Structural insights of mechanically induced aluminum-doped hydroxyapatite nanoparticles by Rietveld refinement☆
2017-05-28AbbasFahamiBahmanNasiriTabriziGaryBeallWanJefreyBasirun
Abbas Fahami,Bahman Nasiri-Tabrizi*,Gary W.Beall,Wan Jefrey Basirun
1 Materials Science,Engineering and Commercialization Program,Texas State University,San Marcos 78666,TX,USA
2 Advanced Materials Research Center,Materials Engineering Department,Najafabad Branch,Islamic Azad University,Najafabad,Isfahan,Iran
3 Department of Chemistry and Biochemistry,Texas State University,San Marcos 78666,TX,USA
4 Physics Department,Faculty of Science,King Abdulaziz University,Jeddah 21589,Saudi Arabia
5 Department of Chemistry,Faculty of Science,University of Malaya,50603 Kuala Lumpur,Malaysia
1.Introduction
In recentdecades,a greatinterestin the use of bioceramics to improve the quality of human life has occurred.This is largely due to the inventive use of specially designed bioceramics for the repair and reconstruction of diseased or damaged parts of the human body.These classes of ceramics are potential candidates in the manufacturing of bone-like scaffolds and may also be designed to deliver biologically active substances aimed at repairing,maintaining,restoring or improving the function of organs and tissues in the organism[1–4].However,insertion of implants to replace the injured parts often gives discomfort to patients,due to the brittleness and insufficient biological properties of the bioceramic implants[5].Therefore,many attempts have been made to improve the functional specifications of ceramic implants and to regenerate old and deteriorating bones with a biomaterial that can be substituted by a new mature bone without transient loss of a mechanical support[6–9].
Atomic doping or substitution in apatite is one of the strategies to overcome some intrinsic properties of bioceramic implants or coating[10].Many recent studies have reported that Ca2+sites in bioceramics can be replaced with various cations such as monovalent(K+,Na+,Ag+),divalent(Cd2+,Zn2+,Eu2+,Sr2+,Mg2+,Ga2+etc.),trivalent(Bi3+,La3+,Y3+,Al3+etc.),tetravalent(Zr4+)and pentavalent(Ta5+,V5+,Nb5+),which exactly determine the implant-environment interactions after the implantation[11–15].Moreover,some studies have focused on the incorporation of monovalent anions(F−,Cl−,Br−)which can replace OH−ions in the anion channel without charge imbalance and some anionic complexes(CO32−,SO43−,SiO43−,etc.)which can substitute the phosphate groups.These structural modifications can alter the solubility,thermal stability,and mechanical properties as well as the invitro bioactivity of HA crystals[16–19].Over the past decades,many different methods have been introduced for the production of various substituted apatites with precise control of the microstructure,particle shape and size[20–24].Among them,powders synthesized by mechanochemical reactions typically possess a well-de fined structure due to the perturbation of surface-bonded species as a result of pressure,which enhances the thermodynamic and kinetic reactions between solids[25–27].On the other hand,it has been shown that the Rietveld refinement analysis of XRD data allows for quantitative probing of the structural modifications of biomaterials[28,29].However,to the best of our knowledge,apart from the absence of a comprehensive study on mechanically induced aluminum doping of HA nanoparticles,there are no reports on the effects of the dopant loading on the structural refinement of Aldoped HA by Rietveld analysis.
Kandoriet al.[30]examined the effects of modification of HA by trivalent metalions(Al(III),La(III),and Fe(III))on protein adsorption behavior using bovine serum albumin(BSA).Their results indicated that both the mean particle length and surface hydrophilicity of Al(III)-,La(III)-,and Fe(III)-substituted HA particles were the determining factors of the adsorption of BSA.Kaygiliet al.[31]also investigated the HA and HA-based bioceramics with high Al contents by using the sol–gel method.They found that with the increase of Al content,the crystallinity dramatically decreased,and the HA transformed into the new phases,including aluminum calcium phosphate and/or aluminum phosphate.Here,the possibility of using a one-pot mechanochemical reaction to prepare nanosize Al-doped HA was studied for the first time.The microstructural features and doping mechanism were investigated using microscopic and spectroscopic techniques.Besides,the mechanosynthesized nanopowders were systematically treated by Rietveld refinement to extract the quantitative phase analysis and the structural parameters,to refine the composition of the Al-substituted HA.This unique top-down approach offers a framework for the future design of substituted apatites.
2.Materials and Methods
2.1.Chemicals
Calcium hydroxide(Ca(OH)2,≥96%),phosphorus pentoxide(P2O5,99%),and aluminum hydroxide(Al(OH)3)(all from Sigma-Aldrich Co.,USA)were used as the precursors without further purification.
2.2.Clari fi cation
With regards to the milling conditions,the mechanochemical reactions can be classified into two main categories:(1)progressive mode where the reaction may expand to a very smallvolume during each collision and(2)mechanically induced self-sustaining reactions(MSRs)wherein a self-propagating combustion reaction can initiate and eventually lead to the instantaneous formation of irreversible crystalline phases.It should be mentioned that the second mode could happen if the reaction enthalpy is sufficiently high[14,32].In this mode,the reaction mechanism can be predicted by calculating the adiabatic temperature or by the parameter(−ΔH298/∑Cp).Accordingly,for self sustaining reaction to take place,it is imperative that these quantities be at leastTad>1800 K and–ΔH298/∑Cp>2000 K[33].In the present case,due to the lack of high exothermic reactions,it could be considered that the mode of the reaction is gradual,which includes the following steps:(1)the interaction of particles and the reactive absorption of atmospheric carbon dioxide during the early stage of milling,and(2)the mechanically induced reaction between calcium phosphate and the dopant.Therefore,it is necessary to mention the following assumptions:(1)milling vial is sealed and isolated(2)all the reactions occur in the standard conditions(ΔG= ΔG°)(3)the activity coefficient is equal to 1,and(4)the reactions occur in the same physical conditions.
2.3.Mechanochemical process
A series of HA:Al3+powders with different doping levels and general formula of Ca10-xAlx(PO4)6(OH)2(0≤x≤1)were synthesized by mechanical activation of reagents for 1 h,using a high-energy ball mill(8000M Mixer/Mill)with hardened chromium steel vials(vol.65 ml)under air atmosphere without any process control agent(PCA).In all cases,the(Ca+Al)/P ratio was fixed at 1.67,while the Al/(Ca+Al)ratio ranged from 0 to 0.10.The ball-to-powder(BPR)weight ratio,total powder mass and rotational speed were 10:1,2.2 g,and 1725 r·min−1(115 V 60 HZ),respectively.The details of the substitution degree are summarized in Table 1.
2.4.Characterization
2.4.1.X-ray powder diffraction(XRD)
X-ray powder diffraction(Bruker D8 Advance ECO(XRD))using a CuKαradiation over a 2θ range from 10°to 70°was employed to determine the phase purity.The “PANalytical X'Pert HighScore”software was used to analyze the XRD pro files,where the patterns were compared to standards compiled by the Joint Committee on Powder Diffraction and Standards(JCPDS),which involved card#24-0033 for HA and#029-0041 for Al(OH)3.The crystallinity degree was assessed using the well-known equation(Eq.(1))[34]:

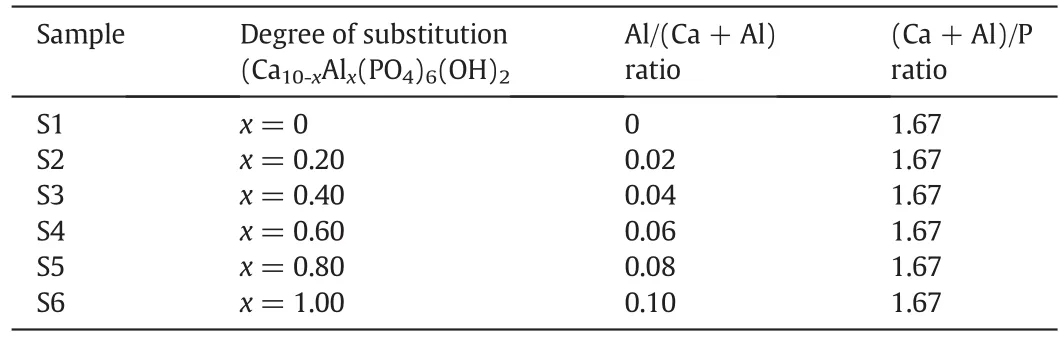
Table 1Details of the substitution degree
2.4.2.Structural refinement
In addition to the above-mentioned approach,Match!,a user friendly software was employed for the phase identification process of the powder diffraction data.It compares the diffraction pattern of the sample to a database containing reference patterns to recognize the phases which are present.The additional information about the sample such as phases,elements or density can also be determined easily.Besides,a quantitative analysis using the Rietveld refinement can be performed as well.The Rietveld refinements can be easily run fromMatch!,and the actual calculations being performed automatically,using the wellknown FullProf program[35].
