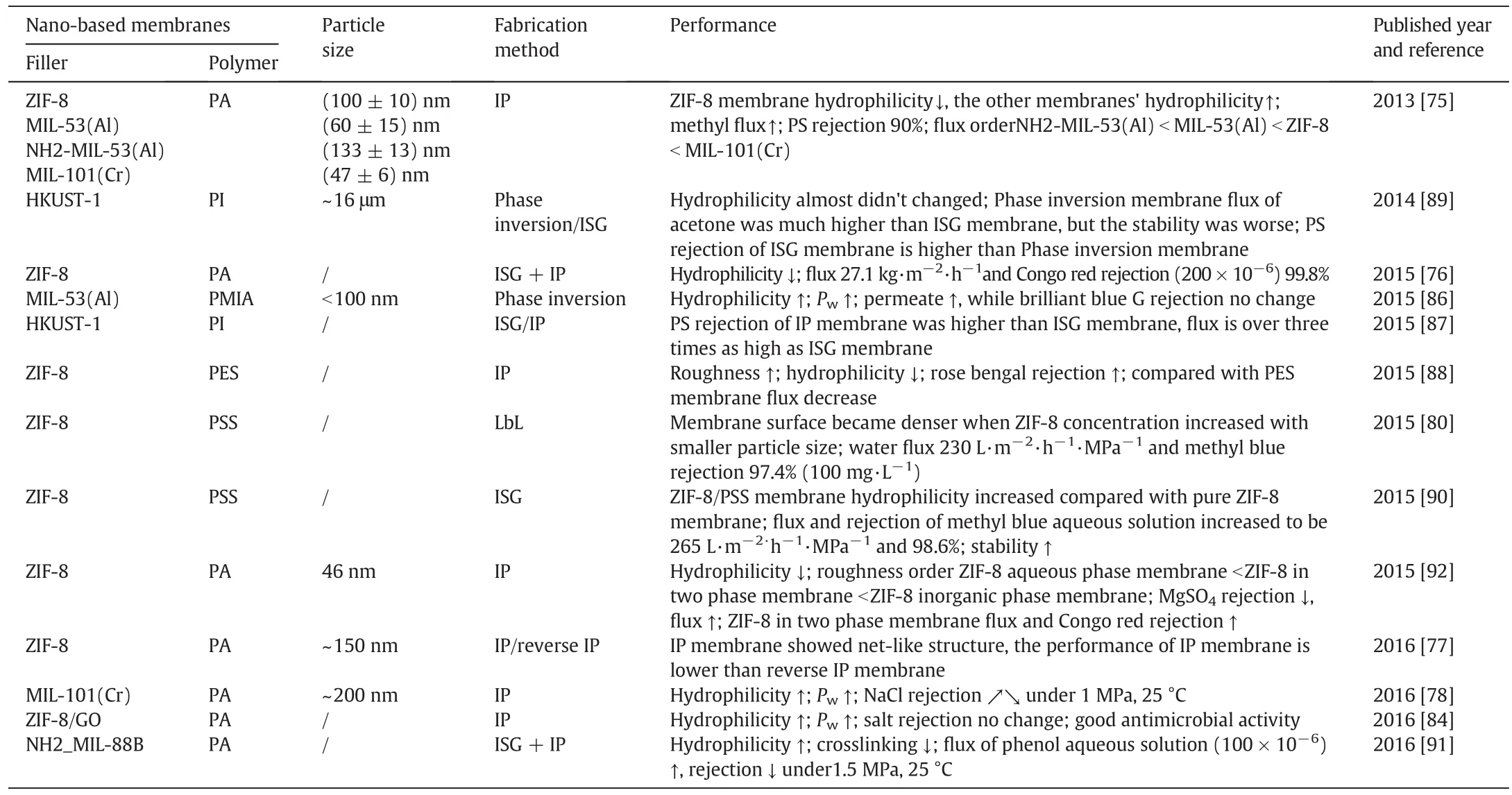
Table 3Summary of nanofiltration membranes prepared with MOFs
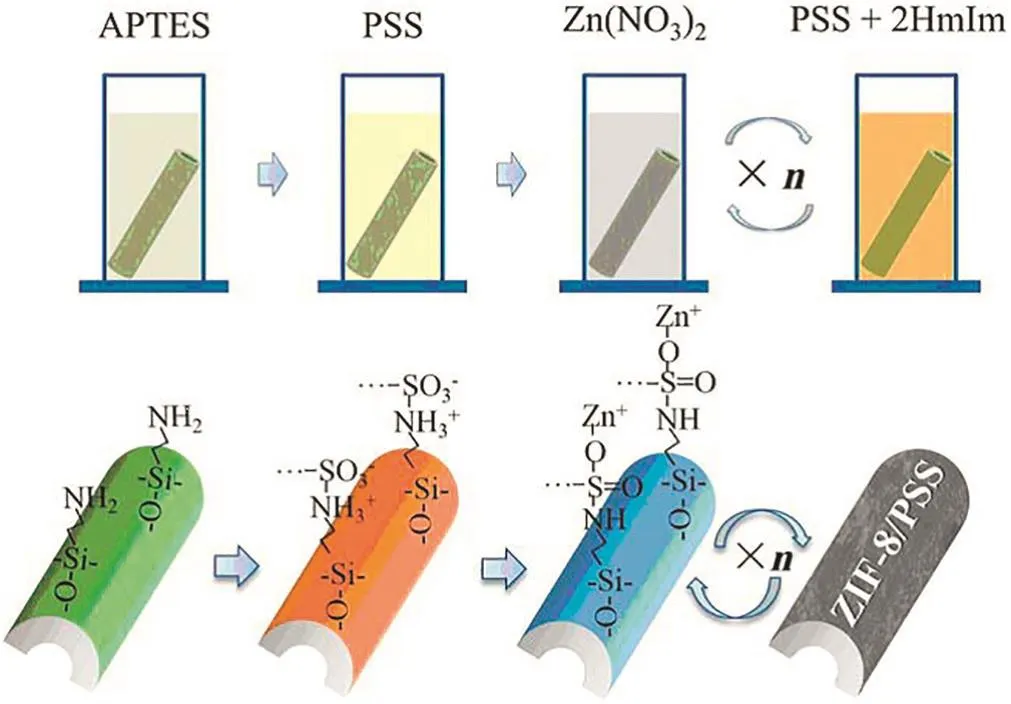
Fig.7.Schematic illustration of preparing ZIF-8/PSS membrane on tubular ceramic substrate by LbL assembly method.Reprinted with permission from Ref.[80].Copy right(2016)Wiley-VCH.
2.4.Nanofiltration membranes prepared with water channel proteins
State-of-the-art synthetic membrane separation performance has been improved a lot,the energy consumption of which is about 15-20%of that used for the early membranes[93].However there is a requirement for membranes with advanced perm-selectivity.Cell membranes exhibit ultrahigh perm-selectivity,the substances like water,ions or small organic molecules can transport through the lipid bilayer regulated by the water channel proteins.In 1993,Agreet al.first discovered the Aquaporin-1(AQP1)water channel proteins and won the Nobel Prize[94].It is found that the water molecules can move freely and quickly at rates of 2×109-8×109molecules per second through single aquaporins[95].Biological lipid bilayers containing aquaporins exhibit much higher water permeability and substance selectivity than all of common commercial membranes.With the unique perm-selectivity,AQP water channel proteins have attracted research interest as functional building blocks of biomimetic membranes for water treatment and substance separation.
