TiO2–SA–Arg nanoparticles stabilized Pickering emulsion for photocatalytic degradation of nitrobenzene in a rotating annular reactor
2017-05-28ShiguangZhangLeiLiYouzhiLiuQiaolingZhang
Shiguang Zhang,Lei Li,Youzhi Liu*,Qiaoling Zhang
Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering,North University of China,Taiyuan 030051,China
1.Introduction
There is an increasing concern about the impact of high concentration organic wastewater on human health and ecological environment[1–2].Organic wastewater discharged from chemical industries may contain a variety of chemical compounds and have a very high chemicaloxygen demand[3].However,conventional wastewater treatment technologies such as physicochemical treatment[4–6],biological treatment[7]and chemical treatment[8],are plagued with a number of problems such as complex operation,large investment,and low efficiency[9].Semiconductor photocatalysis has emerged recently as an environmentally friendly and highly efficient method for the organic wastewater treatment[10–14].However,as photocatalytic degradation often follows a pseudo first order reaction kinetics,the degradation rate decreases considerably with the decrease of pollutant concentration[15].Therefore,increasing the concentration of pollutants or the contact area between the pollutants and the photocatalysts may contribute to improving the degradation efficiency of organic pollutants.
Pickering emulsion is a new kind of emulsion stabilized by solid particles instead of traditional surfactants,and it has a significantly higher stability than conventional emulsions as these solid particles can be strongly adsorbed at the liquid–liquid interface,and thus the Gibbs free energy to remove one solid particle away from the interface is high[16–17].Meanwhile,the contactarea between oil drops and catalysts can be effectively improved by self-assembly of nanoparticles,and the catalysts can be easily removed from water by centrifugal separation or precipitation.Thus,the catalytic reaction at the oil–water interface is environmentally friendly and highly efficient[18–19].The system also has the potential to be applied in the treatment of high concentration organic wastewater.The integration of photocatalysis and Pickering emulsion is expected to further improve the degradation of organic pollutants.A variety of emulsions stabilized by solid particles,such as modified ZnO[20],layered niobate[21],modified TiO2[22–23],and carbon nanotubes loaded with silver phosphate[24–25],have been developed for the photocatalytic degradation of organic contaminants in water,and the results are satisfactory because(a)self-assembled nanoparticles can be adsorbed at the water/oil interface,and thus the photocatalytic system has a relatively large interfacial area;(b)the difference in the solubility between oil and water allows the products to be transferred quickly across the nanoparticle film,and thus increase the photocatalytic reaction rate;and(c)the catalysts can be recycled simply by centrifugal separation[25].
However,despite the potential advantages of Pickering emulsion photocatalytic system(PEPS),some problems remain to be solved.Nisb[22]found that PEPS was effective in the degradation of chlorobenzene,but the maximum degradation rate was only 80%.This can be explained by the following three reasons:(a)the turbidity of the Pickering emulsion is too high,which can affect the propagation velocity of photons;(b)the consumption of the oil phase can lead to poor thermodynamic stability of the emulsion;and(c)insufficient dissolved oxygen(DO).However,these problems can be readily solved with the use of an appropriate reactor.Rotating annular reactor has been considered one of the high efficient photocatalytic reactors[26].The centrifugal force induced by the rotation results in thin liquid films inside the rotating rube,which improves the utilization of UV illumination.Continuous aeration can increase the DO concentration in the PEPS,and the rotation can also promotes the mixing of the particles,pollutants and DO[27].In addition,the shear force resulting from the rotation may continuously emulsify the Pickering emulsions during the reaction,and a stable emulsion structure and large interface area are maintained.
In this study,amphiphilic titanium dioxide(TiO2)nanoparticles modified by salicylic acid(SA)and arginine(Arg)(TiO2–SA–Arg)with excellent photocatalytic performance were prepared,and then tested by high-resolution transmission electron microscopy(HRTEM),contact angle measurement,and UV–Vis diffuse reflectance spectroscopy(UV–Vis DRS).An O/W Pickering emulsion stabilized by these TiO2–SA–Arg nanoparticles was prepared,and then the effects of various design parameters of the rotating annular reactor,initial nitrobenzene concentration,catalystamount,and solution pHon the photocatalytic degradation of nitrobenzene were investigated.In addition,the degradation mechanism of nitrobenzene was also proposed by investigating the effect oftert-butyl alcohol(a hole scavenger)on the degradation rate of nitrobenzene.
2.Materials and Experimental Procedures
2.1.Materials
Nitrobenzene and methanol(chromatographic pure)were purchased from Sinopharm Chemical Reagent Co.Ltd.(China);TiO2(industrial grade)was purchased from Evonik Degussa(Germany);Salicylic acid,arginine and ethanol(analytically pure)for surface modification were purchased from Tianjing Guangfu Chemical Co.(Tianjing,China);hydrochloric acid and sodium hydroxide solution(0.1 mol·L−1;analytically pure)used for adjusting pH were purchased from Tianjing Beichen Fangzheng Chemical Co.(Tianjing,China)and prepared for immediate use.Deionized water was used in all experiments and prepared using the GWA-UN water purification system(Beijing PEPSEE,China).UV lamps with a wavelength of 254 nm(ZW18D17W;Cnlight,Guangdong,China)were used for UVradiation;Ultrasonic cell crusher with a frequency of 21 kHz was used in the preparation of modified catalysts(SCIENTZIID;Xinzhi Bio.,Ningbo,China);high-speed dispersion apparatus was used for the preparation of Pickering emulsions at a rotational speed of 16,000 rpm(Type GF-1;Hangzhou Lohand Biological,China);and conductivity meter(DDSJ-308A)was used for measuring the electrical conductivity of the emulsion.The emulsion is a water-in-oil(W/O)emulsion if the electrical conductivity of the emulsion is nearly 0;otherwise,it is an oil-in-water(O/W)emulsion[22,28–29].
2.2.Experimental set-up
The photocatalytic reactor used in this study consists of a rotatable cylinder and a stationary concentric cylinder,as shown in Fig.1.The rotatable inner cylinder is made of quartz glass with an external diameter of 20 cm;while the stationary outer cylinder is made of stainless steel with an internal diameter of 22,24,or 26 cm.The device is driven by a 220 V,800 W motor with a rotational speed of 0–1200 r·min−1and controlled by a variable frequency driver.The dimensions of the photocatalytic reactor are shown in Table 1.Eight circular tubes of 3 mm in diameter are arranged in a circle of 20.5 cm in diameter and used for supplying air/oxygen.The air/oxygen flow rate is controlled by a rotameter.Four UV lamps(17 W)are installed at the center of the inner cylinder,and the heat generated is removedviathe opening at the upper end of the cylinder.
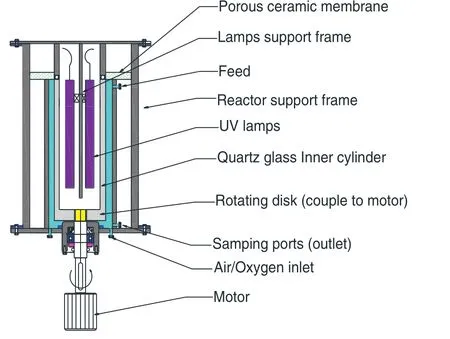
Fig.1.Schematic of the experimental set-up.
