Preparation of cross-linked enzyme aggregates of nitrile hydratase ES-NHT-118 from E.coli by macromolecular cross-linking agent☆
2017-05-28LiyaZhouHaixiaMouJingGaoLiMaYingHeYanjunJiang
Liya Zhou ,Haixia Mou ,Jing Gao ,*,Li Ma ,Ying He ,Yanjun Jiang ,*
1 School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300130,China
2 Hebei Provincial Key Lab of Green Chemical Technology and High Efficient Energy Saving,Hebei University of Technology,Tianjin 300130,China
1.Introduction
Nitrile substrates can be readily converted to the corresponding high value amides under mild conditions through the catalysis of nitrile hydratase(NHase)[1].For example,acrylonitrile and 3-cyanopyridine could be translated into acrylamide(Mitsubishi-Rayon process)and vitamin nicotinamide(Lonza process)through the catalysis of free microbial cells and free isolated NHase,respectively[2–4].Although the whole-cell catalyzed processes have been widely used in nitrile and amide biotransformation,the transformations thatcatalyzed by isolated enzymes are usually preferred.Compared with the whole cells,isolated enzymes can exhibit stringent chemo-,regio-and enantio-selectivity and broad substrate specificity[5,6].However,the use of free isolated NHase is often hampered by several limitations such as high costs,low pH,thermal and operational stability,and difficulties in recovery and reuse.
Immobilization provides one of the mostattractive concepts to overcome these drawbacks and to simplify the biocatalyst recycling and downstream processing[7].There are a few methods or supports reported with respect to the immobilization of NHase,i.e.cross-linked enzyme aggregates(CLEAs)[8],poly(vinylalcohol)(PVA)/chitosan complex[9],Eupergit® resins[10]and sol–gels[2].CLEAs technique is a carrier-free immobilization method and can be applicable to the immobilization of almost any enzyme,and can afford stable,recyclable biocatalysts with high retention of activity[11–15].Until now,CLEAs method has been widely used in immobilizing various kinds of enzymes(i.e.penicillin amidohydrolase[12],formate dehydrogenase[16],lipase[17,18],α-amylase[19],nitrilases[1,8],etc.).In the formation process of CLEAs,glutaraldehyde is commonly used as cross-linking agentbecause it is inexpensive and readily available in commercial quantities.However,with some enzymes,the phenomena of low or no retention of activity were observed when glutaraldehyde was used as the cross-linker[20,21].In order to circumvent this problem,some macromolecular cross-linkers(i.e.bulky polyaldehydes,obtained by periodate oxidation of dextrans)were employed to prepare several CLEAs of enzymes(hydroxynitrile lyase,alcohol dehydrogenase,nitrilases,penicillin acylase,β-mannanase)[22,23].Compared to CLEAs formed with glutaraldehyde,the recovery activities of CLEAs formed with dextran polyaldehyde were improved obviously.Therefore,dextran polyaldehyde may be an efficient cross-linker in the preparation of CLEAs.
NHase ES-NHT-118(EC 4.2.1.84),produced fromEscherichia coli(E.coli)strain that carries the cloned NHase gene,has great potential in organic chemical processing because of its ability to convert nitriles to amides under physiological conditions.However,NHase is a multimeric enzyme that limits its stability outside the cell due to dissociation[24].Therefore,developing an effective immobilized method for this kind of NHase with increased stability is a prerequisite for its industrial applications(i.e.acrylamide production).NHase CLEAs can hold the catalytically active multimer together,so that CLEAs formation can have a beneficial effect on increased stability of NHase.Additionally,NHase has a low Lys residue content which can result in insufficiently cross-linking.Adding polymers or proteins containing numerous free amino groups can overcome this problem.Egg white,a low cost,easily available and nontoxic nature protein,is rich in lysine amino residues[25,26],thus,can be used as a effective additive in CLEAs preparation process.On the other hand,lysozyme in egg white can improve enzymes' stability[27,28]and bacteriolytic activity[29].
In this study,the NHase CLEAs were prepared by using dextran polyaldehyde as cross-linking agent and egg white as protein feeder.Meanwhile,the preparation parameters were optimized.The properties of the NHase CLEAs such as pH and thermal stabilities,storage stability and reusability were also investigated.
2.Experimental
2.1.Materials
NHase ES-NHT-118(EC 4.2.1.84)fromE.colistrain was purchased from Hangzhou Biosci Biotech Co.(China).Dextran and sodium metaperiodate were purchased from Dingguo Biotech Co.(China).Acetonitrile(chromatography grade)was from Concord Sci Tech Ltd.(China).Ammonium sulfate and other reagents were of analytical grade.