2.4.3.Fourier transformed infrared spectroscopy(FTIR)
Fourier transformed infrared spectroscopy(FTIR,Bruker Tensor 27,USA)was employed to assess the functional groups and structural changes during the mechanical activation in a frequency range of 4000–400 cm−1.
2.4.4.Electron microscopy
Specimens were prepared by ultrasonically dispersing the mechanosynthesized powders in ethanol and then depositing them onto carbon coated copper grids.Scanning electron microscopy(FEI Helios Nanolab 400 SEM)was applied to observe the size and morphology of the samples.Energy dispersive X-ray spectroscopy(EDS)and elemental mapping analysis coupled to SEM were also employed to identify the elemental compositions and the spatial distributions of elements.Transmission electron microscopy(JEOL JEM 1200 EXII)and high-resolution transmission electron microscopy(HT-7700,Hitachi,Japan)were performed to investigate the morphological features and lattice fringes of the nanoparticles,respectively.A full particle size distribution can be obtained based on a count of many particles using automated counting techniques.These experimental data are typically represented as a number average[14]:

wherenirepresents the number of particles with a diameter,di,which in practice is typically a mean value within a specified size.However,of greater use is the surface-weighted average diameter:

which allows the comparison of crystallite sizes determined by chemisorption methods,and the volume-weighted average diameter:



whereNis the total number of particles and σ is the standard deviation from the average particle size.
3.Results and Discussion
3.1.Effects of the dopant loading
Fig.1 shows the XRD pro file of the dopant agent.As can be seen,the dopant precursor is highly pure gibbsite,Al(OH)3,which exhibits a monoclinic structure(space groupP1 21/n1)with lattice parameters:a,b,andcof 0.86635,0.50675 and 0.97138 nm,respectively.The XRD patterns of the 1 h milled specimens in the absence(S1)and presence of different amounts of dopant(S2–S6)are shown in Fig.2.In the absence of dopant,due to the fast completion of the mechanochemical reaction,a crystalline form of HA was produced after only 1 h of milling.Similar patterns were perceived in the presence of different values of dopant and therefore the XRD pro files showed a major Al-doped HA(Ca10-xAlx(PO4)6(OH)2;where 0≤x≤0.8)phase.The main peaks attributed to HA:Al3+are as follows:(0 0 2)plane at 2θ =25.82°,(1 0 2)plane at 2θ =28.04°,(2 1 0)plane at 2θ =28.91°,(2 1 1)plane at 2θ =31.75°,(3 0 0)plane at 2θ =32.84°,(2 0 2)plane at 2θ =33.99°,(1 3 0)plane at 2θ =39.75°,(2 2 2)plane at 2θ =46.75°,(2 1 3)plane at 2θ =49.44°,and(0 0 4)plane at 2θ =53.07°.This suggests that Al doping not only favors the HA:Al3+formation,but also assists to stabilize it at room temperature.It should be mentioned that the nonexistence of any characteristic peaks for Al,or Al compounds such as gibbsite and a strong similarity of the XRD patterns recorded for the undoped and doped samples corroborate the substitution ofCa2+by Al3+in the apatite lattice.With an increase in the dopant content to 10%(S6),substantial changes were observed in the XRD reflection.In this case,the high intensity of the strongest peak attributed to gibbsite[(0 0 2)plane at 2θ=18.29°]serves as proof that there is an imperfect substitution due to the intrinsic structural constrains of HA.With increased dopant loading,the sharpness and intensity of the apatite-derived XRD peaks decreased.This behavior can be explained on the basis of the mechanically induced amorphization,which is associated with a significant increase in the lattice imperfections.Accordingly,the crystallinity degree of the milled samples declined drastically from 63%±6%to 24%±2%with the increase of the Al content from 0 to 10%(Fig.3).This result is consistent with the findings of Kandoriet al.[30],showing that the crystallinity of the substituted HA materials decreases with the increase of trivalent metal ions such as Al(III),La(III),and Fe(III).
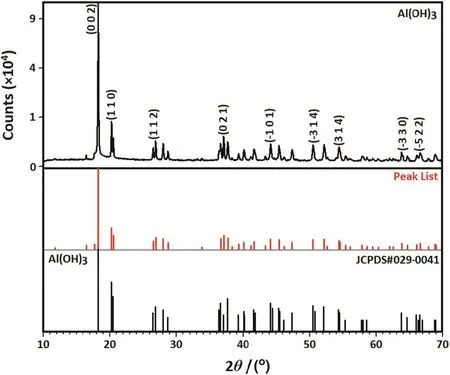
Fig.1.XRD pro file of the dopant agent.
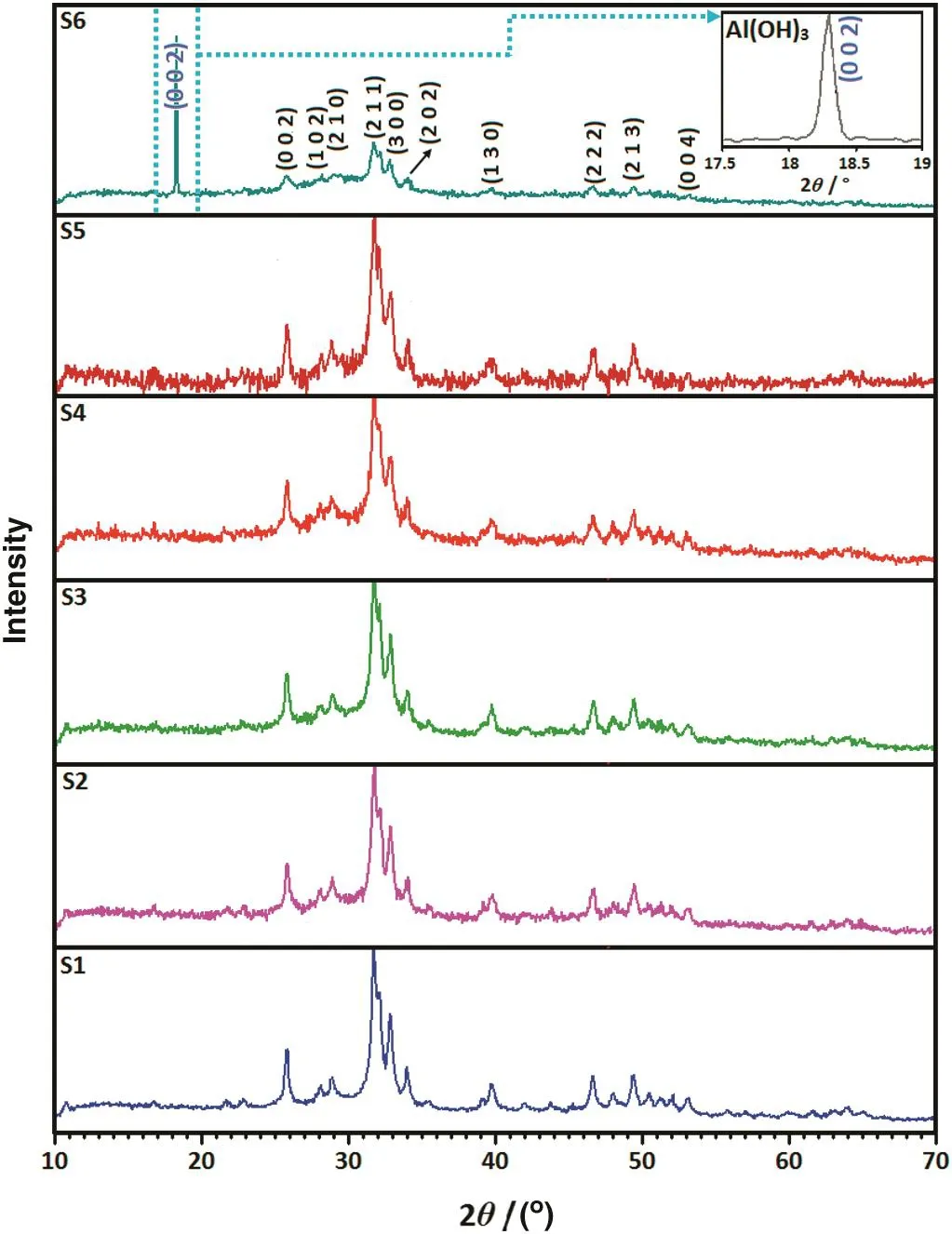
Fig.2.XRD patterns of the 1 h milled specimens in the absence(S1)and presence of different amounts of dopant(S2–S6).
