Assessing the kinetic model of hydro-distillation and chemical composition of Aquilaria malaccensis leaves essential oil
2017-05-28MahtabSamadiZurinaZainalAbidinRobiahYunusDayangRadiahAwangBiakHiroyukiYoshidaEngHaiLok
Mahtab Samadi,Zurina Zainal Abidin ,2,*,Robiah Yunus ,Dayang Radiah Awang Biak ,Hiroyuki Yoshida ,Eng Hai Lok
1 Department of Chemical and Environmental Engineering,Faculty of Engineering,University Putra Malaysia,Serdang,Selangor,Darul Ehsan 43400,Malaysia
2 Aerospace Manufacturing Research Centre(AMRC),Level 7,Tower Block,Faculty of Engineering,University Putra Malaysia,43400 Serdang,Selangor,Malaysia
3 Forest Research Institute Malaysia,52109 Kepong,Selangor,Darul Ehsan,Malaysia
1.Introduction
In the recent years,the market for natural and organic products has expanded significantly.Tremendous numbers of today's natural products used for anti-parasitical,bactericidal,fungicidal,cosmetic,and medicinal purposes,contain essential oils extracted from aromatic plants[1].Essential oil is an intricate combination of volatile compounds,which typically exist in low amount within the plant[2–4].
Aquilaria malaccensis,also known as Agarwood or Gaharu,is one of the 23 species of Indo-Malaysian genusAquilaria,familyThymeleceaeand classMagnoliopsida.As a result of over-harvestingA.malaccensistrees,this specie is now categorized as a “vulnerable”plant in IUCN Red List and it has been included in the World List of Threatened Trees(WLTT;[5–7]).A.malaccensisis a tall,ever-green tree that can be found in Bangladesh,Bhutan,India,Indonesia,Malaysia,Myanmar,Philippines,Singapore,and Thailand[5,6].Because of its high usage(e.g.in medicine,perfume,and wood chips),A.malaccensisis regarded as a valuable and important tree in the southeast Asian countries,and it is commonly used as a traditional medicine to relieve pain,fever,rheumatism,arrest vomiting,and asthma[6].The resin,which is extracted fromA.malaccensistrunk through fungal infection,is the main reason for the high value of this tree[6].Thereby,it is not surprising that almost all theA.malaccensis-related studies have focused on the bark of the tree.This valuable tree has lots of green leaves during the whole year that,similar to the bark,it may have abundant benefits.In addition,the probability of finding rich amount of metabolites,aromatic substances,and microorganisms in different parts of higher plants is significantly higher compare to lower plants[8–10].These materials can be used as medicinal compounds for treatment of a variety of illnesses[8–10].For instance,Wilet al.[11]and Hudaet al.[12]noted that solvent extraction ofA.malaccensisleaves shows significant antioxidant activity,and therefore,it can be considered as a natural antioxidant,which can be used for treating cancer.Additionally,by characterizing methanolic extract ofA.malaccensisleaves,Khalilet al.[13]reported the presence of phytochemicals,which can be used for medicinal and anti-corrosive purposes.In spite of the fact that these studies have assessed the phytochemical properties ofA.malaccensisleaves,they only focused on the non-volatile extracts.Whereas,A.malaccensisleaves oil(i.e.volatile compounds)may also possess important and useful properties.So,given the importance ofA.malaccensisand the fact that no study has been conducted neither on analysis of the essential oil ofA.malaccensisleaves nor on the optimization or kinetic modeling of the extraction,this study attempts to fill this gap in two ways.First,by analyzing the essential oil ofA.malaccensisleaves,this study attempts to identify its chemical compositions in order to provide a foundation for further investigations.Secondly,by kinetic-modeling of the extraction of the essential oil,this study tries to get fuller understanding of the mechanism of hydro-distillation process,and consequently,to identify the optimum condition,in which higher yield of essential oil is obtained.
2.Materials and Methods
2.1.Sample preparation
Leaves ofA.malaccensiswere collected from Forest Research Institute Malaysia(FRIM)garden in September of 2013.Samples were air-dried in a dark room without sunlight,and then they were stored in a cool room(4°C)for subsequent experiments.
2.2.Extraction of essential oil
Air-dried leaves ofA.malaccensiswere grinded right before the extraction process.In order to protect the plant materials from overheating or charring by direct steam,grinded leaves were immersed in distilled water in a round bottom flask on a heater[14,15].Essential oil of the leaves was extracted by hydro-distillation Clevenger apparatus method(i.e.recommended by pharmacopeia;Fig.1)at the boiling range of water and atmospheric pressure[15,16].The extraction process was optimized with respect to time,heating power(250,300,350 W)and solid(g)to solvent(ml)ratio(1:3,1:10,1:12).
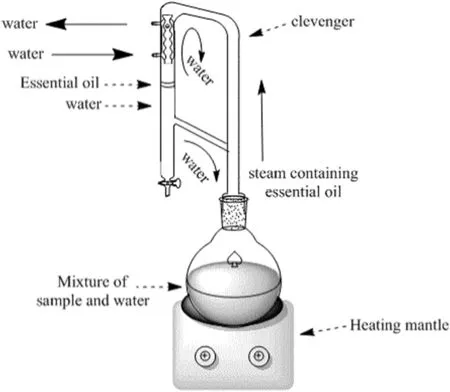
Fig.1.Hydro-distillation Clevenger apparatus system.
Extracted essential oil was dried over anhydrous sodium sulfate to remove all the water and then stored it in dark-sealed-vial at 4°C for further tests.The yield of essential oil was calculated by the following equation:

whereyis the yield of essential oil(%),Vis the volume of collected essential oil(ml)andWis the weight of the plant material(g).
2.3.Gas Chromatography Mass Spectroscopy(GC/MS)
The extracted essential oil was analyzed using Shimadzu autoinjector GC/MS equipped with a FID detector and a BP5 capillary column.20 μ l of essential oil was diluted in 500 μl ofn-hexane as solvent and injected with helium as the carrier gas for identification.The running time was set for 90 min and the temperature and pressure increased during the test from 50 to 250°C and 37.1 to 100 kPa respectively.