Kumaret al.[96]have prepared a MMM with the Aquaporin-Z water channel proteins extracted fromE.colibacterial cells(Fig.8).A triblock copolymer with a high hydrophobic to hydrophilic block ratio was selected,and the resultant protein-polymer membrane demonstrated a remarked high water permeability of 2.5 × 10-11MPa-1·s-1,which is an order of magnitude higher than the purely polymeric membrane and commercial seawater RO membranes.However the major issue is how to fabricate stable biomimetic membrane in scale-up for industrial applications.To overcome this issue,Kaufmanet al.[97]proposed to use a commercial nanofiltration(NTR-7450)membrane as support layer for preparing biomimetic lipid bilayer to render them robust capability to withstand the required pressures.There is an electrostatic interaction between nanofiltration membrane surface and the oppositely charged protein vesicles,however,there is a decrease in permeability from~3× 10-11to~2× 10-12MPa-1·s-1.Zhonget al.[98]fabricated biomimetic membranes consisting of Aquaporin Z(AqpZ)by vesicle rupture of triblock copolymer(ABA)vesicles and UV polymerization on a cellulose acetate substrate membrane.The effect of AqpZ:ABA ratio on nanofiltration performance was investigated.It is found that the membranes comprising AqpZ:ABA ratio of 1:50 exhibited an impressive water permeability of 340 L·m-2·h-1·MPa-1and more than 30%NaCl rejection.
Zhaoet al.[99]prepared an aquaporin-based biomimetic membrane(ABM)by the interfacial polymerization method,where AqpZ with proteoliposomes was added to the MPD aqueous solution and interfacial reacted with TMC on the polysulfone substrate.The resulting membrane ABMwith more than 200 cm2area,had good mechanical stability.High water permeability(40 L·m-2·h-1·MPa-1)and good NaCl rejection(~97%)were observed,which is higher than that of commercial RO membranes(BW30/SW30HR),indicating the potential of ABM for desalination applications.Recently,Dinget al.[100]developed a new kind of biomimetic membranes,which were prepared through an amidation reaction to form covalent bonds between the AqpZ-incorporated supported lipid bilayer(SLB)and a polydopamine(PDA)layer on the porous polysulfone substrate(Fig.9).The nanofiltration results showed that there was a high water permeability of 63.1 L·m-2·h-1·MPa-1with relatively low solute permeability of 17 L·m-2·h-1·MPa-1for the AqpZ incorporated membrane.Nowadays,there is a great improvement in preparation of biomimetic MMMs,however,it is still hard to attain large quantities of water channel proteins and produce large areas of biomimetic membranes without defects for actual applications.
2.5.Nanofiltration membranes prepared with organic micro/nanoparticles
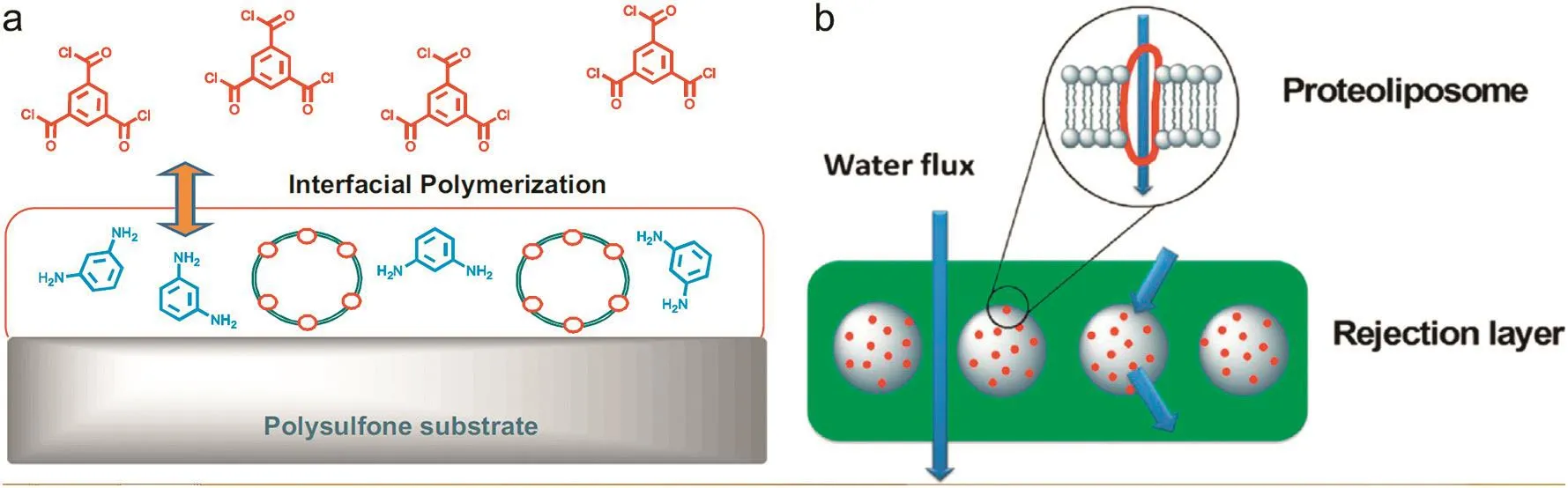
Fig.8.Schematics showing the synthesis of an aquaporin-based biomimetic membrane(ABM)(a)and the conceptual model of ABM(b).Reprinted with permission from Ref.[98].Copy right(2012)Elsevier.
With the development of techniques for synthesizing nanoparticles,it is becoming an alternative research direction for preparation of nanocomposite membranes with polymeric micro/nanoparticles.Compared to inorganic nanoparticles,polymeric micro/nanoparticles have greater flexibility for membrane formation,better compatibility with polymeric matrix,and easier tunability in particle structure,making them particularly suitable for preparing multifunctional MMMs[101,102].Some polymeric micro/nanoparticles,such as PEI particles,hyperbranched/dendrimer polymers and polymeric nanospheres,have been used for the preparation of MMMs.Kotteet al.[103], first prepared a PVDF MMMwith in-situ synthesized polyethylenimine(PEI)particles,wherein the branched PEIas precursor with epichlorohydrin crosslinker was added in the PVDF solution,thus PEI particles(~400 nm to 3 μm)were formed and incorporated into PVDF matrix.
Hyperbranched and dendrimer polymers are a unique kind of macromolecules with three-dimensional network structure and have numerous reactive terminal groups to take part in the membrane formation[104,105].They have been used as additive,precursor,and cross-linking agents in the preparation of membranes.Weiet al.[106]prepared nanofiltration membranes with hydroxyl-ended hyperbranched polyester(HPE)and TMC by in-situ interfacial polymerization on polysulfone membrane.Chianget al.[107]have also used the hyperbranched PEI and TMC to prepare nanofiltration membrane,and compared it with those linear amine membranes.They found that the amine groups could shift inside the pores of hyperbranched PEI,which facilitate water molecules transporting through the membrane and increase the salt rejection.However,because the diffusion of hyperbranched polymers from one phase into the other phase is more difficult than the traditional amine monomers,as a result there is a decrease in the reactivity of hyperbranched polymers and a loose membrane structure.Qinet al.[108]chose 4-dimethylaminopyridine as a catalyst to facilitate the phase transfer of hyperbranched polyesteramide in interfacial polymerization and driven to the formation of a dense,rough,and hydrophilic thin film composite membrane(Fig.10).In addition,due to the existence of numerous terminal groups in the hyperbranched polymer,a new type of β-cyclodextrindendrimer/TMC nanocomposite membranes loaded with Fe/Ni nanoparticles was prepared[109].The dendrimer-membrane was immersed in a Fe3+and Ni2+solution,then the Fe/Ni nanoparticles(40-66 nm)were formed and uniformly dispersed in the membrane.This study indicated that dendrimers are promising building blocks for fabrication of multi-nanocomposite membranes used for removal of organic pollutants from water.
