Structural evolution of chars from biomass components pyrolysis in a xenon lamp radiation reactor☆
2017-05-28HaizhouLinShurongWangLiZhangBinRuJinsongZhouZhongyangLuo
Haizhou Lin,Shurong Wang*,Li Zhang,Bin Ru,Jinsong Zhou,Zhongyang Luo
State Key Laboratory of Clean Energy Utilization,Zhejiang University,Hangzhou 310027,China
1.Introduction
Fast pyrolysis is recognized as a promising thermal–chemical technology for effectively converting biomass into liquid bio-oil,which can be a substitution for fossil vehicle fuels[1,2].Therefore,the production of bio-oilhas aroused extensive attention[3–6].However,attention on structural evolution of bio-char,a solid by-product,is much limited although it is important to reveal the pyrolysis behavior of biomass.Moreover,bio-char is underutilized,and it is very critical to the high value-added utilization of bio-chair before elucidating its structural characterization[7].
Biomass mainly consists of three major components,namely 40 wt%–50 wt%cellulose,20 wt%–40 wt%hemicellulose and 15 wt%–30 wt%lignin[8,9].The formation of bio-char is deeply influenced by the major components due to their different structures.So knowledge of the char from individual component is important for a better understanding of bio-char properties and biomass pyrolysis mechanism.Pastorovaet al.[10]found that the char precursor of cellulose was a polymer based on furanoid skeletons and hydroxyaromatic skeletons.Wootenet al.[11]analyzed the formation of char from cellulose pyrolysis by13C-CPMAS-NMR,suggesting that cellulose firstly converted to an amorphous intermediate cellulose,then to a disordered final carbohydrate,and finally to aromatic carbons.Xinet al.[7]indicated that the cellulose char formation involved a series of complex reactions,including dehydration,decarbonylation,ring-opening and aromatization to form a disordered three-dimensional network precursor,and deoxygenation and dehydrogenation to form a highly aromatic char.
In particular,studies on the char properties from hemicellulose and lignin pyrolysis are scarce.Lvet al.[12]observed that the surface morphology of hemicellulose charshifted from severe irregular agglomeration to smoothness and porousness with temperature increasing by SEM.Xinet al.[13]analyzed the surface property of xylan char by BET,and suggested that the relatively high average pore size of the char could be ascribed to the branched structure in xylan.Sharmaet al.[14]performed the structural characterization of lignin char by FTIR and13C-CPMAS-NMR and found that high temperature could reduce the content of hydroxyl and methoxyl groups,leading to the improvement of aromaticity.Lou and Wu[15]characterized the pyrolysis char of EMAL lignin by FTIR,and the results showed that the aromatic substitution decreased gradually with the temperature increasing,and the aromatic nature of char completely disappeared at 700°C.
Although there are some studies on the structural analysis of the chars from biomass components as mentioned above,the structural evolution of component chars during the whole pyrolysis process is rarely analyzed.Additionally,the structural differences of the chars from biomass components are seldom compared.In this study,the chars(solid residues)of biomass components(cellulose,hemicellulose and lignin)pyrolysis at different pyrolysis stages in a xenon lamp radiation reactor were collected.Then the structural evolution of chars was analyzed and compared using elemental analysis,FTIR,XRD and SEM,aiming at providing a comprehensive understanding of the formation of chars from biomass components pyrolysis.
2.Experimental
2.1.Materials
Microcrystalline cellulose,xylan and organic lignin were used as the model compounds of cellulose,hemicellulose and lignin,respectively.These materials were purchased from Sigma-Aldrich Corporation.
2.2.Apparatus
The diagram of the xenon lamp radiation reactor is shown in Fig.1.The radiation source was a spherical high pressure short arc xenon lamp(3 kW).The xenon lamp was located on the inner focus of the ellipsoidal speculum to converge the light on the other focus after reflection.The diameter of the ellipsoidal speculum was 360 mm,and the focal lengths were 60 mm and 800 mm,respectively.A plane speculum was arranged between the two focuses to change the light path,so that the light was gathered in the quartz glass reaction tube(inner diameter of 40 mm)to realize the radiation heating of the sample.A rotating shutter at a response time of 0.01 s was arranged between the plane speculum and the reaction tube to control the heating time.After reaction,the sample was quenched by liquid nitrogen immediately.The operation of the reaction system was controlled by a programmable logic controller(PLC).In the pyrolysis experiments,0.1 g sample was put on a quart glass slice in the reaction tube.Then the reactor was purged with pure N2gas ata flow of200 ml·min−1for 3 min to provide an inert atmosphere.The output power of the xenon lamp was kept at3 kW.The heating time was in range of 15–120 s,which was controlled by the rotating shutter.

Fig.1.Xenon lamp radiation pyrolysis apparatus.