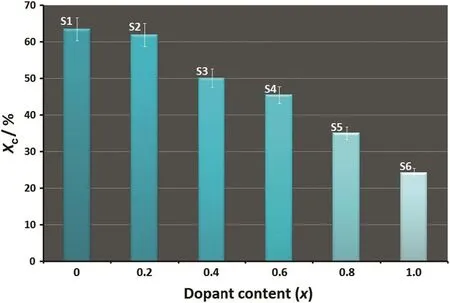
Fig.3.Crystallinity degree of the 1 h milled samples as a function of the dopant loading.
3.2.Rietveld re fi nement results
HA is in the space groupP63/mand its unit cell parameters area=b=0.94347 nm andc=0.68893 nm[36].HA have two different binding sites(C and P sites)on the particle surface.After the dispersion of HA particles in aqueous media,the calcium atoms(Ca(2))are exposed on the HA surface by the dissolution of OH−ions,to produce calcium ions or positively charged sites on the particle surface,so-called C sites which are arranged on theacorbcparticle face.On the contrary,the P sites,negatively charged adsorbing sites,each formed by six oxygen atoms corresponding to three crystal phosphate ions,are arranged hexagonally on theabparticle face with a minimal interdistance,in both theaandbdirections[30].Therefore,it is quite clear that the HA structure presents two nonequivalent Ca2+sites,where the smallest distance between a cation and a coordinated oxygen is observed at site(2)and the smallest Ca–Ca distances are found between Ca2+ions at site(1)[14].This suggests that small ions are preferentially substituted at Ca(1),while large cations are preferably exchanged at site(2).Consequently,in the present case,Al3+ions would first replace Ca2+at site(1)as the ionic radius of Al3+(0.0535 nm)is smaller than that of Ca2+(0.099 nm).
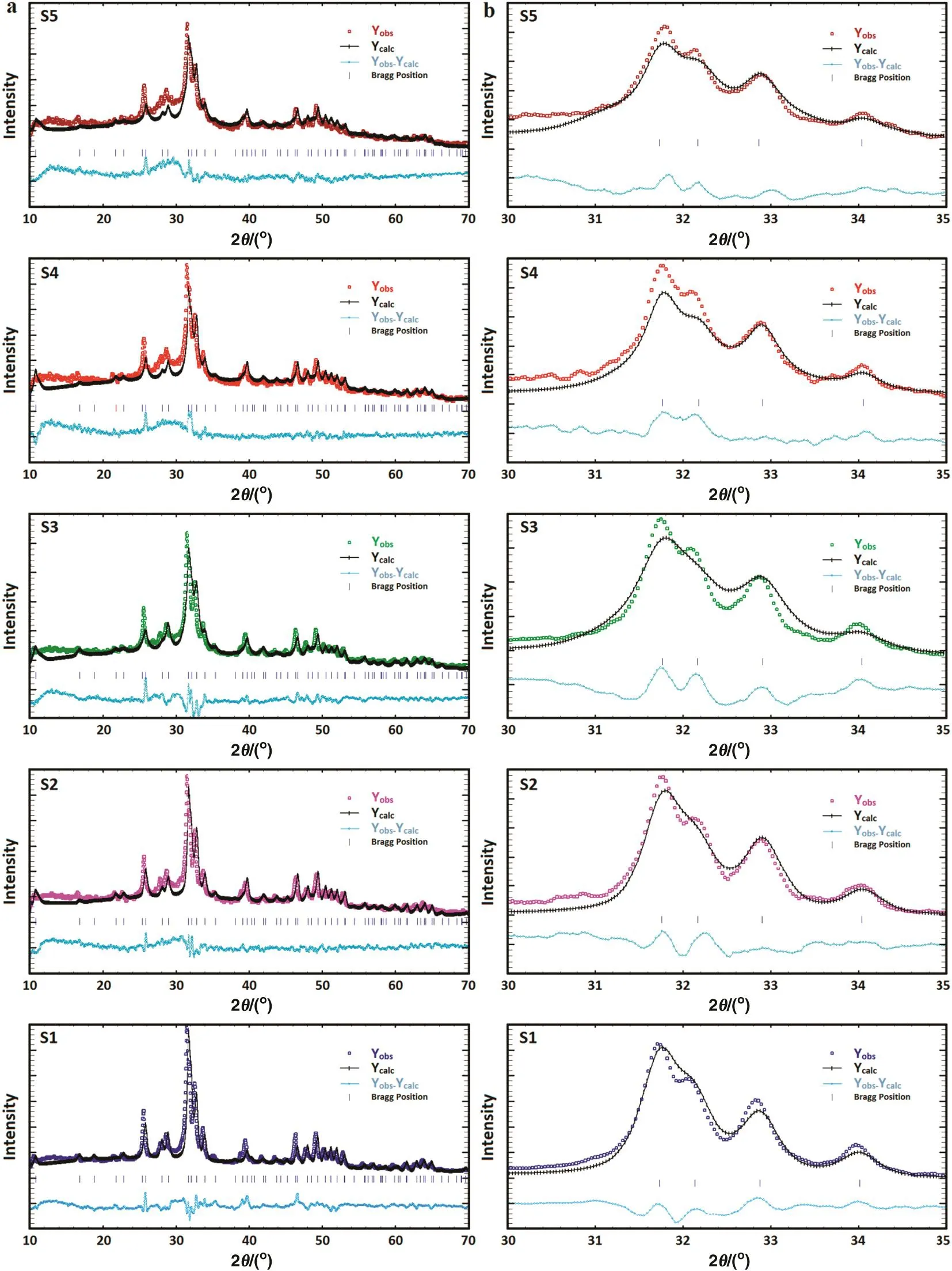
Fig.4.(a)Rietveld refinement plots of the 1 h milled specimens in the absence(S1)and presence of different amounts of dopant including S2(2%),S3(4%),S4(6%),and S5(8%);(b)magnified XRD-Rietveld refinement pro files(2θ =30o–35o).