Zwitterionic polymers,bearing both anionic and cationic groups in the same monomer unit,are promising materials for preparing water treatment membranes.In particular,zwitterionic polymers exhibit distinctive water binding ability,which not only facilitates the water transport through the membrane,but also improves their antifouling properties[110,111].Preliminary studies showed that the incorporation of zwitterionic groups into nanofiltration membranes by means of interfacial polymerization[112-114]and surface modification[115,116]could improve both the water permeation and antifouling performances of the membranes.Recently we found that zwitterionic polymers could be assembled into colloid particles for constructing a unique class of nanomaterials for preparing nanofiltration membranes.We fabricated a novel type of TFNMs containing zwitterionic polyelectrolyte nanoparticles(ZPNPs)by interfacial polymerization.The ZPNPs particles were prepared through ionic crosslinking between zwitterionic copolymers and sodium carboxymethyl cellulose[117].By modulating the zwitterionic group content and ionic cross-linking degree of ZPNPs,an optimized nanofiltration performance was obtained by the zwitterionic nanocomposite membrane.It showed a high water permeability(109.7 L·m-2·h-1·MPa-1)and enhanced NaCl/Na2SO4selectivity(28.4),respectively;these values were 191%and 125%of those for the pristine polyamide membrane(Fig.11).In addition,zwitterionic nanogel(ZNG)particles were synthesizedviaa facile and straightforward surfactant-free emulsion polymerization method with 3-dimethyl(methacryloyloxyethyl)ammonium propane sulfonate and 2-hydroxyethyl acrylate[118].The size of free-volume and nano-cavity in ZNG/PIP-TMC nanocomposite membranes could be conveniently tuned with the ZNG loading content.Positron annihilation results demonstrated that the enhanced water permeability of the zwitterionic TFNMs is mainly due to the high hydrophilicity of zwitterionic nanoparticles and the existence of nanoscale channels in membranes,which provide preferential flow paths for the water molecules.The high salt selectivity was achieved by the strong electrostatic repulsion and the appropriate free-volume within membranes.
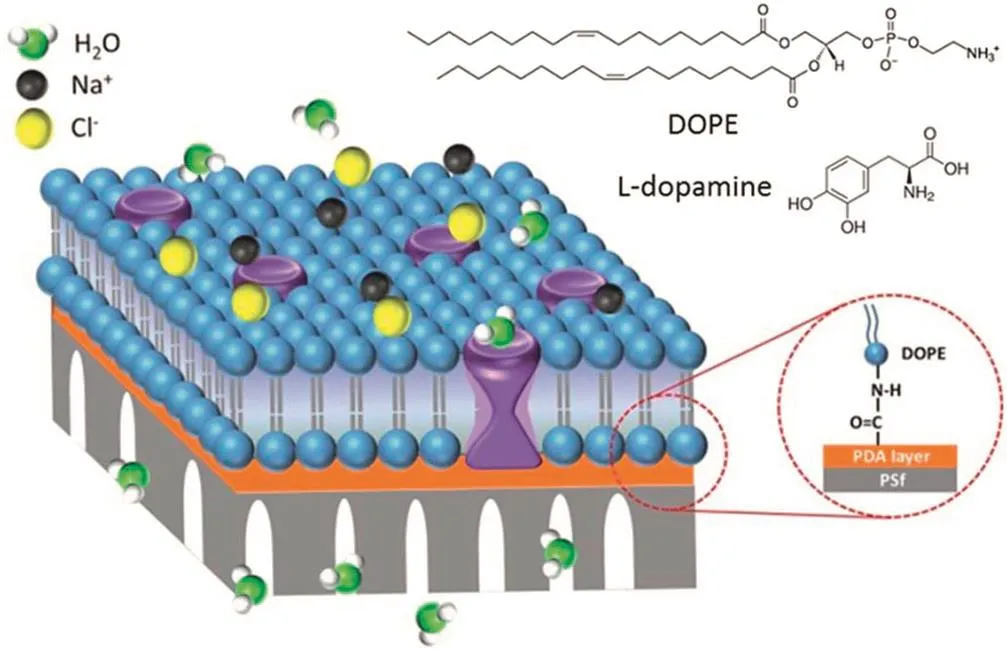
Fig.9.Schematic presentation of the structure of the AqpZ-incorporated SLB membrane with covalent bonds.By using EDC/S-NHS as the catalyst,the amide bond linked DOPE SLB is established on top of a PDA layer(brown)coated porous PSf(gray)support.Reprinted with permission from Ref.[100].Copy right(2015)Royal Society of Chemistry.

Fig.10.Schematic illustration of the interfacial polymerization in the preparation process of the hyperbranched polyesteramide composite membrane.Reprinted with permission from Ref.[108].Copy right(2013)American Chemical Society.
Polyaniline as conductive polymer is a new kind of nanomaterials suitable for preparing nanofiltration membranes due to its tunability in hydrophobic/hydrophilic property and charged character,and the stability and processibility for membrane formation[119].Liet al.[120]have prepared nanocomposite nanofiltration membranes incorporated with polyaniline by interfacial polymerization between PIP and TMC on polysulfone membrane.The results showed that water flux of the nanocomposite membrane was increased from 45 to 70 L·m-2·h-1,meanwhile,the membrane had good salt separation performance,retentions of Na2SO4,MgSO4,MgCl2and NaCl were 99.4%,98.5%,85.4%and 59.2%,respectively.Recently,polyhedral oligomeric silsesquioxane(POSS)was chosen as nanoparticles for preparing nanofiltration membranes,which has flexible backbone,small size(1-3 nm),and high degree of dispersibility and compatibility in polymeric matrix[121].Dalwaniet al.[122]used POSS ammonium salt as aqueous functional materials and interfacial polymerized with TMC to prepare a thin POSS-polyamide membrane.Heet al.[123]reported a TFNM comprising POSS nanoparticles.The resultant membrane had a good water permeability of 54 L·m-2·h-1·MPa-1and high rejections of SeO32-,SeO42-and HAsO42-toward 93.9%,96.5%,and 97.4%,respectively.The high rejections against selenium and arsenic anions are attributed to the small membrane pore size(0.65 nm,smaller than the hydrated diameters of these anions)and highly negatively charged density,consequently,combining the Donnan and size exclusion effect,the membrane could successfully reject anions.
3.Conclusions and Future Perspectives
Nano filtration has been widely used forseawaterand brackish water desalination,pure water and wastewater treatment,and the substance separation and purification in industrials.It has become one of the main technologies to tackle the globalissues of water scarcity and environmental pollution.Remarkable progress has been made in nanofiltration membrane preparation and application because of its good separation performance.The demand to further reduce energy consumption and operation cost has continually motivated researchers exploring advanced nanofiltration membranes with superior permeability and selectivity,and discovering structural stable and fouling resistant nanofiltration membranes for actual application.
