Effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions☆
2017-05-28TongbaoZhangYangchengLuGuangshengLuo
Tongbao Zhang,Yangcheng Lu*,Guangsheng Luo
State Key Laboratory of Chemical Engineering,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
1.Introduction
Interest in iron phosphate compounds has always been intensive due to their impressive performance and wide application in the fields of battery[1–2],catalysis[3–5],wastewater purification[6,7],ferroelectrics[8],andetc.[9].It has been well recognized that the manipulation of the structure and size of iron phosphate materials is of great importance for achieving various and competitive functions[10–12].For examples,as cathode materials for lithium/sodium ion batteries,iron phosphates with amorphous or orthorhombic heterosite structure were found to exhibit much better electrochemical performance due to the existence of plenty of migration routes for carrier ions[13–15].Although α-Quartz iron phosphate is inert in electrochemical activity,it shows high performance to catalyze the gas-phase selective oxidation of benzene to phenol[16],in which the tetrahedral-coordinated Fe is the active center.As for enhancing the electrochemical performance or the catalytic activity,a choice is to facilitate both electron and carrier ions migration or expose more active sites by reducing the size of iron phosphate materials mentioned above.
The synthesis of iron phosphate compounds commonly starts from versatile precipitation reactions between dissolved iron(III)and phosphate ions in aqueous solution[17–23].The reaction conditions,especially in terms of temperature and pH,directly determine the reactants participating in the fast precipitation process.In following,the growth process of the as-prepared precipitates affords products with diversity in size,morphology and crystalline structure[6,24].The solubilities of iron phosphate materials are important controlling factors throughout the conversion process.Chang and Jackson firstly determined the solubility of strengite(FePO4·2H2O)in water at room temperature after 8 to 21 days equilibrium experiments[25].Assuming that FePO4·2H2O dissociates into Fe3+,H2PO4−and OH−,the calculated solubility product(Ksp)of strengite was between 10–33.5and 10−35.According to the heat capacities at temperatures between 8 and 310 K and the heat of formation at 298 K,Eganet al.obtained similar value(10–34.56)by thermodynamic calculation[26].The measurements on strengite solubility in dilute H3PO4by Nriaguet al.[27]also con firmed the scale ofKsp(10–34.88).Besides,Dean assumed that the amorphous iron phosphate(FePO4·2H2O)dissociates into Fe3+,PO43−and H2O in water,and reportedKspof 9.92×10−29at 298 K[28].
However,there exist complex dissociations and complexations in the aqueous solution of iron phosphate materials,and the calculation of solubility fromKspis difficult.On the other hand,considering versatile iron phosphate materials and broad pH/temperature window for synthesis,the measurements on their solubilities are still scarce.In this work,we selected amorphous iron phosphate dihydrate(the direct product of precipitation)as solute,phosphoric acid as a pH regulator with foreign ions free,and systematically investigate the effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions.The concentration of phosphoric acid and temperature varied from 1.13 wt%to 10.7 wt%and 298.15 to 363.15 K,respectively.The quantitative variation laws as well as fundamental data of solubilities were of great importance for understanding and designing iron phosphate materials synthesis processes.
2.Experimental Section
2.1.Chemicals
The iron(III)phosphate dihydrate(FePO4·2H2O)was purchased from the Sigma-Aldrich.The orthophosphoric acid(H3PO4,aq.soln.)was purchased from Alfa Aesar.The hydrochloric acid(HCl,36 wt%–38 wt%)was purchased from Beijing Chemical Works.All of the reagents were used as received without further purification.Ultrapure deionized water(18.2 MΩ)was used throughout the experiments for solution preparation and vessel cleaning.
2.2.Solution preparation
All the vessels used throughout the experiments were thoroughly cleansed.They were firstly washed by 1 mol·L−1HCl solution under sonication for 2 h to remove any possible impurities.Then the vessels were thoroughly rinsed by ultrapure deionized water and further sonicated for another 1 h.The water cleaning procedure was repeated for three times.Finally,they were dried at 105°C in air overnight.
The H3PO4solutions with various concentrations were prepared by diluting purchased 85%H3PO4solution and stored in volumetric flasks.The exact concentrations of H3PO4,determined by an inductively coupled plasma-optical emission spectrometer,were listed in Table 1,in which the pH value and density measured at room temperature were also provided.
2.3.Equilibrium procedure
A typical equilibrium procedure was described as follows.Firstly,50 ml of phosphoric acid solution was transferred to conical flask added with sufficient amount of FePO4·2H2O powders in advance.And then,the conical flask was sealed and placed in a thermostatic oven(DHG-9035A,HONGHUA)at temperature above 308.15 K,or in a thermostatic bath equipped with a refrigeration system(LSHZ-300,HAOCHENG)at temperature below 308.15 K.The temperature uncertainty for equilibrium was±0.1 K.According to Chang's results[25],the equilibrium time was set as long as 30 days to approach the liquid–solid equilibrium,after which the supernatant was carefully drawn out by a single-use syringe.The syringe nozzle was equipped with a 0.22μm filter head to remove any possible solid and get clearliquid samples for solubility measurement.The residual solid was isolated by suction filtration,washed by water,and dried at 105°C in air overnight for crystalline structure characterization.