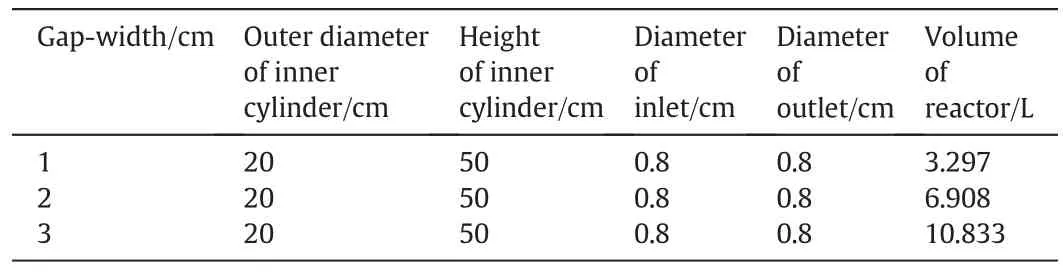
Table 1Dimensions of the rotating annular reactor
2.3.Preparation of surface-modi fi ed TiO2 NPs and Pickering emulsions
TiO2–SA–Arg nanoparticles were prepared by a two-step approach[28]:(1)5 g of TiO2nanoparticles was added into 20 ml of saturated SA solution and then maintained for 20 min in an ultrasonic bath;and(2)2.974 g of Arg was added into the suspension and subjected to ultrasonic treatment for 24 h.After centrifugal separation,the mixture was washed three times with water and ethanol and then dried under vacuum at 105 °C,yielding light yellow TiO2–SA–Arg nanoparticles.
Pickering emulsions were prepared using a high-speed homogenizer:(1)the pH of deionized water was adjusted using HCl and NaOH solution,and then a given amount of catalysts was added and dispersed under ultrasonic vibration for 20 min;and(2)the suspension was transferred to a high-speed homogenizer,and a given volume of oil phase nitrobenzene was added dropwise and dispersed for 20 min at a rotational speed of 16,000 r·min−1.An O/W Pickering emulsion was obtained as determined by the electrical conductivity.
2.4.Photodegradation of NB
The emulsion was filled into the reactor in a discontinuous mode,and rotated at a given speed for 30 min in dark until adsorption equilibrium was reached.Per milliliter of sample was taken from the reactor and diluted 10 times with methanol,and then filtered through a 0.45 mm syringe-like filter.The product was stored in the dark for future analysis,and the initial concentration(C0)for the pollutant was obtained.Then UV lamps were turned on,and samples were collected every 30 min for 3 h and stored as described previously.In a continuous mode,Pickering emulsion was recycled into the reactor through a metering pump,and a 5 L reservoir was installed between the feed and the outlet of the reactor.
2.5.Characterization
The surface morphologies and particle sizes were characterized using HRTEM(JEM-2100;JEOL,Japan).The contact angles of nanoparticles before and after photocatalytic reaction were measured using a contact angle goniometer.The solid particles were pressed into pellets and the contact angles of water droplets on the surface were measured three times,and the mean of three measurements was reported.The absorption wavelength was determined using a UV–Vis spectrophotometer equipped with an integrating sphere.TiO2powders before and after surface modification were dispersed in toluene,and then cast on the glass slide and dried.A drop of emulsion was placed on the glass slide,and its morphology and size were observed and photographed under an optical microscope.The particle size of the emulsion droplet was indicated by the number-average diameterDn,and the dispersity was indicated by the Sauter average diameterd32[30]:

The concentration of nitrobenzene was determined by high performance liquid chromatograph on a C18 reversed phase column(250 mm × 4.6 mm,5 μm).Mobile phase:methanol–water;volume ratio:70:30; flow velocity:0.9 ml·min−1;column temperature:20 °C;and injection volume:20 μl.UV light intensity was measured using a PM100VA UV Irradiation Instrument(Thorlabs Inc.,USA).
3.Results and Discussion
3.1.Characterization of photocatalysts
3.1.1.TEM characterization
The morphologies of photocatalysts were characterized by HRTEM(Fig.2).A severe agglomeration of nanoparticles is observed before modification,and the particle size is approximately 30 nm.However,modified photocatalysts have an irregular flaky shape with a particle size of 35 nm.The boundary of these nanoparticles becomes more distinct after modification,indicating that the organic layer formed on the surface can prevent the agglomeration of nanoparticles.The interplanar distance of the anatase phase(101)and the rutile phase(101)is 0.350 and 0.249 nm,respectively,indicating no significant change in the TiO2crystal structure after modification.An organic layer of about1 nm thick is formed on the surface of the modified nanoparticles.The contact angles for different photocatalysts are shown in the right upper corner of each picture.It shows that the contact angle increases from 11.3°before modification to 79.2°after modification,because the reaction of SA and Arg with the hydrophilic hydroxyl groups on the TiO2surface results in the grafting of lipophilic fatty chains and benzene rings onto the TiO2surface[30,32].Therefore,it is concluded that modification with SA and Arg can reduce the surface hydrophilicity of the photocatalysts,thus improving the ability to stabilize the oil–water mixture[33].
3.1.2.UV–Vis DRS
The UV–Vis diffuse reflectance spectra of modified nanoparticles are shown in Fig.3.Pure TiO2is strongly absorbed only in the UV region with an absorption maximum of415 nm.A large red shift of the absorption edge is observed for the modified nanoparticles,and its absorption maximum(600 nm)is higher than that of SA modified TiO2(500 nm)[32].Thus,modification with SA and Arg contributes to improving the visible light absorption ability of photocatalysts.Assume that doped samples and TiO2are indirect semiconductors,(αhν)1/2is plotted against light energy(hν)according to the Kubelka–Munk function,as shown in the inset in Fig.3.The band gap energy of unmodified and modified TiO2samples is 3.05 and 2.97 eV,respectively,indicating that modification with SA and Arg extends the optical response range of TiO2to the visible region[34].
3.2.The effects of operating parameters
3.2.1.The effects of aeration
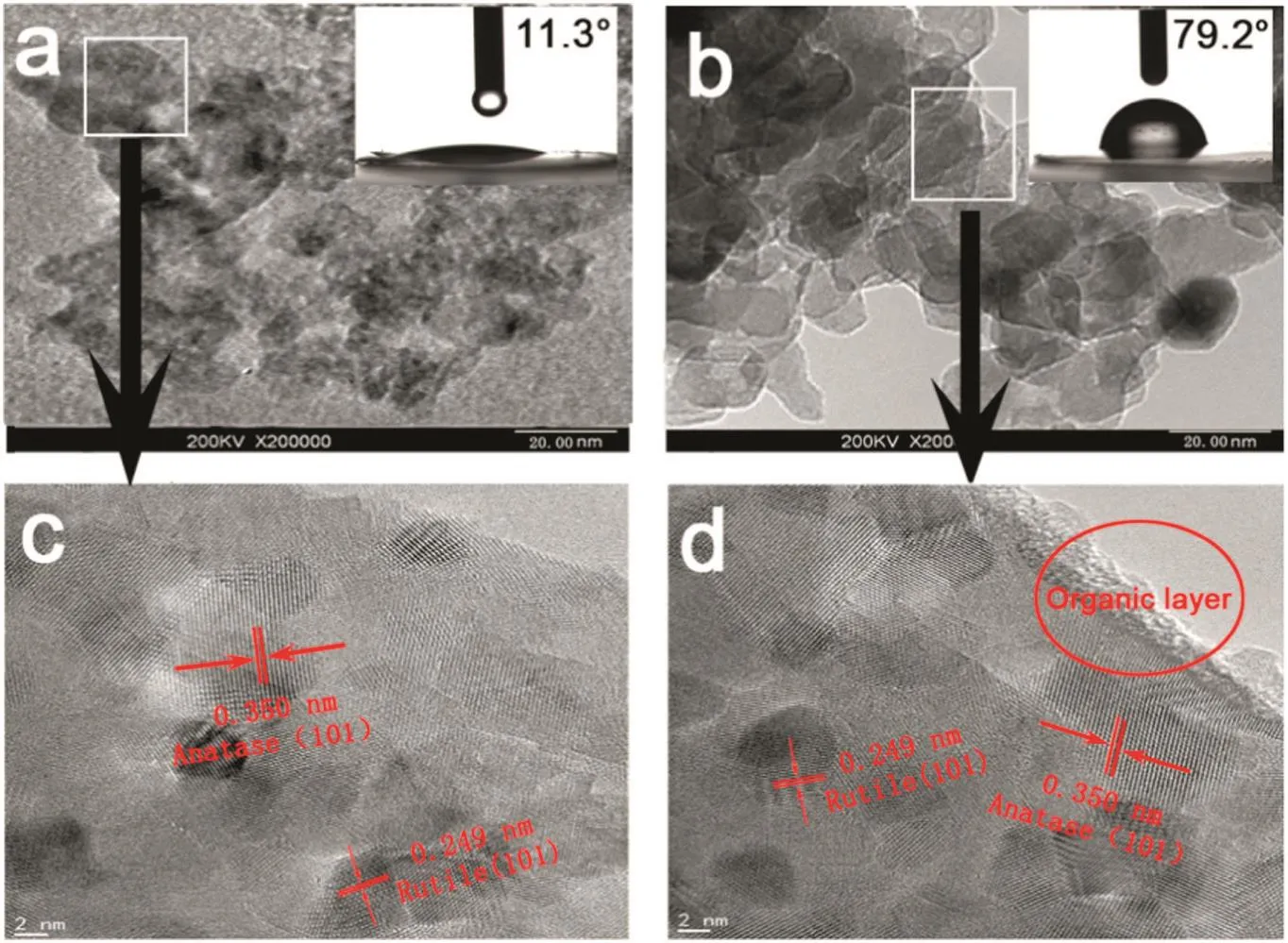
Fig.2.HRTEM images of TiO2 and TiO2–SA–Arg nanoparticles(CAs are shown in the right upper corner).
