Development of a new cleaner production process for cassava ethanol☆
2017-05-28KeWangXinchaoYangXidongRenJianhuaZhangZhongguiMao
Ke Wang*,Xinchao Yang,Xidong Ren*,Jianhua Zhang,Zhonggui Mao
The Key Laboratory of Industrial Biotechnology,Ministry of Education,School of Biotechnology,Jiangnan University,Wuxi 214122,China
1.Introduction
Ethanol used as a fuel additive or directly as a fuel source has grown in popularity in recent decades[1].Global fuel ethanol production reached about 25800 million gal in 2015[2].However,the industry is facing criticism due to the small positive net energy balance achieved and problems associated with wastewater management[3,4].Decreasing energy consumption and improving wastewater treatment are therefore crucial to sustainable development of the fuelethanol industry.
In China,cassava is becoming more significant as the feedstock for fuel ethanol production because of its low cost,high productivity,and lack of competition for arable land[5].In the conventional cassava ethanol fermentation process(Fig.1(a))[6,7],stillage is generated at the rate of 8–15 L stillage per L ethanol,and is usually treated by solid–liquid separation,followed by anaerobic–aerobic digestion and possible further processing in order to achieve the national discharge standard.This requires high capital investment,with significant operating costs because of the aerobic digestion.To resolve these problems,an integrated ethanol–methane fermentation process has been developed[8,9].This integrated process can eliminate wastewater discharge and reduce freshwater and energy consumption(Fig.1(b)).However,since the digestate formed has a high buffer capacity with a pH of approximately 8.0,sulfuric acid is needed to adjust the slurry to an optimum pH(5.5–6.0)for the action of α-amylase.In addition,Saccharomyces cerevisiaein the ethanol fermentation seldom utilizes the sulfate,and therefore this remains in the stillage.In addition,during anaerobic digestion sulfate in the stillage can be reduced to H2S.This pathway outcompetes methane producing bacteria for substrates;furthermore,H2S is highly toxic and also increases the methane purification cost.Therefore,an alternative approach is required to eliminate sulfuric acid usage.
An additional problem is that proteins and other nitrogen containing organic matter are degraded in the anaerobic digestion process to produce ammonium.Only a small part of the produced ammonium is assimilated by microorganisms during anaerobic digestion,and therefore the majority is left in the digestate.Previous work showed that when the ammonium concentration exceeded a critical concentration of 200 mg·L−1,ethanol production was reduced and greater amounts of glycerol were produced[10].Therefore,in the integrated process,an ammoniumremoval process is required when its concentration exceeds 200 mg·L−1.At present,the most common ammonium removal methods include biological consumption,air-stripping,break point chlorination and chemical precipitation[11–13].The biological method is generally used for treating wastewater containing low concentration of ammonium.The common problem for the air stripping,break point chlorination and chemical precipitation methods is that reagents are required.Reagent addition not only increases the cost,but also negatively influences the integrated process.Therefore,none of these methods are suitable for the integrated process.