2.2.Preparation of dextran polyaldehyde
Dextran polyaldehyde was prepared according to the previous report[20].Typically,1.65 g of dextran(10 kDa,100 kDa or 500 kDa)was dissolved in 50 mL distilled water and then 3.85 g of sodium metaperiodate was added.The resulting solution was stirred at room temperature for 90 min.Subsequently,the solution was dialyzed five times under stirring.Then,the liquid was lyophilized to obtain dextran polyaldehyde.
2.3.Preparation and characterization of NHase CLEAs
1 mL of NHase solution (the protein concentration was 0.4 mg·mL−1)and 100 μL of egg white were mixed under stirring in an Eppendorf tube at 4°C.Then 2 mL of precipitant was slowly added to precipitate the enzyme.After 30 min,0.1 mL of cross-linker solution was added and incubated for a certain time under vortex agitation.The solution was centrifuged at 5000 r·min−1for 5 min,and the precipitation defined as NHase CLEAs was washed three times with phosphate buffer(pH 7.0,0.05 mol·L−1).The activity of NHase was determined using acrylonitrile as substrate and the product(acrylamide)concentration in the reaction mixture(Fig.1)was analyzed by HPLC.
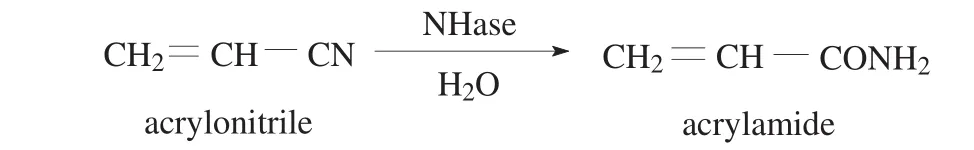
Fig.1.Reaction scheme for enzymatic conversion of acrylonitrile to acrylamide.
The standard activity test was as follows:a certain amount of enzyme was added into 0.5 mL of acrylonitrile solution(125 mmol·L−1)at 25 °C to start the reaction.After 5 min,1 mL of HCl(1 mol·L−1)was added into the reaction mixture to end the reaction.After filtration,the sample was analyzed by HPLC(Agilent Technologies)using a 4.6×100 Eclipase Plus C18 column,eluent H2O–acetonitrile(Chromatography grade),70:30(v/v)at 1 mL·min−1and column temperature of 25°C.Acrylamide was detected using a DAD detector at a wavelength of 230 nm.Activity was calculated according to the standard curve.One unit(U)of enzyme activity was defined as the amount of enzyme that catalyzed the production of1μmolacrylamide per min under assay conditions.
The activity recovery of NHase CLEAs was calculated by the ratio of the total activity of NHase CLEAs to the total free NHase activity used for CLEAs preparation.
The effects of precipitant,cross-linker and cross-linking time on the activity recovery were investigated.
The morphology of NHase CLEAs was analyzed by using scanning electron microscopy(SEM),and the micrograph was taken on a Nova NanoSEM450 instrument.The Michaelisconstant(Km)was determined at various substrate concentrations and the data were fitted to the Michaelis–Menten equation.
2.4.Effect of pH and temperature on the activity of NHase CLEAs
An amount of free NHase and NHase CLEAs were incubated in different buffers with pH values from 3.0–12.0 at 25 °C for 1 h.After incubation,the activity was determined using a standard activity test.
An amount of free NHase and NHase CLEAs were incubated in phosphate buffer(pH 7.0,0.05 mol·L−1)at20–60°C for 1 h.After incubation,the mixture was cooled to 25°C,and then the activity was determined.
The results for effect of pH and temperature on the activity of NHase CLEAs were expressed in relative form with the highest value being 100%activity.
2.5.Catalytic performance of NHase CLEAs under high concentrations of substrate
Different concentrations of acrylonitrile(722–1980 mmol·L−1)were catalyzed by free NHase or NHase CLEAs(10 U)at 25°C.Every 30 min,a sample was withdrawn and quenched with HCl,and then analyzed by HPLC.
2.6.Reusability and storage stability of NHase CLEAs
Reusability of NHase CLEAs was determined as follows:10 U of NHase CLEAs were mixed with buffer to a volume of 2 mL,then 60 μL of acrylonitrile(722 mmol·L−1)was added at 25 °C under stirring condition to start the reaction.After 6 h,the NHase CLEAs were separated and washed three times using phosphate buffer.After washing,another cycle of reaction was carried out and the residual activity was determined.
Storage stability was determined as follows:free NHase and NHase CLEAs were stored at 4 °C in phosphate buffer(pH 7.0,0.05 mol·L−1).