To authenticate the crystallographic data,the standard XRDpatterns of nanostructured materials do not provide the necessary sufficient intensity data,and so further actions are needed to complete the structural analysis.In the present case,the structural refinements were done by the Rietveld method using the Fullpr of program as well as the Crystallography Open Database of all possible occurring phases.The peak shapes were modeled on a pseudo-Voigt distribution,and an asymmetry parameter was refined.At first,all the parameters were fixed according to the literature values and subsequently they were all refined during the successive refinement cycles.Fig.4a shows the Rietveld refinement plots of the 1 h milled specimens in the absence(S1)and presence of different amounts of dopant including S2(2%),S3(4%),S4(6%),and S5(8%).From these reflections,the refined XRD patterns demonstrate that all the specimens are in single phase form.In addition,these plots con firm the achievement of the Rietveld refinement,as reflected by small difference between calculated and observed.As the refinement progressed,the relative percentage of the hexagonal phase remained stable as the dopant content increased to 8%.However,with the increase of the dopant loading to 10%(S6),the percentage of the hexagonal structure corresponding to HA decreased severely to 77.5%while that of the monoclinic structure belonging to gibbsite increased to 22.5%.As can be seen in the magnified XRD pro files(2θ =30°–35°)(Fig.4b),by increasing the dopant content,the apatite-derived XRD peaks declined in both sharpness and intensity which can be linked to the mechanically induced amorphization.The variousRfactors obtained from the Rietveld refinements are summarized in Table 2.The values ofRfactors likeRp,RwpandRexpare found to be large.Similar high values ofRfactors for the nanostructured materials have been reported by the previous studies[37,38].It can be derived from the poorcounting statistics with a XRD fasts can.However,the goodness of fit(G.O.F.)values given byRwp/Rexpwere relatively low,i.e.,all approximately less than 2%,which is considered as acceptable in accordance with basic principle of G.O.F.less than 4%[39].It has been reported that diffuse scattering in the diffraction pattern of the nanocrystalline materials is more prominent than that of bulk crystalline materials.This effect is due to the large ratio of surface to volume atoms.In fact,the diffuse scattering becomes significant at the nanoscale while Bragg scattering gets diminished,which leads to decline in crystallinity and largeRfactors[40].
Fig.5 illustrates the refined lattice constants and unit cell volume for samples S1,S2,S3,S4,and S5 based on the XRD-Rietveld analysis.Fig.5b shows the variation of thea-axis parameter of the HA phase with the dopant loading.For the undoped HA,thea-axis tends to decrease compared to the standard.A similar trend was observed in the case of the doped powders as dopant content increased to 6%(S4).In fact,for the doped powders,thea-axis was shorter than the standard HA,but with a further increase in the dopant content to 8%,thea-axis rose to 0.94335 nm.On the other hand,measurement of thec-axis(Fig.5c)shows that the pure HA (S1)is increased slightly in length(0.68945 nm)as compared to the standard.This finding is contrary to the doped cases.It is obvious that the HA decreases faintly in length with the dopant loading and reached a minimum of 0.68835 nm in the case of S5(8%).The change in the unit cell volume of the hexagonal phase as a function of the dopant content is shown in Fig.5d.A decrease in the unit cell volume of the hexagonal phase with an increase in the dopant loading indicates the incorporation of Al3+into the HA lattice.These findings reveal that only about 8%of the dopant(Al3+)probably incorporates into the HA lattice and the rest remains as the unwanted phase(see Fig.2).
3.3.FTIR analysis
The FTIR spectra of the mechanosynthesized powders were recorded to assess the functional groups of the undoped and doped samples as shown in Fig.6.Besides,the corresponding assignments of the 1 hmilled specimens are listed in Table 3.The bands attributed to apatite are recognized from Fig.6a and Table 3.It is obvious that the functional groups in the doped samples are very similar to the HA.However,some differences can be observed in the position and shape of the bands in the short-range FTIR spectra(Fig.6b and c).The characteristic bands for PO43−groups appear at 1097,1044,961,603,569 and 471 cm−1[6,7,13,14].The asymmetrical stretching(ν3)and bending(ν4)modes of the PO4-derived bands are detected at around 1097 and 1044,and 603 and 569 cm−1,respectively.The symmetric stretching modes(ν1and ν2)of the PO43−ions were also observed at around 961 and 471 cm−1,respectively.In addition,the stretching and vibration modes of the OH−ion are identified at around 3576 and 670 cm−1,respectively[41].The lower intensity of both the OH-and PO4-derived bands in the doped samples could be explained on the basis of the carbonate content within the synthesized powders[14].Lafonet al.[42]found that the intensity of the OH-derived bands decreased as the carbonate content increased in the HA lattice and these bands disappeared fully at high carbonate contents.In the present case,the level of carbonate substitution increased as the dopant content rose to 10%(S6),signifying the mechanically-induced substitution of Al can stimulate the solidstate amorphization in some bioceramics.As shown in Fig.6c,the two regions of the carbonate vibrations in the HA:Al3+powders are:(i)1463–1423 cm−1attributed to ν3(asymmetric stretch vibration)and(ii)CO3-derived bands at 874 cm−1ascribed to ν2(out-of-plane bending vibration).The position of the CO3-derived bands implies that the CO32−-for-PO43−substitution(B-type)dominates in the HA:Al3+powders.However,some fraction of the OH−groups might be substituted by the CO32−groups(A-type),which is usually observed in carbonated HAp powders.The increase in the concentration of dopant along with the carbonate(withx)in the HA:Al3+powders,which raises the lattice disorder,can be partially justified by the broadening of the XRD reflections,with increasingx(see Fig.4).It is important to note that the de ficit in the negative charge,caused by the replacement of PO43−by CO32−,can be compensated by the loss of a positive charge through the removal of Ca2+from the lattice[10].This anionic complex can improve the mechanical properties,biological responses as well as the densification of HA and also reduces the sintering temperature required to achieve a near-full density compared to the stoichiometric HA[43].It should be noted that all the experiments were done in an open atmosphere,therefore the atmospheric CO2absorption was unavoidable.Based on the FTIR spectra,the position and shape of the characteristics bands of apatite may be locally controlled by the dopant loading.
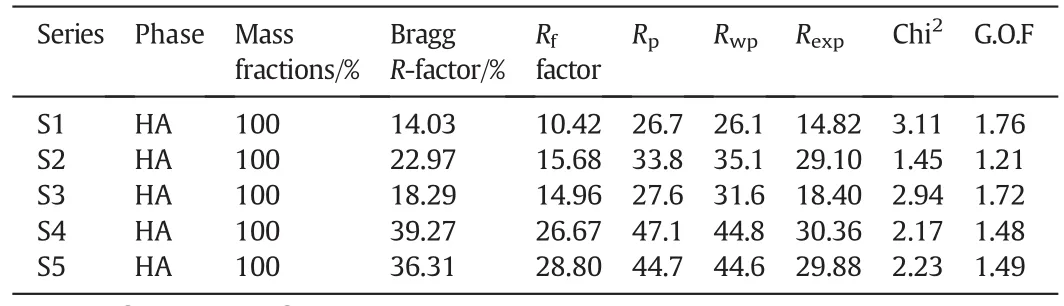
Table 2R factors obtained from the Rietveld refinements of the Al substituted HA

Fig.5.The refined(a-c)lattice constants and(d)unit cell volume of the 1 h milled samples as function of the dopant loading 1Å=0.1 nm.
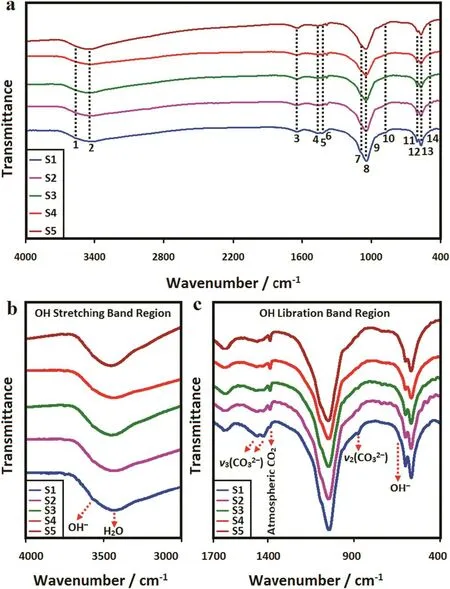
Fig.6.(a)FTIR spectra of the 1 h milled samples as well as some changes in the position and shape of the bands in the short-range FTIR spectra(b)OH stretching band region and(b)OH libration band region.