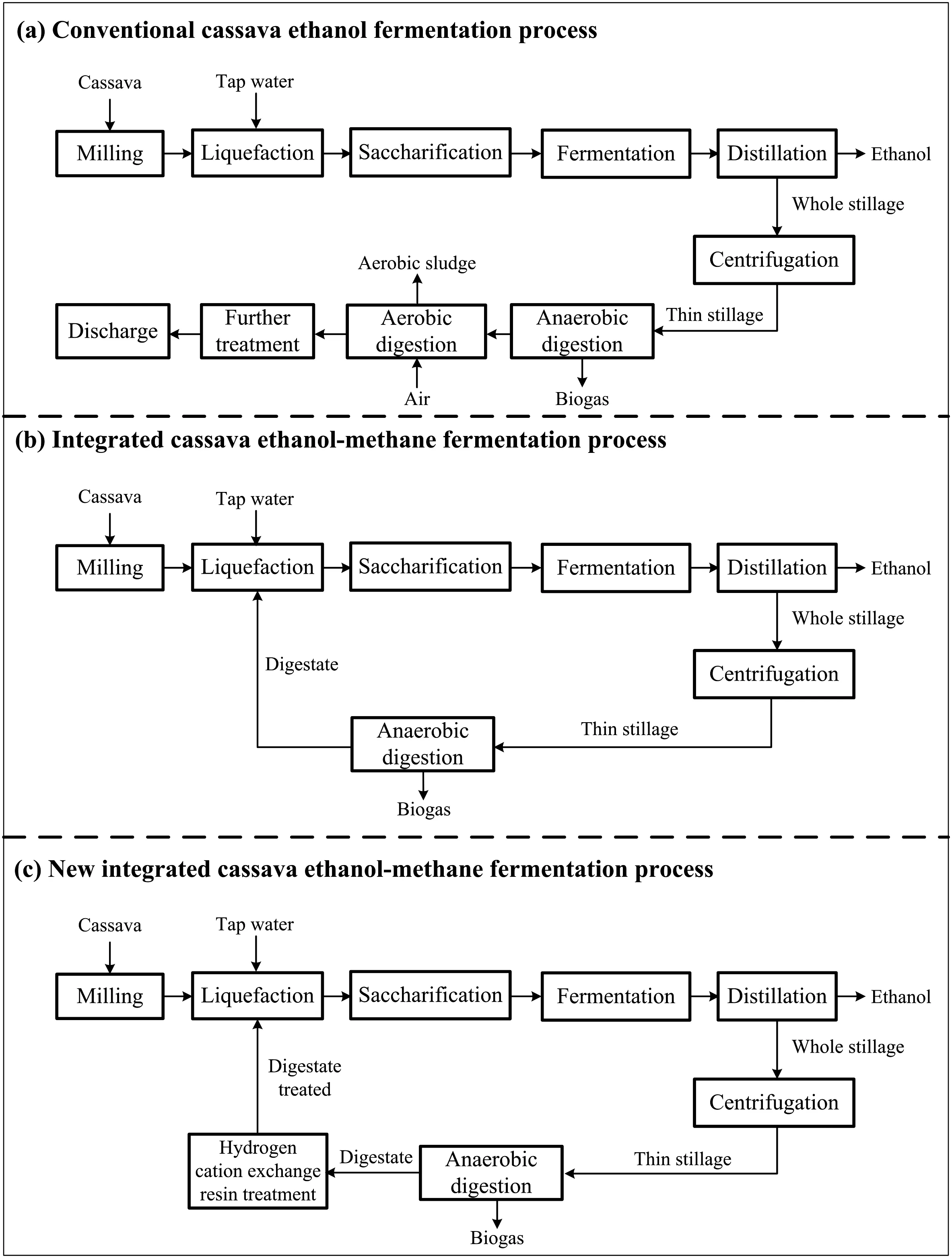
Fig.1.Comparison of the conventional process(a),integrated process(b)and new integrated process(c)for cassava ethanol production.
To resolve these problems,a new and improved process has been proposed(Fig.1(c)),where the digestate is treated using hydrogen form cation exchange resin to reduce both the ammonium concentration and pH of the digestate.In this study,the new process was evaluated at the lab scale.
2.Materials and Methods
2.1.Hydrogen-form cation exchange resin
The hydrogen-form cation exchange resin(001×7)(Tianjin Nankai Resin Co.Ltd.,China)contains sulfonic acid(−SO3H)group covalently fixed to the styrene-based polymer.The moisture content and particle diameter are in the range of 45%–52%and 0.42–1.2 mm,respectively.The operating pH is in the range of 1–14,and the maximum operating temperature is below 100°C.The total ion exchange capacity is above 4.5 mmol·g−1dry resin and 1.8 mmol·g−1wet resin,respectively.Before use,the resin was washed several times with DI water,and then treated by 1 mol·L−1HCl whose volume was four times of the resin for 1 h.Lastly,it was washed with DI water to remove all the excess acid.
2.2.Outline of the new integrated process method
For the first ethanol fermentation batch,DI water was used as process water to make mash.When the ethanol fermentation stopped,the broth was transferred from the fermenter into a distillation column and distilled.The stillage was separated by centrifugation(4000×gfor 20 min)into solids and liquids.The supernatant(thin stillage)was then treated by two-stage anaerobic digestion.The effluent(digestate)of the digester was treated using hydrogen-form cation exchange resin(001×7)by batch process.The pretreated 001×7 resin was added to the digestate(1 ml resin per 35 ml digestate)in a conical flask which was agitated in an orbital shaker.When the pH of the digestate decreased to 6.0,the 001×7 resin was recycled by filtration using a Buchner funnel.The treated digestate was used as process water for the following fermentation batch.Due to water loss in the stillage treatment process,DI water was added as necessary,but no wastewater was discharged.
2.3.Microorganism,cassava and enzymes
An industrial strain ofS.cerevisiae(supplied by Hubei Angel Yeast Co.Ltd.,China)was used.Cassava powder(starch content of 68 wt%–72 wt%;particle size about 0.45 mm)was provided by Henan Tianguan Fuel Ethanol Co.Ltd.,China.Thermotolerant α-amylase(20000 IU·ml−1)and glucoamylase(100000 IU·ml−1)were obtained from Genencor China Co.Ltd.
2.4.Inocula preparation and ethanol fermentation
Seed mediumwas prepared containing(per liter)20 g glucose,8.5 g yeast extract,1.3 g(NH4)2SO4,0.1 g MgSO4·7H2O,and 0.06 g CaCl2·2H2O.Yeast was inoculated into the seed medium and incubated at 30 °C for 19 h,in a shaker rotating at 100 r·min−1,before use as inoculum.