The results for reusability and storage stability of NHase CLEAs were given in relative form with the initial activity assigned as 100%.
3.Results and Discussions
3.1.Selection of precipitant
Enzymes could be precipitated from their aqueous solutions and aggregated together by noncovalent bonding under the effect of salts,water miscible organic solvents,or nonionic polymers without damage of their original tertiary structures[12].The nature of the precipitant has an important effect on the activity recovery of the enzymes;hence,it is necessary to select a suitable precipitant for preparation of NHase CLEAs.As shown in Fig.2, five kinds of precipitants(methanol,ethanol,acetone,isopropanol and saturated ammonium sulfate solution)were used to prepare NHase aggregates.The obtained aggregates were redissolved,and then the activity recovery was measured.When saturated ammonium sulfate solution was used as precipitant,about 80%of activity recovery was obtained which was better than other precipitants.The possible reason was that the essential water of NHase was much easier lost in water miscible organic solvents(methanol,ethanol,acetone,isopropanol).When essential water was lost,the structure of NHase molecules would be changed and the activity would be decreased.Additionally,NHase was also easily denaturalized in the presence of polar,hydrophilic solvents.Therefore,saturated ammonium sulfate solution was used in the subsequent experiments.

Fig.2.Effect of precipitant on the activity recovery of redissolved NHase aggregates.The ratio of NHase solution to precipitant with 1:2(v:v),and NHase was precipitated for 30 min at 4°C.
3.2.Selection of cross-linker
The type of cross-linker predictably has an important effect on the activity recovery of CLEAs.Thus,glutaraldehyde and three types of dextran polyaldehyde with different molecular weight(10 kDa(D-10 kDa),100 kDa(D-100 kDa)or 500 kDa(D-500 kDa))were used to prepare NHase CLEAs.After precipitation(0.5 h)and cross-linking(4 h),the NHase CLEAs were obtained.As shown in Fig.3,when glutaraldehyde as traditional cross-linker was used,the activity recovery of the resulting NHase CLEAs was only 13.79%.While dextran polyaldehyde as a macromolecular cross-linker was used,the activity recoveries of the NHase CLEAs(36.89%,38.56%and 39.37 for D-10 kDa,D-100 kDa and D-500 kDa,respectively)were generally much higher than that obtained with glutaraldehyde.The increased activity recovery was attributed to the large dimensions of the dextran polyaldehyde,which precluded its possible reaction with catalytically relevant amino groups in the active site of the enzyme[21].Therefore,compared with glutaraldehyde as a small cross-linker,the use of dextran polyaldehyde resulted in a significantly lower loss of active sites and,consequently,enzyme activity.In addition,the recovery activities were increased with the increase of the molecular weight of cross-linkers,but the differences were not obvious.
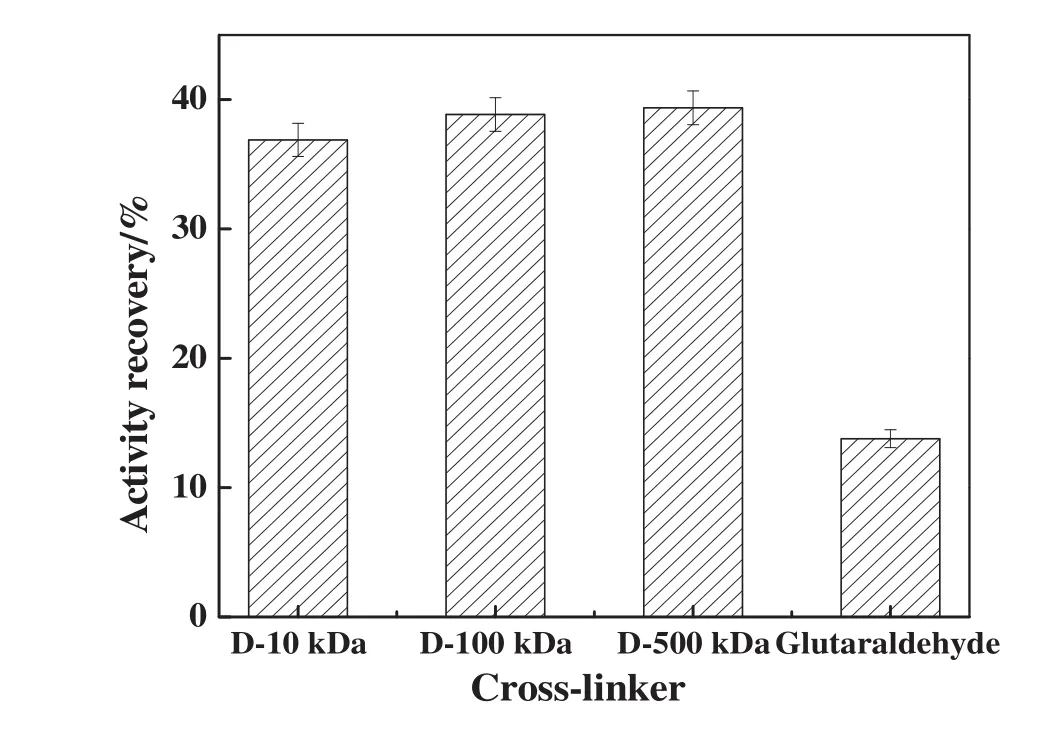
Fig.3.Effect of cross-linker on the activity recovery of NHase CLEAs.Experiments were performed using saturated ammonium sulfate solution as precipitant,and NHase was precipitated for 30 min at 4 °C and cross-linked for 4 h at 4 °C.