2.4.Analysis and characterization
The iron and phosphorus contents in liquid samples were analyzed by an inductively coupled plasma-optical emission spectrometer(ICPOES,iCAP6300,ThermoFisher)or an inductively coupled plasma-mass spectrometer(ICP-MS,iCAPQ,ThermoFisher).The limit of detection of ICP-OES for iron is 10−8g·g−1.The power of the plasma was 1150 W.Peristaltic pump rotation speed was 100 r·min−1.The limit of detection of ICP-MS foriron is10−12g·g−1.The power of the plasma was 1548 W.Argon gas in high-purity(99.9999%)was used as the carrier gas with flow rate 0.952 L·min−1.Each sample was measured for three times or more,and the average value was reported.Some selected samples were also characterized by UV–vis spectrometer(UV-2450,Shimadzu Corporation)with 5 nm slit width.The solid phase was characterized by X-ray diffraction(XRD,D8-Advance,40 kV,40 mA)using Cu Kαradiation at a 5°min−1scanning rate.
3.Results and Discussion
3.1.Solubility of FePO4·2H2O in deionized water
The solubility data of FePO4·2H2O in deionized water(18.2 MΩ)at temperatures from 298.15 to 363.15 K and atmosphere pressure are presented in Table 2.Herein,we assume that FePO4·2H2O dissociates to Fe3+and PO43−,and the solubility product(Ksp)of FePO4·2H2O is expressed as Eq.(1).

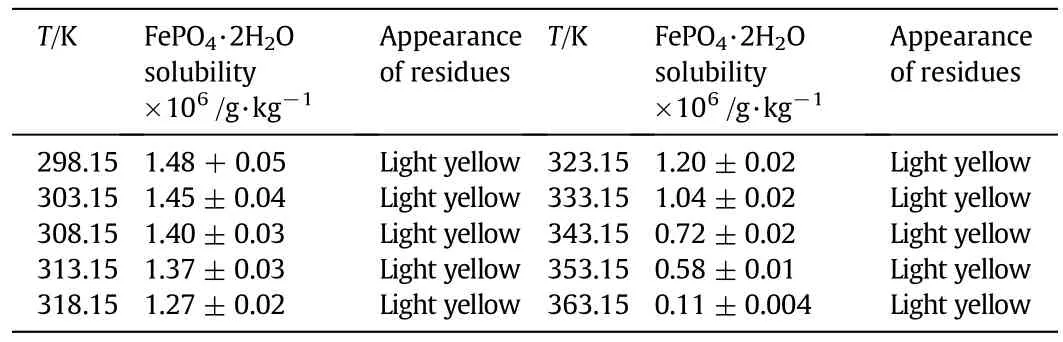
Table 2Solubility of FePO4·2H2O in deionized water at 298.15 to 363.15 K and atmospheric pressure①
In the aqueous solution,Fe3+could be hydrolyzed to Fe(OH)2+and Fe(OH)2+;phosphoric acid has a three-step dissociation process generating various phosphate anions[29].When using deionized water as solvent,the content of phosphorus equals to that of iron in solution;the interaction between iron species and phosphate species could be neglected because the concentration of OH−is much higher than that of phosphate species.As the contents of phosphorous and iron in solution being measured,the concentrations of Fe3+and PO43−can be calculated according to the hydrolysis constant of Fe3+and the dissociation constant of phosphoric acid previously reported[30,31]directly.In this work,theKspof FePO4·2H2O at 298.15 K was calculated as 5.39× 10−29,close to Dean's report(9.92×10−29)[28].It indicates the reliability of our solubility measurements.From Table 2,it could be found that the solubility of FePO4·2H2O monotonously decreases with the temperature increasing.The solubility at 343.15 K is only half of that at 298.15 K.

Table 1The composition,pH value and density of various solvents
3.2.Solubility of FePO4·2H2O in various H3PO4 solutions
2.8.In other words,doubling the H3PO4concentration could increase the solubility of FePO4·2H2O by 5 to 7 times.As for the effect of temperature on the solubility of FePO4·2H2O,the case of using H3PO4solution as solvent is similar with that of using deionization water as solvent,i.e.,the solubility of FePO4·2H2O decreases with the increasing of
The dissolution behavior of FePO4·2H2O in H3PO4solutions is complex due to the formation of various complex ions at acidic environment.The composition of complex ion is significantly dependent on the amount of H3PO4acid.Considering the interactions between one iron ion and one phosphate ion only,Stummet al.indicated that FeHPO4+was of majority when the concentration of H+was below 2 × 10−2mol·L−1,otherwise FeH2PO42+was the main species[32].While,when the concentration of H3PO4was largely excessive over that of iron ions,multi-ligands complex may become dominant,such as Fe(H2PO4)2+,FeH3(PO4)2,FeH5(PO4)22+,Fe(H2PO4)3,and FeH7(PO4)3+[33].The FePO4·2H2O crystals with varied morphologies and structures were usually synthesized under such conditions,but until now,little thermodynamic knowledge is available about these complexes,and there is no report on the solubility data of FePO4·2H2O at high H3PO4concentration(≥0.1 mol·L−1).Herein,we measured the solubility of FePO4·2H2O in H3PO4solutions with its concentration from 1.13 wt%to 10.7 wt%and temperatures from 298.15 to 363.15 K.All the results are listed in Table 3.