The research of nanofiltration membranes incorporated with nanomaterials has attracted increasing attention during the last two decades.In this paper,the progress of new generation nanofiltration membrane based on nanomaterials was reviewed,classified into different types of nano-based nanofiltration membranes with various nanomaterials.It has been reported that the nanomaterial based nanofiltration membranes have great potential in overcoming the “trade-off”problem between permeability and selectivity.Meanwhile,the mechanical/thermal-stability and antifouling properties could be improved with incorporation of specific nanomaterials.Despite the significant research achievements,there are still some challenges encountered during nanomaterial based nanofiltration membrane fabrication:
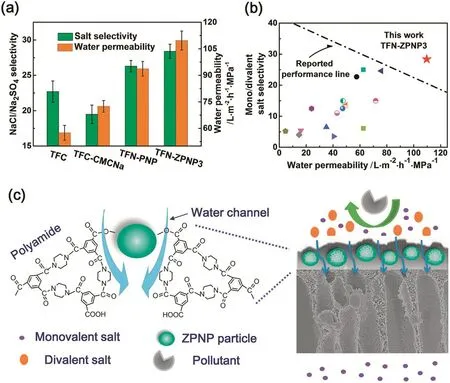
Fig.11.(a)Separation performance of TFC,TFC-CMCNa,TFN-PNP,and TFN-ZPNP3 membranes tested with pure water and 1 g·L-1 aqueous Na2SO4 and NaCl solutions(pH=7.0)at25°C and 0.6 MPa;(b)comparison of the TFN-ZPNP3 membrane with various polyamide membranes reported in previous studies;(c)schematic illustration of the microstructure and separation performance of the TFN-ZPNP membrane.Reprinted with permission from Ref.[117].Copy right(2015)Royal Society of Chemistry.
Nanocomposite membranes incorporated with inorganic nanomaterials have been studied a lot,however,the relatively poor dispersion of inorganic nanomaterials and their agglomeration in polymeric matrix,and lack of chemical interaction with matrix/support layer,easily lead to performance degradation.It is needed to develop nanocomposite membranes based on advanced nanomaterials with suitable chemical/physical characteristics that could homogenously distribute and stably exist in polymeric matrix membranes.Comparing with the traditional introduction methods for metal and metal oxide nanoparticles,the in-situ preparative technique has demonstrated superiority in controlling dispersibility,compatibility and stability of nanomaterials in/on the membrane matrix,such nano-based membranes may have high and stable nanofiltration performance.
With the introduction of carbon-based nanomaterials,e.g.,CNT and graphene,the nanocomposite membranes normally show an improved permeability,mechanical/thermal-stability and antifouling properties.In order to fully play the role of CNT and graphene in the construction of nano-channelwithin membranes,the efficient and convenient orientation membrane preparation method should be developed.Moreover,graphene has advantages in the fabrication of ultra-thin separation membrane,but the membrane cross-linking density and stability should be further enhanced for satisfying application requirements.
MOFs as a novel kind of porous nanomaterials with tunable chemical structure and physical characteristics have shown prospects in fabricating nanofiltration membranes.Compared with the inorganic porous nanomaterials,MOFs have not only high surface area and pore volume but also an organic and flexible backbone,there would be a good compatibility between MOFs and polymeric matrix membrane.However,the in-situ growth regularity of MOFs and their stability within membranes also need to be well controlled to achieve high nanofiltration performance.
The membranes embedded with water channel proteins have shown an extremely high water permeability,but their actual applications were still limited by the expensive and complex methods of extracting proteins and maintaining their activity in the whole membrane preparation and application process.Additionally,some new kinds of organic particles have been synthesized and adopted to fabricate nanocomposite nanofiltration membranes,which exhibit good compatibility and perm-selectivity.But it also requires consideration of their pressure resistance in nanofiltration process,and the practicality and suitability with current membrane modules and manufacturing process as well as the cost for up-scale fabrication.
Nanomaterials based nanofiltration membranes show high separation performance and antifouling properties,it is highly recommended to develop advanced instruments and simulation techniques to characterize and analyse the variation of membrane microstructure in actual applications.It is very important to understand the interaction between the matrix membrane and nanomaterials as well as the transport and antifouling mechanism of the nano-based nanofiltration membranes.It would provide more fundamental knowledge for exploiting advanced nanofiltration membranes for water treatment and substance separation applications.
Nomenclature
AAO anodic aluminum oxide
ABM aquaporin-based biomimetic membranes
Ag NPs silver nanoparticles
AQP1 Aquaporin-1
AqpZ Aquaporin Z
CA cellulose acetate
CCG chemically converted graphene
CNT carbon nanotube
CS chitosan
DOPE dioleoyl phosphoethanolamine
DMAP 4-dimethylaminopyridine
ECH epichlorohydrin
EDCN-ethyl-N′-(3-dimethylaminopropyl)carbodiimide
GCNTm graphene and multiwalled carbon nanotubes membrane
GFD gallons per square foot per day
GO graphene oxide
Hheight,nm
HBPs hyperbranched polymers
HPE hydroxyl-ended hyperbranched polyester
IP interfacial polymerization
ISG in-situ growth
Llong,μm
LbL layer-by-layer
MMMs mixed matrix membranes
MOFs metal-organic frameworks
MPD metaphenylene diamine
MWCNTs multiwalled carbon nanotubes
NaA naphthylacetic acid
NCC nanoconfined composite
OCMCO-(carboxymethyl)-chitosan
OD out diameter
OMC ordered mesoporous carbon
PA polyamide
PAA polyacrylic acid
PDA polydopamine
PEC polyelectrolyte complex
PEI polyethyleneimine
PES polyether sulfone
PI polyimide
PIP piperazine
PMIA polyisophthaloyl metaphenylene diamine
PMMA polymethyl methacrylate
POSS polyhedral oligomeric silsesquioxane
PTFE polytetra fluoroethylene
PVA polyvinyl alcohol
PVDF polyvinylidene fluoride
Pwpermeate of water,L·m-2·h-1·MPa-1
SA sodium alginate
SiO2silica
SLB supported lipid bilayer
S-NHS sulfo-N-hydroxysuccinimide
SPSF sulfonated polysulfone
SWNTs single-walled carbon nanotubes
TEOA triethanolamine
TFNM thin film nanocomposite membrane
TiO2titanium oxide
TMC trimesoyl chloride
uGNM ultrathin graphene nanofiltration membranes
ZIF-8 zeolitic imidazolate framework-8
ZNGs zwitterionic nanogels
ZPNPs zwitterionic polyelectrolyte nanoparticles
[1]M.A.Shannon,P.W.Bohn,M.Elimelech,J.G.Georgiadis,B.J.Marinas,A.M.Mayes,Science and technology for water purification in the coming decades,Nature452(2008)301-310.
[2]M.Elimelech,W.A.Phillip,The future of seawater desalination:Energy,technology,and the environment,Science333(2011)712-717.
[3]K.P.Lee,T.C.Arnot,D.Mattia,Areview of reverse osmosis membrane materials for desalination-development to date and future potential,J.Membr.Sci.370(2011)1-22.
[4]P.Vandezande,L.E.M.Gevers,I.F.J.Vankelecom,Solvent resistant nanofiltration:separating on a molecular level,Chem.Soc.Rev.37(2008)365-405.
[5]R.W.Baker,Membrane Technology and Applications,second ed.John Wiley&Sons,NY,2004.