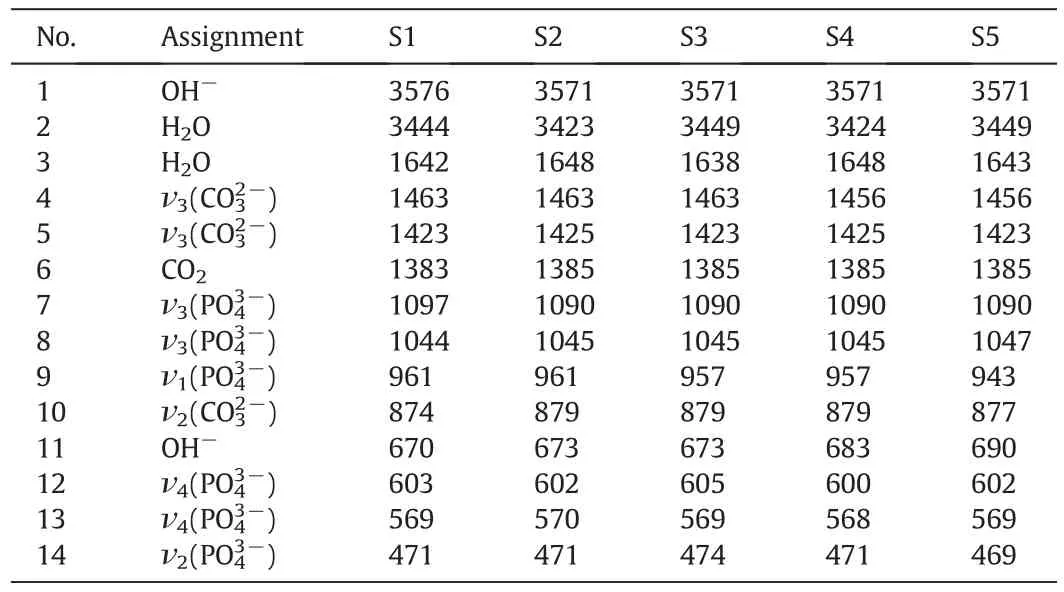
Table 3The revealed bands and their assignments for FTIR spectra
3.4.SEM/TEM observations and EDS analysis
Given that the morphological features,chemical composition and spatial distribution of elements in nanostructured bioceramics play an important role in biomedical functionality,the size and morphology,as well as the chemical constituents of the mechanosynthesized powders were analyzed by SEM,TEM/HRTEM and EDS techniques.Fig.7 shows the SEM images and the edge mode of the SEM micrographs of the 1 h milled HA:Al3+powder.As shown in Fig.7a,the doped nanoparticles exhibit a high tendency to agglomerate due to their large surface to volume ratio.A closer examination at higher magnification illustrates that each agglomerates comprises of many fine round particles(Fig.7b).Generally,the deformation of the smaller particles and convection processes can control the coalescence of liquid nanoparticles.On the other hand,solid state diffusion dominates the coalescence of solid-like particles[44].Accordingly,in the present case,the coalescence of the milled particles is due to the solid state diffusion.Fig.7c and d shows a high level of the volume fraction of grain boundary in the doped case.This feature suggests an ultra fine microstructure formed in the presence of the dopant.In fact,the incorporation of Al in the HA lattice led to a grain refinement and a decrease of particle size during the mechanical activation.It is likely that the doped particles in grain boundaries led to an intensive work hardening as well as an increase in the lattice imperfections and subgrain boundaries,and therefore resulted in a decrease in the grain size.

Fig.7.(a,b)SEM images and(c,d)the edges mode of the SEM micrographs of the 1 h milled HA:Al3+powder.
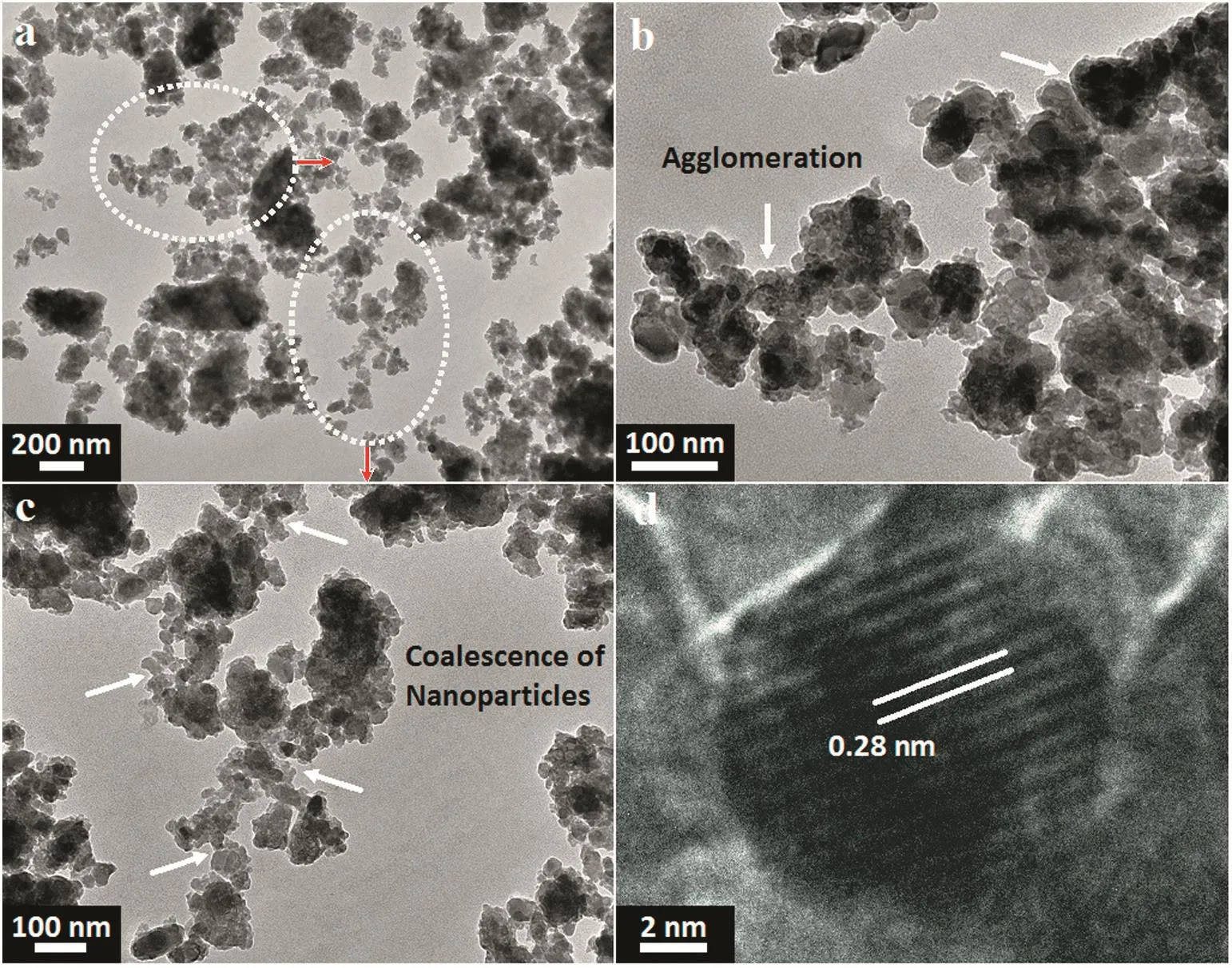
Fig.8.(a-c)TEM observations at different magnifications accompanied by(d)HRTEM image of the 1 h milled HA:Al3+powder.