2.4.Kinetic model
Modeling of the extraction process is used for evaluation of the variable-conditions affecting the extraction[17].It is also considered as a fundamental step for devising an efficient process.In this study,two of the most widely-used models for hydro-distillation extraction from plant particles(i.e.first-order kinetic model and non-stationary model;[18])were compared to one another in order to find the best kinetic model for describing the extraction ofA.malaccensis'leaves oil.
2.4.1.Non-stationary diffusion model
The mass transport of essential oil through plant particles during the hydro-distillation process occurs as unsteady-state diffusion.So,Milojevicet al.[15]contended that a batch hydro-distillation with no chemical reaction can be described by model of non-stationary diffusion model,which is based on the unsteady-state essential oil diffusion through plant material(i.e.modification of Fick's second law of one dimensional unsteady state diffusion;Eq.(2)).This model comprises two successive stages:washing and diffusion.

whereqis the yield ofA.malaccensisleaves essential oil at timet,q0is the initial average concentration of essential oil in theA.malaccensisleaves,and e is a constant.In this model,b,characterizes the fast oil distillation(washing)stage and,k,characterizes the slow oil distillation(diffusion)stage[15,19–21].
The assumptions for this model are as follows:
(a)The plant particles are isotropic and equal in shape,size,and initial oil content.The shape and size of plant particles do not change during the hydro-distillation.
(b)The essential oil is considered as a pseudo-component.
(c)A fraction of the essential oil is located at the external surfaces of the broken leaves ofA.malaccensisand the rest is uniformly distributed in the plant particles.
(d)The effective diffusion coefficient is constant.
(e)The concentration of essential oil on the external surfaces of the plant particles at any moment during the hydro-distillation is zero because of its instantaneous“washing”from the surfaces.
(f)There is no resistance to the mass transport of essential oil from the external surfaces of the plant particles.
2.4.2.Model of pseudo-first order kinetics
By taking into account that first order kinetic desorption model doesn't encompass the washing stage,it is appropriate for describing the processes,which are controlled by the intra-particle diffusion[18,22].
So,the assumptions for this model were as follows:
(a)The analyte is uniformly distributed within the matrix.
(b)The concentration of compound at the matrix surfaces is zero,as soon as extraction begins(corresponding to no solubility limitation).
(c)The plant particles are isotropic and uniform in size and shape.
Thus,the model for spherical matrix of uniform size is Eq.(3).

whereqis the mass of analyte extracted after timet(mg·g−1),q0is the initial total mass of the analyte in the matrix(mg·g−1)andkis the first order rate constant describing the extraction efficiency(min−1).
3.Results and Discussion
For the first time,essential oil ofA.malaccensisleaves was extracted by hydro-distillation Clevenger apparatus,which is a common conventional method for extraction of essential oil from plant particles.Since the extraction procedure significantly varies in different power,solid/liquid ratio,and time,thereby,these parameters were studied in order to determine the effect of each factor and consequently find the optimum condition,in which highest yield of essential oil is extracted.Also,two common kinetic models were assessed to find the best fitting model for explaining the hydro-distillation extraction process ofA.malaccensisleaves essential oil.Moreover,the chemical components of the essential oil were identified using Gas Chromatography Mass Spectroscopy(GC–MS).
3.1.Effect of operational condition
3.1.1.Power
Among the parameters affecting hydro-distillation process,power was found to be one of the key influential factors.The minimum power for the experiment was the lowest power,at which water could reach the boiling point(i.e.250 W).On the other hand,the highest power for the experiment was the maximum power that didn't have any adverse effects on the yield and quality of the extracted oil(i.e.350 W).So,the yield variation ofA.malaccensisleaves essential oil during 4 h of extraction was observed at different powers(250,300,and 350 W),while the sample to solvent ratio was constant at 1:10 g·ml−1(recommended by European Pharmacopeia[23]).
The result indicated that at higher powers the rate of extraction was faster compared to lower powers,although the total amount ofA.malaccensisleaves oil remained nearly constant(the lowest yield was achieved at the minimum power and the highest yield was achieved at medium power;Fig.2).It is likely that at the minimum power,the heat transfer between the surface and the core of the flask containing the sample and water was slower in comparison to other powers.The slower heat-transfer,on the other hand,might have influenced the vapor formation process(i.e.process essential for diffusion of oil from the cells)resulting in incomplete extraction and lower yield.The result also provided evidence that the yield of extraction in 350 W was almost similar to that in 300 W at 3 h.However,after 3 h the yield slightly decreased in the extraction process at350 W.The decrease of oil in 350 Wafter 3 h may be accounted for by the charring or decomposition of the sample as the result of rapid and fast heating for a long period of time.
In short,the extraction process was more efficient at higher powers(i.e.300 or 350 W)as more energy was transferred to suspension ofA.malaccensisleaves.As the result,the oil was better extracted from the plant particles.
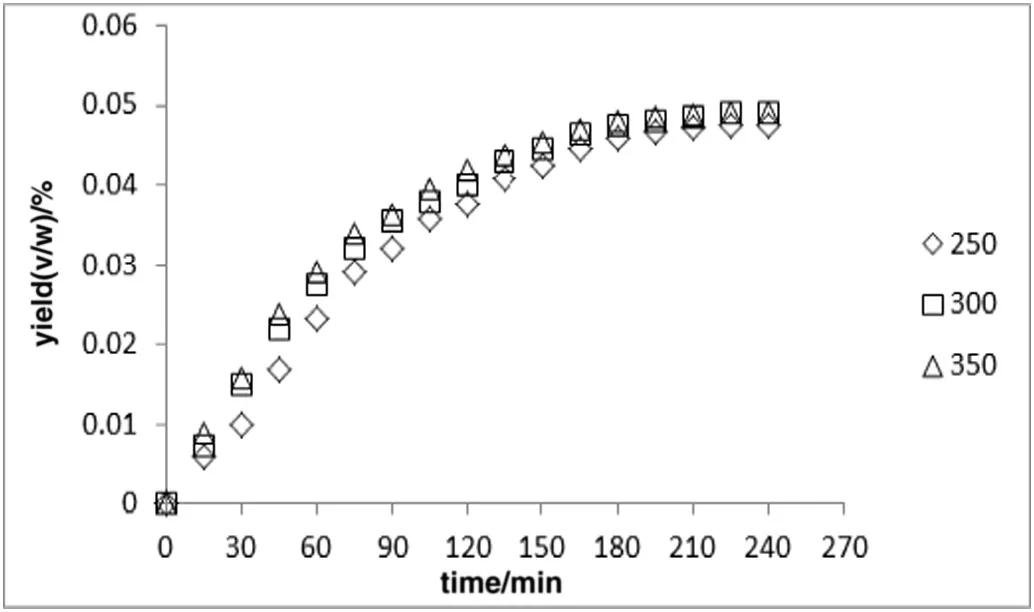
Fig.2.The amount of extracted essential oil from Aquilaria malaccensis'leaves at different heating powers(constant solid to solvent ratio of 1:10).