3.3.Selection of cross-linker's concentration and cross-linking time
Dextran polyaldehyde(500 kDa)was selected as the cross-linker,and then the cross-linker's concentration and cross-linking time were optimized.As shown in Fig.4,the activity recovery increased with the increase of cross-linker's concentration,and then decreased with further increase of cross-linker's concentration.When the cross-linker's concentration was low,the provided aldehyde group was insufficient for cross-linking reaction.So a little amount of NHase CLEAs was obtained and the NHase CLEAs were unstable in the reactions.However,excessive amounts of cross-linker could lead to excessive cross-linking of enzyme aggregates and then decrease the enzyme activity[30,31].In addition,under a certain concentration of cross-linker,the activity recovery increased with the extension of cross-linking time firstly,then decreased slightly with time further extended.Thus,based on the results in Fig.4,the optimal final concentration of cross-linker was 2.5 mg·mL−1and the cross-linking time was 2 h.Under these conditions,the activity recovery of the NHase CLEAs was 49.64%.

Fig.4.Effect of cross-linker's concentration and cross-linking time on the activity recovery of NHase CLEAs.Experiments were performed using saturated ammoniumsulfate solution as precipitant and dextran polyaldehyde(500 kDa)as cross-linker,and NHase was precipitated for 30 min at 4°C.
3.4.SEM image of NHase CLEAs
As shown in Fig.5,a porous structure of the NHase CLEAs was observed,which was consistent with the result of previous report[21].Thanks to the porous structure,the mass transfer limitation could be reduced effectively,and the substrates could enter into the inner NHase CLEAs in reaction,and then the increased activity recovery could be obtained[21].
3.5.Effect of pH and temperature on the activity of free NHase and NHase CLEAs
As shown in Fig.6a,NHase CLEAs showed a clearsuperiority over the pH range of3.0–12.0 compared to free NHase.For example,atacidic pHs such as pH 5.0,free NHase only maintained 34.44%of its original activity,while NHase CLEAs retained about 67%.The same behavior was also observed under basic condition.The activity of free NHase was less than 29%at pH 10.0,while that of NHase CLEAs retained more than 79%of original activity under the same condition.These results were similar to that reported by van Pelt[8],and also indicated that cross-linking had a positive effect on pH stability of NHase.The enhanced pH stability was attributed to multipoint attachment by covalent bonds between NHase molecules and dextran polyaldehyde,which restricted inactivation and conformational changes of NHase molecules[32].
As shown in Fig.6b,when the temperature was above 35°C,the activity of free NHase decreased rapidly with the increase of temperature,only 25.97%of the initial activity was retained at 45°C after 1 h heat treatment.A main reason of its reduced activity was due to the fast denaturation of free NHase at temperatures above 35°C.However,NHase CLEAs could maintain high activity at the temperature range of 20–45 °C.For example,86.27%of the initial activity was still retained at 45°C.Therefore,NHase CLEAs had higher stability than free NHase at elevated temperatures which indicated that the thermal stability of NHase was improved significantly after immobilization.Similar results were obtained by immobilizing NHase on mesoporous onion-like silica[1].According to the literature,the higher thermal stability of NHase CLEAs compared to free NHase was attributed to the higher resistivity of NHase against the denaturation through the covalent bonds between NHase molecules and dextran polyaldehyde[32].