[6]R.J.Petersen,Composite reverse-osmosis and nanofiltration membranes,J.Membr.Sci.83(1993)81-150.
[7]X.Zheng,Y.S.Wei,Z.W.Wang,Z.X.Zhang,Report for sustainable development strategy of china water treatment industry,Membrane and Industry II,China Renmin University Press,Beijing,2016(in Chinese).
[8]M.Ulbricht,Advanced functional polymer membranes,Polymer47(2006)2217-2262.
[9]J.R.Werber,C.O.Osuji,M.Elimelech,Materials for next-generation desalination and water purification membranes,Nat.Rev.Mater.16018(2016)1-15.
[10]D.Li,H.T.Wang,Recent developments in reverse osmosis desalination membranes,J.Mater.Chem.20(2010)4551-4566.
[11]A.G.Fane,R.Wang,M.X.Hu,Synthetic membranes for water purification:Status and future,Angew.Chem.Int.Ed.54(2015)3368-3386.
[12]A.W.Mohammad,Y.H.Teowa,W.L.Ang,Y.T.Chung,D.L.Oatley-Radcliffe,N.Hila,Nano filtration membranes review:Recent advances and future prospects,Desalination356(2015)226-254.
[13]M.M.Pendergast,E.M.V.Hoek,A review of water treatment membrane nanotechnologies,Energy Environ.Sci.4(2011)1946-1971.
[14]D.Li,Y.S.Yan,H.T.Wang,Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes,Prog.Polym.Sci.61(2016)104-155.
[15]J.Yin,B.L.Deng,Polymer-matrix nanocomposite membranes for water treatment,J.Membr.Sci.479(2015)256-275.
[16]J.H.Jhaveri,Z.V.P.Jhaveri,V.Murthy,A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes,Desalination379(2016)137-154.
[17]P.S.Goh,A.F.Ismail,N.Hilal,Nano-enabled membranes technology:Sustainable and revolutionary solutions for membrane desalination,Desalination380(2016)100-104.
[18]B.H.Jeong,E.M.V.Hoek,Y.S.Yan,A.Subramani,X.F.Huang,G.Hurwitz,A.K.Ghosh,A.Jawor,Interfacial polymerization of thin film nanocomposites:A new concept for reverse osmosis membranes,J.Membr.Sci.294(2007)1-7.
[19]M.L.Lind,B.H.Jeong,A.Subramani,X.Huang,E.M.V.Hoek,Effect of mobile cation on zeolite-polyamide thin film nanocomposite membranes,J.Mater.Res.24(2009)1624-1631.
[20]M.L.Lind,A.K.Ghosh,A.Jawor,X.Huang,W.Hou,Y.Yang,E.M.V.Hoek,Influence of zeolite crystal size on zeolite-polyamide thin film nanocomposite membranes,Langmuir25(2009)10139-10145.
[21]H.Huang,X.Qu,H.Dong,L.Zhang,H.Chen,Role of NaA zeolites in the interfacial polymerization process towards a polyamide nanocomposite reverse osmosis membrane,RSC Adv.3(2013)8203-8207.
[22]L.X.Dong,X.C.Huang,Z.Wang,Z.Yang,X.M.Wang,C.Y.Tang,A thin- film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles,Sep.Purif.Methods166(2016)230-239.
[23]H.Q.Wu,B.B.Tang,P.Y.Wu,Optimizing polyamide thin film composite membrane covalently bonded with modified mesoporous silica nanoparticles,J.Membr.Sci.428(2013)341-348.
[24]Y.F.Li,H.Mao,H.Q.Zhang,G.H.Yang,R.Ding,J.T.Wang,Tuning the microstructure and permeation property of thin film nanocomposite membrane by functionalized inorganic nanospheres for solvent resistant nanofiltration,Sep.Purif.Methods165(2016)60-70.
[25]K.Sunada,Y.Kikuchi,K.Hashimoto,A.Fujishima,Bactericidal and detoxification effects of TiO2thin film photocatalysts,Environ.Sci.Technol.32(1998)726-728.
[26]S.H.Kim,S.Y.Kwak,B.H.Sohn,T.H.Park,Design of TiO2nanoparticle self-assembled aromatic polyamide thin- film-composite(TFC)membrane as an approach to solve biofouling problem,J.Membr.Sci.211(2003)157-165.
[27]S.Y.Kwak,S.H.Kim,S.S.Kim,Hybrid organic/inorganic reverse osmosis(RO)membrane for bactericidal anti-fouling.1.Preparation and characterization of TiO2nanoparticle self-assembled aromatic polyamide thin- film-composite(TFC)membrane,Environ.Sci.Technol.35(2001)2388-2394.
[28]S.Madaeni,N.Ghaemi,Characterization of self-cleaning RO membranes coated with TiO2particles under UV irradiation,J.Membr.Sci.303(2007)221-233.
[29]R.A.Damodar,S.J.You,H.H.Chou,Study the self cleaning,antibacterial and photocatalytic properties of TiO2entrapped PVDF membranes,J.Hazard.Mater.172(2009)1321-1328.
[30]Z.N.Song,M.Fathizadeh,Y.Huang,K.Chu,Y.Yoon,L.Wang,W.W.Xu,M.Yu,TiO2nanofiltration membranes prepared by molecular layer deposition for water purification,J.Membr.Sci.510(2016)72-78.
[31]W.L.Chou,D.G.Yu,M.C.Yang,The preparation and characterization of silver loading cellulose acetate hollow fiber membrane for water treatment,Polym.Adv.Technol.16(2005)600-607.
[32]K.Zodrow,L.Brunet,S.Mahendra,D.Li,A.Zhang,Q.L.Li,P.J.J.Alvarez,Polysulfone ultra filtration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal,Water Res.43(2009)715-723.
[33]A.Mollahosseini,A.Rahimpour,A new concept in polymeric thin- film composite nanofiltration membranes with antibacterial properties,Biofouling29(2013)537-548.
[34]X.Liu,S.Qi,L.Yang,B.Cao,C.Y.Tang,Synthesis and characterization of novel antibacterial silver nanocomposite nanofiltration and forward osmosis membranes based on layer-by-layer assembly,Water Res.47(2013)3081-3092.
[35]J.Yin,Y.Yang,Z.Q.Hu,B.L.Deng,Attachment of silver nanoparticles(AgNPs)onto thin- film composite(TFC)membranes through covalent bonding to reduce membrane biofouling,J.Membr.Sci.441(2013)73-82.
[36]S.S.Liu,F.Fang,J.J.Wu,K.S.Zhang,The anti-biofouling properties of thin- film composite nanofiltration membranes grafted with biogenic silver nanoparticles,Desalination375(2015)121-128.
[37]Y.Zhang,Y.Wan,Y.T.Shi,G.Y.Pan,H.Yan,J.Xu,M.Guo,L.X.Qin,Y.Q.Liu,Facile modification of thin- film composite nanofiltration membrane with silver nanoparticles for anti-biofouling,J.Polym.Res.23(2016)1-9.
[38]S.Y.Lee,H.J.Kim,R.K.Patel,S.J.Im,J.H.Kim,B.R.Min,Silver nanoparticles immobilized on thin film composite polyamide membrane:Characterization,nano filtration,antifouling properties,Polym.Adv.Technol.18(2007)562-568.