3.1.2.Solid/liquid ratio
Another key parameter that affects hydro-distillation is solid/liquid ratio,which is the amount of sample(g)per amount of water as solvent(ml).Given the fact that according to European Pharmacopeia the best ratio of sample to solvent is usually 1:10[23],the range of sample to solvent ratio for the experiment was determined in a way that it includes the above-mentioned ratio.On the other hand,lower and upper limits of sample to solventratio for the experiment(i.e.1:7 and 1:12)were selected so that the solvent covers the sample to protect it from burning but does not over flow the flask.Since the results of the current study provided evidence that 300 W was the optimum heating power,therefore,the effect of sample-to-solvent ratio was studied at constant heating power of 300 W for 4 h.
The result indicated that,as expected,lower and upper range-limits of sample to solventratio(i.e.1:7 and 1:12)resulted in lower yield of essential oil in comparison to the middle sample to solvent ratio(i.e.1:10;Fig.3).The lower yield of essential oil in lower sample to water ratio(1:7)may be explained by the fact that there was not enough water to protect the sample from charring and overheating.On the other hand,in higher range limit(i.e.1:12)the lower yield may be accounted for by the fact that the heat was wasted on heating water rather than the sample itself.Additionally,the lower yield may also be explained by the“hydrolytic effect”[22].
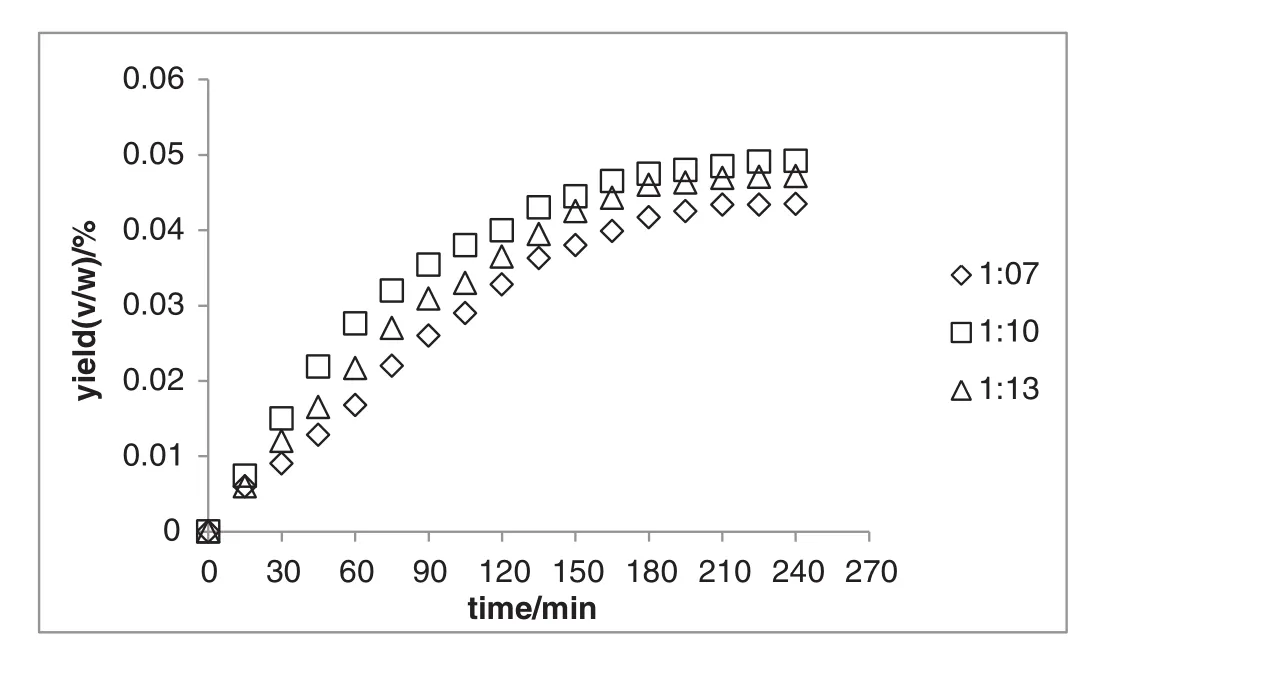
Fig.3.The amount of extracted essential oil from Aquilaria malaccensis'leaves at different sample to solvent ratios(constant heating power of 300 W).
Findings suggest that the amount of water should be sufficient for covering the entire plant sample in order to protect it from degradation caused by high temperature.According to the result,the best solid/liquid ratio was 1:10,because water covers the sample completely and bonds with compounds of the sample while protecting it from degradation.