For mash preparation,cassava powder,process water and thermotolerant α-amylase(3 ml water and 10 IU α-amylase/g cassava powder)were added to a liquefaction tank.For experiments involving untreated digestate,sulfuric acid or sodium hydroxide was added as necessary to adjust the pH(6.0)to the optimum for α-amylase.For experiments involving treated digestate,no pH control was required.The slurry was then heated to 95 °C for 2 h,cooled to 60 °C,and glucoamylase(130 IU/g cassava powder)added.The temperature was further decreased to 30°C and the slurry transferred into a 10-L fermenter(8 L working volume,Baoxing Bio-engineering Co.Ltd.,China).Urea(0.3 g·L−1)and 10 vol%inoculum were added to start the ethanol fermentation.The temperature was maintained at 30°C.Total retention time in the fermenter was 48 h.At the end of fermentation,the broth was sampled and analyzed.Experiments in flasks were carried out based on the above procedure.
2.5.Two-stage anaerobic digestion treatment of the thin stillage
Operation of the two-stage anaerobic reactor was as described by Wanget al.[14].Thin stillage obtained by centrifugation(4000×gfor 20 min)was fed by peristaltic pump into the thermophilic anaerobic sequencing batch reactor(ASBR)and then into the mesophilic ASBR.The digestate flowing from the outlet of the mesophilic ASBR was collected and stored at−20 °C until use.
2.6.Analytical methods
Ethanol,glycerol,acetic acid,lactic acid were analyzed by high performance liquid chromatography(Dionex U3000,USA)using a Bio-Rad HPX-87H Aminex ion exclusion column operated at 65°C.A refractive index detector was employed(Shodex RI-101,Japan).The mobile phase was 0.005 mol·L−1sulfuric acid at a flow rate of 0.6 ml·min−1.COD,ammonium,volatile fatty acids(VFAs)and alkalinity were determined by standard methods[15].Metals(K,Na,Mg,Ca,Fe,Zn and Cu)were analyzed on a SpectrAA-220 atomic absorption spectrometer(Varian,Australia).Statistical analysis(one-way analysis of variance(ANOVA)with Student'st-test)was performed with Excel and Origin 8.5(Microsoft Corp.,USA).
3.Results and Discussion
3.1.Optimum amount of hydrogen-form cation exchange resin(001×7)
In the digestate,alkalinity consists mainly of NH4HCO3,NaHCO3,and other bicarbonates.H+in the hydrogen-form cation exchange resin can exchange the cations(NH4+,Na+etc.)in the digestate and decompose the bicarbonate,thereby reducing the concentration of ammonium and alkalinity of the digestate.This method has the added advantage that no sulfate is introduced to the digestate.However,because it is not necessary to remove the ammonium and alkalinity completely,dosage of the hydrogen-form cation exchange resin should be controlled to a suitable level.In this experiment,sodium bicarbonate solution(5 g·L−1,60 mmol·L−1)was used to simulate the digestate.The alkalinity was equivalent to 2976 mg CaCO3·L−1.Wet resin(5 ml)was used to treat the sodium bicarbonate solution and this was found to decrease the pH of 150 ml sodium bicarbonate solution to about 6.0,which would meet the demand of the integrated process(Fig.2).Thus,to treat digestate with a 3000 mg CaCO3·L−1of alkalinity,the ratio between wet resin and digestate was standardized as 1:35(v/v).Using this optimum dosage,001×7 resin was added to the digestate to investigate the effects of the ion exchange resin on the composition of the digestate.Table 1 shows the alkalinity,ammonium and metal ion contents before and after treatment as well as the corresponding amounts removed.By treatment with 001×7 resin,the pH of the digestate was reduced to 6.0,and 54.7 mmol·L−1of alkalinity was removed,in good agreement with the total of 53.3 mmol·L−1cations also removed.Ammonium was depleted by 82%,and its concentration in the effluent reduced to 8.9 mmol·L−1(125 mg·L−1).It can be seen that 001×7 resin treatment meets the requirements of the integrated process for control of ammonium concentration and pH of the digestate.
3.2.Effect on ethanol fermentation of the digestate treated by 001×7 resin
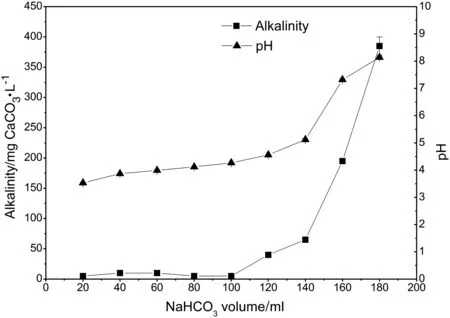
Fig.2.Treatment capacity of 001×7 resin.