2.3.Structural characterization
The elemental composition of the sample was measured on a Vario MICRO Elemental Analyzer(Elementar Analysensysteme GmbH,Germany).The functional groups of the sample were analyzed on a Nicolet 5700 FTIR spectrometer(Thermo Fisher Scientific Corporation,USA).The spectra were recorded from 400 to 4000 cm−1with a resolution of 4 cm−1by averaging 36 scans.The surface morphology of sample was analyzed by a scanning electron microscope(SEM)(SIRION-100 FEI).The sample was pretreated by coating an Au film prior to scanning.
The accelerating voltage was 20 kV and the magnification was 2500.The crystalline index(CrI)of sample was calculated from X-ray diffraction patterns via Eq.(1)[16].

whereI002was the peak intensity of 002 lattice plane(at 2θ =22.5°),andIamwas the peak intensity of the amorphous phase(at 2θ =18.5°).
3.Results and Discussion
3.1.Evolution of elemental composition
As shown in Fig.2(a)–(c),the evolution of elemental composition for the chars from the three major components presented similar tendency.The C content increased while the H and O contents decreased with the heating time prolonging.The elemental composition of cellulose chars changed slightly before heating time of 95 s,indicating that cellulose needed relatively long time to preheat the sample prior to initiating decomposition.Subsequently,the C contents increased sharply from 44.68%at 95 s to 80.14%at 100 s,while the corresponding H and O contents decreased from 6.26%and 49.06%to 3.72%and 16.14%,respectively.This suggested that cellulose suffered severe decomposition in a very short time,releasing a large amount of oxygenic volatiles such as acids,ketones,and aldehydes.After that,cellulose underwent a carbonization process,showing that the C content increased slowly at the expense of H and O contents.This was consistent with the TG result of cellulose pyrolysis that the DTG curve appeared a single sharp peak at about 350°C[4].In comparison to cellulose,hemicellulose and lignin needed shorter preheating time as their elemental composition changed obviously at heating time less than 15 s.This might be due to the existence of weak branches and linkages in their structure.For example,the acetyl groups in hemicellulose and β-O-4 linkage in lignin were easy to decompose at low temperature[5,6].In addition,the loose structure and deep color of the hemicellulose(yellow)and lignin(brown)made their radiation absorption efficiency much higher than that of the white crystal cellulose.Therefore,hemicellulose and lignin could absorb a lot of heat within a short time to initiate the pyrolysis.The elemental composition for hemicellulose and lignin mainly changed at the heating time of 15–30 s,suggesting that the matrix of hemicellulose and lignin decomposed at a relatively long time compared to cellulose.Correspondingly,the C contents of hemicellulose and lignin increased from 37.40%and 66.63%at 15 s to 58.06%and 79.87%at 30 s,respectively.These changes of elemental composition for hemicellulose and lignin were not as severe as that for cellulose at the main pyrolysis stage.This was consistent with the appearance of low and wide peak in DTG curve for hemicellulose and lignin[17].When the heating time was beyond 30 s,both hemicellulose and lignin underwent a slow carbonization process.Due to the aromatic nature of lignin structure,the C content of lignin char was as high as 86%at 120 s,which was much higher than that of hemicellulose char(less than 70%).
From the van Krevelan diagram(Fig.2(d)),it could be easily found that the ratios of H/C and O/C for the chars decreased with the heating time increased,revealing that the aromaticity of char was gradually strengthened.The values of ΔH/C/ΔO/Cfor cellulose and hemicellulose were 1.74 and 1.94,respectively,which were close to 2.This indicated that dehydration was primary during the char formation due to the abundanthydroxyl groups in the sugarunits of cellulose and hemicellulose[18].Meanwhile,the decarbonylation and decarboxylation to form CO and CO2were the main reason for the ΔH/C/ΔO/Cvalue of less than 2[19].The value of ΔH/C/ΔO/Cfor lignin was 3.04,which was much higher than that for cellulose and hemicellulose.This was due to the abundant methoxyl groups and aliphatic branch chains in the lignin structure and they could decompose during lignin pyrolysisviademethylation and demethoxylation[20,21].In addition,the condensation of the aromatic rings to form polycyclic aromatics could lead to the release of hydrocarbons and hydrogen,which also contributed to the high value of ΔH/C/ΔO/C[14].
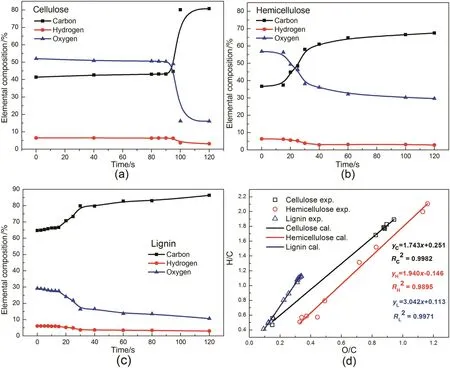
Fig.2.Evolution of elemental composition of chars((a)cellulose,(b)xylan,(c)lignin and(d)van Krevelan plot depicting the changes in O/C and H/C ratio and the linear fitting of the changes).