Electron–hole pairs are formed in large numbers on the surface of photocatalysts illuminated by UVlamps.However,the presence of trapping agents or surface defects can inhibit the recombination of electrons with holes.Holes have high reaction activity,and can react with H2O or OH−ions adsorbed on the surface to form highly oxidative hydroxyl free radicals.The oxygen molecules adsorbed on the surface cannot only participate in the reduction reaction,but also constitute an important source of hydroxyl free radicals[35].Fig.4 shows the degradation of nitrobenzene under the conditions of no aeration,aeration and oxygenation,respectively.It is clear that oxygenation leads to a higher degradation rate of nitrobenzene,as it allows the DO concentration to be maintained at a high level in the Pickering emulsion,thus resulting in an increase in the clearance of electrons and the oxidative free radicals,and consequently a higher photocatalytic degradation rate.Increasing the aeration rate can further improve the degradation efficiency by providing more oxidative free radicals and improving the stirring of Pickering emulsion.It can also improve the stability of the system and the mass transfer due to the rotation of inner cylinder,which will be discussed in Section 3.2.3.No aeration leads to the reduction of the photocatalytic degradation of nitrobenzene.However,nitrobenzene can still degrade slightly in this case probably due to the photochemical reaction of the nitrobenzene molecules themselves.The reaction rate under aeration is between that under oxygenation and that under no aeration.Thus,continuous oxygen supply is essential for the photocatalytic reaction in a closed reactor.However,as air aeration is more cost effective in industrial applications,in what follows we will concentrate on the air aeration.
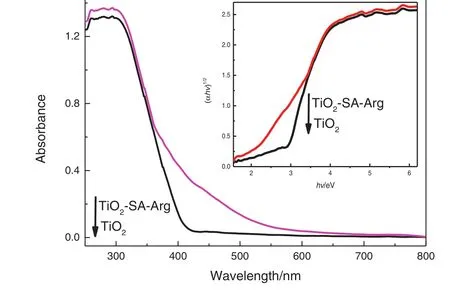
Fig.3.UV–Vis absorption spectra and plot of(αhν)1/2 versus hν for TiO2 and TiO2–SA–Arg samples.
3.2.2.The effects of rotational speed of the inner cylinder
The rotational speed of the inner cylinder is set based on the following considerations:(1)to ensure an adequate shear rate in order to maintain the emulsion state and prevent irreversible stratification;(2)to ensure the turbulence of the emulsion and high mass and heat transfer efficiency;and(3)lower energy consumption.In this study,the rotational speed is 0–250 r·min−1,the solid particle concentration is 2.5 wt%,the oil-phase concentration is 3000 mg·L−1,and the distance between the inner and outer cylinders is 2 cm,respectively.As the use of TiO2–SA–Arg nanoparticles could also result in good photocatalytic activities,a comparative test was conducted to evaluate the additional benefit provided by emulsion.In doing so,the oily suspension system without emulsifying was add in the rotating annular reactor and the photocatalytic degradation reaction was carried out at 0 r·min−1.Fig.5 shows that the Pickering emulsion can significantly improve the photocatalytic efficiency,and the degradation rate of Pickering emulsion is almost twice that of the oily suspension system.Furthermore,it is clear that the degradation rate is higher at 50 r·min−1than at 0 r·min−1.This is because the trajectory of oxygen is a straight line and the oxygen bubbles have a larger size under no rotation,but the trajectory of oxygen is a spiral under mechanical rotation,resulting in an increase in the residence time of oxygen and a decrease in the bubble size,and consequently an increase in DO.However,a further increase of rotational speed can lead to the shear deformation of oxygen to smaller bubbles.In addition,mechanical rotation can increase the mixing in the reactor,and thus catalyst particles are more likely to be exposed to UV radiation.Increasing the rotational speed can also help to maintain the particle size and the stability of the emulsion,as shown in Table 2.Although aeration promotes the mixing of Pickering emulsions,it is less effective than the mechanical stirring caused by the rotation of innercylinder.Thus,the aeration accompanied by the rotation of inner cylinder can further increase the utilization rate of the oxygen.
To investigate the effects of rotational speed on the droplet size and distribution of emulsions,Pickering emulsions collected under different conditions were observed and photographed.Table 2 shows the number average diameterDnand the Sauter average diameterd32of emulsion droplets,and Fig.6 shows some of the microscope photos.As the rotational speed of the inner cylinder increases in the dark,the number average diameter and the Sauter average diameter decrease.Especially,in the later stage of the program,the average particle size increases and the distribution becomes wider under low speed conditions due to the collision and coalescence of the Pickering emulsion droplets.However,the shear force caused by high-speed rotation can emulsify the Pickering emulsions again,and thus the emulsion droplets show low average size and narrow distribution(the smaller the Sauter average diameter,the larger the surface area of the liquid of the same volume,and the higher the dispersity[31]),indicating that increasing the rotational speed helps to improve the emulsion stability and the photocatalytic efficacy.Furthermore,after UV irradiation for 120 min,the Pickering emulsions become a suspension system due to the consumption of oil-phase,as shown in Table 2.The shear force induced by rotation is helpful for the stabilization of the emulsions at the initial stage,but the emulsions become less stable at the later stage due to the consumption of the oilphase.Thus,it is concluded that the shearforce is conducive to the suspension of the photocatalysts.
3.2.3.The effects of light intensity and spacing width
The light intensity can be varied by changing the number of UV lamps,and the film thickness can be varied by changing the spacing between the two cylinders.For immobilized photocatalytic reactor,the reaction occurs at the catalyst surface,and the intensity of UV light absorbed by the catalysts is measured by a radiometer.However,for rotating annular reactor,the light intensity decreases as the thickness of the liquid film increases[36].Sczechowskiet al.[37]found that 99%of incident light was attenuated in the slurry at a distance of 1 mm depth from the light exposed surface.This system has poorer transparency than the suspension system,and the liquid film that can absorb the exciting light is thinner.The light intensity absorbed by the system can be approximately represented by that at the outer surface of the inner cylinder.Fig.6 shows that for a given amount of catalysts and pollutants,the initial photocatalytic reaction rate is almost linearly related to the light intensity.This is because despite the poor transparency of the Pickering emulsion,good mixing is achieved in the reactor due to the aeration and rotation of inner cylinder.The Pickering emulsion can be transferred quickly from the dark region to the bright region,and the increase in light intensity results in the production of more oxidative free radicals in unit time,thus facilitating the photocatalytic reaction.