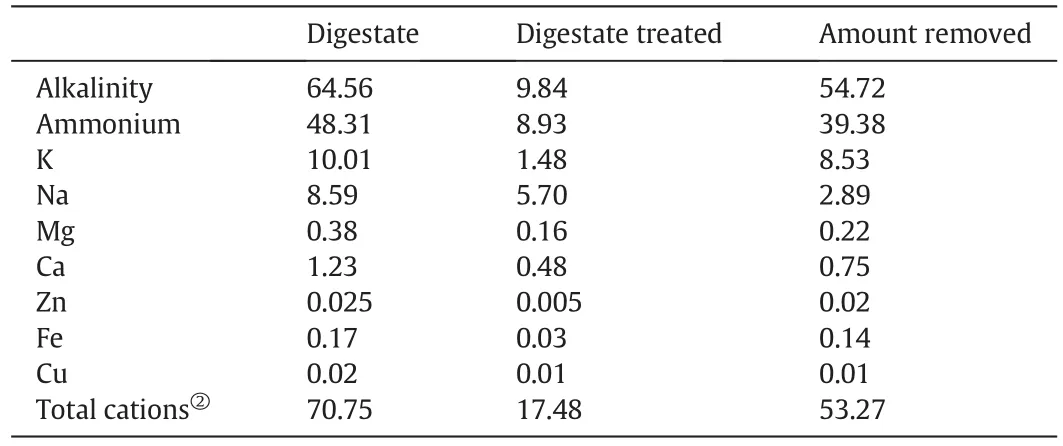
Table 1Effect of the 001×7 resin treatment on the alkalinity,ammonium and metal ions of the digestate①
The ion exchange resin-treated digestate was recycled as process water for ethanol fermentation,with controls using DI water and digestate without treatment.Before liquefaction of the cassava slurry,the pH was adjusted to 5.5–6.0 using 30 wt%H2SO4or 10 wt%NaOH as necessary.When resin-treated digestate was used,the pH of the slurry was 5.53 and 6.08 before and after liquefaction(Table 2),and therefore no pH adjustment was required.However,when digestate without resin treatment was used,it was necessary to add about 40 mmol·L−1H2SO4to the slurry to adjust its initial pH to 6.0 and the pH increased to 6.51 after liquefaction.It should be noted that when DI water alone was employed the slurry pH decreased to 5.08 after cassava was added.Although the pH of DI water was 6.49,it had no buffering capacity,unlike treated digestate.NaOH was therefore added to adjust the slurry pH to 5.90.Ethanol fermentation results are shown in Fig.3.It can be seen that compared with DI water,when treated digestate was employed as process water the fermentation rate was higher,and the cycle time reduced(Fig.3(a)).This indicates that components in the treated digestate promoteS.cerevisiaegrowth.Total ethanol production was similar in both experiments,however,higher than in the fermentation with untreated digestate(Fig.3(b)).Although ethanol fermentation with untreated digestate had the highest fermentation rate,ethanol production in this case was lowest because the higher ammonium concentration caused more glycerol to be produced(Fig.3(b)).
3.3.Regeneration of the 001×7 resin
Economic effectiveness of employing ion exchange resin depends on its long-term reuse.We examined the capacity of the resin over ten cycles,with the results shown in Fig.4.There was an overall drop in capacity of 7.9%,mostly over the first five cycles,after which there was little change,with a final value of 1.76 mmol·ml−1wet resin.

Table 2Change of pH in the process of preparing medium using different process water

Fig.4.Effect of regeneration cycle on exchange capacity of 001×7 resin.
3.4.Evaluation of the new process
3.4.1.Performance of anaerobic digestion and digestate treatment by the hydrogen-form cation exchange resin
Anaerobic digestion is a complex and multi-step process involving many different microorganisms.Synergistic effects of these microorganisms guarantee stable operation and high efficiency of the anaerobic digestion,thereby producing a high-quality digestate,which iscrucialto the integrated process[8,10].When the hydraulic retention time(HRT)is too short or the input contains inhibitory materials,the anaerobic digester could deteriorate,resulting in increased concentrations of organic acids in the digestate or other negative impacts.In this study,the HRTs for the thermophilic and mesophilic anaerobic digesters were set as 22 and 6 days respectively.Table 3 summarizes the data on the performance of the anaerobic digestion.VFAs,mainly acetic acid and propionic acid,are very important inter mediates in the anaerobic digestion process and their concentrations are also important parameters that determine the efficiency of the anaerobic digestion.Only low levels of VFAs are found in the effluent when the anaerobic digester performs efficiently and stably[16].Although it has been reported that acetic acid and propionic acid can inhibitS.cerevisiaegrowth,low concentrations of acetic acid and propionic acid increased ethanol production[17–20].It can be seen(Table 3)that the concentration of VFAs was 103 mg·L−1on average.At this low level there may have been a positive influence on ethanol production.The consistent pH value and high alkalinity also indicate a stable operation of the anaerobic digestion[21].Although the average COD concentration of thin stillage was 95.9 g·L−1,the effluent contained only about 1.9 g·L−1,for a COD removal rate of 98%.This indicated that the anaerobic digestion performed with high efficiency.The average methane yield was 322 ml per gram of COD removed,which also remained at a high level.