3.2.Evolution of functional groups
Fig.3(a)shows the FTIR spectra of the cellulose chars at different heating time.At the initial preheating stage(0–80 s),there was no obvious change in the structure of cellulose as the spectra were almost the same.As the heating time increased from 85 s to 95 s,the FTIR peaks began to weaken,such as the peaks at 897 cm−1,1047 cm−1and 1163 cm−1,corresponding to the glycosidic bond in cellulose[22,23].This suggested that cellulose began to decompose to intermediate cellulose with low degree of polymerization(DP).Meanwhile,the weakening peaks at 3400 cm−1,1320 cm−1and 1060 cm−1corresponding to hydroxyl groups implied the occurrence of dehydration.At heating time of 95 s,the intensity of the peaks at 1457 cm−1and 1320 cm−1(–CH2– in sugar rings),1382 cm−1(–CH– in sugar rings)and 1113 cm−1(C–O–C in the pyran rings)decreased notably[22,23].This suggested the ringopening of sugar units in low DP cellulose.The followed dehydration and decarbonylation might lead to the formation of char precursor[11].With the heating time prolonged to 100 s,the peaks corresponding to the original cellulose structure almost disappeared.At the same time,new peaks at 1586 cm−1and 873 cm−1corresponding to aromatic structure appeared,suggesting the transformation from original cellulose structure to aromatic structure[24,25].This was in accordance with the results obtained by Xinet al.[7]that cellulose char evolved from the disordered three-dimensional network precursor composed of oligosaccharides,aliphatic hydrocarbons and aromatics.
From the FTIR spectra of the hemicellulose chars(see Fig.3(b)),it could be found that the hemicellulose structure mainly changed at the heating time of 15–30 s.The peaks at 1045 cm−1and 1168 cm−1corresponding to glycosidic bonds in xylan gradually diminished and finally disappeared,revealing the severe depolymerization of xylan due to the cleavage of glycosidic bonds[5,26].The peaks at 1441 cm−1and 1378 cm−1(–CH2–and –CH–in xylose units)became weak but still existed after the reaction,in contrast to the disappearance of that in cellulose pyrolysis.This was because xylose ring was easy to be retained in the char structure as the xylosylcation formed during xylan depolymerization was more likely to polymerize to char[27].For cellulose,the glucosyl cation formed during cellulose depolymerization could undergo intramolecular dehydroxy reaction to formation of 1,6-anhydride due to the existence of a free primary hydroxyl group at C-6,and the 1,6-anhydride was easy to form other volatiles[27].With the heating time prolonging,new peaks at1586 cm−1and 874 cm−1corresponding to aromatic structure were observed,suggesting the evolution from sugar rings to aromatic rings during char formation[24,25].
In Fig.3(c),the spectra of the lignin chars showed that the intensity of main peaks evidently decreased at heating time of 25 s.The weak peaks at 1218 cm−1and 1030 cm−1were assigned to the ether bonds and the primary alcohol hydroxyl groups,respectively,suggesting the cleavage of ether linkages(β-O-4)and the removal of hydroxyl groups in side chains[21].Peaks at 1328 cm−1and 1125 cm−1were assigned to C–O and C–H of guaiacyl units,while peaks at 1268 cm−1and 1113 cm−1were assigned to C–O and C–H of syringyl units[28].These diminishing peaks revealed that the major structure of lignin suffered severe damage at heating time of25 s.At the same time,the peaks at 2861 cm−1(–OCH3)and 2923 cm−1(–CH2– and –CH–)almost disappeared,implying that the cracking of aliphatic linkages and branches and the removal of methoxyl groups were intensive.The peaks at 1702 cm−1corresponding to unconjugated C=O disappeared,while the peaks at 1605 cm−1assigning to C=O conjugated with aromatic rings just became weak.This was in agreement with the previous study that the reaction activity of the unconjugated carbonyl group was higher than that of the conjugated one,because the unconjugated carbonyl group existed in the branches and was easy to decompose to COand CO2[21].Since the reaction time was 30 s,most of the peaks corresponding to the oxygenic functional groups were nearly removed,showing an absorption pattern of aromatic structure.This was consistent with the result of elemental analysis that the C content was over 80%at this time.In particular,the absorption corresponding to the aromatic skeleton was shifted from1513 cm−1for raw lignin to 1498 cm−1for char.Meanwhile,new peaks at 1585 cm−1and 874 cm−1corresponding to aromatic structure appeared.These observations could be due to the formation of polycyclic aromatic structure via condensation at high temperature[25].