3.1.3.Time
Another important parameter in the hydro-distillation process is time.Obviously,the time of the extraction process should be long enough to extract all the existing essential oil from the plant sample.The required extraction time varies depending on the type of plants and extraction equipment.In order to find the time suitable for extraction ofA.malaccensisleaves oil,the yield of essential oil during the extraction time was screened and measured from the start to the end of extraction,when no increase in amount of essential oil was observed(Figs.2,3).The yield of essential oil increased from nothing(at the beginning),to around 5%(after about 3 and half hours).Since the amount of essential oil did not increase after 4 h,extraction process was considered to be done after the 4th hour.Therefore,as it was depicted in Fig.2,the highest amount of essential oil fromA.malaccensisleaves was extracted after around 3 and half hours,when the figure reaches the steady state stage.Most of the essential oil was extracted in 2 1/2 h,although for the complete extraction at least 3 h was needed.As mentioned earlier,once the power(i.e.extraction rate)was increased during the extraction,the total extraction time reduced slightly.The result of experiment showed that at the power of 300 and 350 W,the highest yield was obtained after 3 h.The maximum yield at 250 W was acquired after 4 h,although the yield was slightly lower compared to that at 300 and 350 W.
3.2.Kinetic model
As mentioned earlier,so far,there have been no studies on the kinetic modeling or optimization of the essential oil extraction fromA.malaccensisleaves by hydro-distillation.Therefore,this study tried to assess the kinetic extraction of essential oil fromA.malaccensisleaves using the model of simultaneous washing and diffusion,and first order kinetic model.
The yield of essential oil during the extraction period was observed at different powers(i.e.250,300,and 350 W;Fig.2)and solid(g)to solvent(ml)ratio(i.e.1:7,1:10,and 1:12;Fig.3).As depicted in Figs.2 and 3,the yield of essential oil increased with time.The linearized form of Eqs.(2)and(3)versustime was used in order to verify the proposed kinetic models and mechanism of oil isolation(Figs.4–7).The parameters of the kinetic models were calculated using the linear regression method(Tables 1 and 2).
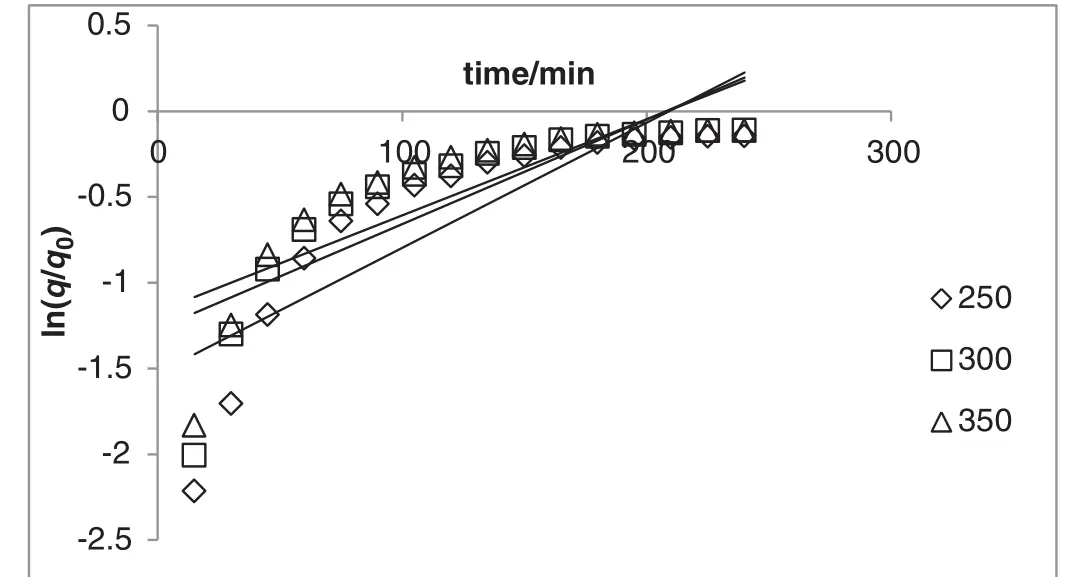
Fig.4.One site kinetic model of extraction A.malaccensis'leaves oil by hydro-distillation at various heating powers(constant solid to solvent ratio of 1:10).
The result indicated that in comparison with first-order kinetic model(Figs.4 and 5),the model of simultaneous washing and diffusion or non-stationary diffusion model(Figs.6 and 7)better described the extraction process as it was strongly correlated with the experiment results(the higherR2and lower amount of error functions,Tables 1 and 2).
Since the model of simultaneous washing and diffusion showed better fit with the result of the experiment,two conclusions can be made.First,the kinetics for the extracted oil fromA.malaccensisleaves was described using a two-parameter model of unsteady-state diffusion through the plant particles.Second,the kinetic model for extracting the essential oil by hydro-distillation consisted of two stages of washing and diffusion(i.e.dominant stage).
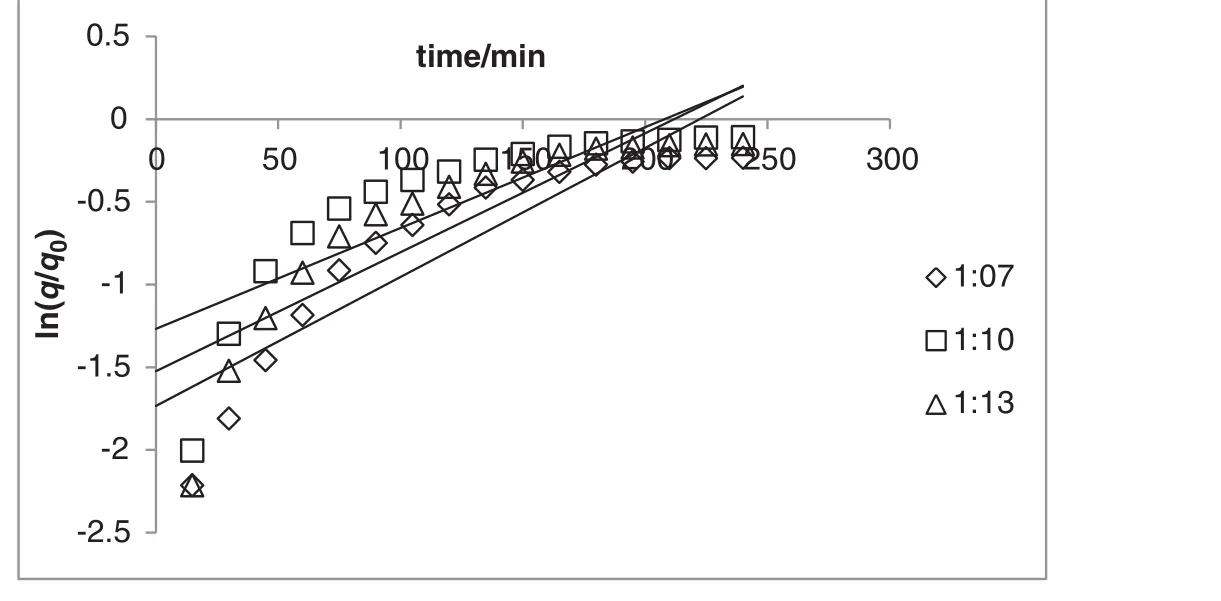
Fig.5.One site kinetic model of extraction A.malaccensis'leaves oil by hydro-distillation at various sample to solvent ratios(constant heating power of 300 W).