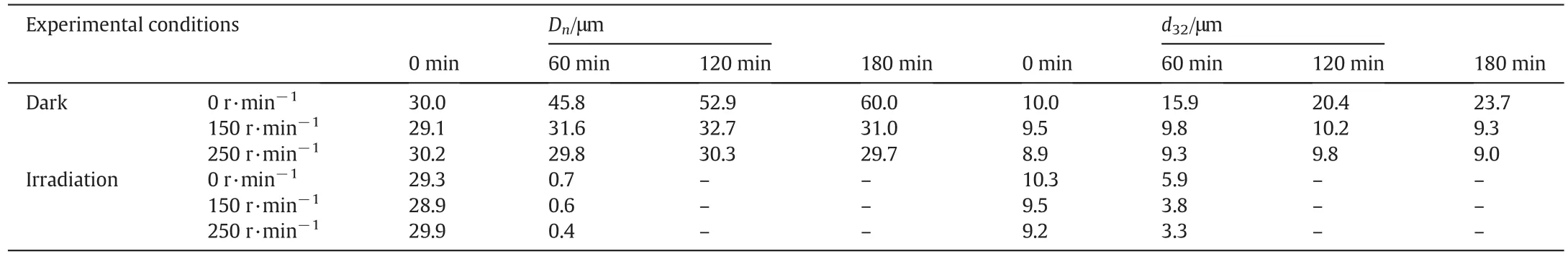
Table 2Effects of rotational speed on the droplet size and distribution of emulsions(2.5 wt%TiO2,3000 mg·L−1 NB,medium-spacing reactor)
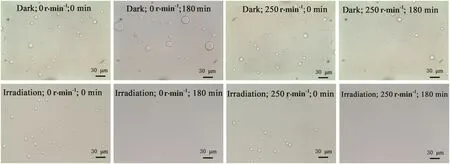
Fig.6.The microscope pictures of emulsions for different rotational speeds under dark/irradiation conditions.
We investigated the effect of the spacing between the two cylinders on the photocatalytic degradation of nitrobenzene(Fig.7).The highest photocatalytic degradation rate is obtained at the small spacing.A desirable degradation rate can be obtained even with the use of a single UV lamp,but the volume of Pickering emulsion in the rotating annular reactor reaches a minimum in such a case,and thus the treatment capacity is low.At the medium and large spacing,a single UV lamp is insufficient to produce good photocatalytic degradation,and the use of four UV lamps can attain only 93.7%of the total degradation rate.Thus,increasing the spacing can lead to an increase in the treatment capacity,but at the expense of a slight decrease in the photocatalytic reaction rate.As a semi-continuous operation mode is most suitable for industrial applications,we investigated the degradation of nitrobenzene in a small-spacing reactor with a circulation volume of 5 L.Table 3 shows that the degradation efficiency is not as good as that in the full residence reactor.This is mainly because the residence time of the liquid in the reactor is decreased,thus resulting in a reduction of the time available for photon excitation.
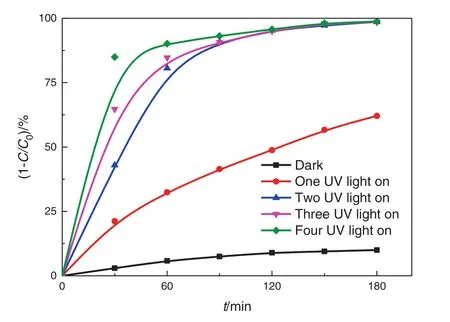
Fig.7.Effects of UV light intensity on the photocatalytic degradation of nitrobenzene.(2.5 wt%TiO2,3000 mg·L−1 nitrobenzene,medium-spacing reactor).

Table 3Effect of gap-width(2.5 wt%TiO2,3000 mg·L−1 NB)
The total quantum yield is used for comparing the performance of different reactors,which is defined as[38–39]:

where γ0is the initial degradation rate of the pollutant,andIis the absorption rate of photons measured by the ferric oxalate in the presence of catalysts.
In general,the initial reaction rate is proportional to the light intensity under low lightintensity,but to the square root of the light intensity under high light intensity,respectively.As a consequence,the quantum yield is constant under low light intensity,but increases with the decrease of light intensity under high light intensity[40].Fig.6 shows that the initial degradation rate is linearly related to the light intensity,which is similar to that under low light intensity.Thus,for a given amount of catalysts,light intensity can be the major factor affecting the quantum yield.
3.3.Dynamic analysis of degradation
3.3.1.The effects of initial concentration of nitrobenzene
The initial concentration of nitrobenzene is 2000–4000 mg·L−1(the saturation concentration of the nitrobenzene solution is 1900 mg·L−1under the experimental conditions)(Fig.9).The removal rate of nitrobenzene in the Pickering emulsion for different initial concentrations of nitrobenzene is shown in Fig.8.
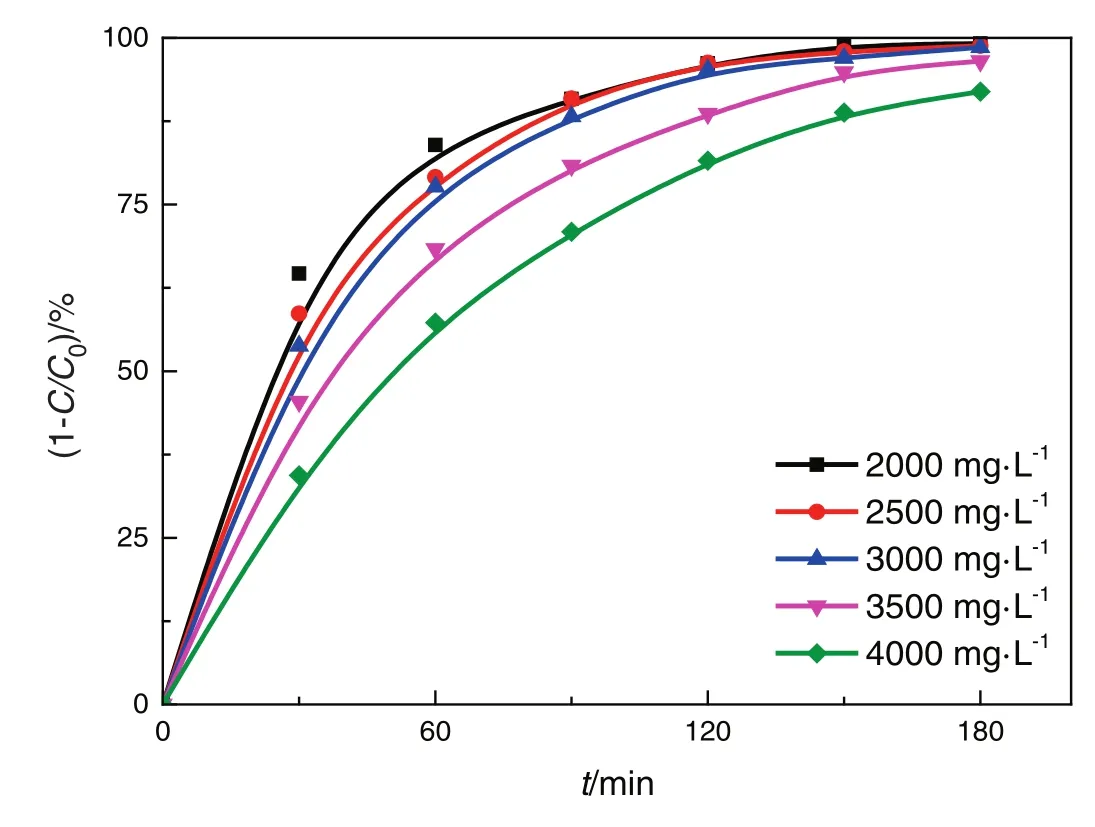
Fig.8.Effects of initial concentration of nitrobenzene.(2.5 wt.%TiO2,medium-spacing reactor,all four lamps illuminated).