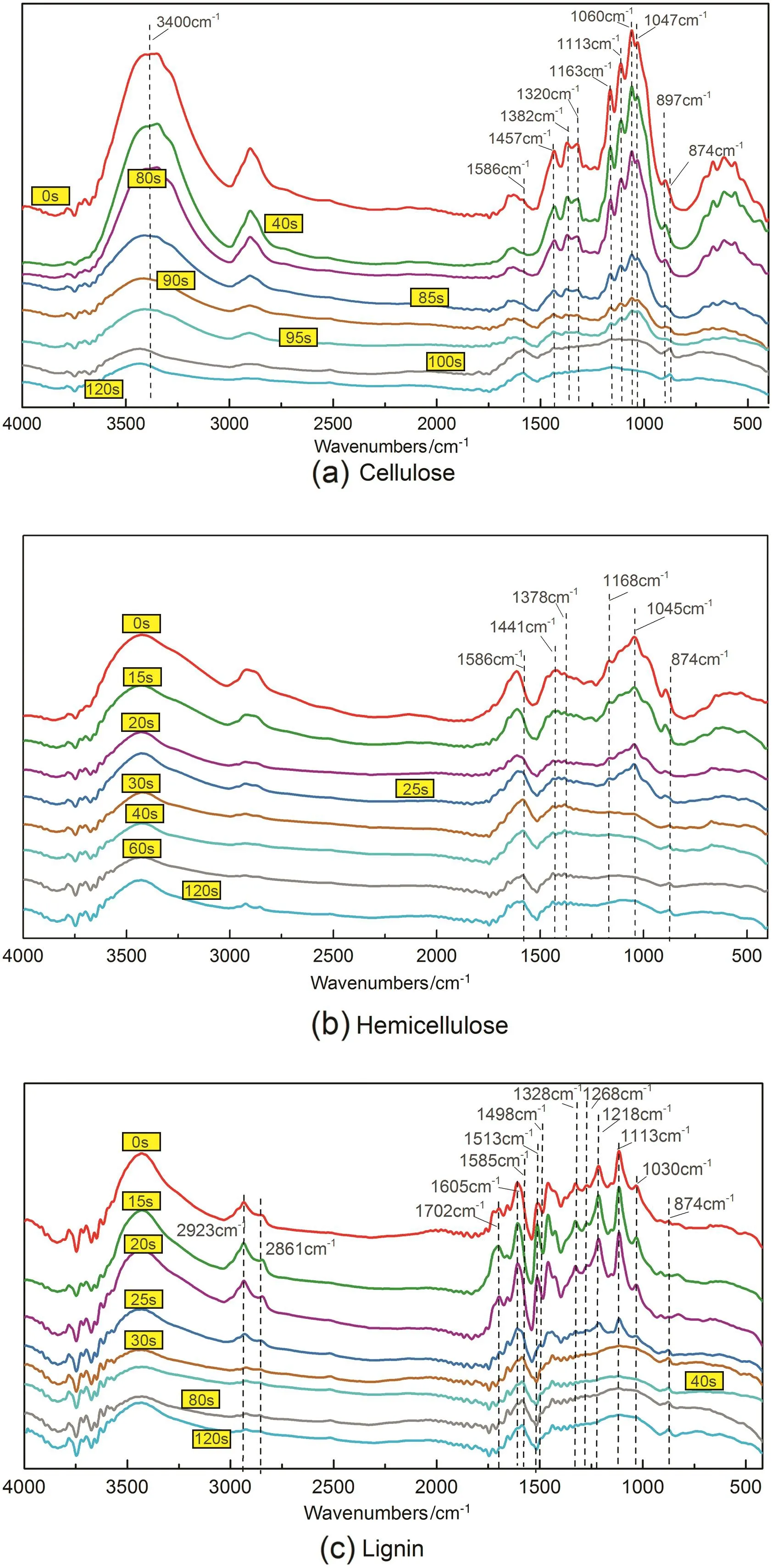
Fig.3.FTIR spectra of chars from biomass components pyrolysis.
3.3.Evolution of cellulose crystalline
Cellulose has a stable crystal structure due to the strong intermolecular and intramolecular hydrogen bonds induced by the abundant hydroxyl groups,which is very different from the amorphous structure of hemicellulose and lignin.As shown in Fig.4,the X-ray diffraction pattern of the raw cellulose had four typical diffraction peaks at 2θ=15.0°,16.0°,22.4°and 34.3°,corresponding to plane of 101,101,002 and 040,respectively,and the 101 plane and 101 plane were overlapped with each other,suggesting that the crystal structure of the cellulose belonged to cellulose I[29,30].At the heating time of 90 s,the X-ray diffraction pattern of the sample still presented four diffraction peaks,indicating that the crystal structure of the cellulose was not alerted in the preheating pyrolysis stage.At heating time of100 s,the four diffraction peaks nearly disappeared,implying that crystal structure was destroyed.Meanwhile,the weak diffraction peak at 22.9°disclosed the formation of a small amount of disordered graphite structure evolved from the non-crystal structure[7].