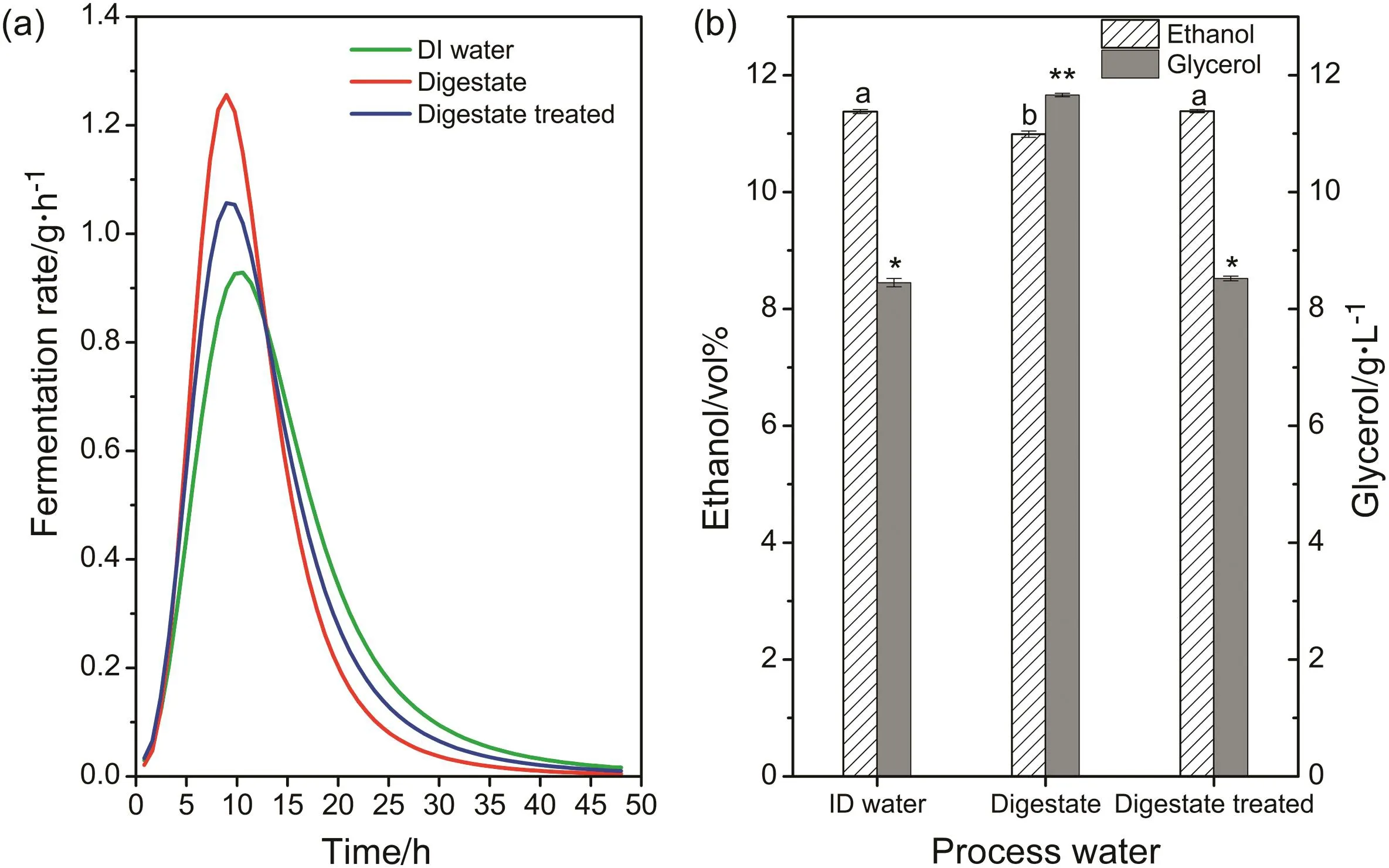
Fig.3.Effect of different process waters on ethanol fermentation(a.fermentation rate;b.ethanol and glycerol production).Different letters or number of asterisks show significant difference,with p<0.05.