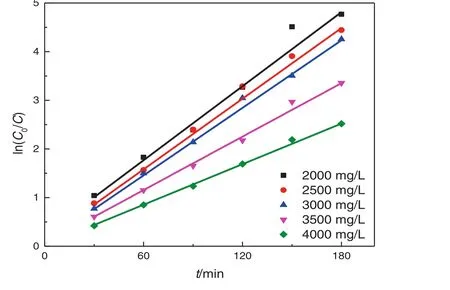
Fig.9.The effects of initial concentration of nitrobenzene on the degradation kinetics of nitrobenzene.
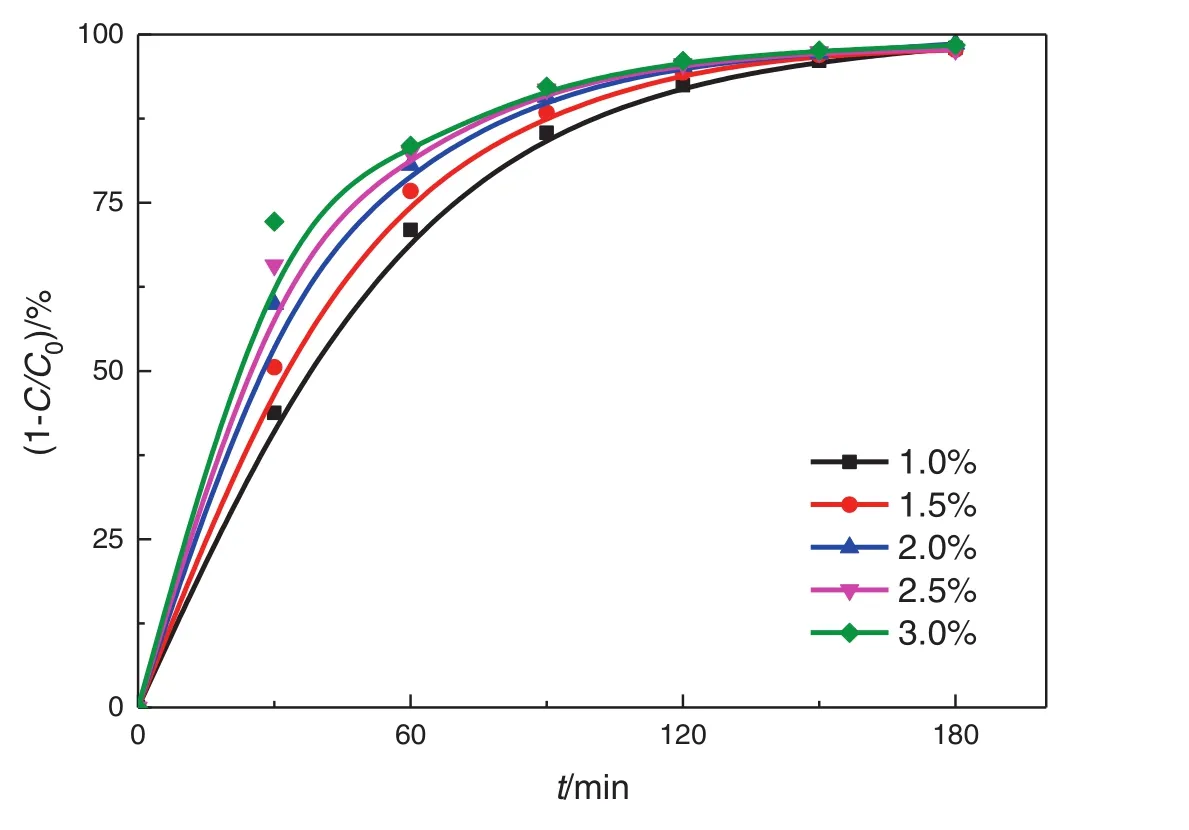
Fig.10.Effects of concentration of TiO2–SA–Arg.(3000 mg·L−1 NB,medium-spacing reactor,all four lamps illuminated).
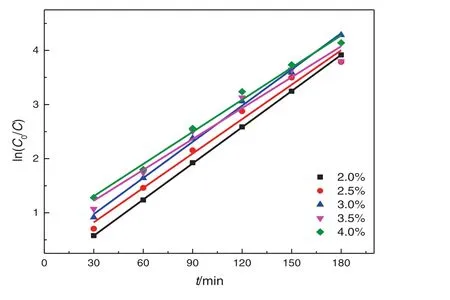
Fig.11.Effects of concentration of TiO2–SA–Arg on the degradation kinetics of nitrobenzene.
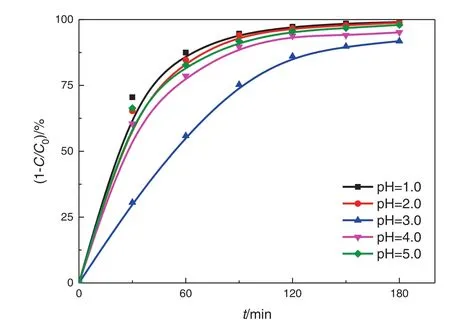
Fig.12.Effect of pH values.(2.5 wt%TiO2,medium-spacing reactor,all four lamps illuminated).
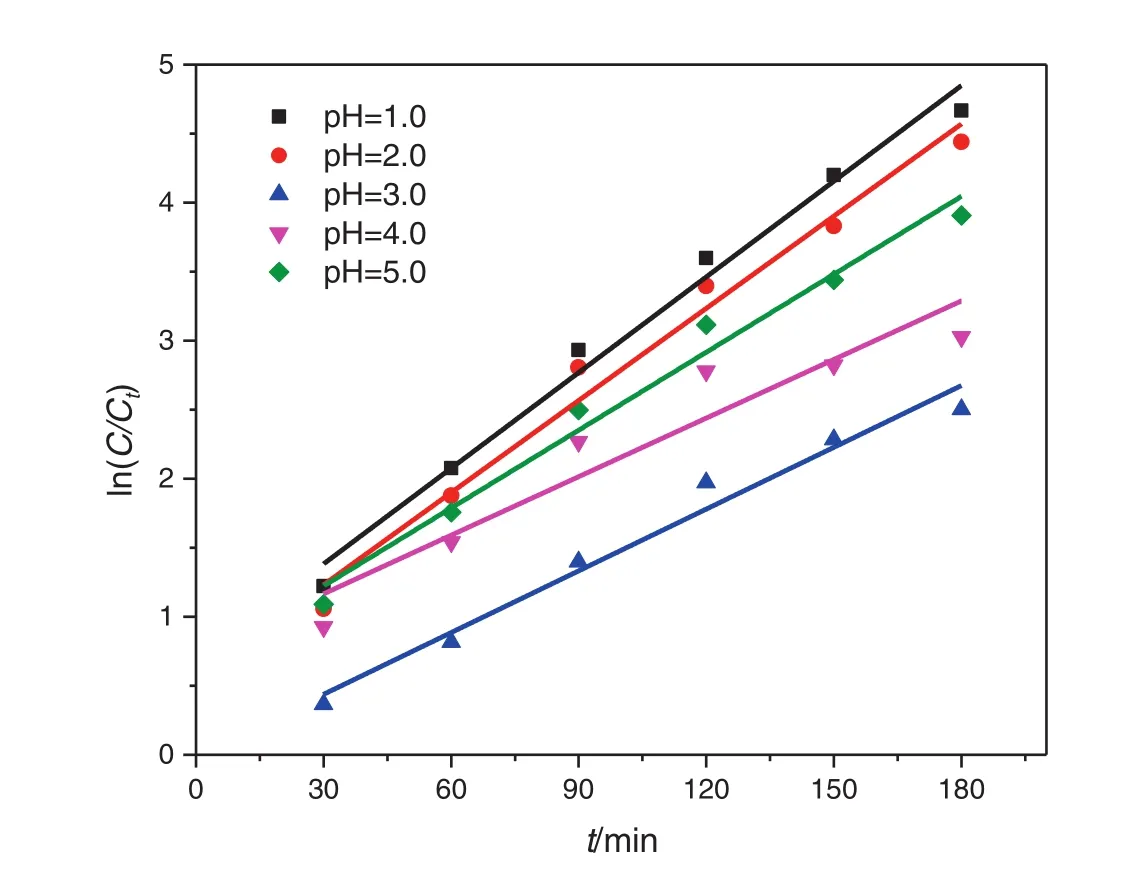
Fig.13.Effects of pH values on the degradation kinetics of nitrobenzene.