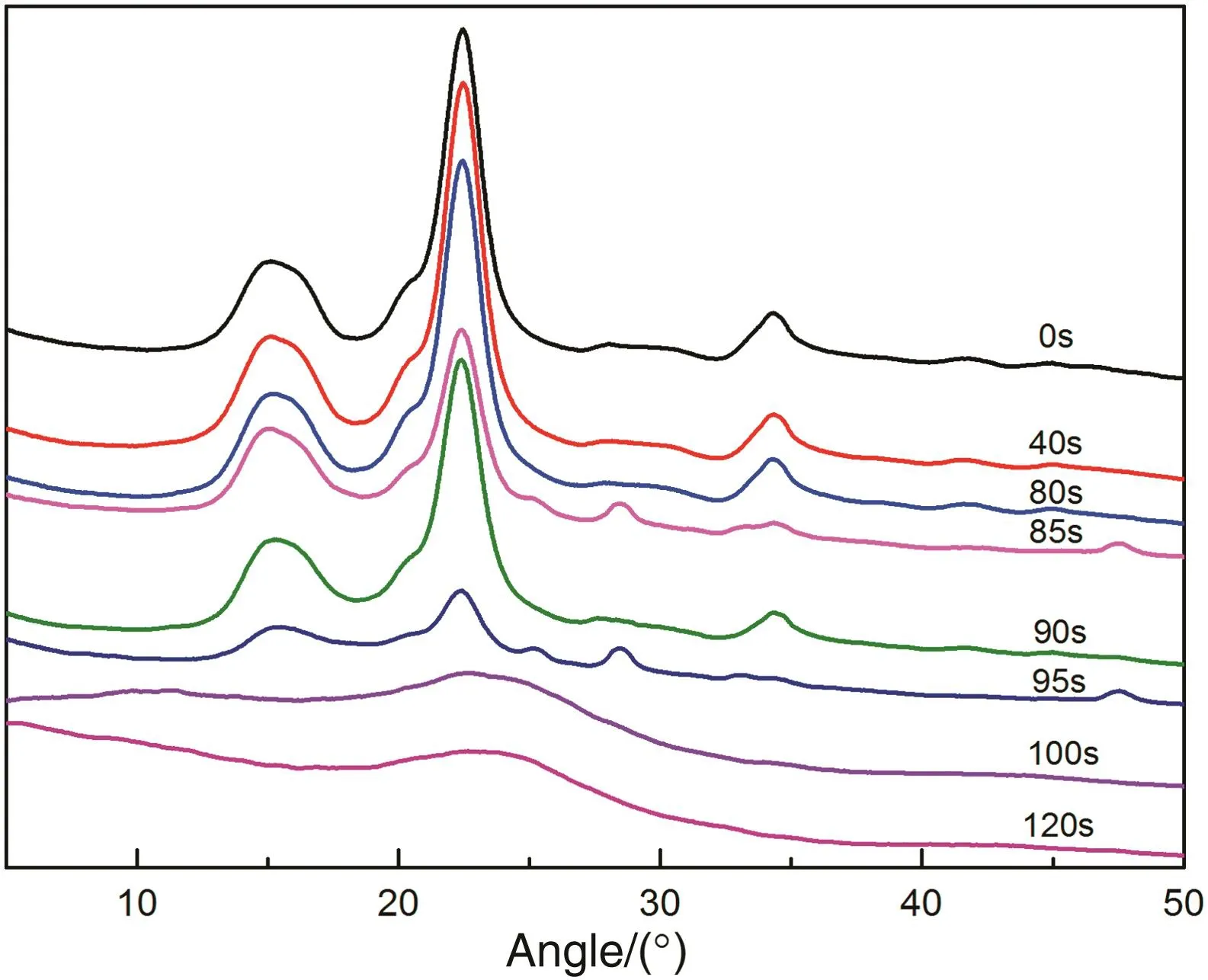
Fig.4.X-ray diffractograms of cellulose chars.