The average ammonium concentration of the digestate was 736 mg·L−1,much higher than the critical concentration of 200 mg·L−1.Therefore to reduce the pH and the ammonium concentration to a suitable value,001×7 ion exchange resin was used to treat the digestate.The results in Table 4 show that pH,alkalinity and ammonium were significantly reduced by the resin treatment.The pH and ammonium concentration were 6.07 and 120 mg·L−1,respectively,on average.Due to the use of ion exchange resin,sulfuric acid was not required to adjust the pH of the digestate,and the ammonium concentration was sufficiently reduced not to affect ethanol fermentation.It should be noted that metal ions were also significantly removed,thus avoiding the accumulation of these ions and their inhibition toS.cerevisiaeat high concentration.
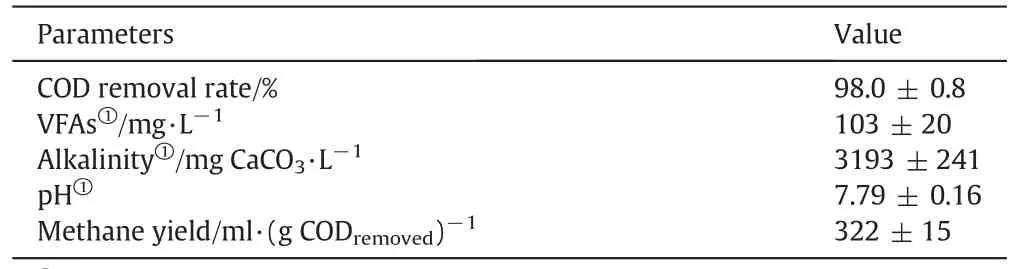
Table 3Performance of the two-stage anaerobic digestion of thin stillage(average value)
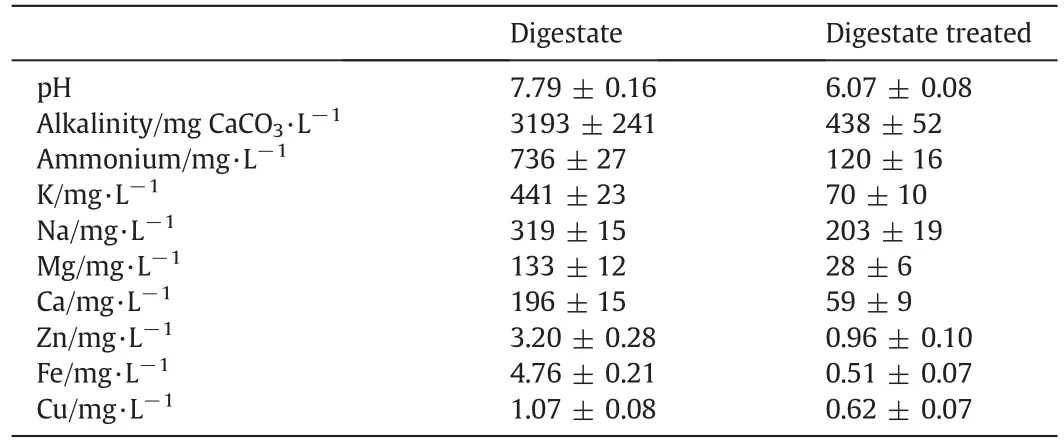
Table 4Chemical characterization of the digestate before and after treatment by the 001×7 resin
3.4.2.Performance of ethanol fermentation

Fig.5.The production in different fermentation batches of(a)ethanol and by-products(b)glycerol;(c)acetic acid;and(d)lactic acid.
Using this new integrated process,a total of 10 consecutive ethanol fermentation batches were carried out.For the first batch,tap water was used as the process water for ethanol fermentation.The subsequent batches(2–10 batches)were operated in a closed system where the digestate was recycled after resin treatment.As shown in Fig.5(a),ethanol production in consecutive batches fluctuated slightly,mainly due to the error of measurement and a slight change of total sugar.The average ethanol production(11.43 vol%)of the recycling batches was similar to the initial(DIwater)batch(11.39 vol%).Acomparable result was found for glycerol concentration(Fig.5(b)).Glycerol plays a role in osmotic regulation and maintaining an intracellular redox balance of the cell[22].The majority of the ammonium contained in the digestate was removed by the resin,thereby eliminating its effects on the intracellular redox balance and glycerol production ofS.cerevisiae.In addition,removal of metal ions avoided their accumulation and consequent increase of osmotic pressure in the recycled digestate and thus glycerol production was not affected.The by-products,acetic acid and lactic acid,were also determined at the end of ethanol fermentation and their concentrations remained broadly similar across the batches(Fig.5(c,d)).These results demonstrate that the new integrated process ran successfully with no negative effects arising from hydrogen-form cation exchange resin treatment of the recycled digestate.