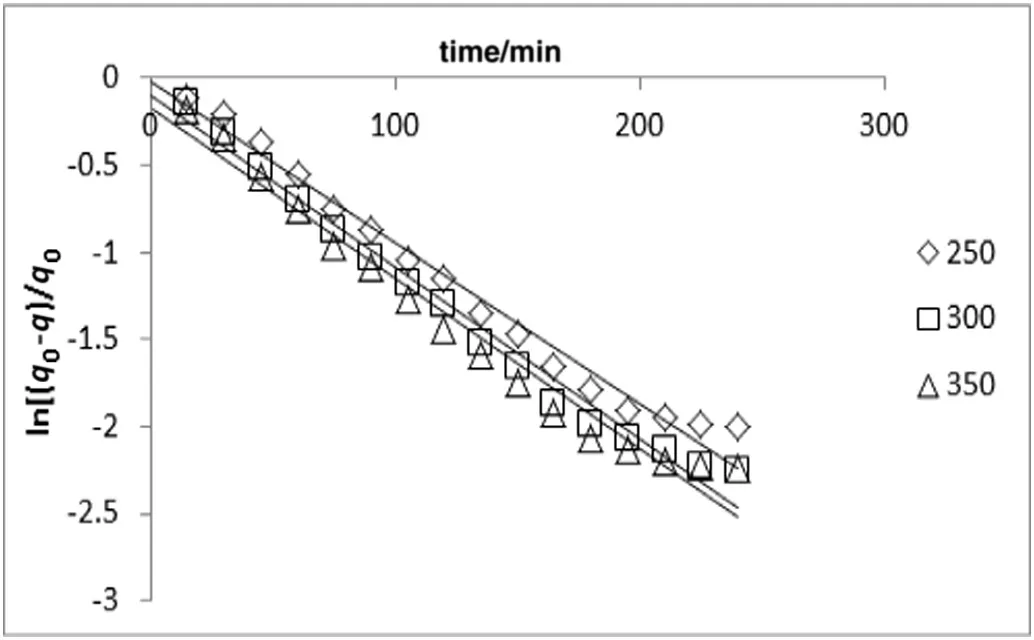
Fig.6.Simultaneous washing and diffusion kinetic model of extraction A.malaccensis'leaves oil by hydro-distillation at various heating powers(constant sample to solvent ratio of 1:10).
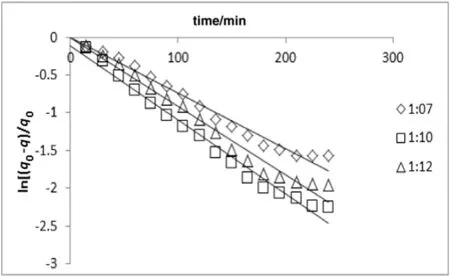
Fig.7.Simultaneous washing and diffusion kinetic model of extraction A.malaccensis'leaves oil by hydro-distillation at various sample to solvent ratios(constant heating power of 300 W).
Washing(i.e.fast-oil distillation process)refers to the stage,during which the essential oil is washed from external and near the external surface of the plant.The washing stage is characterized by a rapid increase in the yield of essential oil at the very beginning of the extraction process[15].Diffusion refers to slow oil distillation.During the diffusion stage,essential oil is diffused from the interior parts of the plant particles towards external surfaces and then it is followed by its distillation.The diffusion stage is characterized by a slow exponential increase in the yield of essential oil during distillation[15].As it can be observed from Figs.2 and 3,at first,the yield ofA.malaccensisleaves oil increased rapidly(i.e.fast oil distillation stage)and then,the rate of oil distillation slowed down until a nearly constant oil yield was reached(i.e.slow-oil distillation stage).
As it can be seen in Tables 1 and 2,with the increase of distillation power(from 250 to 350 W)and solid to solvent ratio(from 1:7 to 1:10),both mass transfers kinetic coefficients(i.e.kandb)of nonstationary model increased.The result provided evidence that at higher distillation power(i.e.300 and 350)and higher solid to solvent ratio(i.e.1:10)the oil was washed and diffused from theA.malaccensisleaves faster and easier.The higher level of oil extraction at that condition may be accounted for the increase of diffusivities and driving forces of solute(oil)and solvent.
Since the slow and fast distillation coefficients(bandk)were affected by both power and solid-to-solvent ratio,it suggests that the non-stationary model explains the extraction process.