Table 1 shows the variation of the CrI during cellulose pyrolysis.The CrI decreased from 78.50%at 0 s to 64.84%at 85 s.According to the results above,the elemental composition and functional group distribution almostremained unchanged at this stage.Thus,it could be deduced that cellulose needed to absorb a lot of heat to destroy the hydrogen bond network,leading to the break of the stable crystal structure,prior to the severe depolymerization.This was con firmed by the results obtained by Yanget al.[4]that the DSC curve of cellulose pyrolysis showed a huge endothermic peak before the main stage of pyrolysis.It was notable that the CrI increased to 70.0%at 95 s,and then sharply decreased to 37.53%at 95 s and to zero after 100 s.The increase of CrI at 95 s might be ascribed to the consumption of amorphous structureof celluloseviadecomposing to volatiles,which caused the increase of crystal structure proportion.
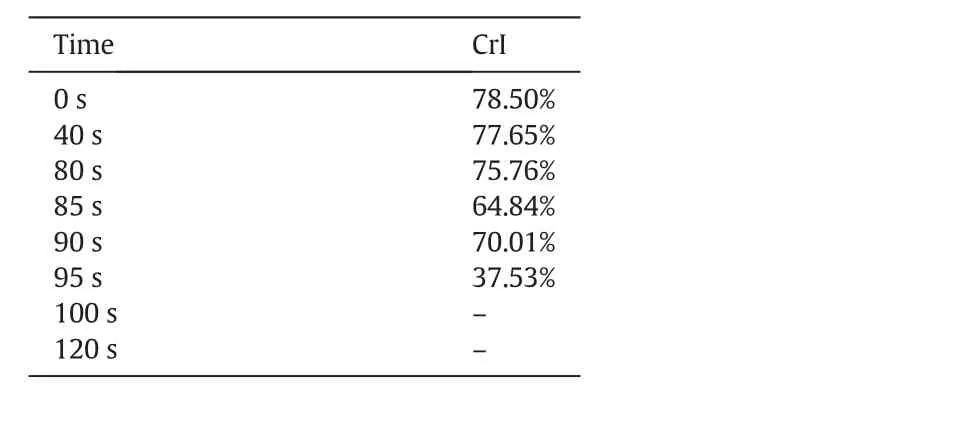
Table 1Evolution of cellulose crystallinity in pyrolysis process
3.4.Evolution of surface morphology
Fig.5(a)shows the evolution of surface morphology of chars during cellulose pyrolysis.The raw cellulose had clear particle pro file and wrinkled surface.At 95 s,it could be clearly observed that the surface of cellulose particle arose molten trace and some small holes.This corresponded to the observation above that the crystal structure suffered damage and began to release volatiles.At 100 s,the cellulose particle was completely molten and the holes enlarged obviously.Finally,the residue of cellulose pyrolysis evolved to porous char with smooth surface.
As displayed in Fig.5(b),the raw hemicellulose particle had rough surface,and they molten at a heating time of 20 s.Meanwhile,a large amount of tiny holes formed.This was consistent with the decomposition of unstable branches in hemicellulose at low temperature.At 25 s,the surface of hemicellulose was dilapidated and many large cavities generated.This was because of the rupture of bubbles induced by the releasing of a large amount of volatiles from the inner hemicellulose.After the followed carbonization,the hemicellulose char showed porosity like honeycomb.This resulted from the branched and amorphous structure of hemicellulose,as the morphology of char was closely related to the formation and release of volatiles[31].
Unlike cellulose and hemicellulose,lignin retained its original granular pro file instead of molten surface in the initial stage of pyrolysis at 20 s(see Fig.5(c)).At the same time,there were many pores at various sizes on the surface.This might be caused by the decomposition of the external surface structure due to the relative high temperature on surface.With the heating time prolonging to 25 s,many cavities and wreckage were observed,which indicated that a large amount of volatiles formed in lignin inner and their fastrelease made lignin matrix collapse.This was in accordance with the thermal ejection theory that the lignin fragments were cracked and expelled from the lignin matrix during lignin pyrolysis[32,33].At 120 s,the lignin char showed smooth surface and abundant large size pores.Lvet al.[34]also observed that lignin char had many large pores with a diameter of more than 10 μm.
4.Conclusions
The biomass components(cellulose,hemicellulose and lignin)were pyrolyzed in a xenon lamp radiation reactor and the solid residues at different stages were collected and subjected to investigate the structural evolution of char.The analysis of the elemental composition of the chars showed that the C content increased while the H and O contents decreased with the heating time prolonging.Meanwhile,the values of ΔH/C/ΔO/Cfor cellulose and hemicellulose were close to 2,while that for lignin was more than 3.These indicated that dehydration was the dominant reaction in cellulose and hemicellulose pyrolysis,while demethoxylation was prevalent in lignin pyrolysis.The results of FTIR,XRD and SEM showed that cellulose pyrolysis needed to absorb a lot of heatto break down the crystalstructure prior to the occurrence of severe decomposition.In contrast,hemicellulose and lignin underwent the damage of instable branches and linkages before suffering the intensive devolatilization.The formation and release of volatiles were different during pyrolysis of the three components,which led to various surface morphologies of the chars.