As seen,the solubility of FePO4·2H2O in 1.13 wt%H3PO4is 0.490 g·kg−1at 298.15 K,even more than three hundred thousand times of that in deionized water(1.48×10−6g·kg−1).The jump of solubility with H3PO4addition implies that some complex interactions between Fe3+and phosphate ions become significant to convert Fe3+to soluble coordination species,and boost the dissolving of FePO4·2H2O.In detail,H2PO4−is of the main anionic species in the solution containing 1.13 wt%H3PO4,so the iron in the soluble(FeH2PO4)2−can be much more than that as Fe3+and results in abnormal growth of solubility.When the H3PO4concentration increases from 1.13 wt%to 10.7 wt%,the solubility of FePO4·2H2O increases further,but the trend becomes smooth.Fig.1 shows the phenomena intuitively,in which the variance of the solubility of FePO4·2H2O could reach one to two orders of magnitude.We attempt to calibrate the solubility of FePO4·2H2O(y)with the H3PO4concentration(x)by equationy=A·xb.Table 4 lists the calibration results.At every temperature except for 298.15 K,the correlation coefficients are over 0.95,indicating that the calibration results agree with the measurements well.The exponentbdetermines the sensitivity of the solubility of FePO4·2H2Oon the H3PO4concentration.As the temperature is 308.15 K or higher,balmost keeps in the range from 2.4 to temperature.Fig.2 shows two typical curves reflecting the effect of temperature.The solubility seems to approach a constant value with the increasing of temperature,so we attempt to exploit equationy=y0+A·e−x/tto calibrate the solubility(y)with temperature(x).Table 5 lists the calibration results.The correlation coefficients can reach 0.90 to 0.95.With the increasing of H3PO4concentration,y0almost increases monotonously as expected,andtincreases in general.The increasing oftimplies that the sensitivity on temperature decreases.Considering the effects of temperature and H3PO4concentration comprehensively,high temperature and low H3PO4concentration are appropriate to enlarge the relative variance of solubility with temperature or H3PO4concentration adulation.Otherwise,low temperature and high H3PO4concentration should be selected to shrink the relative variance of solubility.The two groups of calibrating equations,with the H3PO4concentration and temperature as independent variables,respectively,could be used to predict the solubility of FePO4·2H2O in H3PO4solutions with concentration from 1.13 wt%to 10.7 wt%at temperatures from 298.15 K to 363.15 K.
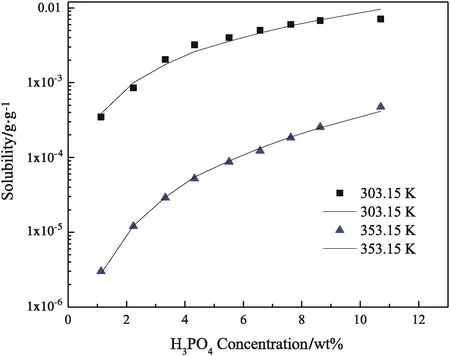
Fig.1.The dependence of solubility of FePO4·2H2O on the concentration of H3PO4 at atmospheric pressure.The dots and lines correspond to the experimental data and calculated results,respectively.