It shows that the removal rate of nitrobenzene is high at the initial stage,but decreases gradually with the degradation time.ln(C0/C)is plotted against the degradation timet,as shown in Fig.8.It shows that the photocatalytic degradation of nitrobenzene in the rotating annular reactor follows the first-order reaction,that is,the reaction rate is proportional to the reactant concentration and decreases with the progression of the reaction(Table 4).
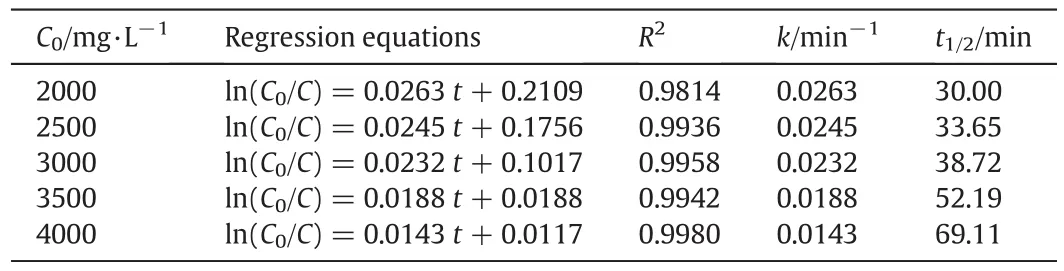
Table 4Kinetic equations and parameters for different initial concentrations of nitrobenzene
The heterogeneous photocatalytic degradation of organic contaminants can be expressed by the Langmuir–Hinshelwood equation(L–H equation)[41]:

whererLHis the total reaction rate of the organic compounds in the reactor(mg·L−1·min−1),κ is the Langmuir rate constant of the organic compounds(mg·L−1·min−1),andKis the apparent adsorption equilibrium constant(L·mg−1).
Eq.(3)can be rewritten as:

Eq.(4)shows that 1/Cis linearly related to 1/−rLH.When the concentration of organic compounds is too low,KC≪1 and−rLH≈κKC,indicating first-order reaction;when the concentration is too high,KC≫1 and−rLH≈κ,indicating zero-order reaction;while when the concentration is moderate,the reaction order is between 0 and 1.Thus,as the reactant concentration increases,the reaction order decreases from 1 to 0.It has been shown that most photocatalytic reactions follow the L–H equation at the initial stage.However,the L–H equation becomes inapplicable at the later stage because as the reactant concentration decreases,the diffusion of reactants to the catalystsurface is reduced.Another important reason is the inactivation of the catalysts at the later stage of the photocatalytic reaction.The linear regression of 1/C0on 1/r0(r0=k·C0)at the initial stage reveals thatR2=0.2848,indicating no linear relationship.Thus,although the photocatalytic degradation of nitrobenzene in the rotating annular reactor follows the pseudo- first-order reaction,it is not well described by the L–H equation because the absorption sites are not fixed due to the formation of solid nanoparticle film by the catalysts.
3.3.2.The effects of catalyst concentration
The catalyst concentration is 1.0–3.0 wt%in this study,which allows to maintain a high stability of the Pickering emulsion and to achieve a high photocatalytic degradation rate,and the oil-phase concentration is 3000 mg·L−1.Fig.10 clearly shows that the removal rate of nitrobenzene increases with the increase of catalyst concentration,because(1)increasing catalyst concentration can lead to an increase in the emulsion stability and a decrease in the particle size of emulsion and nitrobenzene droplets,and thus an increase in the inter facial area.Suppose that all the particles can be irreversibly adsorbed at the oil–water interface(no free particles in the continuous phase),the amount of particles that form a dense film is inversely proportional to the average radius of the liquid droplets.However,when the content of solid particles is low,the emulsion particles have a large size and are dispersed.As the particle concentration increases,the size of liquid droplets decreases,and the emulsion stability increases[42];and(2)the production of photon-generated oxidants is increased.However,itis noted thatthe turbidity increases when the catalyst concentration is too high,leading to the scattering of the incident light,and thus poor light permeability and photon efficacy.However,too thick nanoparticle film can hinder the mass transfer of oxygen across the film,and thus hinder the photocatalytic oxidation reaction.The plot of ln(C0/Ct)~tunder different catalyst concentrations is shown in Fig.11,and the kinetics equations and parameters are displayed in Table 5.The analysis of variance shows that the degradation of nitrobenzene under different catalyst concentrations follows the pseudo first-order reaction kinetics.
3.3.3.The effects of pH values
Low pH value may have an effect on the degradation of nitrobenzene and the formation of acidic intermediates.Thus,we investigated the degradation dynamics of nitrobenzene under low pH conditions,and the removal rate of nitrobenzene in the Pickering emulsion under different pH conditions is shown in Fig.12.The pH value can have a significant effect on the electric charges on the particle surface and thus the agglomeration of nanoparticles.The agglomeration of nanoparticles depends critically on the van der Waals attractive force and the electrostatic repulsive force,which is indicated by the ζ-potential.Liet al.[30]showed that the isoelectric point(pHPZC)of TiO2–SA–Arg particles was about 3.0,and serious agglomeration occurred in this case.However,increasing or decreasing pH promoted the dispersion of these solid particles.The Zeta potential of modified TiO2particles is positive at pH=1–3 and its absolute value decreases gradually,which is not good for the dispersion of particles.As a result,as the pH value increases,the degradation rate of nitrobenzene decreases.However,at pH=3–5,as the pH value increases,the agglomeration of particles decreases,the emulsion stability becomes better,and the degradation rate of nitrobenzene increases.The pH value can also have an effect on the adsorption amount of nitrobenzene,and the lower the pH value,the higher the adsorption amount of nitrobenzene.ln(C0/Ct)is plotted against the timetunder different pH conditions,as shown in Fig.13.The reaction rate constantk(0.149–0.256 min−1)is calculated from the slope.The analysis of variance shows that the degradation of nitrobenzene under acidic conditions follows the pseudo first-order reaction kinetics(Table 6).