[39]C.L.Kong,A.Koushima,T.Kamada,T.Shintani,M.Kanezashi,T.Yoshioka,T.Tsuru,Enhanced performance of inorganic-polyamide nanocomposite membranes prepared by metal-alkoxide-assisted interfacial polymerization,J.Membr.Sci.366(2011)382-388.
[40]E.S.Kim,B.Deng,Fabrication of polyamide thin- film nano-composite(PATFN)membrane with hydrophilized ordered mesoporous carbon(H-OMC)for water purifications,J.Membr.Sci.375(2011)46-54.
[41]E.S.Kim,G.Hwang,M.G.El-Din,Y.Liu,Development of nanosilver and multiwalled carbon nanotubes thin- film nanocomposite membrane for enhanced water treatment,J.Membr.Sci.394-395(2012)37-48.
[42]D.Hu,Z.L.Xu,Y.M.Wei,A high performance silica- fluoropolyamide nanofiltration membrane prepared by interfacial polymerization,Sep.Purif.Technol.110(2013)31-38.
[43]B.Rajaeian,A.Rahimpour,M.O.Tade,S.M.Liu,Fabrication and characterization of polyamide thin film nanocomposite(TFN)nano filtration membrane impregnated with TiO2nanoparticles,Desalination313(2013)176-188.
[44]P.Daraei,S.S.Madaeni,E.Salehi,N.Ghaemi,H.S.Ghari,M.A.Khadivi,E.Rostami,Novel thin film composite membrane fabricated by mixed matrix nanoclay/chitosan on PVDF micro filtration support:Preparation,characterization and performance in dye removal,J.Membr.Sci.436(2013)97-108.
[45]M.Majumder,N.Chopra,R.Andrews,B.J.Hinds,Colloid science:Non-spherical bubbles,Nature438(2005)930.
[46]H.Q.Wu,B.B.Tang,P.Y.Wu,MWNTs/polyester thin film nanocomposite membrane:An approach to overcome the trade-off effect between permeability and selectivity,J.Phys.Chem.C114(2010)16395-16400.
[47]A.Tiraferri,C.D.Vecitis,M.Elimelech,Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties,ACS Appl.Mater.Interfaces3(2011)2869-2877.
[48]H.D.Lee,H.W.Kim,Y.H.Cho,H.B.Park,Experimental evidence of rapid water transport through carbon nanotubes embedded in polymeric desalination membranes,Small10(2014)2653-2660.
[49]F.Y.Zhao,Y.L.Ji,X.D.Weng,Y.F.Mi,C.C.Ye,Q.F.An,C.J.Gao,High- flux positively charged nanocomposite nanofiltration membranes filled with poly(dopamine)modified multiwall carbon nanotubes,ACS Appl.Mater.Interfaces8(2016)6693-6700.
[50]F.Y.Zhao,Q.F.An,Y.L.Ji,C.J.Gao,A novel type of polyelectrolyte complex/MWCNT hybrid nanofiltration membranes for water softening,J.Membr.Sci.492(2015)412-421.
[51]M.Terrones,N.Grobert,J.Olivares,J.P.Zhang,H.Terrones,K.Kordatos,W.K.Hsu,J.P.Hare,P.D.Townsend,K.Prassides,A.K.Cheetham,H.W.Kroto,D.R.M.Walton,Controlled production of aligned-nanotube bundles,Nature388(1997)52-55.
[52]K.Bubke,H.Gnewuch,M.Hempstead,J.Hammer,M.L.H.Green,Optical anisotropy of dispersed carbon nanotubes induced by an electric field,Appl.Phys.Lett.71(1997)1906-1908.
[53]M.R.Maschmann,A.D.Franklin,P.B.Amama,D.N.Zakharov,E.A.Stach,T.D.Sands,T.S.Fisher,Vertical single-and double-walled carbon nanotubes grown from modified porous anodic alumina templates,Nanotechnology17(2006)3925-3929.
[54]W.F.Chan,H.Y.Chen,A.Surapathi,M.G.Taylor,X.H.Shao,E.Marand,J.K.Johnson,Zwitterion functionalized carbon nanotube/polyamide nanocomposite membranes for water desalination,ACS Nano7(2013)5308-5319.
[55]H.Y.Zhao,Z.J.Zhou,H.Dong,L.Zhang,H.L.Chen,L.Hou,A facile method to align carbon nanotubes on polymeric membrane substrate,Sci.Rep.3(2013)3480-3484.
[56]G.P.Liu,W.Q.Jin,N.P.Xu,Graphene-based membranes,Chem.Soc.Rev.44(2015)5016-5030.
[57]N.X.Wang,S.L.Ji,G.J.Zhang,J.Li,L.Wang,Self-assembly of graphene oxide and polyelectrolyte complex nanohybrid membranes for nanofiltration and pervaporation,Chem.Eng.J.213(2012)318-329.
[58]S.Bano,A.Mahmood,S.J.Kim,K.H.Lee,Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties,J.Mater.Chem.A3(2015)2065-2071.
[59]M.Hu,B.X.Mi,Enabling graphene oxide nanosheets as water separation membranes,Environ.Sci.Technol.47(2013)3715-3723.
[60]G.Cicero,J.C.Grossman,E.Schwegler,F.Gygi,G.Galli,Water confined in nanotubes and between graphene sheets:a first principle study,J.Am.Chem.Soc.130(2008)1871-1878.
[61]S.K.Kannam,B.D.Todd,J.S.Hansen,P.J.Daivis,Slip length of water on graphene:limitations of non-equilibrium molecular dynamics simulations,J.Chem.Phys.136(2)(2012)140-151.
[62]L.Qiu,X.H.Zhang,W.R.Yang,Y.F.Wang,G.P.Simon,D.Li,Controllable corrugation of chemically converted graphene sheets in water and potential application for nanofiltration,Chem.Commun.47(2011)5810-5812.
[63]Y.Han,Z.Xu,C.Gao,Ultrathin graphene nanofiltration membrane for water purification,Adv.Funct.Mater.23(2013)3693-3700.
[64]Y.Han,Y.Q.Jiang,C.Gao,High- flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes,ACS Appl.Mater.Interfaces7(2015)8147-8155.
[65]W.S.Hung,C.H.Tsou,M.D.Guzman,Q.F.An,Y.L.Liu,Y.M.Zhang,C.C.Hu,K.R.Lee,J.Y.Lai,Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing,Chem.Mater.26(2014)2983-2990.
[66]F.Perreault,M.E.Tousley,M.Elimelech,Thin- film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets,Environ.Sci.Technol.Lett.(2013)71-76.
[67]S.B.Liu,T.H.Zeng,M.Hofmann,E.Burcombe,J.Wei,R.R.Jiang,J.Kong,Y.Chen,Antibacterial activity of graphite,graphite oxide,graphene oxide,and reduced graphene oxide:membrane and oxidative stress,ACS Nano5(2011)6971-6980.
[68]J.L.Wang,X.L.Gao,J.Wang,Y.Wei,Z.K.Li,C.J.Gao,O-(Carboxymethyl)-chitosan nanofiltration membrane surface functionalized with graphene oxide nanosheets for enhanced desalting properties,ACS Appl.Mater.Interfaces7(2015)4381-4389.
[69]S.Roy,S.A.Ntim,S.Mitra,K.K.Sirkar,Facile fabrication of superior nanofiltration membranes from interfacially polymerized CNT-polymer composites,J.Membr.Sci.375(2011)81-87.