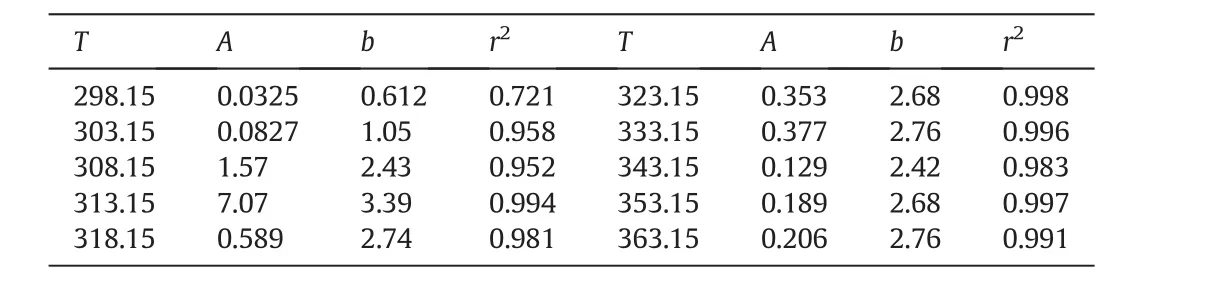
Table 4Parameters for calibrating the solubility of FePO4·2H2O with H3PO4 concentration
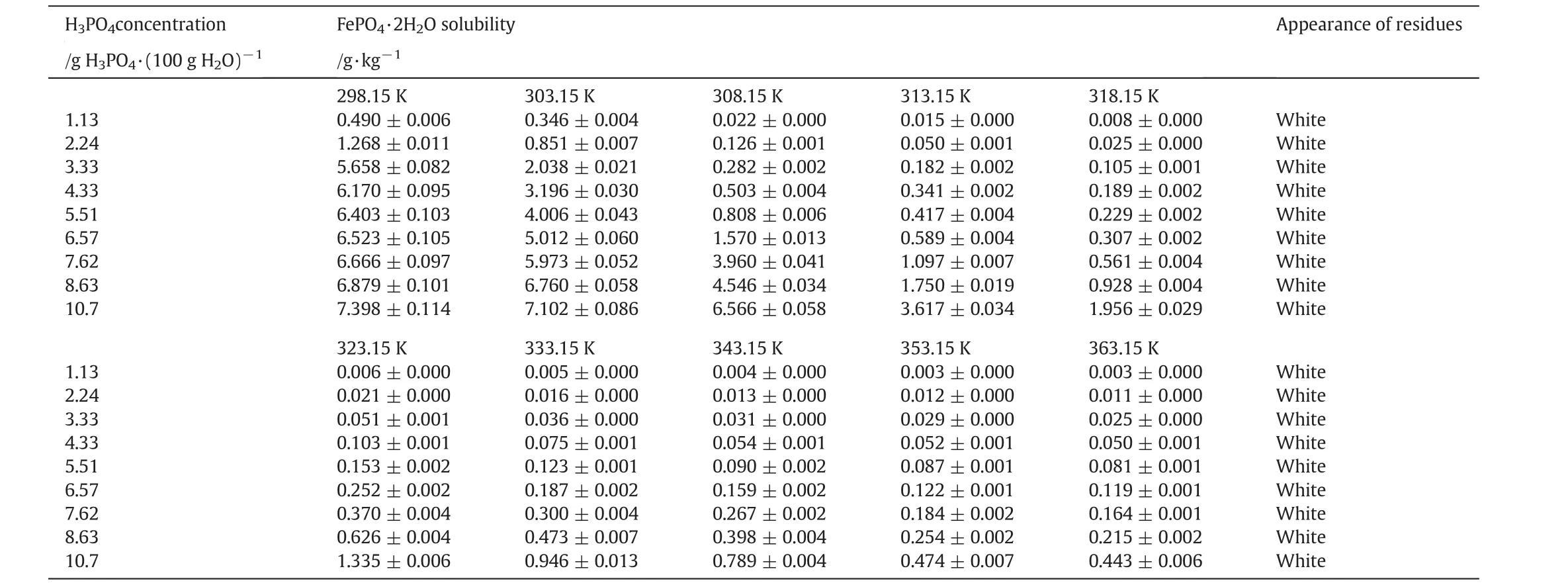
Table 3Solubility of FePO4·2H2O in various concentrations of H3PO4 solutions at 298.15 to 363.15 K and atmospheric pressure①
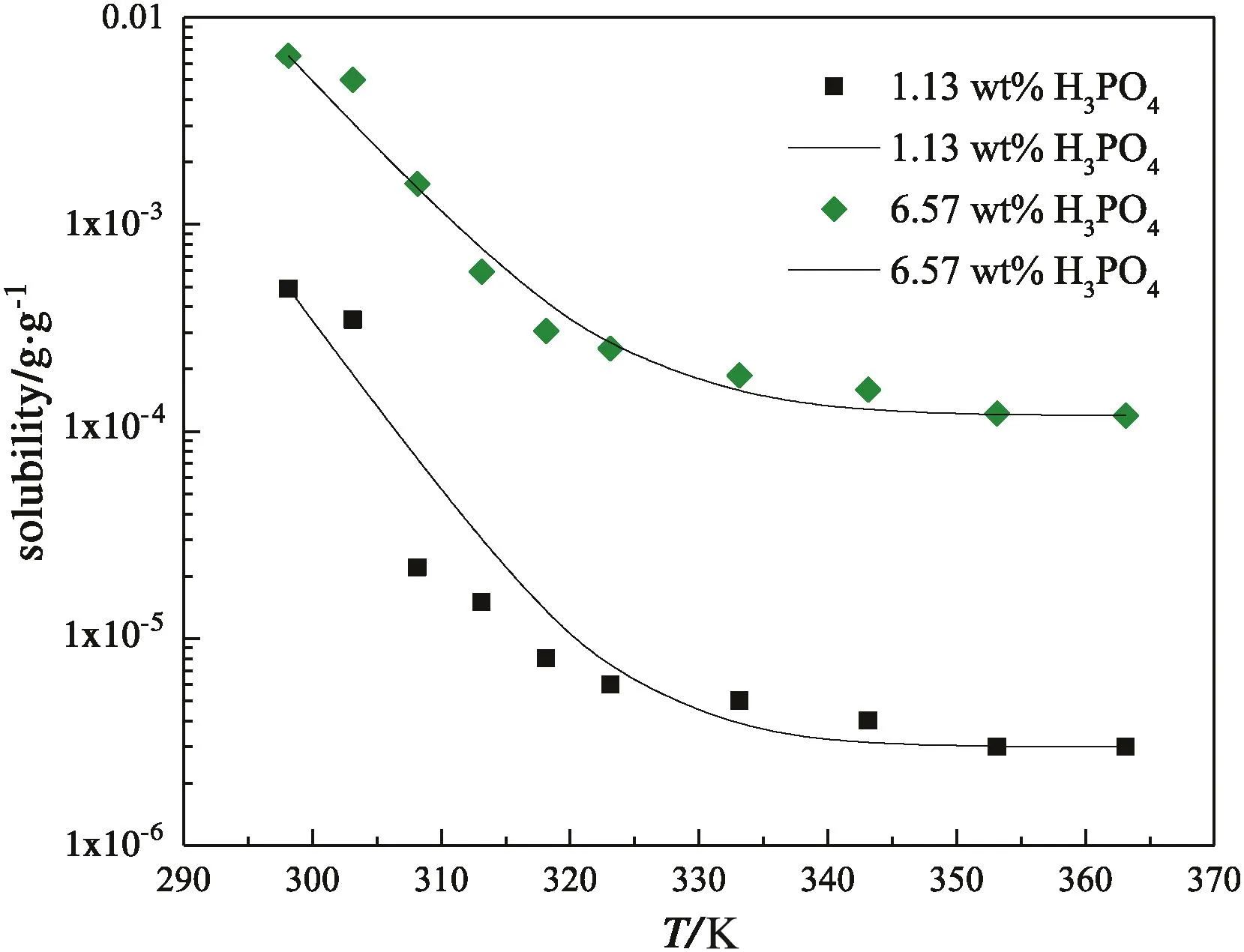
Fig.2.The dependence of solubility of FePO4·2H2O on temperature at atmospheric pressure.The dots and lines correspond to the experimental data and calculated results,respectively.
3.3.The identi fi cation of the solid phase
It should be noticed that the solid residues have different appearances in Tables 1 and 2.In Table 1,the residues obtained in deionized water were light yellow,the same with the original amorphous FePO4·2H2O.In Table 2,all the residues obtained in H3PO4solutions turn to be white without exception.We used X-ray diffraction technique to check whether crystalline phase transition takes place in powder samples,Fig.3 presents XRD patterns for two typical samples.One corresponds to the light yellow powders obtained in water at 353.15 K.The other corresponds to the white powders obtained in 5.51 wt%H3PO4solution at 353.15 K.As seen,there is no diffraction peak detected for the light yellow powders,indicating the amorphous feature of residual FePO4·2H2O,the same with the original.Differently,after long residence in H3PO4solutions,the white powders obtained in 5.51 wt%H3PO4addition at 353.15 K show sharp diffraction peaks in good agreement with orthorhombic FePO4·2H2O standard data of PDF no.33-0667.The results evidence that the FePO4·2H2O in H3PO4solutions experiences the dissolution of amorphous FePO4·2H2O and followed recrystallization of orthorhombic FePO4·2H2O.Compared with the amorphous FePO4·2H2O,the orthorhombic FePO4·2H2O is more stable in thermodynamics in aqueous solution.Since the transformation from amorphous FePO4·2H2O to orthorhombic FePO4·2H2O abides a dissolution–recrystallization mechanism,the solubility of amorphous FePO4·2H2O may determine the rate of the whole process.In water,the extremely low solubility of amorphous FePO4·2H2O leads that the transformation seems to be blocked during 30 days of experiment.Vice versa,the addition of H3PO4makes the transformation process accelerates much and becomes remarkable.