Fig.8 demonstrates the TEM micrographs accompanied by the HRTEM image of the 1 h milled HA:Al3+powder.From the TEM images in Fig.8a–c,the doped powder consists of nanospheroids with an average size of(44±20)nm and so it is very promising for bone tissue regeneration.As can be seen in Fig.8b and c,the coalescence of nanospheroids during the milling influences the formation of cluster like agglomerates.It is quite evident that the milled sample has a high tendency to agglomerate,as shown in SEMimages.As soon astwo adjacent primary particles collide,the coalescence may happen on the basis that these two particles share a common crystallographic orientation.Accordingly,the two primary particles are connected and combined to generate a secondary one.Given that the sizes of the secondary particles are still very small,it is reasonable that they will continue to collide and interact,which may ultimately lead to the agglomeration.The HRTEM image in Fig.8d reveals a distance of 0.28 nm between the adjacent lattice fringe,which implies the growth control along the(100)crystallographic plane.Based on the SEM/TEM observations,the agglomeration mechanism is proposed as shown in Fig.9.It is most likely that the agglomeration occurs in three certain stages as follows[45]:(1)Rittinger stage,where the particles interaction can be neglected and the energy input is nearly proportional to the new surface area formation;(2)aggregation stage,wherein the formed new surface area is not congruent to the energy input.Nonetheless,the dispersion degree is still rising considerably.In this stage,the particles stick to each other without any chemical reaction and therefore the resultant aggregates can be separated by slight mechanical intervention;(3)agglomeration stage,where a rise in the dispersion first drops to a negligible value,then stops altogether;it may even give way to a decrease of the surface area due to the particle interaction(agglomeration).In this stage,the particles are grown together by chemical bonds and separation becomes impossible and thus it is most likely that the mechanochemical reaction and structural modifications mostly happen at this stage.
The EDS spectra and elemental mapping images of the 1 h milled HA:Al3+powder is shown in Fig.10.It is clear that the main elements are calcium,phosphorus,oxygen and aluminum.From the EDS results,the(Ca+Al)/P ratio of the doped sample is around 1.75,more than 1.67 as a result of a decrease in the Ca and P concentration.This increase in(Ca+Al)/P ratio can be linked to the CO32−-for-PO43−substitution(B-type),which is in good agreement with the FTIR results.Moreover,contamination due to the excessive wearing of the vial and balls was absent,which con firmed the purity of the as-prepared powders.With regards to the elemental mapping images,an appropriate spatial distribution of the elements in the nanopowders was detected,indicating the formation of the HA:Al3+powder with a homogenous microstructure during an efficient mechanochemical process.
4.Conclusions
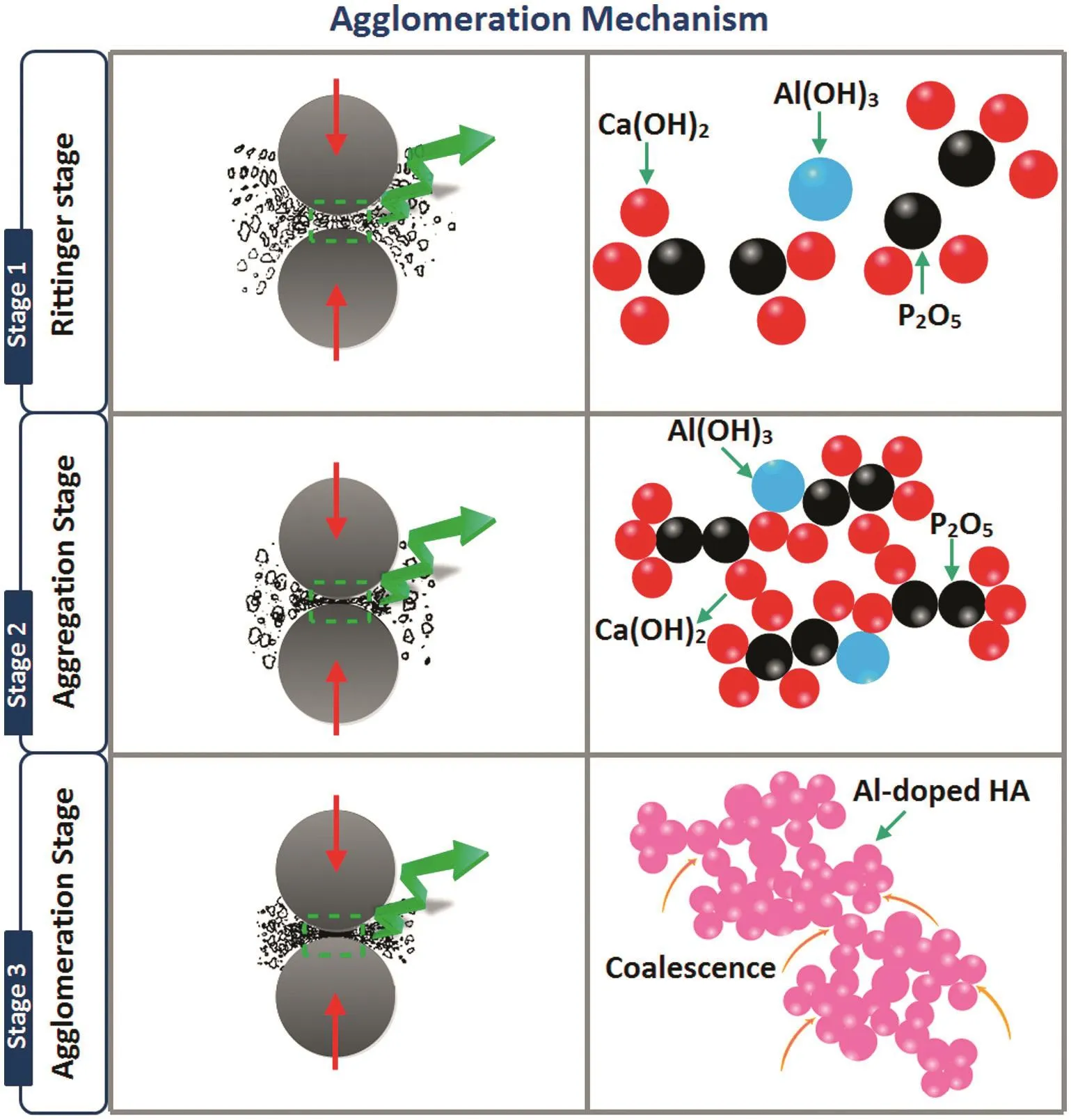
Fig.9.The proposed agglomeration mechanism on the basis of SEM/TEM observations.
In summary,HA:Al3+nanopowders were successfully synthesized using a one-pot mechanochemical reaction.The resultant nanopowders were systematically treated by Rietveld refinement to extract the quantitative phase analysis and the structural parameters.Results showed that an increase in dopant loading induced the amorphization of HA and consequently the crystallinity degree of the milled samples decreased drastically from 63%±6%to 24%±2%as the dopant content increased from 0 to 10%.Moreover,a-and c-axis directions and unit cell volume exhibited a decreasing mode with increasing dopant content owing to the smaller ionic size of Al3+as compared to Ca2+.The structural refinements revealed that only about 8%of the dopant probably goes into the HA lattice and the rest stays as unwanted phase.The position of the CO3-derived bands in FTIR spectra indicated that the CO32−-for-PO43−substitution(B-type)was dominant in the HA:Al3+powders.From the SEM and TEM observations,the doped nanoparticles had a high tendency to agglomerate and closer examination at higher magnification demonstrated that each agglomerate consisted of nanospheroids with an average size of(44±20)nm.According to the edges mode of the SEM micrographs,a high level of the volume fraction of grain boundary was detected,which showed the mechanically induced incorporation of Al in to the HA lattice may lead to a grain refinement and a decrease of particle size.The elemental mapping images con firmed the formation of the HA:Al3+nanopowders with a homogenous microstructure.The proposed mechanochemical reaction offers an outline for the future design of nanocrystalline substituted apatites.