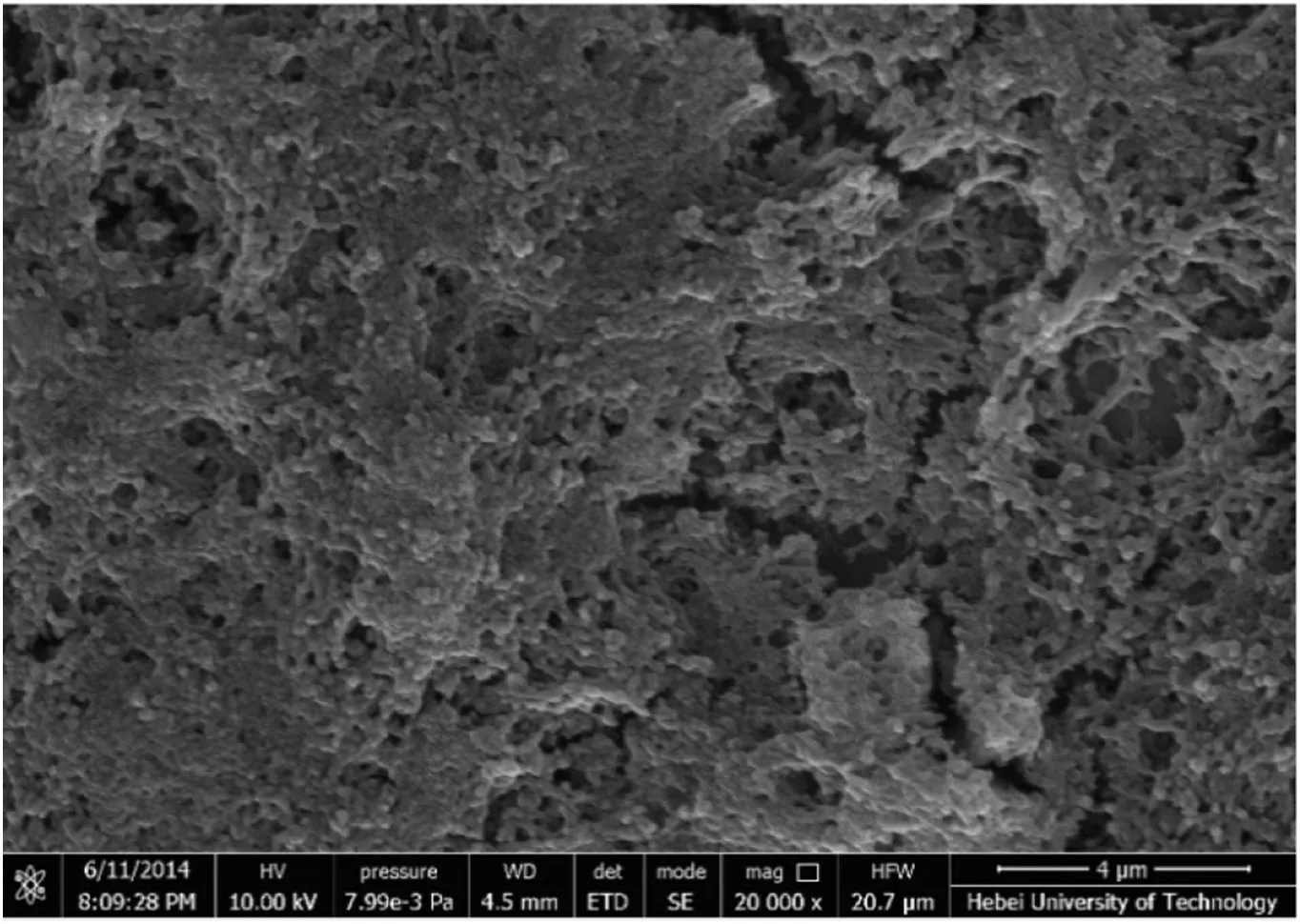
Fig.5.The morphology of NHase CLEAs.
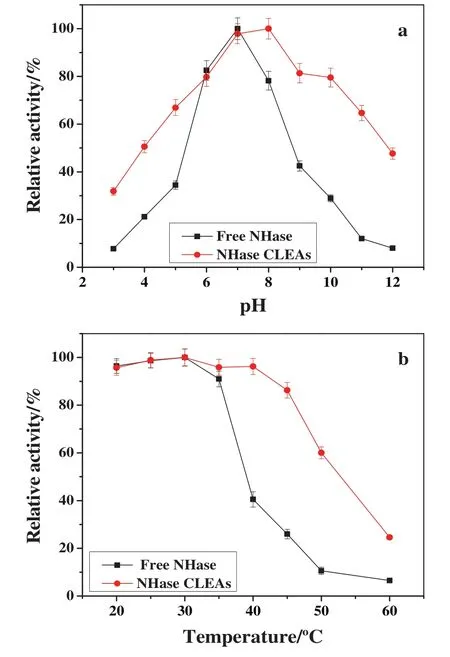
Fig.6.Effect of pH(a)and temperature(b)on the activity of free NHase and NHase CLEAs.