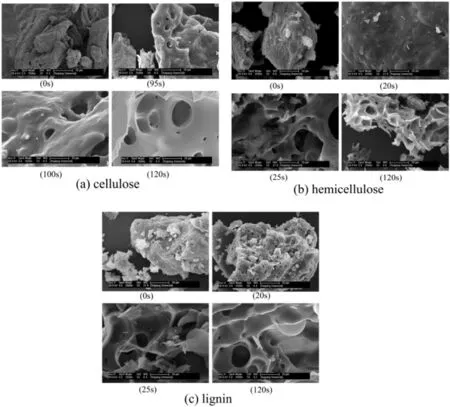
Fig.5.SEM micrographs of chars from biomass components pyrolysis.
[1]S.R.Wang,B.Ru,H.Z.Lin,Z.Y.Luo,Degradation mechanism of monosaccharides and xylan under pyrolytic conditions with theoretic modeling on the energy pro files,Bioresour.Technol.143(0)(2013)378–383.
[2]A.V.Bridgwater,Review of fast pyrolysis of biomass and productupgrading,Biomass Bioenergy38(2012)68–94.
[3]X.J.Guo,S.R.Wang,Q.Wang,Z.G.Guo,Z.Y.Luo,Properties of bio-oil from fast pyrolysis of rice husk,Chin.J.Chem.Eng.19(1)(2011)116–121.
[4]H.P.Yang,R.Yan,H.P.Chen,D.H.Lee,C.G.Zheng,Characteristics of hemicellulose,cellulose and lignin pyrolysis,Fuel86(12–13)(2007)1781–1788.
[5]S.R.Wang,B.Ru,H.Z.Lin,W.X.Sun,Pyrolysis behaviors of four O-acetyl-preserved hemicelluloses isolated from hardwoods and softwoods,Fuel150(2015)243–251.
[6]S.R.Wang,B.Ru,H.Z.Lin,W.X.Sun,Z.Y.Luo,Pyrolysis behaviors of four lignin polymers isolated from the same pine wood,Bioresour.Technol.182(2015)120–127.
[7]S.Z.Xin,H.P.Yang,Y.Q.Chen,M.F.Yang,L.Chen,X.H.Wang,H.P.Chen,Chemical structure evolution of char during the pyrolysis of cellulose,J.Anal.Appl.Pyrolysis116(2015)263–271.
[8]P.McKendry,Energy production from biomass(part 1):Overview of biomass,Bioresour.Technol.83(1)(2002)37–46.
[9]F.X.Collard,J.Blin,A review on pyrolysis of biomass constituents:mechanisms and composition of the products obtained from the conversion of cellulose,hemicelluloses and lignin,Renew.Sust.Energ.Rev.38(2014)594–608.
[10]I.Pastorova,R.E.Botto,P.W.Arisz,J.J.Boon,Cellulose char structure—A combined analytical Py-Gc-Ms,Ftir,and Nmr-study,Carbohydr.Res.262(1)(1994)27–47.
[11]J.B.Wooten,J.I.Seeman,M.R.Hajaligol,Observation and characterization of cellulose pyrolysis intermediates by C-13 CPMAS NMR.A new mechanistic model,Energy Fuel18(1)(2004)1–15.
[12]G.J.Lv,S.B.Wu,R.Lou,Characteristics of corn stalk hemicellulose pyrolysis in a tubular reactor,Bioresources5(4)(2010)2051–2062.
[13]S.Z.Xin,H.P.Yang,Y.Q.Chen,X.H.Wang,H.P.Chen,Assessment of pyrolysis polygeneration of biomass based on major components:product characterization and elucidation of degradation pathways,Fuel113(0)(2013)266–273.
[14]R.K.Sharma,J.B.Wooten,V.L.Baliga,X.H.Lin,W.G.Chan,M.R.Hajaligol,Characterization of chars from pyrolysis of lignin,Fuel83(11–12)(2004)1469–1482.
[15]R.Lou,S.B.Wu,Products properties from fast pyrolysis of enzymatic/mild acidolysis lignin,Appl.Energy88(1)(2011)316–322.
[16]L.Segal,J.J.Creely,A.E.Martin,C.M.Conrad,An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer,Text.Res.J.29(10)(1959)786–794.
[17]S.D.Stefanidis,K.G.Kalogiannis,E.F.Iliopoulou,C.M.Michailof,P.A.Pilavachi,A.A.Lappas,A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose,hemicellulose and lignin,J.Anal.Appl.Pyrolysis105(2014)143–150.