The relationships between kinetic parameters and the hydrodistillation rate are as follows(x:power/extraction rate):
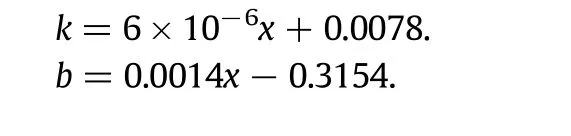
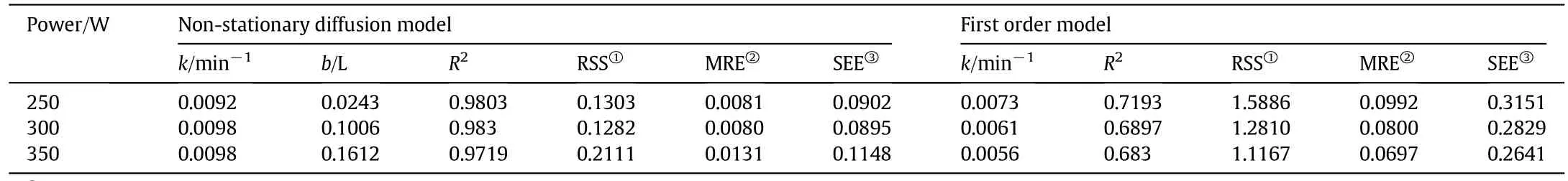
Table 1Values of the kinetic parameters for different powers
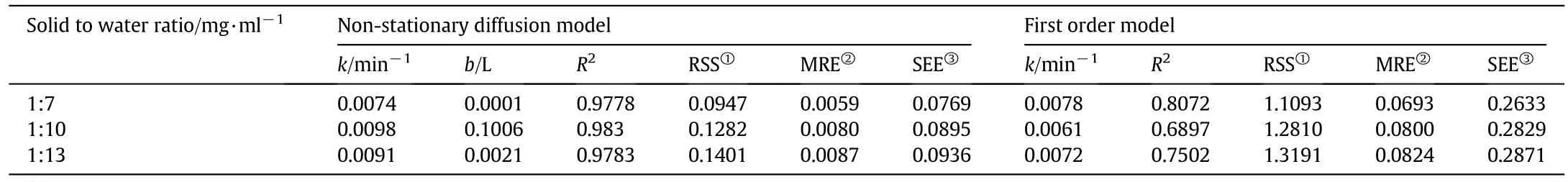
Table 2Values of the kinetic parameters for different solid to solvent ratios
As it can be deduced from the above-mentioned equations,the level of power had much more influence on fast oil distillation coefficient compared to the slow oil distillation coefficient.Thereby,it can be concluded that the level of energy input has more impact on oil washing process rather than to the oil diffusion distillation.
3.3.Gas Chromatography Mass Spectroscopy(GC–MS)
The essential oil ofA.malaccensis'leaves(yellow color and strong smell)was extracted in optimum condition(i.e.power 300 W,solid per liquid ratio 1:10,and time 210 min)with the yield of 5%and its chemical compositions were characterized by GC/MS(Fig.8).
Gas Chromatography Mass Spectroscopy(GC/MS)identified 42 chemical components(Table 3),constitutes 93%of essential oil,by using their retention indices relative ton-alkane(i.e.standard)and mass-spectra comparison with Wiley and NIST library,and gas chromatography/mass spectroscopy reference book(i.e.identification of essential oil components by gas chromatography/mass spectroscopy,[24]).The identified major compounds were Pentadecanal(32.082%),9-Octadecenal,(Z)-(15.894%),and Tetradecanal(6.927%;Fig.9).
Fig.8.GC/MS of the essential oil of A.malaccensis'leaves by hydro-distillation.
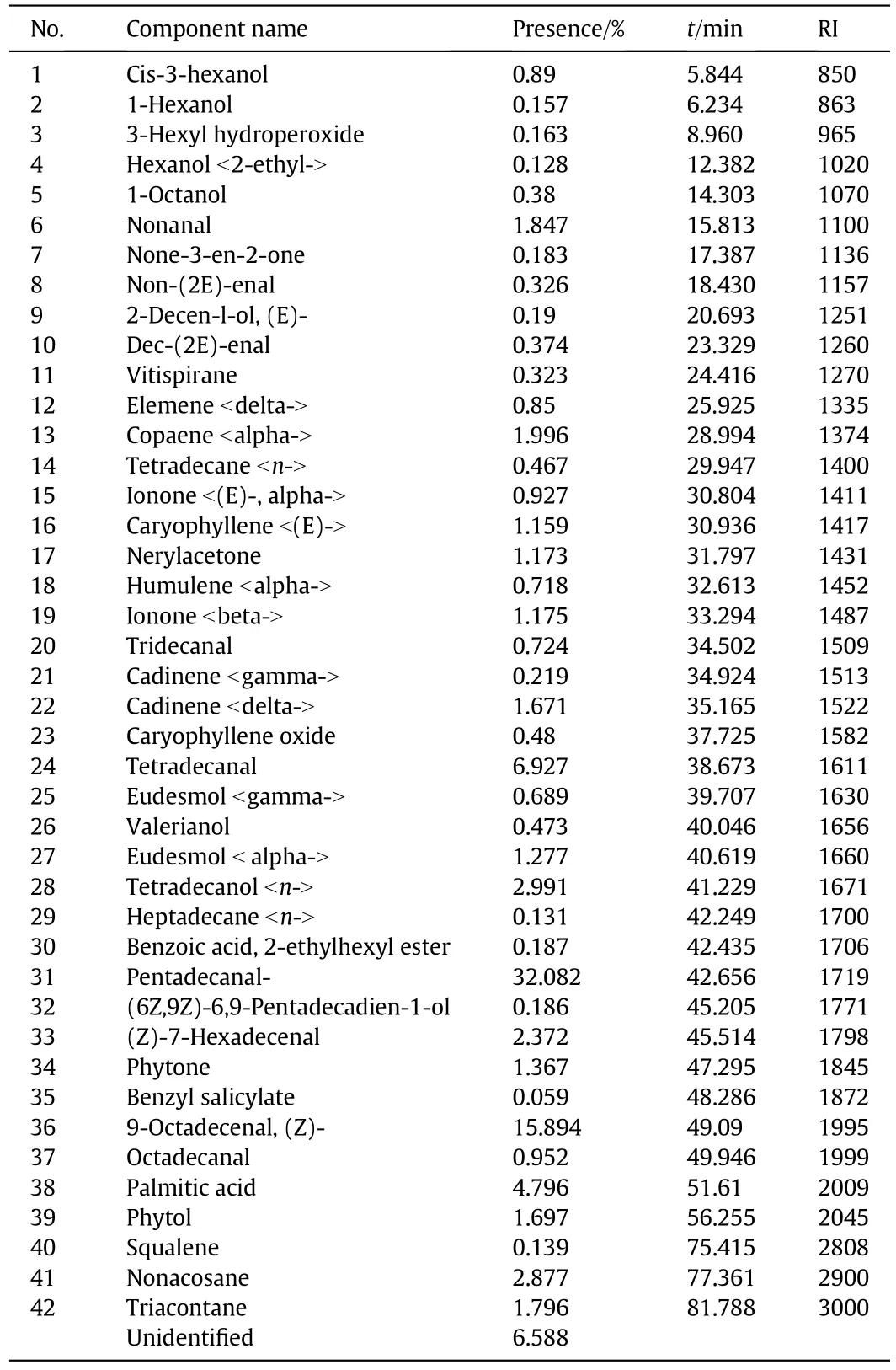
Table 3Chemical compositions of essential oil of Aquilaria malaccensis'leaves
Pentadecanal(i.e.major compound)is a long chain fatty aldehyde,and it acts as ovipositional deterrent[25].According to Pathak&Khan[26],Pentadecanal inhibits oviposition and disturbs the insect's growth and development.It can also adversely affect eggs,larval,and pupal stages.In addition,it is identified as an effective attractant for female mosquitoes[27].