Achieving an efficient balance between the energy consumed for bioethanol production and management of waste for other valueadded products is a tough challenge faced by the bioethanol industry[23].Previous studies have evaluated possible solutions to this challenge.It has been reported that thin stillage can be used to irrigate farmland,which can improve the physical and chemical properties of the soil,increase water and salinity retention,and restore and maintain soil fertility.However,wide use of this approach was limited by seasonal and regional problems.Directly recycling thin stillage as the process water for subsequent ethanol fermentation is economical,and a number of studies have focused on this method[24–26],but ethanol fermentation was negatively influenced when the recycling ratio was greater than 50%.Since the thin stillage contains abundant organics and inorganics,some researchers have used it to produce single cell protein(SCP)and bioproducts,including organic acids,alcohols,enzymes,aliphatic ketones,astaxanthin,and biopolymers[27–31].However,these studies still remain at the lab scale.
Gao and Li replaced freshwater by digestate as the process water for ethanol fermentation,and found that digestate can enhance ethanol production by 18%[32].However,sulfuric acid was required and,as previously described,ethanol production was eventually inhibited by the increasing concentration of ammonium present in the digestate.
4.Conclusions
A new cleaner production process for cassava ethanol fermentation has been developed.The problems encountered when directly recycling digestate were resolved by treated it with hydrogen-form cation exchange resin to bring ammonium concentration,pH and alkalinity within acceptable levels,permitting its reuse as process water for the next cassava ethanol fermentation batch.
Application of the new process would eliminate wastewater discharge and reduce energy and water consumption in the cassava ethanol production process,achieving the goal of sustainable development of the cassava ethanol industry.
[1]S.I.Mussatto,G.Dragone,P.M.R.Guimarães,J.P.A.Silva,L.M.Carneiro,I.C.Roberto,A.Vicente,L.Domingues,J.A.Teixeira,Technological trends,global market,and challenges of bio-ethanol production,Biotechnol.Adv.28(6)(2010)817–830.
[2]Renewable Fuels Association.Ethanol industry outlook 2016,http://www.ethanolrfa.org/pages/annual-industry-outlook,2016.
[3]J.Hill,E.Nelson,D.Tilman,S.Polasky,D.Tiffany,Environmental,economic,and energetic costs and bene fits of biodiesel and ethanol biofuels,Proc.Natl.Acad.Sci.U.S.A.103(30)(2006)11206–11210.
[4]R.Leng,C.Wang,C.Zhang,D.Dai,G.Pu,Life cycle inventory and energy analysis of cassava-based fuel ethanol in China,J.Clean.Prod.16(3)(2008)374–384.
[5]O.J.Sanchez,C.A.Cardona,Trends in biotechnological production of fuel ethanol from different feedstocks,Bioresour.Technol.99(13)(2008)5270–5295.
[6]H.J.Jördening,J.Winter,Environmental Biotechnology:Concepts and Applications,John Wiley&Sons,Germany,2005.
[7]J.S.Kim,B.G.Kim,C.H.Lee,S.W.Kim,H.S.Jee,J.H.Koh,A.G.Fane,Development of clean technology in alcohol fermentation industry,J.Clean.Prod.5(4)(1997)263–267.
[8]C.M.Zhang,Z.G.Mao,X.Wang,J.H.Zhang,F.B.Sun,L.Tang,H.J.Zhang,Effective ethanol production by reutilizing waste distillage anaerobic digestion effluent in an integrated fermentation process coupled with both ethanol and methane fermentations,Bioprocess Biosyst.Eng.33(9)(2010)1067–1075.
[9]Q.H.Zhang,X.Lu,L.Tang,Z.G.Mao,J.H.Zhang,H.J.Zhang,F.B.Sun,A novel full recycling process through two-stage anaerobic treatment of distillery wastewater for bioethanol production from cassava,J.Hazard.Mater.179(1–3)(2010)635–641.
[10]K.Wang,J.H.Zhang,P.Liu,Z.G.Mao,Suitability of anaerobic digestion effluent as process water for corn fuel ethanol fermentation,Water Sci.Technol.69(9)(2014)1894–1899.
[11]M.Lay-Son,C.Drakides,New approach to optimize operational conditions for the biological treatment of a high-strength thiocyanate and ammonium waste:pH as key factor,Water Res.42(3)(2008)774–780.
[12]S.K.Marttinen,R.H.Kettunen,K.M.Sormunen,R.M.Soimasuo,J.A.Rintala,Screening of physical–chemical methods for removal of organic material,nitrogen and toxicity from low strength land fill leachates,Chemosphere46(6)(2002)851–858.
[13]T.Zhang,L.Ding,H.Ren,X.Xiong,Ammonium nitrogen removal from coking wastewater by chemical precipitation recycle technology,Water Res.43(20)(2009)5209–5215.
[14]K.Wang,J.H.Zhang,L.Tang,H.J.Zhang,G.Y.Zhang,X.Z.Yang,P.Liu,Z.G.Mao,Establishment and assessment of a novel cleaner production process of corn grain fuel ethanol,Bioresour.Technol.148(8)(2013)453–460.