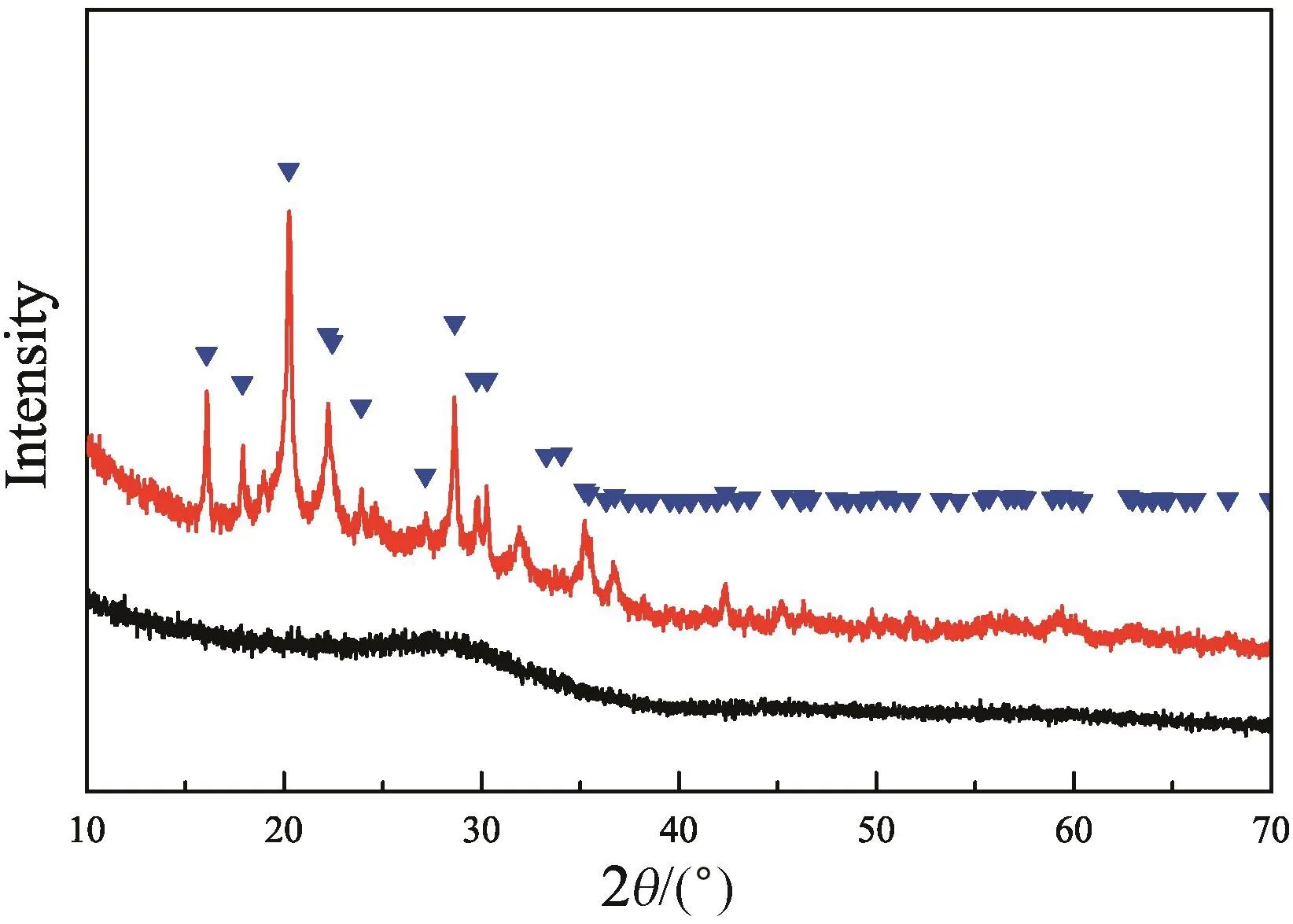
Fig.3.XRD spectra of solid residues in water(bottom)and in 5.51 wt%H3PO4 solution(top)at 353.15 K.
3.4.Understanding iron phosphate materials preparation based on the solubility data
The soluble iron and phosphorus,determined by solubility in thermodynamics,is of iron source and phosphorus source for iron phosphate materials preparation.So,the information on the solubility and dissolving process of FePO4·2H2O can help to understand and design the preparation of various iron phosphate materials.In detail,for amorphous iron phosphate materials,the long residence of as prepared products in H3PO4solution should be avoided to inhibit the crystalline phase transition,and high temperature is suitable to decrease the size of products by decreasing the solubility as well as increasing the supersaturation.For the preparation of iron phosphate crystalline materials by dissolution–recrystallization mechanism,high temperature and high H3PO4concentration are appropriate conditions,since high H3PO4concentration can improve the dissolution of precursors and high temperature can decrease the solubility of final products and increase the yield.When using FePO4·2H2O as the controlled iron or phosphoroussources,high H3PO4concentration and low temperature could accelerate the providing of iron or phosphorous,while low H3PO4concentration and high temperature have the opposite effect.