The other major compound,9-Octadecenal(also known as Olealdehyde),is a fatty aldehyde as well.Strong body of research has identified 9-Octadecenal,(Z)as an effective and important attractant compound,which can be used as a trap for male moth[28,29].Moreover,it can be used in control of human pathogens,pests,termites and maggots[30,31].
Tetradecanal,which can be categorized as a bioluminescent aldehyde[32–34],was the third major component of the essential oil.Ruanoet al.[35]noted that Tetradecanal(i.e.a defensive compound used by ant queens)can be used as a good general ant repellent.
In short,GC/MS indicated that the essential oil ofA.malaccensisleaves has a great potential to use as a natural pesticide based on its identified compounds.The pesticidal property ofA.malaccensisleaves oil was expected as Zaridah and colleagues[36]had been provided evidence for pesticidal characteristic of oil extracted from the wood ofA.malaccensis.
4.Conclusions
In this study,the kinetic model of oil extraction from theA.malaccensisleaves using the hydro-distillation was investigated to understand the extraction mechanism and to identify the optimum condition,in which the maximum extracted yield and the minimum of water consumption and extraction time can be achieved.The result showed that non-stationary diffusion model comprising two stages of washing and diffusion better describes the extraction of oil fromA.malaccensisleaves.Both the hydro-distillation rate and theA.malaccensisleaves to-water ratio were identified as influential factors affecting the extraction period,distillation rate,and oil yield.The highest yield of the essential oil was extracted at power of around 300–350 W,solid to solvent ratio of 1:10,and around 3 h of extraction.Also,using GC/MS,42 chemical components ofA.malaccensisleaves essential oil were identified for the first time.Among these components,pentadecanal,9-octadecenal,(Z),and tetradecanal were the most concentrated components.Given the possibility of usingA.malaccensisleaves as a natural source of pesticides,it is recommended for future studies to practically test(e.g.larvicidal and mosquitocidal)the identified major components for developing new organic pesticidal products.
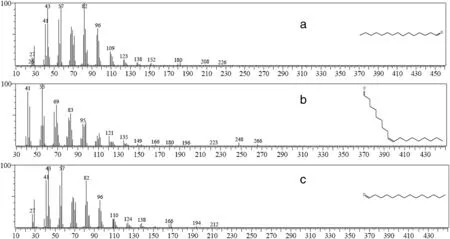
Fig.9.Mass spectra of identified major components of essential oil of Aquilaria malaccensis'leaves:Pentadecanal(a),9-octadecenal,(Z)(b),tetradecanal<n-> (c).
[1]J.Jiao,Q.-Y.Gai,Y.-J.Fu,Y.-G.Zu,M.Luo,C.-J.Zhao,et al.,Microwave-assisted ionic liquids treatment followed by hydro-distillation for the efficient isolation of essential oil from Fructus forsythiae seed,Sep.Purif.Technol.107(2013)228–237.
[2]E.Reverchon,Supercritical fluid extraction and fractionation of essential oils and related products,J.Supercrit.Fluids(10)(1997)1–37.
[3]Y.H.Hui,Encyclopedia of Food Science and Technology,Wiley,New York,1992.
[4]M.E.Lucchesi,F.Chemat,J.Smadja,Solvent-free microwave extraction of essential oil from aromatic herbs:Comparison with conventional hydro-distillation,J.Chromatogr.A1043(2004)323–327.
[5]M.C.Eurlings,B.Gravendeel,TrnL-trnF sequence data imply paraphyly of Aquilaria and Gyrinops(Thymelaeaceae)and provide new perspectives for agarwood identification,Plant Syst.Evol.1(2005)1–12.
[6]A.Barden,N.A.Anak,T.Mulliken,M.Song,Heart of the Matter:Agarwood Use and Trade and CITES Implementation forAquilaria malaccensis,TRAFFIC International,Cambridge,UK,2000.
[7]S.Old field,A.Newton,G.Fragoso,P.Mathew,L.Miles,M.Edwards,Towards a Global Tree Conservation Atlas:Mapping the Status and Distribution of the World's Threatened Tree Species,UNEP-WCMC,FFI,Cambridge,UK,1998.
[8]P.Morris,A.H.Scragg,A.Stafford,M.W.Fowler,Secondary Metabolites in Plant Cell Culture,PhD Thesis,Cambridge University,London,2011.
[9]K.Suresh,S.S.Babu,R.Harisaranraj,Studies on in vitro antimicrobial activity of ethanol extract ofRauvolfia tetraphylla,Ethnobotonical Leafets,2008 586–590.
[10]A.H.Ibrahim,S.S.Al-Rawi,A.M.Abdul Majid,N.N.Rahman,K.M.Abo-Salah,M.O.Ab Kadir,Separation and fractionation ofAquilaria malaccensisoil using supercritical fluid extraction and the cytotoxic properties of the extracted oil,Procedia Food Sci.1(2011)1953–1959.