[15]A.Eaton,L.Clesceri,A.Greenberg,Standard Methods for the Examination of Water and Wastewater,American Public Health Association,New York,1995.
[16]R.E.Speece,Anaerobic Biotechnology for IndustrialWastewaters,Vanderbilt University,Archae Press,Tennessee,1996.
[17]D.A.Abbott,W.M.Ingledew,Buffering capacity of whole corn mash alters concentrations of organic acids required to inhibit growth ofSaccharomyces cerevisiaeand ethanol production,Biotechnol.Lett.26(16)(2004)1313–1316.
[18]M.J.Taherzadeh,C.Niklasson,G.Lidén,Acetic acid—Friend or foe in anaerobic batch conversion of glucose to ethanol bySaccharomyces cerevisiae?Chem.Eng.J.52(15)(1997)2653–2659.
[19]K.C.Thomas,S.H.Hynes,W.M.Ingledew,In fluence of medium buffering capacity on inhibition ofSaccharomyces cerevisiaegrowth by acetic and lactic acids,Appl.Environ.Microbiol.68(4)(2002)1616–1623.
[20]C.M.Zhang,L.Jiang,Z.G.Mao,J.H.Zhang,L.Tang,Effects of propionic acid and pH on ethanol fermentation bySaccharomyces cerevisiaein cassava mash,Appl.Biochem.Biotechnol.165(3–4)(2011)883–891.
[21]S.Sung,H.Santha,Performance of temperature-phased anaerobic digestion(TPAD)system treating dairy cattle wastes,Water Res.37(7)(2003)1628–1636.
[22]E.Albers,C.Larsson,G.Lidén,C.Niklasson,L.Gustafsson,In fluence of the nitrogen source onSaccharomyces cerevisiaeanaerobic growth and product formation,Appl.Environ.Microbiol.62(9)(1996)3187–3195.
[23]B.Hickey,M.Motylewski,Sustainable alternatives for whole stillage management,Fuel Ethanol Workshop,St.Louis,2007.
[24]W.Białas,D.Szymanowska,W.Grajek,Fuel ethanol production from granular corn starch usingSaccharomyces cerevisiaein a long term repeated SSF process with full stillage recycling,Bioresour.Technol.101(9)(2010)3126–3131.
[25]D.Szymanowska-Powałowska,G.Lewandowicz,P.Kubiak,W.Błaszczak,Stability of the process of simultaneous saccharification and fermentation of corn flour.The effect of structural changes of starch by stillage recycling and scaling up of the process,Fuel119(119)(2014)328–334.
[26]J.S.Wall,R.J.Bothast,A.A.Lagoda,K.R.Sexson,Y.V.Wu,Effect of recycling distillers' solubles on alcohol and feed production from corn fermentation,J.Agric.Food Chem.31(4)(1983)770–775.
[27]A.Bhattacharyya,J.Saha,S.Haldar,A.Bhowmic,U.K.Mukhopadhyay,J.Mukherjee,Production ofpoly-3-(hydroxybutyrate-co-hydroxyvalerate)byHaloferax mediterraneiusing rice-based ethanol stillage with simultaneous recovery and re-use of medium salts,Extremophiles18(2)(2014)463–470.
[28]A.P.Djukić-Vuković,L.V.Mojović,B.M.Jokić,S.B.Nikolić,J.D.Pejin,Lactic acid production on liquid distillery stillage byLactobacillus rhamnosusimmobilized onto zeolite,Bioresour.Technol.135(2)(2013)454–458.
[29]M.H.Selim,A.M.Elshafei,A.I.El-Diwany,Production of single cell protein from yeast strains grown in Egyptian vinasse,Bioresour.Technol.36(2)(1991)157–160.
[30]T.P.West,B.Strohfus,Pullulan production byAureobasidium pullulansgrown on ethanol stillage as a nitrogen source,Microbios88(354)(1995)7–18.
[31]F.C.Yang,I.Lin,Production of acid protease using thin stillage from a rice-spirit distillery byAspergillus niger,Enzym.Microb.Technol.23(6)(1998)397–402.
[32]T.Gao,X.Li,Using thermophilic anaerobic digestate effluent to replace freshwater for bioethanol production,Bioresour.Technol.102(2)(2011)2126–2129.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Suppressing secondary reactions of coal pyrolysis by reducing pressure and mounting internals in fixed-bed reactor☆
- An adaptive neuro-fuzzy sliding mode controller for MIMO systems with disturbance
- Transformation mechanism of nutrient elements in the process of biochar preparation for returning biochar to soil☆
- Preparation of cross-linked enzyme aggregates of nitrile hydratase ES-NHT-118 from E.coli by macromolecular cross-linking agent☆
- Multi-objective regulation in autohydrolysis process of corn stover by liquid hot water pretreatment
- Modelling of adsorption of textile dyes over multi-walled carbon nanotubes:Equilibrium and kinetic