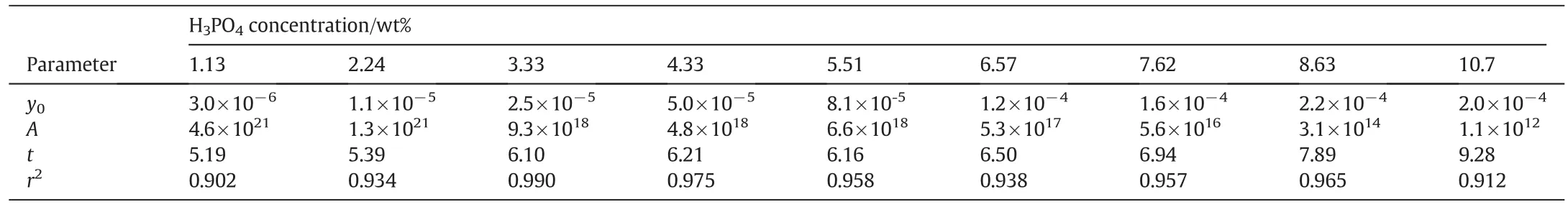
Table 5Parameters for calibrating the solubility of FePO4·2H2O with temperature
4.Conclusions
The solubilities of iron phosphate dihydrate in deionized water and phosphoric acid solutions at high mass concentrations from 1.13%to 10.7%were systematically measured at temperature from 298.15 to 363.15 K and atmosphere pressure.The solubility was found to increase 5 orders of magnitude or more with increasing the concentration of phosphoric acid,and decrease 1 to 2 orders of magnitude with increasing the equilibrium temperature.The phosphoric acid addition and temperature were found to affect the solubility of iron phosphate dihydrate by the formation or dissociation of coordination species,which could further accelerate the phase transformation from the amorphous iron phosphate dihydrate to orthorhombic iron phosphate dehydrate by dissolution–recrystallization mechanism.The high dependences of the solubility of iron phosphate materials on H3PO4concentration and temperature could be well predicted by calibration equations and effectively exploited to regulate the precipitation,crystallization and growth processes between iron(III)and phosphate ions in aqueous solutions for synthesizing iron phosphate materials with diversified crystalline structure and morphology.
[1]Y.J.Lee,H.Yi,W.Kim,K.Kang,D.S.Yun,M.S.Strano,G.Ceder,A.M.Belcher,Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes,Science324(2009)1051–1055.
[2]C.X.Guo,Y.Q.Shen,Z.L.Dong,X.D.Chen,W.X.Lou,C.M.Li,DNA-directed growth of FePO4nanostructures on carbon nanotubes to achieve nearly 100%theoretical capacity for lithium-ion batteries,Energy Environ.Sci.5(2012)6919–6922.
[3]T.B.Zhang,Y.C.Lu,G.S.Luo,Synthesis of single-crystal dendrictic iron hydroxyl phosphste as s Fenton catalyst,CrystEngComm15(2013)9104–9111.
[4]Y.Wang,X.Wang,Z.Su,Q.Guo,Q.Tang,Q.Zhang,H.Wan,SBA-15-supported iron phosphate catalyst for partial oxidation of methane to formaldehyde,Catal.Today93–95(2004)155–161.
[5]D.Yu,J.Qian,N.Xue,D.Zhang,C.Wang,X.Guo,W.Ding,Y.Chen,Mesoporous nanotubes of iron phosphate:Synthesis,characterization,and catalytic property,Langmuir23(2007)382–386.
[6]T.B.Zhang,Y.C.Lu,G.S.Luo,Iron phosphate prepared by coupling precipitation and aging:Morphology,crystal structure,and Cr(III)adsorption,Cryst.Growth Des.13(2013)1099–1109.
[7]X.X.Zhang,S.S.Tang,M.L.Chen,J.H.Wang,Iron phosphate as a novel sorbent for selective adsorption of chromium(III)and chromium speciation with detection by ETAAS,J.Anal.At.Spectrom.27(2012)466–472.
[8]S.Benmokhtar,H.Belmal,A.E.Jazouli,J.P.Chaminade,P.Gravereau,S.Pechev,J.C.Grenier,G.Villeneuve,D.Waal,Synthesis,structure,and physicochemical investigations of the new α Cu0.50TiO(PO4)oxyphophate,J.Solid State Chem.180(2007)772–779.
[9]Y.M.Lai,X.F.Liang,S.Y.Yang,J.X.Wang,L.H.Cao,B.Dai,Raman and FTIR spectra of iron phosphate glasses containing cerium,J.Mol.Struct.992(2011)84–88.
[10]P.Moreau,D.Guyomard,J.Gaubicher,F.Boucher,Structure and stability of sodium intercalated phases in olivine FePO4,Chem.Mater.22(2010)4126–4128.
[11]Y.Yin,P.Wu,H.Zhang,C.Cai,Enhanced cathode performance of amorphous FePO4hollow nanospheres with tunable shell thickness in lithium ion batteries,Electrochem.Commun.18(2012)1–3.
[12]Y.Song,P.Y.Zavalij,M.Suzuki,M.S.Whittingham,New iron(III)phosphate phases:Crystal structure and electrochemical and magnetic properties,Inorg.Chem.41(2002)5778–5786.
[13]Y.Fang,L.Xiao,J.Qian,X.Ai,H.Yang,Y.Cao,Mesoporous amorphous FePO4nanospheres as high-performance cathode material for sodium-ion batteries,Nano Lett.14(2014)3539–3543.