[11]N.N.Wil,N.A.Omar,N.A.Ibrahim,S.N.Tajuddin,In vitro antioxidant activity and phytochemical screening ofAquilaria malaccensisleaf extracts,J.Chem.Pharm.Res.6(12)(2014)688–693.
[12]A.Huda,M.Munira,S.Fitrya,M.Salmah,Antioxidant activity ofAquilaria malaccensis(thymelaeaceae)leaves,Pharmacogn.Res.1(5)(2009)270–273.
[13]A.S.Khalil,A.A.Rahim,K.B.Abdallah,Characterization of methanolic extracts of agarwood leaves,J.Appl.Ind.Sci.1(3)(2013)78–88.
[14]H.Sovová,S.Aleksovski,Mathematical model for hydrodistillation of essential oils,Flavour Fragrance J.21(2006)881–889.
[15]S.Z.Milojevic,T.D.Stojanovic,R.Palic,M.L.Lazic,V.B.Veljkovic,Kinetics ofdistillation of essential oil from comminuted ripe juniper(Juniperus communisL.)berries,Biochem.Eng.J.39(2008)547–553.
[16]K.H.Baser,G.Buchbauer,Handbook of Essential Oils:Science,Technology,and Applications,CRC Press,New York,2009.
[17]E.Cassel,R.Vargas,N.Martinez,D.Lorenzo,E.Dellacassa,Steam distillation modeling for essential oil extraction process,Ind.Crop.Prod.29(2009)171–176.
[18]S.Milojević,D.Radosavljević,V.Pavićević,S.Pejanović,V.Veljković,Modeling the kinetics of essential oil hydrodistillation from plant materials,Hem.Ind.5(2013)843–859.
[19]I.Stanisavljevi'c,M.Lazi'c,V.Veljkovi'c,Kinetics of hydrodistillation and chemical composition of essential oil from cherry laurel(Prunus laurocerasusL.var.serbica Pan ˇci´c)leaves,J.Essent.Oil Res.22(2010)564–567.
[20]T.Peng,M.Don,M.Tahrel,Optimisation and kinetics studies on the extraction of essential oil fromZingiber cassumunar,J.Phys.Sci.23(1)(2012)65–82.
[21]J.Pornpunyapat,P.Chetpattananondh,C.Tongurai,Mathematical modeling for extraction of essential oil fromAquilaria crassnaby hydrodistillation and quality of agarwood oil,J.Bangladesh Pharmacol.Soc.6(2011)18–24.
[22]M.Desai,J.Parikh,A.Kumar De,Modelling and optimization studies on extraction of lemongrass oil fromCymbopogonflexuosus(Steud.)Wats,Chem.Eng.Res.Des.92(2014)793–803.
[23]Councilof Europe,European Pharmacopoeia Commission,European Pharmacopoeia,Council Of Europe,Strasbourg,2008.
[24]R.P.Adams,Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry,4th Edition Allured Publishing Corporation,Illinois,2007.
[25]H.Sharma,Biotechnological Approaches for Pest Management and Ecological Sustainability,CRC Press,USA,2008.
[26]M.D.Pathak,Z.Khan,Insect Pests of Rice,Int.Rice Res.Inst.,ICIPE,Manila,Philippines,1994.
[27]M.Cooperband,J.McElfresh,J.Millar,R.Cardé,Attraction of femaleCulex quinquefasciatusSay(Diptera:Culicidae)to odors from chicken feces,J.Insect Physiol.54(7)(2008)1184–1192.
[28]S.H.Tatsuki,Identification of possible sex pheromone of the yellow stem borer mothScirpophaga incertulas,Appl.Entomol.Zool.20(1985)357–359.
[29]G.Ho,V.L.Hao,D.Hall,T.Le,D.Nguyen,J.Cross,et al.,(Z)-11-hexadecenyl acetate and(Z)-13-octadecenyl acetate improve the attractiveness of the standard sex pheromone of the yellow rice stem borerScirpophaga incertulas(Lepidoptera:Pyralidae)in northern Vietnam,J.Fac.Agric.Kyushu Univ.59(1)(2014)85–89.
[30]A.Manilal,S.Sujith,B.Sabarathnam,G.Kiran,J.Selvin,C.Shakir,et al.,Biological activity of the red algaLaurencia brandenii,Acta Bot.Croat.70(1)(2011)81–90.
[31]A.Abd El-Aty,A.Mohamed,F.Samhan,In vitro antioxidant and antibacterial activities of two fresh water Cyanobacterial species,Oscillatoria agardhiiandAnabaena sphaerica,J.Appl.Pharm.Sci.4(07)(2014)069–075.
[32]A.H.Rose,Adv in Microbial Physiology APL,Volume 34,Academic Press,NY,1993.
[33]H.Watanabe,H.Inaba,J.W.Hastings,Effects of aldehyde and internal ions on bioluminescence expression ofPhotobacterium phosphoreum,Arch.Microbiol.156(1991)1–4.
[34]S.Ulitzur,J.W.Hastings,Evidence for tetradecanal as the natural aldehyde in bacterial bioluminescence,Proc.Natl.Acad.Sci.U.S.A.76(1979)265–267.
[35]F.Ruano,A.Hefetz,A.Lenoir,W.Francke,A.Tinaut,Dufour's gland secretion as a repellent used during usurpation by the slave-maker antRossomyrmex minuchae,J.Insect Physiol.51(2005)1158–1164.
[36]M.Z.Zaridah,M.A.Azah,A.Rohani,Mosquitocidal activities of Malaysian plants,J.Trop.For.Sci.18(1)(2006)74–80.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Structural insights of mechanically induced aluminum-doped hydroxyapatite nanoparticles by Rietveld refinement☆
- Structural evolution of chars from biomass components pyrolysis in a xenon lamp radiation reactor☆
- TiO2–SA–Arg nanoparticles stabilized Pickering emulsion for photocatalytic degradation of nitrobenzene in a rotating annular reactor
- Effects of temperature and phosphoric acid addition on the solubility of iron phosphate dihydrate in aqueous solutions☆
- A novel model for multi-plant mixed heavy crude oils refinery planning
- A self-tuning control method for Wiener nonlinear systems and its application to process control problems☆