[18]B.Ru,S.R.Wang,G.X.Dai,L.Zhang,Effect of Torrefaction on biomass physicochemical characteristics and the resulting pyrolysis behavior,Energy Fuel29(9)(2015)5865–5874.
[19]J.Zhang,M.W.Nolte,B.H.Shanks,Investigation of primary reactions and secondary effects from the pyrolysis of different celluloses,ACS Sustain.Chem.Eng.2(12)(2014)2820–2830.
[20]S.R.Wang,K.G.Wang,Q.Liu,Y.L.Gu,Z.Y.Luo,K.F.Cen,T.Fransson,Comparison of the pyrolysis behavior of lignins from different tree species,Biotechnol.Adv.27(5)(2009)562–567.
[21]S.R.Wang,H.Z.Lin,B.Ru,W.X.Sun,Y.R.Wang,Z.Y.Luo,Comparison of the pyrolysis behavior of pyrolytic lignin and milled wood lignin by using TG–FTIR analysis,J.Anal.Appl.Pyrolysis108(0)(2014)78–85.
[22]M.Kacurakova,P.Capek,V.Sasinkova,N.Wellner,A.Ebringerova,FT-IR study of plant cell wall model compounds:pectic polysaccharides and hemicelluloses,Carbohydr.Polym.43(2)(2000)195–203.
[23]J.Bian,F.Peng,X.P.Peng,X.Xiao,P.Peng,F.Xu,R.C.Sun,Effect of Emim Ac pretreatment on the structure and enzymatic hydrolysis of sugarcane bagasse cellulose,Carbohydr.Polym.100(2014)211–217.
[24]D.W.Rutherford,R.L.Wershaw,C.E.Rostad,C.N.Kelly,Effectof formation conditions on biochars:compositional and structural properties of cellulose,lignin,and pine biochars,Biomass Bioenergy46(2012)693–701.
[25]R.K.Sharma,J.B.Wooten,V.L.Baliga,P.A.Martoglio-Smith,M.R.Hajaligol,Characterization of char from the pyrolysis of tobacco,J.Agric.Food Chem.50(4)(2002)771–783.
[26]S.R.Wang,T.Liang,B.Ru,X.J.Guo,Mechanism of xylan pyrolysis by Py-GC/MS,Chem.Res.Chin.Univ.29(4)(2013)782–787.
[27]G.R.Ponder,G.N.Richards,Thermal synthesis and pyrolysis of a xylan,Carbohydr.Res.218(0)(1991)143–155.
[28]B.Scholze,D.Meier,Characterization of the water-insoluble fraction from pyrolysis oil(pyrolytic lignin).Part I.PY-GC/MS,FTIR,and functional groups,J.Anal.Appl.Pyrolysis60(1)(2001)41–54.
[29]J.X.Zhang,J.Luo,D.M.Tong,L.F.Zhu,L.L.Dong,C.W.Hu,The dependence of pyrolysis behavior on the crystal state of cellulose,Carbohydr.Polym.79(1)(2010)164–169.
[30]U.J.Kim,S.H.Eom,M.Wada,Thermal decomposition of native cellulose:Influence on crystallite size,Polym.Degrad.Stab.95(5)(2010)778–781.
[31]H.P.Yang,R.Yan,H.P.Chen,D.H.Lee,D.T.Liang,C.G.Zheng,Mechanism of palm oil waste pyrolysis in a packed bed,Energy Fuel20(3)(2006)1321–1328.
[32]E.Fratini,M.Bonini,A.Oasmaa,Y.Solantausta,J.Teixeira,P.Baglioni,SANS analysis of the microstructural evolution during the aging of pyrolysis oils from biomass,Langmuir22(1)(2006)306–312.
[33]R.Bayerbach,D.Meier,Characterization of the water-insoluble fraction from fast pyrolysis liquids(pyrolytic lignin).Part IV:structure elucidation of oligomeric molecules,J.Anal.Appl.Pyrolysis85(1–2)(2009)98–107.
[34]G.J.Lv,S.B.Wu,G.H.Yang,J.C.Chen,Y.Liu,F.G.Kong,Comparative study of pyrolysis behaviors of corn stalk and its three components,J.Anal.Appl.Pyrolysis104(2013)185–193.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Structural insights of mechanically induced aluminum-doped hydroxyapatite nanoparticles by Rietveld refinement☆
- TiO2–SA–Arg nanoparticles stabilized Pickering emulsion for photocatalytic degradation of nitrobenzene in a rotating annular reactor
- Assessing the kinetic model of hydro-distillation and chemical composition of Aquilaria malaccensis leaves essential oil
- Effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions☆
- A novel model for multi-plant mixed heavy crude oils refinery planning
- A self-tuning control method for Wiener nonlinear systems and its application to process control problems☆