[14]M.Moradi,Z.Li,J.Qi,W.Xing,K.Xiang,Y.M.Chiang,A.M.Belcher,Improving the capacity of sodium ion battery using a vireo-templated nanostructured composite cathode,Nano Lett.15(2015)2917–2921.
[15]V.Mathew,S.Kim,J.Kang,J.Gim,J.Song,J.P.Baboo,W.Park,D.Ahn,J.Han,L.Gu,Y.Wang,Y.S.Hu,Y.K.Sun,J.Kim,Amorphous iron phosphate:Potential host for various charge carrier ions,NPG Asia Mater.6(2014),e138.
[16]P.Nagaraju,C.Srilakshmi,N.Pasha,N.Lingaiah,I.Suryanarayana,P.S.Sai Prasad,Effect of P/Fe ratio on the structure and ammoxidation functionality of Fe–P–O catalysts,Appl.Catal.A Gen.334(2008)10–19.
[17]Y.C.Lu,T.B.Zhang,Y.Liu,G.S.Luo,Preparation of FePO4nano-particles by coupling fast precipitation in membrane dispersion microreactor and hydrothermal treatment,Chem.Eng.J.210(2012)18–25.
[18]S.Scaccia,M.Carewska,P.P.Prosini,Thermoanalytical study of iron(III)phosphate obtained by homogeneous precipitation from different media,Thermochim.Acta413(2004)81–86.
[19]E.Pierri,E.Dalas,The precipitation of ferric phosphate on porous polymer,Colloids Surf.A Physicochem.Eng.Asp.139(1998)335–340.
[20]E.Pierri,D.Tsamouras,E.Dalas,Ferric phosphate precipitation in aqueous media,J.Cryst.Growth213(2000)93–98.
[21]M.L.Escoda,F.Torre,V.Salvadó,The formation of mixed ligand complexes of Fe(III)with phosphoric and citric acids in 0.5 M NaNO3aqueous solutions,Polyhedron18(1999)3269–3274.
[22]T.Roncal-Herrero,J.D.Rodríguez-Blanco,L.G.Benning,E.H.Oelkers,Precipitation of iron and aluminum phosphates directly from aqueous solution as a function of temperature from 50 to 200°C,Cryst.Growth Des.9(2009)5197-5205.
[23]K.Kandori,T.Kuwae,T.Ishikawa,Control on size and adsorptive properties of spherical ferric phosphate particles,J.Colloid Interface Sci.300(2006)225–331.
[24]T.Zhang,Y.Lu,G.Luo,Size adjustment of iron phosphate nanoparticles by using mixed acids,Ind.Eng.Chem.Res.52(2013)6962–6968.
[25]S.C.Chang,M.L.Jackson,Solubility product of iron phosphate,Soil Sci.Soc.Am.Proc.21(1957)265–268.
[26]E.P.Egan,Z.T.Wake field,B.B.Luff,Low temperature heatcapacity and heatofformation of crystalline and colloidal ferric phosphate dihydrate,65(1961)1265–1270.
[27]J.O.Nriagu,Solubility equilibrium constant of strengite,Am.J.Sci.272(1972)476–484.
[28]R.J.Xie,M.J.Gomez,Y.J.Xing,P.S.Klose,Fouling assessment in a municipal water reclamation reverse osmosis system as related to concentration factor,J.Environ.Eng.Sci.3(2004)61–72.
[29]T.Zhang,Y.Lu,D.Xin,G.Luo,Direct precipitation for a continuous synthesis of nanoiron phosphate with high purity,Ind.Eng.Chem.Res.53(2014)6723–6729.
[30]A.Stefansson,T.M.Seward,A spectrophotometric study of iron(III)hydrolysis in aqueous solution to 200 °C,Chem.Geol.249(2008)630–645.
[31]R.E.Mesmer,C.F.Bases,Phosphoric acid dissociation equilibria in aqueous solutions to 300 °C,J.Solut.Chem.3(1974)307–322.
[32]H.Galal-Gorchev,W.Stumm,The reaction of ferric iron with ortho-phosphate,J.Inorg.Nucl.Chem.25(1963)567–574.
[33]M.Iuliano,L.Ciavatta,G.D.Tommaso,On the solubility constant of strengite,Soil Chem.71(2007)1137–1140.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Structural insights of mechanically induced aluminum-doped hydroxyapatite nanoparticles by Rietveld refinement☆
- Structural evolution of chars from biomass components pyrolysis in a xenon lamp radiation reactor☆
- TiO2–SA–Arg nanoparticles stabilized Pickering emulsion for photocatalytic degradation of nitrobenzene in a rotating annular reactor
- Assessing the kinetic model of hydro-distillation and chemical composition of Aquilaria malaccensis leaves essential oil
- A novel model for multi-plant mixed heavy crude oils refinery planning
- A self-tuning control method for Wiener nonlinear systems and its application to process control problems☆