[70]G.N.Baroña,M.Choi,B.Jung,High permeate flux of PVA/PSf thin film composite nanofiltration membrane with aluminosilicate single-walled nanotubes,J.Colloid Interface Sci.386(2012)189-197.
[71]J.N.Shen,C.C.Yu,H.M.Ruan,C.J.Gao,B.V.Bruggen,Preparation and characterization of thin- film nanocomposite membranes embedded with poly(methyl methacrylate)hydrophobic modified multiwalled carbon nanotubes by interfacial polymerization,J.Membr.Sci.442(2013)18-26.
[72]C.C.Yu,H.W.Yu,Y.X.Chu,H.M.Ruan,J.N.Shen,Preparation thin film nanocomposite membrane incorporating PMMA modified MWNT for nanofiltration,Key Eng.Mater.565(2013)882-886.
[73]H.Q.Wu,B.B.Tang,P.Y.Wu,Optimization,characterization and nanofiltration properties test of MWNTs/polyester thin film nanocomposite membrane,J.Membr.Sci.428(2013)425-433.
[74]B.Seoane,J.Coronas,I.Gascon,M.E.Benavides,O.Karvan,J.Caro,J.Gascon,Metalorganic framework based mixed matrix membranes:a solution for highly efficient CO2capture,Chem.Soc.Rev.44(2015)2421-2454.
[75]S.Sorribas,P.Gorgojo,C.Tellez,J.Coronas,A.G.Livingston,High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration,J.Am.Chem.Soc.135(40)(2013)15201-15208.
[76]L.Y.Wang,M.Q.Fang,J.Liu,J.He,J.D.Li,J.D.Lei,Layer-by-layer fabrication of highperformance polyamide/ZIF-8 nanocomposite membrane for nanofiltration applications,ACS Appl.Mater.Interfaces7(2015)24082-24093.
[77]C.V.Goethem,R.Verbeke,S.Hermans,R.Bernstein,I.F.J.Vankelecom,Controlled positioning of MOFs in interfacially polymerized thin- film nanocomposites,J.Mater.Chem.A4(2016)16368-16376.
[78]Y.Xu,X.L.Gao,Q.Wang,X.Y.Wang,Z.Y.Ji,C.J.Gao,Highly stable MIL-101(Cr)doped water permeable thin film nanocomposite membranes for water treatment,RSC Adv.6(2016)82669-82675.
[79]R.Zhang,S.Ji,N.Wang,L.Wang,G.Zhang,J.R.Li,Coordination-driven in situ selfassembly strategy for the preparation of metal-organic framework hybrid membranes,Angew.Chem.Int.Ed.53(2014)9775-9779.
[80]N.X.Wang,T.J.Liu,H.P.Shen,S.L.Ji,J.R.Li,Ceramic tubular MOF hybrid membrane fabricated through in situ layer-by-layer self-assembly for nanofiltration,AIChE J.62(2016)538-546.
[81]J.Campbell,J.D.S.Burgal,G.Szekely,R.P.Davies,D.C.Braddock,A.Livingston,Hybrid polymer/MOF membranes for organic solventnano filtration(OSN):Chemical modification and the quest for perfection,J.Membr.Sci.503(2016)166-176.
[82]A.S.Huang,Q.Liu,N.Y.Wang,Y.Q.Zhu,J.Caro,Bicontinuous zeolitic imidazolate framework ZIF-8@GO membrane with enhanced hydrogen selectivity,J.Am.Chem.Soc.136(2014)14686-14689.
[83]Y.Hu,J.Wei,Y.Liang,H.Zhang,X.Zhang,W.Shen,H.Wang,Zeolitic imidazolate framework/graphene oxide hybrid nanosheets as seeds for the growth of ultrathin molecular sieving membranes,Angew.Chem.Int.Ed.55(2016)2048-2052.
[84]J.Wang,Y.M.Wang,Y.T.Zhang,A.Uliana,J.Y.Zhu,J.D.Liu,B.V.Bruggen,Zeolitic imidazolate framework/graphene oxide hybrid nanosheets functionalized thin film nanocomposite membrane for enhanced antimicrobial performance,ACS Appl.Mater.Interfaces8(2016)25508-25519.
[85]N.X.Wang,X.T.Li,L.Wang,L.L.Zhang,G.J.Zhang,S.L.Ji,Nanoconfined zeolitic imidazolate framework membranes with composite layers of nearly zero thickness,ACS Appl.Mater.Interfaces8(2016)21979-21983.
[86]L.F.Zhu,H.W.Yu,H.J.Zhang,J.N.Shen,L.X.Xue,C.J.Gao,B.V.Bruggen,Mixed matrix membranes containing MIL-53(Al)for potential application in organic solvent nanofiltration,RSC Adv.5(89)(2015)73068-73076.
[87]J.Campbell,R.P.Davies,D.C.Braddockb,A.G.Livingston,Improving the permeance of hybrid polymer/metal organic framework(MOF)membranes for organic solvent nanofiltration(OSN)—development of MOF thin films via interfacial synthesis,J.Mater.Chem.A3(18)(2015)9668-9674.
[88]Y.B.Li,L.H.Wee,A.Volodin,J.A.Martensa,I.F.J.Vankelecom,Polymer supported ZIF-8 membranes prepared via an interfacial synthesis method,Chem.Commun.51(5)(2015)918-920.
[89]J.Campbell,G.Szekely,R.P.Davies,D.C.Braddock,A.G.Livingston,Fabrication of hybrid polymer/metal organic framework membranes:Mixed matrix membranes versus in situ growth,J.Mater.Chem.A2(2)(2014)9260-9271.
[90]R.Zhang,S.L.Ji,N.X.Wang,L.Wang,G.J.Zhang,J.R.Li,Coordination-driven in situ self-assembly strategy for the preparation of metal-organic framework hybrid membranes,Angew.Chem.Int.Ed.126(37)(2015)9933-9937.
[91]L.Y.Wang,S.X.Duan,M.Q.Fang,J.Liu,J.He,J.D.Li,J.D.Lei,Surface modification route to prepare novel polyamide@NH2_MIL-88B nanocomposite membranes for water treatment,RSC Adv.6(75)(2016)71250-71261.
[92]L.Y.Wang,M.Q.Fang,J.Liu,J.He,L.H.Deng,J.D.Li,J.D.Lei,The influence of dispersed phases on polyamide/ZIF-8 nanofiltration membranes for dye removal from water,RSC Adv.5(63)(2015)50942-50954.
[93]R.Truby,Seawater desalination by ultralow-energy reverse osmosis,Adv.Membr.Tech.Appl.(2008)87-100.
[94]P.Agre,S.Sasaki,M.J.Chrispeels,Aquaporins:a family of water channel proteins,Am.J.Physiol.Ren.Physiol.265(1993)F461.
[95]D.Kozono,M.Yasui,L.S.King,P.Agre,Aquaporin water channels:atomic structure and molecular dynamics meet clinical medicine,J.Clin.Invest.109(2002)1395-1399.
[96]M.Kumar,M.Grzelakowski,J.Zilles,M.Clark,W.Meier,Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z,Proc.Natl.Acad.Sci.U.S.A.104(2007)20719-20724.
[97]Y.Kaufman,A.Berman,V.Freger,Supported lipid bilayer membranes for water purification by reverse osmosis,Langmuir26(2010)7388-7395.