3.6.The kinetic parameters of free NHase and NHase CLEAs
The kinetic parameters of free NHase and NHase CLEAs were determined at final concentration of acrylonitrile ranging from 0 to 50 mmol·L−1.TheKmof NHase was determined by the non-linear regression analysis[33].TheKmvalues of free NHase and NHase CLEAs were 1.598 mmol·L−1and 2.075 mmol·L−1,respectively.The result indicated that there was no big difference betweenKmof free NHase and NHase CLEAs.So after immobilization,the substrate affinity of NHase was notevidently decreased.This may be attributed to porous structure of NHase CLEAs,which showed low steric hindrance and favored the diffusion of the substrate and product molecules[9,34].
3.7.Tolerance to high concentrations of substrate
The tolerance to high concentrations of substrate was an important factor in application process.As can be seen in Fig.7,when free NHase was used as catalyst,the product(acrylamide)concentration was decreased from 116.7 to 37.85 mmol·L−1after 6 h of incubation with substrate concentration increased from 722 to 1980 mmol·L−1.In contrast,the acrylamide concentration catalyzed by NHase CLEAs was increased from 359.6 mmol·L−1to 823.9 mmol·L−1under the same conditions.These results indicated that NHase CLEAs had higher tolerance and was more stable than free NHase especially exposure to high concentrations of substrate,which was in agreement with previous reports[8].The increased stability of NHase CLEAs could be explained by that the NHase molecules were quite rigidly bound to each other,which can prevent subunits from the dissociation in high concentrations of substrate[8,35].The dissociation of subunits could result in NHase deactivation and stability decreasing.Additionally,the existence of concentration gradients inside the NHase CLEAs could also improve the stability of NHase.Because the inner layer of the CLEAs was protected from deactivation in high concentrations of substrate,only the outside part of NHase was more susceptible to denaturation and deactivation[8].

Fig.7.The tolerance of free NHase(solid)and NHase CLEAs(blank)to high concentration of substrate.
3.8.Reuse and storage stability of free NHase and NHase CLEAs
Reusability was another important parameter of enzyme and a good reusability could reduce the cost of enzyme in applications.The reusability of NHase CLEAs was studied with eight cycles.After each cycle,the NHase CLEAs were separated from reaction mixture and reused in next reaction.As shown in Fig.8a,the NHase CLEAs showed 97.89%of the original activity in the second reaction cycle.Further decreases in activity were less significant during the first four cycles,and more than 89%of the original activity was still retained in the fourth cycle.After recycling eight times,the NHase CLEAs could retain about 52%of its original activity.Compared with previous reports[8],the NHase CLEAs had lower reusability due to leakage of enzyme and denaturation of NHase under rigorous shaking[36].
The storage stabilities of free NHase and NHase CLEAs were compared in Fig.8b.As can be seen,the storage stability of the NHase was improved after immobilization.About 50%of the initial activity was maintained for NHase CLEAs in 30 days,which was better than that of free NHase(maintained 35%of its initial activity).The improved storage stability was due to multipoint attachment by covalent bonds between NHase molecules and dextran polyaldehyde.But,compared to other enzymes,the NHase CLEAs inactivate relatively fast during storage which could probably be largely attributed to conformational changes in the NHase molecules.The cross-linking of the NHase could be relaxed during the storage resulting in unfavorable conformations and a poorer access of the substrates to the active site of the enzyme.Additionally,the microbial degradation was the other reason[37].

Fig.8.Reusability(a)and storage stability(b)of free NHase and NHase CLEAs.
Therefore,from all the above results,it was reasonable to believe that the NHase CLEAs had great potential in industrial applications.
4.Conclusions
Immobilization of nitrile hydratase ES-NHT-118 fromE.colias CLEAs has proven to be a very effective and mild method to get highly porous,active,and water-insoluble biocatalyst.Besides simplicity,the attractive of this immobilization method is the use of dextran polyaldehyde as cross-linker,which can improve the activity recovery.Under the optimal preparation conditions,about 50%of activity recovery can be obtained with dextran polyaldehyde as cross-linker,which was better than that of glutaraldehyde(only 13.79%).The robustness against thermal and pH denaturation,the tolerance to high concentrations of substrate,and the good reusability and storage stability allow NHase CLEAs exploited in industrial applications.
[1]J.Gao,Q.Wang,Y.J.Jiang,J.K.Gao,Z.H.Liu,L.Y.Zhou,Y.F.Zhang,Formation of nitrile hydratase cross-linked enzyme aggregates in mesoporous onion-like silica:Preparation and catalytic properties,Ind.Eng.Chem.Res.54(2015)83–90.