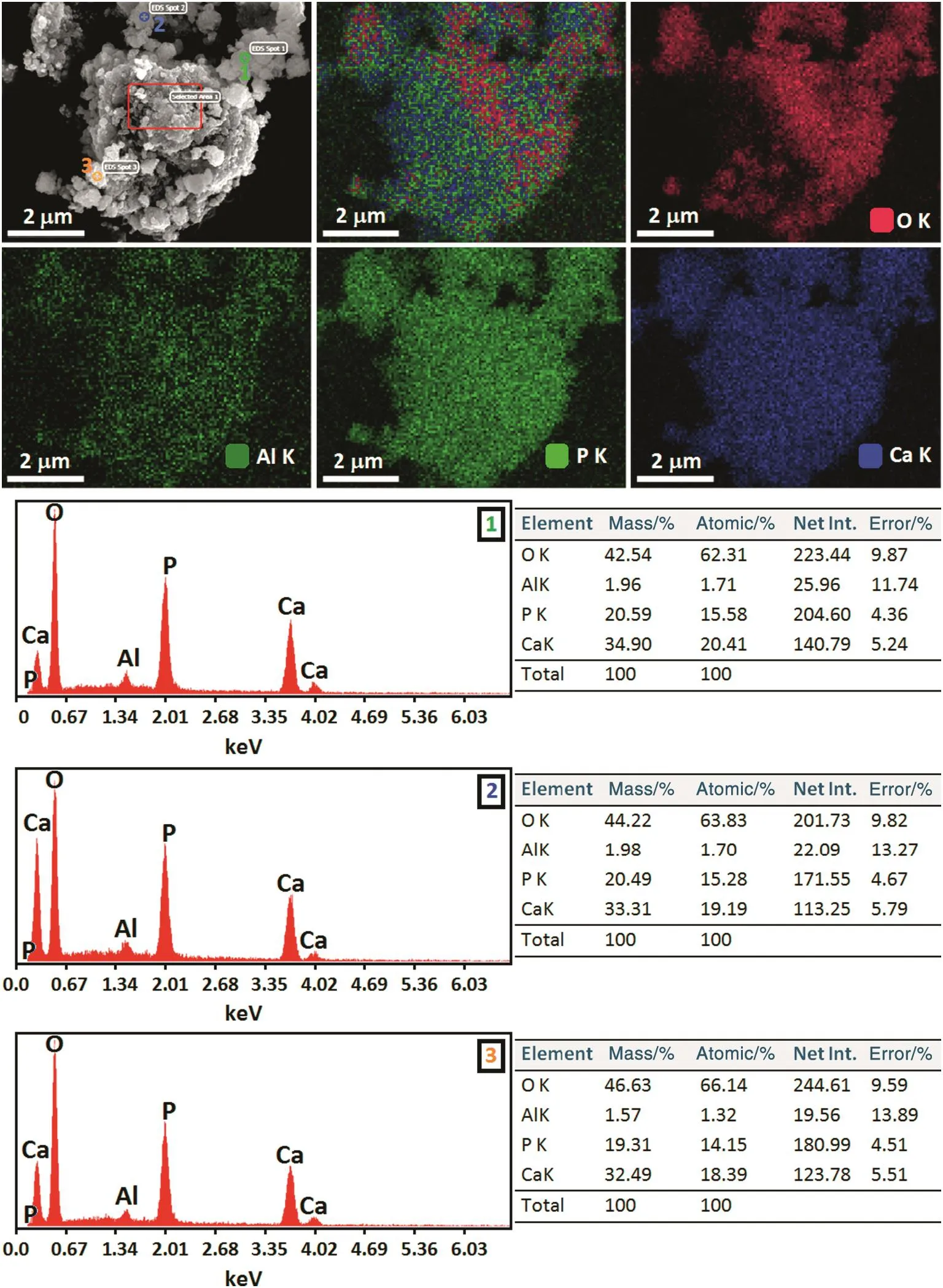
Fig.10.EDS spectra and elemental mapping images of the 1 h milled HA:Al3+powder.
Acknowledgment
The authors are also grateful to University of Malaya grant number RP005B-13AET and Research Affairs of Islamic Azad University,Najafabad Branch for supporting of this research.
[1]L.L.Hench,Bioceramics:from concept to clinic,J.Am.Ceram.Soc.74(1991)1487–1510.
[2]S.M.Best,A.E.Porter,E.S.Thian,J.Huang,Bioceramics:past,present and for the future,J.Eur.Ceram.Soc.28(2008)1319–1327.
[3]M.Vallet-Regí,E.Ruiz-Hernández,Bioceramics:from bone regeneration to cancer nanomedicine,Adv.Mater.23(2011)5177–5218.
[4]H.Liu,G.W.Xu,Y.F.Wang,H.S.Zhao,S.Xiong,Y.Wu,B.C.Heng,C.R.An,G.H.Zhu,D.H.Xie,Composite scaffolds of nano-hydroxyapatite and silk fibroin enhance mesenchymal stem cell-based bone regeneration via the interleukin 1alpha autocrine/paracrine signaling loop,Biomaterials 49(2015)103–112.
[5]F.S.Shirazi,M.Mehrali,B.Nasiri-Tabrizi,S.Baradaran,S.Gharehkhani,H.S.C.Metselaar,N.A.Kadri,N.A.Abu Osman,Mechanochemical synthesis and characterization of silver(Ag+)and tantalum(Ta5+)doped calcium silicate nanopowders,Sci.Adv.Mater.7(2015)2664–2671.
[6]S.Baradaran,E.Moghaddam,B.Nasiri-Tabrizi,W.J.Basirun,M.Mehrali,M.Sookhakian,M.Hamdi,Y.Alias,Characterization of nickel-doped biphasic calcium phosphate/graphene nanoplatelet composites for biomedical application,Mater.Sci.Eng.C 49(2015)656–668.
[7]S.Baradaran,E.Moghaddam,W.J.Basirun,M.Mehrali,M.Sookhakian,M.Hamdi,M.R.Nakhaei Moghaddam,Y.Alias,Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite,Carbon 69(2014)32–45.
[8]F.S.Shirazi,M.Mehrali,A.A.Oshkour,H.S.C.Metselaar,N.A.Kadri,N.A.Abu Osman,Mechanical and physical properties of calcium silicate/alumina composite for biomedical engineering applications,J.Mech.Behav.Biomed.Mater.30(2014)168–175.
[9]S.F.S.Shirazi,S.Gharehkhani,H.S.C.Metselaar,B.Nasiri-Tabrizi,H.Yarmand,M.Ahmadi,N.A.A.Osman,Ion size,loading,and charge determine the mechanical properties,surface apatite,and cell growth of silver and tantalum doped calcium silicate,RSC Adv.6(2016)190–200.
[10]M.Šupová,Substituted hydroxyapatites for biomedical applications:a review,Ceram.Int.41(2015)9203–9231.
[11]T.J.Webster,E.A.Massa-Schlueter,J.L.Smith,E.B.Slamovich,Osteoblast response to hydroxyapatite doped with divalent and trivalent cations,Biomaterials 25(2004)2111–2121.
[12]S.Dasgupta,S.S.Banerjee,A.Bandyopadhyay,S.Bose,Zn-and Mg-doped hydroxyapatite nanoparticles for controlled release of protein,Langmuir 26(2010)4958–4964.