[98]P.S.Zhong,T.S.Chung,K.Jeyaseelan,A.Armugam,Aquaporin-embedded biomimetic membranes for nanofiltration,J.Membr.Sci.407(2012)27-33.
[99]Y.Zhao,C.Q.Qiu,X.S.Li,A.Vararattanavech,W.M.Shen,J.Torres,C.Nielsen,R.Wang,X.Hu,A.Fane,C.Tang,Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization,J.Membr.Sci.423(2012)422-428.
[100]W.D.Ding,J.Cai,Z.Y.Yu,Q.H.Wang,Z.N.Xu,Z.N.Wang,C.J.Gao,Fabrication of an aquaporin-based forward osmosis membrane through covalent bonding of a lipid bilayer to a microporous support,J.Mater.Chem.A3(2015)20118-20126.
[101]W.J.Lau,S.Gray,T.Matsuura,D.Emadzadeh,J.P.Chen,A.F.Ismail,A review on polyamide thin film nanocomposite(TFN)membranes:History,applications,challenges and approaches,Water Res.80(2015)306-324.
[102]X.Li,A.Sotto,J.S.Li,B.V.Bruggen,Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles,J.Membr.Sci.524(2017)502-528.
[103]M.R.Kotte,M.Cho,M.S.Diallo,A facile route to the preparation of mixed matrix polyvinylidene fluoride membranes with in-situ generated polyethyleneimine particles,J.Membr.Sci.450(2014)93-102.
[104]Y.C.Zheng,S.P.Li,Z.L.Weng,C.J.Gao,Hyperbranched polymers:Advances from synthesis to applications,Chem.Soc.Rev.44(12)(2015)4091-4130.
[105]H.J.Huang,S.Ramaswamy,U.W.Tschirner,B.V.Ramarao,A review of separation technologies in current and future biore fineries,Sep.Sci.Technol.62(2008)1-21.
[106]X.Z.Wei,L.P.Zhu,H.Y.Deng,Y.Y.Xu,B.K.Zhu,Z.M.Huang,New type of nanofiltration membrane based on crosslinked hyperbranched polymers,J.Membr.Sci.323(2008)278-287.
[107]Y.C.Chiang,Y.Z.Hsub,R.C.Ruaan,C.J.Chuang,K.L.Tung,Nano filtration membranes synthesized from hyperbranched polyethyleneimine,J.Membr.Sci.326(2009)19-26.
[108]J.X.Qin,S.S.Lin,S.Q.Song,L.Zhang,H.L.Chen,4-Dimethylaminopyridine promoted interfacial polymerization between hyperbranched polyesteramide and trimesoyl chloride for preparing ultralow-pressure reverse osmosis composite membrane,ACS Appl.Mater.Interfaces5(2013)6649-6656.
[109]S.P.Malinga,O.A.Arotiba,R.W.M.Krause,S.F.Mapolie,M.S.Diallo,B.B.Mamba,Composite polyester membranes with embedded dendrimer hosts and bimetallic Fe/Ni nanoparticles:Synthesis,characterisation and application to water treatment,J.Nanopart.Res.15(2013)2-15.
[110]Y.L.Ji,Q.F.An,Q.Zhao,W.D.Sun,K.R.Lee,H.L.Chen,C.J.Gao,Novel composite nanofiltration membranes containing zwitterions with high permeate flux and improved anti-fouling performance,J.Membr.Sci.390-391(2012)243-253.
[111]Y.Chang,Y.J.Shih,C.J.Lai,H.H.Kung,S.Y.Jiang,Blood-inertsurfaces via ion-pair anchoring of zwitterionic copolymer brushes in human whole blood,Adv.Funct.Mater.23(2013)1100-1110.
[112]Q.F.An,W.D.Sun,Q.Zhao,Y.L.Ji,C.J.Gao,Study on a novel nanofiltration membrane prepared by interfacial polymerization with zwitterionic amine monomers,J.Membr.Sci.431(2013)171-179.
[113]Y.F.Mi,Q.Zhao,Y.L.Ji,Q.F.An,C.J.Gao,A novel route for surface zwitterionic functionalization of polyamide nanofiltration membranes with improved performance,J.Membr.Sci.490(2015)311-320.
[114]X.D.Weng,Y.L.Ji,R.Ma,F.Y.Zhao,Q.F.An,C.J.Gao,Superhydrophilic and antibacterial zwitterionic polyamide nanofiltration membranes for antibiotics separation,J.Membr.Sci.510(2016)122-130.
[115]R.Yang,J.J.Xu,G.Ozaydin-Ince,S.Y.Wong,K.K.Gleason,Surface-tethered zwitterionic ultrathin antifouling coatings on reverse osmosis membranes by initiated chemical vapor deposition,Chem.Mater.23(2011)1263-1272.
[116]X.Li,Y.M.Cao,G.D.Kang,H.J.Yu,X.M.Jie,Q.Yuan,Surface modification of polyamide nanofiltration membrane by grafting zwitterionic polymers to improve the antifouling property,J.Appl.Polym.Sci.131(2014)205-212.
[117]Y.L.Ji,Q.F.An,Y.S.Guo,W.S.Hung,K.R.Lee,C.J.Gao,Bio-inspired fabrication of high perm-selectivity and anti-fouling membranes based on zwitterionic polyelectrolyte nanoparticles,J.Mater.Chem.A4(2016)4224-4231.
[118]Y.L.Ji,Q.Zhao,Q.F.An,L.L.Shao,K.R.Lee,Z.K.Xu,C.J.Gao,Novel separation membranes based on zwitterionic colloid particles:Tunable selectivity and enhanced antifouling property,J.Mater.Chem.A1(2013)12213-12220.
[119]M.Sairam,X.X.Loha,K.Li,A.Bismarck,J.H.G.Steinke,A.G.Livingston,Nanoporous asymmetric polyaniline films for filtration of organic solvents,J.Membr.Sci.330(2009)166-174.
[120]H.Y.Li,D.Zhai,Y.Zhou,C.J.Gao,Polyamide composite NF membrane modified with polyaniline nanoparticles,CIESC J.66(2015)142-148(in Chinese).
[121]J.Duan,E.Litwiller,I.Pinnau,Preparation and water desalination properties of POSS-polyamide nanocomposite reverse osmosis membranes,J.Membr.Sci.473(2015)157-164.
[122]M.Dalwani,J.Zheng,M.Hempenius,M.J.T.Raaijmakers,C.M.Doherty,A.Hill,M.Wessling,N.E.Benes,Ultra-thin hybrid polyhedral silsesquioxane-polyamide films with potentially unlimited 2D dimensions,J.Mater.Chem.22(2012)14835-14838.
[123]Y.R.He,Y.P.Tang,S.C.Tai,Concurrent removal of selenium and arsenic from water using polyhedral oligomeric silsesquioxane(POSS)-polyamide thin- film nanocomposite nanofiltration membranes,Ind.Eng.Chem.Res.55(2016)12929-12938.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Positively charged nanofiltration membrane fabricated by poly(acid-base)complexing effect induced phase inversion method for heavy metal removal☆
- Substrate matters:The influences of substrate layers on the performances of thin- film composite reverse osmosis membranes☆
- Polymer-based membranes for solvent-resistant nanofiltration:A review
- Mass transfer model,preparation and applications of zeolite membranes for pervaporation dehydration:A review☆
- Manipulation of confined structure in alcohol-permselective pervaporation membranes☆
- Monovalent cation perm-selective membranes(MCPMs):New developments and perspectives☆