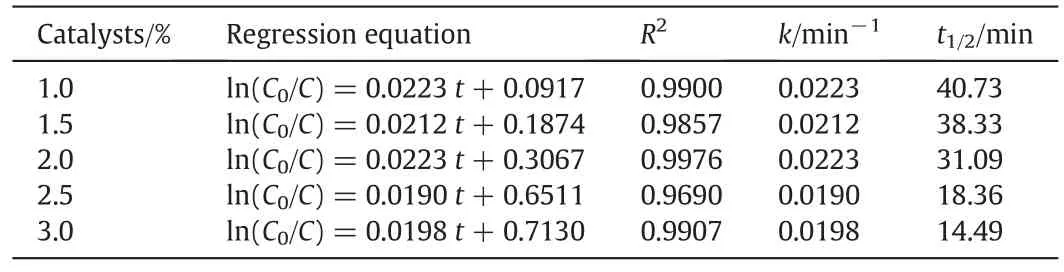
Table 5Kinetics equations and parameters for different amounts of photocatalysts
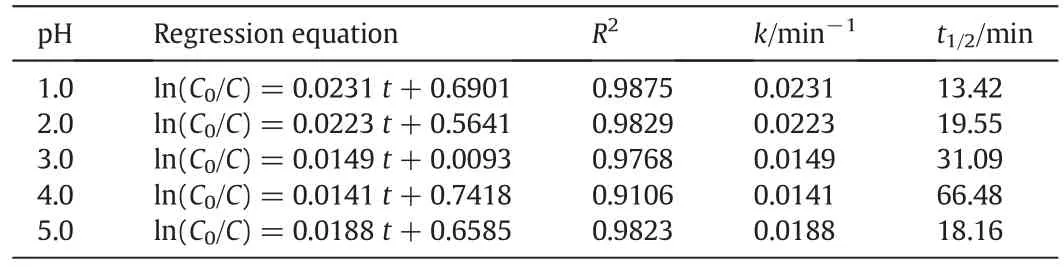
Table 6Kinetics equations and parameters under different pH conditions
3.4.Degradation mechanisms
CH2OH derived fromtert-butyl alcohol under illumination can act as the hole scavenger.The addition oftert-butyl alcohol leads to current multiplication on the TiO2surface,which is beneficial for the photocatalytic reduction of nitrobenzene[43].Table 7 clearly shows thattertbutyl alcohol promotes the photocatalytic degradation of nitrobenzene under no aeration,but inhibits the photocatalytic degradation of nitrobenzene underaeration.In this study,TiO2nanoparticles were modified with SA and Arg.Arg has potent electron-donating capacity and can act as a hole trap to prevent electron–hole recombination.In addition,photoinduced electrons can reduce nitrobenzene to aniline.However,one of the following two conditions musthold:(1)no DO in the system,and(2)the addition of a certain amount of free Arg.Theπ–πconjugated structure formed by SA and TiO2can also lead to the photoreduction of nitrobenzene,but its effect is not significant.
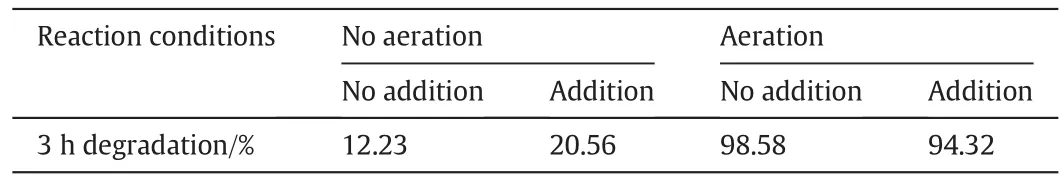
Table 7Effects of tert-butyl alcohol on the removal rate of nitrobenzene
Previous studies have focused primarily on either oxidation or reduction in the photocatalysis,while there have been few studies examining both oxidation and reduction.However,a better understanding of their synergistic effect in the Pickering emulsions is of both theoretical and practical importance.Nitrobenzene can undergo reduction reaction as electron acceptors and oxidative degradation initiated by hydroxylfree radicals.Compared with unmodified catalysts,TiO2–Arg and TiO2–SA catalysts can improve the degradation rate of nitrobenzene,but by different mechanisms.In Section 3.2.1,we show that the photocatalytic degradation of nitrobenzene occurs under no aeration conditions.However,under aeration conditions,DO(an electron-trapping agent)increases and hydroxyl free radicals forms by protonation,thus leading to the oxidation reaction of the system.
4.Conclusions
The main purpose of this study is to investigate the photocatalytic degradation and kinetics of nitrobenzene in the O/W Pickering emulsion in the rotating annular reactor.The results show that this rotating annular reactor significantly contributes to improving the photocatalytic degradation of nitrobenzene.The increase of the aeration rate,rotational speed,and light intensity can effectively increase the degradation of nitrobenzene.Although the photocatalytic degradation of nitrobenzene in the rotating annular reactor follows the pseudo first-order reaction,it is not well described by the L–H equation.In addition,aeration has a significant effect on the photocatalytic degradation pathway of nitrobenzene.Nitrobenzene can undergo reduction reaction as electron acceptors and oxidative degradation initiated by hydroxyl free radicals.
[1]Z.N.Norvill,A.Shilton,B.Guieysse,Emerging contaminant degradation and removal in algal wastewater treatment ponds:Identifying the research gaps,J.Hazard.Mater.313(5)(2016)291–309.
[2]T.C.Li,B.Jiang,X.Feng,D.W.Wang,A.J.Yuan,X.G.Li,Purification of organic wastewater containing Cu2+and Cr3+by a combined process of micro electrolysis and bio film,Chin.J.Chem.Eng.11(2)(2003)146–150.
[3]F.Q.Chen,S.F.Wu,J.Z.Chen,S.X.Rong,COD removal efficiencies of some aromatic compounds in supercritical water oxidation,Chin.J.Chem.Eng.9(2)(2001)137–140.
[4]X.H.Li,B.W.Zhao,K.Zhu,X.P.Hao,Removal of nitrophenols by adsorption using β-cyclodextrin modified zeolites,Chin.J.Chem.Eng.19(6)(2011)938–943.
[5]H.W.Ma,Q.F.Fang,L.M.Wu,M.Q.Wei,Y.H.Zhang,The adsorption of phenol by lignite activated carbon,Chin.J.Chem.Eng.19(3)(2011)380–385.
[6]M.F.F.Sze,G.Mckay,Adsorptive removal of para-chlorophenol using stratified tapered activated carbon column,Chin.J.Chem.Eng.20(3)(2012)444–454.
[7]Y.Jiang,N.Q.Ren,X.Cai,D.Wu,Y.L.Qiao,S.Lin,Biodegradation of phenol and 4-chlorophenol by the mutant strain CTM 2,Chin.J.Chem.Eng.16(5)(2008)796–800.
[8]Y.Y.Lu,Y.Liu,B.W.Xia,W.Q.Zuo,Phenol oxidation by combined cavitation water jet and hydrogen peroxide,Chin.J.Chem.Eng.20(4)(2012)760–767.
[9]J.D.Vela,L.B.Stadler,K.J.Martin,L.Raskin,C.B.Bott,N.G.Love,Prospects for biological nitrogen removal from anaerobic ef fluents during mainstream wastewater treatment,Environ.Sci.Technol.Lett.2(9)(2015)234–244.
[10]H.Yu,X.X.Zheng,Z.Y.Yin,T.Feng,B.B.Fang,K.S.Hou,Preparation of nitrogen-doped TiO2nanoparticle catalyst and its catalytic activity under visible light,Chin.J.Chem.Eng.15(6)(2007)802–807.
[11]C.B.Ratiu,C.Orha,C.Misca,C.Lazau,P.S firloaga,S.Olariu,Photocatalytical inactivation ofEnterococcus faecalisfrom water using functional materials based on natural zeolite and titanium dioxide,Chin.J.Chem.Eng.22(1)(2014)38–43.
[12]Q.S.Yang,Y.J.Liao,L.L.Mao,Kinetics of photocatalytic degradation of gaseous organic compounds on modified TiO2/AC composite photocatalyst,Chin.J.Chem.Eng.20(3)(2012)572–576.
[13]H.F.Liu,S.F.Ji,Y.Y.Zheng,M.Li,H.Yang,Porous TiO2-coated magnetic core-shell nanocomposites:Preparation and enhanced photocatalytic activity,Chin.J.Chem.Eng.20(3)(2012)572–576.
[14]L.W.Shan,J.B.Mi,L.M.Dong,Z.D.Han,B.Liu,Enhanced photocatalytic properties of silver oxide loaded bismuth vanadate,Chin.J.Chem.Eng.22(8)(2014)909–913.
[15]D.Vione,Influence of electron acceptors on the kinetics of metoprolol photocatalytic degradation in TiO2suspension.A combined experimental and theoretical study,RSC Adv.5(2015)54589–54604.