[2]S.Martinez,M.L.Kuhn,J.T.Russell,R.C.Holz,T.E.Elgren,Acrylamide production using encapsulated nitrile hydratase fromPseudonocardia thermophilain a sol–gel matrix,J.Mol.Catal.B Enzym.100(2014)19–24.
[3]J.S.Gong,Z.M.Lu,H.Li,J.S.Shi,Z.M.Zhou,Z.H.Xu,Nitrilases in nitrile biocatalysis:Recent progress and forthcoming research,Microb.Cell Factories11(2012)142.
[4]X.D.Sun,H.M.Yu,Z.Y.Shen,Deactivation kinetics of nitrile hydratase in free resting cells,Chin.J.Chem.Eng.17(2009)822–828.
[5]A.Schmid,J.S.Dordick,B.Hauer,A.Kiener,M.Wubbolts,B.Witholt,Industrial biocatalysis today and tomorrow,Nature409(2001)258–268.
[6]Y.Shen,F.Du,W.Gao,A.Wang,C.Chen,Stereoselective nitrile hydratase,Afr.J.Microbiol.Res.6(2012)6114–6121.
[7]M.Bilal,M.Asgher,Dye decolorization and detoxification potential of Ca-alginate beads immobilized manganese peroxidase,BMC Biotechnol.15(2015)111–124.
[8]S.van Pelt,S.Quignard,D.Kubáč,D.Y.Sorokin,F.van Rantwijka,R.A.Sheldon,Nitrile hydratase CLEAs:The immobilization and stabilization of an industrially important enzyme,Green Chem.10(2008)395–400.
[9]S.V.Pawar,G.D.Yadav,PVA/chitosan-glutaraldehyde cross-linked nitrile hydratase as reusable biocatalyst for conversion of nitriles to amides,J.Mol.Catal.B Enzym.101(2014)115–121.
[10]I.Chiyanzu,D.A.Cowan,S.G.Burton,Immobilization ofGeobacillus pallidusRAPc8 nitrile hydratase(NHase)reduces substrate inhibition and enhances thermostability,J.Mol.Catal.B Enzym.63(2010)109–115.
[11]S.S.Nadar,V.K.Rathod,Magnetic macromolecular cross linked enzyme aggregates(CLEAs)of glucoamylase,Enzym.Microb.Technol.83(2016)78–87.
[12]L.Q.Cao,F.van Rantwijk,R.A.Sheldon,Cross-linked enzyme aggregates:A simple and effective method for the immobilization of penicillin acylase,Org.Lett.2(2000)1361–1364.
[13]J.D.Cui,S.R.Jia,Optimization protocols and improved strategies of cross-linked enzyme aggregates technology:Current development and future challenges,Crit.Rev.Biotechnol.35(2015)15–28.
[14]R.A.Sheldon,R.Schoevaart,L.M.van Langen,Cross-linked enzyme aggregates(CLEAs):A novel and versatile method for enzyme immobilization(a review),Biocatal.Biotransform.23(2005)141–147.
[15]R.A.Sheldon,Cross-linked enzyme aggregates(CLEAs):Stable and recyclable biocatalysts,Soc.Transit.35(2007)1583–1587.
[16]M.H.Kim,S.Park,Y.H.Kim,K.Won,S.H.Lee,Immobilization of formate dehydrogenase fromCandida boidiniithrough cross-linked enzyme aggregates,J.Mol.Catal.B Enzym.97(2013)209–214.
[17]F.Kartal,M.H.A.Janssen,F.Hollmann,R.A.Sheldon,A.Kılınc,Improved esterification activity ofCandida rugosalipase in organic solvent by immobilization as cross-linked enzyme aggregates(CLEAs),J.Mol.Catal.B Enzym.71(2011)85–89.
[18]P.Gupta,K.Dutt,S.Misra,S.Raghuwanshi,R.K.Saxena,Characterization of crosslinked immobilized lipase from thermophilic mouldThermomyces lanuginosausing glutaraldehyde,Bioresour.Technol.100(2009)4074–4076.
[19]A.S.Sahutoglu,C.Akgul,Immobilisation ofAspergillus oryzaeα-amylase andAspergillus nigerglucoamylase enzymes as cross-linked enzyme aggregates,Chem.Pap.69(2015)433–439.
[20]C.Mateo,J.M.Palomo,L.M.van Langen,F.van Rantwijk,R.A.Sheldon,A new,mild cross-linking methodology to prepare cross-linked enzyme aggregates,Biotechnol.Bioeng.86(2004)273–276.
[21]Q.N.Zhen,M.F.Wang,W.Qi,R.X.Su,Z.M.He,Preparation of β-mannanase CLEAs using macromolecular cross-linkers,Catal.Sci.Technol.3(2013)1937–1941.