[13]B.Nasiri-Tabrizi,E.Zalnezhad,B.Pingguan-Murphy,W.J.Basirun,A.M.S.Hamouda,S.Baradaran,Structural and morphological study of mechanochemically synthesized crystalline nanoneedles of Zr-doped carbonated chlorapatite,Mater.Lett.149(2015)100–104.
[14]B.Nasiri-Tabrizi,B.Pingguan-Murphy,W.J.Basirun,S.Baradaran,Crystallization behavior of tantalum and chlorine co-substituted hydroxyapatite nanopowders,J.Ind.Eng.Chem.33(2016)316–325.
[15]N.Kose,A.Otuzbir,C.Pekşen,A.Kiremitçi,A.Doğan,A silver ion-doped calcium phosphate-based ceramic nanopowder-coated prosthesis increased infection resistance,Clin.Orthop.Relat.Res.471(2013)2532–2539.
[16]A.Fahami,G.W.Beall,T.Betancourt,Synthesis,bioactivity and zeta potential investigations of chlorine and fluorine substituted hydroxyapatite,Mater.Sci.Eng.C 59(2016)78–85.
[17]A.Fahami,B.Nasiri-Tabrizi,R.Ebrahimi-Kahrizsangi,Mechanosynthesis and characterization of chlorapatite nanopowders,Mater.Lett.110(2013)117–121.
[18]B.Nasiri-Tabrizi,A.Fahami,Mechanosynthesis of nanosized B-type carbonated fluorapatite,Mater.Lett.134(2014)42–46.
[19]S.M.Toker,A.Tezcaner,Z.Evis,Microstructure,microhardness,and biocompatibility characteristics of yttrium hydroxyapatite doped with fluoride,J.Biomed.Mater.Res.B Appl.Biomater.96(2011)207–217.
[20]G.Renaudin,E.Jallot,J.M.Nedelec,Effect of strontium substitution on the composition and microstructure of sol–gel derived calcium phosphates,J.Sol-Gel Sci.Technol.51(2009)287–294.
[21]C.Shi,J.Gao,M.Wang,J.Fu,D.Wang,Y.Zhu,Ultra-trace silver-doped hydroxyapatite with non-cytotoxicity and effective antibacterial activity,Mater.Sci.Eng.C 55(2015)497–505.
[22]X.Yuan,B.Zhu,G.Tong,Y.Su,X.Zhu,Wet-chemical synthesis of Mg-doped hydroxyapatite nanoparticles by step reaction and ion exchange processes,J.Mater.Chem.B 1(2013)6551–6559.
[23]N.S.V.Capanema,A.A.P.Mansur,S.M.Carvalho,A.R.P.Silva,V.S.Ciminelli,H.S.Mansur,Niobium-doped hydroxyapatite bioceramics:synthesis,characterization and in vitro cytocompatibility,Materials 8(2015)4191–4209.
[24]J.Ma,J.Qin,Graphene-like zinc substituted hydroxyapatite,Cryst.Growth Des.15(2015)1273–1279.
[25]M.Sadat-Shojai,M.T.Khorasani,E.Dinpanah-Khoshdargi,A.Jamshidi,Synthesis methods for nanosized hydroxyapatite with diverse structures,Acta Biomater.9(2013)7591–7621.
[26]B.Nasiri-Tabrizi,A.Fahami,Production of poorly crystalline tricalcium phosphate nanopowders using different mechanochemical reactions,J.Ind.Eng.Chem.20(2014)1236–1242.
[27]B.Nasiri-Tabrizi,A.Fahami,Reaction mechanisms of synthesis and decomposition of fluorapatite–zirconia composite nanopowders,Ceram.Int.39(2013)5125–5136.
[28]S.Gomes,G.Renaudin,E.Jallot,J.-M.Nedelec,Structural characterization and biological fluid interaction of sol–gel-derived Mg-substituted biphasic calcium phosphate ceramics,ACS Appl.Mater.Interfaces 1(2009)505–513.
[29]M.A.Lopes,J.C.Knowles,J.D.Santos,Structural insights of glass-reinforced hydroxyapatite composites by Rietveld refinement,Biomaterials 21(2000)1905–1910.
[30]K.Kandori,S.Toshima,M.Wakamura,M.Fukusumi,Y.Morisada,Effects of modi fication of calcium hydroxyapatites by trivalent metal ions on the protein adsorption behavior,J.Phys.Chem.B 114(2010)2399–2404.
[31]O.Kaygili,C.Tatar,F.Yakuphanoglu,S.Keser,Nano-crystalline aluminumcontaining hydroxyapatite based bioceramics:synthesis and characterization,J.Sol-Gel Sci.Technol.65(2013)105–111.
[32]C.Suryanarayana,N.Al-Aqeeli,Mechanically alloyed nanocomposites,Prog.Mater.Sci.58(2013)383–502.
[33]L.Takacs,Self-sustaining reactions induced by ball milling,Prog.Mater.Sci.47(2002)355–414.
[34]E.Landi,A.Tampieri,G.Celotti,S.Sprio,Densification behaviour and mechanisms of synthetic hydroxyapatites,J.Eur.Ceram.Soc.20(2000)2377–2387.
[35]J.Rodriguez-Carvajal,Recent developments of the program FULLPROF,commission on powder diffraction(IUCr),Newsletter 26(2001)12–19.
[36]J.Terra,E.R.Dourado,J.G.Eon,D.E.Ellis,G.Gonzalez,A.M.Rossi,The structure of strontium-doped hydroxyapatite:an experimental and theoretical study,Phys.Chem.Chem.Phys.11(2009)568–577.
[37]L.Kumar,P.Kumar,A.Narayan,M.Kar,Rietveld analysis of XRD patterns of different sizes of nanocrystalline cobalt ferrite,Int.Nano Lett.3(2013)1–12.
[38]M.Bhagwat,A.V.Ramaswamy,A.K.Tyagi,V.Ramaswamy,Rietveld refinement study of nanocrystalline copper doped zirconia,Mater.Res.Bull.38(2003)1713–1724.
[39]A.Sarkar,S.Kannann,In situ synthesis,fabrication and Rietveld refinement of the hydroxyapatite/titania composite coatings on 316LSS,Ceram.Int.40(2014)6453–6463.
[40]N.G.Jovic,A.S.Masadeh,A.S.Kremenovic,B.V.Antic,J.L.Blanusa,N.D.Cvjeticanin,G.F.Goya,M.V.Antisari,E.S.Bozin,Effect of thermal annealing on structural and magnetic properties of lithium ferrite nanoparticles,J.Phys.Chem.113(2009)20559–20567.
[41]S.H.Rhee,Synthesis of hydroxyapatite via mechanochemicaltreatment,Biomaterials 23(2002)1147–1152.
[42]J.P.Lafon,E.Champion,D.Bernache-Assollant,Processing of AB-type carbonated hydroxyapatite Ca10−x(PO4)6−x(CO3)x(OH)2−x−2y(CO3)yceramics with controlled composition,J.Eur.Ceram.Soc.28(2008)139–147.
[43]I.R.Gibson,W.Bon field,Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite,J.Biomed.Mater.Res.59(2002)697–708.
[44]B.Nasiri-Tabrizi,A.Fahami,Synthesis and characterization of fluorapatite–zirconia composite nanopowders,Ceram.Int.39(2013)4329–4337.
[45]P.Balaz,Mechanochemistry in nanoscience and minerals engineering, first ed.Springer,Berlin Heidelberg,Germany,2008.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Structural evolution of chars from biomass components pyrolysis in a xenon lamp radiation reactor☆
- TiO2–SA–Arg nanoparticles stabilized Pickering emulsion for photocatalytic degradation of nitrobenzene in a rotating annular reactor
- Assessing the kinetic model of hydro-distillation and chemical composition of Aquilaria malaccensis leaves essential oil
- Effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions☆
- A novel model for multi-plant mixed heavy crude oils refinery planning
- A self-tuning control method for Wiener nonlinear systems and its application to process control problems☆