[16]F.Yang,S.Y.Liu,J.Xu,Q.Lan,F.Wei,D.J.Sun,Pickering emulsions stabilized solely by layered double hydroxides particles:The effect of salt on emulsion formation and stability,J.Colloid Interface Sci.302(1)(2006)159–169.
[17]T.Wu,H.T.Wang,B.X.Jing,F.Liu,P.C.Burns,C.Z.Na,Multi-body coalescence in Pickering emulsions,Nat.Commun.6(2015)5929.
[18]Z.Y.Fan,A.Tay,M.Pera-Titus,W.J.Zhou,S.Benhabbari,X.S.Feng,G.Malcouronne,L.Bonneviot,F.D.Campo,L.M.Wang,J.M.Clacenset,Pickering interfacial catalysts for solvent-free biomass transformation:Physicochemical behavior of non-aqueous emulsions,J.Colloid Interface Sci.427(8)(2014)80–90.
[19]D.E.Resasco,Carbon nanohybrids used as catalysts and emulsifiers for reactions in biphasic aqueous/organic systems,Chin.J.Catal.35(6)(2014)798–806.
[20]W.Wu,S.Gao,W.X.Tu,J.F.Chen,P.Y.Zhang,Intensified photocatalytic degradation of nitrobenzene by Pickering emulsion of ZnO nanoparticles,Particuology8(5)(2010)453–457.
[21]T.Nakato,H.Ueda,S.Hashimoto,R.Terao,M.Kameyama,E.Mouri,Pickering emulsions prepared by layered niobate K4Nb6O17intercalated with organic cations and photocatalytic dye decomposition in the emulsions,ACS Appl.Mater.Interfaces4(8)(2012)4338–4347.
[22]M.F.Nsib,A.Maayou fi,N.Moussa,N.Tarhouni,A.Massouri,A.Houas,Y.Chevalier,TiO2modified by salicylic acid as a photocatalyst for the degradation of monochlorobenzeneviaPickering emulsion way,J.Photochem.Photobiol.A251(9)(2013)10–17.
[23]M.Nawaz,D.Kim,W.Miran,A.Kadam,J.Heo,S.Shin,J.Jang,S.R.Lim,D.S.Lee,Effect of toluene,an immiscible pollutant,on the photocatalytic degradation of azo dye,J.Ind.Eng.Chem.30(2015)10–13.
[24]N.Mohaghegh,M.Tasviri,E.Rahimi,M.R.Gholami,A novel p–n junction Ag3PO4/BiPO4-based stabilized Pickering emulsion for highly efficient photocatalysis,RSC Adv.5(17)(2015)12944–12955.
[25]W.Y.Zhai,G.P.Li,P.Yu,L.F.Yang,L.Q.Mao,Silver phosphate/carbon nanotubestabilized Pickering emulsion for highly efficient photocatalysis,J.Phys.Chem.C117(29)(2013)15183–15191.
[26]P.K.Dutta,A.K.Ray,Experimental investigation of Taylor vortex photocatalytic reactor for water purification,Chem.Eng.Sci.59(2004)5249–5259.
[27]M.Subramanian,A.Kannan,Photocatalytic degradation of phenol in a rotating annular reactor,Chem.Eng.Sci.65(9)(2010)2727–2740.
[28]F.F.Lv,L.Q.Zheng,C.H.Tung,Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system,Int.J.Pharm.301(1–2)(2005)237–246.
[29]R.Aveyard,B.P.Binks,J.H.Clint,Emulsions stabilised solely by colloidal particles,Adv.Colloid Interf.Sci.100–102(2)(2003)503–546.
[30]L.Li,Y.J.Feng,Y.Z.Liu,B.Wei,J.X.Guo,W.Z.Jiao,Z.H.Zhang,Q.L.Zhang,Titanium dioxide nanoparticles modified by salicylic acid and arginine:Structure,surface properties and photocatalytic decomposition of p-nitrophenol,Appl.Surf.Sci.363(2016)627–635.
[31]W.Z.Jiao,J.Li,Y.Z.Liu,Q.L.Zhang,W.L.Liu,C.C.Xu,L.Guo,Dispersion performance of methanol-diesel emulsified fuel prepared by high gravity technology,China Pet.Process Petrocehm.16(1)(2014)27–34.
[32]Q.L.Zhang,L.Li,Y.Z.Liu,B.Wei,J.X.Guo,Y.J.Feng,Grafting dynamics,structures and properties of Nano TiO2–SA photocatalytic materials,Acta Phys.-Chim.Sin.31(6)(2015)1510–1524 in Chinese.
[33]L.Li,Q.L.Zhang,H.L.Fan,Y.Z.Liu,B.Wei,Y.J.Feng,Preparation of titanium dioxide particles modified by salicylic acid and arginine and its photocatalytic reaction on oil–water interface[J],J.Inorg.Mater.31(4)(2016)413–420(in Chinese).
[34]H.Q.Jiang,Q.F.Wang,J.S.Li,Q.Y.Wang,Z.Y.Li,Sol-hydrothermal preparation of N–P–TiO2nano-particles for photocatalytic degradation of 4-chlorophenol under sunlight irradiation,Acta Chim.Sin.70(20)(2012)377–381 in Chinese.
[35]D.D.Dionysiou,A.A.Burbano,M.T.Suidan,Effect of oxygen in a thin- film rotating disk photocatalytic reactor,Environ.Sci.Technol.36(17)(2002)3834–3843.
[36]H.Huang,K.C.Huang,D.Tsai,Light absorption measurement of a plasmonic photocatalyst in the circular plane waveguide of a photocatalytic dual light source spinning disk reactor,Opt.Rev.20(2)(2013)236–240.
[37]J.G.Sczechowski,C.A.Koval,R.D.Noble,A Taylor vortex reactor for heterogeneous photocatalysis,Chem.Eng.Sci.50(20)(1995)3163–3173.
[38]J.Zhang,F.T.Lu,S.B.Qi,Y.M.Zhao,K.P.Wang,B.Zhang,Y.Q.Feng,In fluence of various electron-donating triarylamine groups in BODIPY sensitizers on the performance of dye-sensitized solar cells,Dyes Pigments128(2016)296–303.
[39]J.Bolobajev,M.Trapido,A.Goi,Effect of iron ion on doxycycline photocatalytic and Fenton-based autocatatalytic decomposition,Chemosphere153(2016)220–226.
[40]K.Mehrotra,G.S.Yablonsky,A.K.Ray,Kinetic studies of photocatalytic degradation in a TiO2slurry system:Distinguishing working regimes and determining rate dependences,Ind.Eng.Chem.Res.42(11)(2003)2273–2281.
[41]V.Vaiano,O.Sacco,D.Pisano,D.Sannino,P.Ciambelli,From the design to the development of a continuous fixed bed photoreactor for photocatalytic degradation of organic pollutants in wastewater,Chem.Eng.Sci.137(2015)152–160.
[42]S.A.Bon,P.J.Colver,Pickering miniemulsion polymerization using Laponite clay as a stabilizer,Langmuir23(16)(2007)8316–8322.
[43]H.Y.Huang,J.H.Zhou,H.L.Liu,Y.H.Zhou,Y.Y.Feng,Selective photoreduction of nitrobenzene to aniline on TiO2nanoparticles modified with amino acid,J.Hazard.Mater.178(1–3)(2010)994–998.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Structural insights of mechanically induced aluminum-doped hydroxyapatite nanoparticles by Rietveld refinement☆
- Structural evolution of chars from biomass components pyrolysis in a xenon lamp radiation reactor☆
- Assessing the kinetic model of hydro-distillation and chemical composition of Aquilaria malaccensis leaves essential oil
- Effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions☆
- A novel model for multi-plant mixed heavy crude oils refinery planning
- A self-tuning control method for Wiener nonlinear systems and its application to process control problems☆