[22]A.Chmura,S.Rustler,M.Paravidino,F.van Rantwijk,A.Stolz,R.A.Sheldon,The combi-CLEA approach:enzymatic cascade synthesis of enantiomerically pure(S)-mandelic acid,Tetrahedron Asymmetry24(2013)1225–1232.
[23]G.Irazoqui,C.Giacomini,F.Batista-Viera,B.M.Brena,Hydrophilization of immobilized model enzymes suggests a widely applicable method for enhancing protein stability in polar organic co-solvents,J.Mol.Catal.B Enzym.46(2007)43–51.
[24]H.Yamaguchi,M.Miyazaki,Y.Asanomi,H.Maeda,Poly-lysine supported crosslinked enzyme aggregates with efficient enzymatic activity and high operational stability,Catal.Sci.Technol.1(2011)1256–1261.
[25]J.J.Karimpil,J.S.Melo,S.F.D'Souza,Hen egg white as a feeder protein for lipase immobilization,J.Mol.Catal.B Enzym.71(2011)113–118.
[26]Y.J.Jiang,Q.Wang,Y.He,L.Y.Zhou,J.Gao,Co-aggregation of laccase and nature egg white:A simple method to prepare stable and recyclable biocatalyst,Appl.Biochem.Biotechnol.172(2014)2496–2506.
[27]A.Illanes,L.Wilson,C.Altamirano,Z.Cabrera,L.Alvarez,C.Aguirre,Production of cephalexin in organic medium at high substrate concentrations with CLEA of penicillin acylase and PGA 450,Enzym.Microb.Technol.40(2007)195–203.
[28]K.C.Gulla,M.D.Gouda,M.S.Thankur,N.G.Karanth,Enhancement of stability of immobilized glucose oxidase by modification of free thiols generated by reducing disul fide bonds and using additives,Biosens.Bioelectron.19(2004)621–625.
[29]B.S.Kubal,S.F.D'Souza,Immobilization of catalase by entrapment of permeabilized yeast cells in hen egg white using glutaraldehyde,J.Biochem.Biophys.Methods59(2004)61–64.
[30]S.Talekar,A.Joshi,G.Joshi,P.Kamat,R.Haripurkar,S.Kamble,Parameters in preparation and characterization of cross linked enzyme aggregates,RSC Adv.3(2013)12485–12511.
[31]R.Reshmi,S.Sugunan,Superior activities of lipase immobilized on pure and hydrophobic clay supports:Characterization and catalytic activity studies,J.Mol.Catal.B Enzym.97(2013)36–44.
[32]S.Ba,L.Haroune,C.Cruz-Morató,C.Jacquet,I.E.Touahar,J.Bellenger,C.Y.Legault,J.P.Jones,H.Cabana,Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters,Sci.Total Environ.487(2014)748–755.
[33]G.Kemmer,S.Keller,Nonlinear least-squares data fitting in excel spreadsheets,Nat.Protoc.5(2010)267–281.
[34]K.Kannan,R.V.Jasra,Designing of nitrile hydratase from alkaline protease using quanidinehydrochloride and cobalt metal ion,Catal.Today198(2012)353–358.
[35]L.Wilson,L.Betancor,G.Fernandez-Lorente,M.Fuentes,A.Hidalgo,J.M.Guisan,B.C.Pessela,R.Fernández-Lafuente,Cross-linked aggregates of multimeric enzymes:A simple and efficient methodology to stabilize their quaternary structure,Biomacromolecules5(2004)814–817.
[36]S.Talekar,V.Ghodake,T.Ghotage,P.Rathod,P.Deshmukh,S.Nadar,M.Mulla,M.Ladole,Novel magnetic cross-linked enzyme aggregates(magnetic CLEAs)of alpha amylase,Bioresour.Technol.123(2012)542–547.
[37]L.Y.Zhou,C.Wang,Y.J.Jiang,J.Gao,Immobilization of papain in biosilica matrix and its catalytic property,Chin.J.Chem.Eng.21(2013)670–675.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Corrosion performance of Al–Al2O3 cold sprayed coatings on mild carbon steel pipe under thermal insulation☆
- Modelling of adsorption of textile dyes over multi-walled carbon nanotubes:Equilibrium and kinetic
- Integrated ozone–photo–Fenton process for the removal of pollutant from industrial wastewater☆
- Suppressing secondary reactions of coal pyrolysis by reducing pressure and mounting internals in fixed-bed reactor☆
- Multi-objective regulation in autohydrolysis process of corn stover by liquid hot water pretreatment
- Development of a new cleaner production process for cassava ethanol☆
