鸡粪发酵液培养的小球藻水热液化制备生物原油及其特性
2017-05-25朱张兵张源辉李保明张婷婷董泰丽刘志丹
朱张兵,王 猛,张源辉,2,李保明,张婷婷,董泰丽,刘志丹※
鸡粪发酵液培养的小球藻水热液化制备生物原油及其特性
朱张兵1,王 猛1,张源辉1,2,李保明1,张婷婷1,董泰丽3,刘志丹1※
(1. 中国农业大学水利与土木工程学院农业部设施农业工程重点实验室,环境增值能源实验室,北京100083; 2. 美国伊利诺伊大学香槟校区农业与生物工程系,厄巴纳61801;3. 山东民和生物科技有限公司,蓬莱 265600)
为探索沼液资源再利用,以鸡粪沼气发酵液培养的小球藻为原料,采用水热液化技术制备生物原油。采取正交试验,在温度250~330 ℃、时间30~90 min及含固量15%~25%下,探讨了水热反应后各相产物特性及元素回收效率。生物原油产率为13.23%~23.83%,最高产油率在330 ℃、60 min、15%时取得。生物原油中碳、氢及氮回收率分别是16.13%~31.14%、19.18%~34.89%及5.97%~14.32%,最高碳回收率及最低氮回收率分别在330 ℃、60 min、15%及250 ℃、30 min、15%时获得。水热液化各相产物中,碳、氢及氮回收率在水相中占主导地位,分别为48.74%~60.43%、46.81%~62.13%及74.84%~82.67%。热重分析暗示生物原油可能适合制备润滑油。此外,GC-MS分析表明生物原油中烃类物质质量分数为16.14%~24.91%,主要为低碳链烃类,如甲苯及二氢茚等。
回收率;沼气;碳;微藻;热化学;水热液化;生物原油;鸡粪沼液
0 引 言
随着化石能源不断消耗,可再生、清洁的生物质能引起广泛关注。中国是传统的农业大国,产生了大量农业废弃物,如2010年,中国畜禽粪便总量约为22.35亿t[1]。畜禽粪便是一种重要生物质资源,可被用于沼气发酵[2-4]。但畜禽粪便沼气发酵液产量大、氮磷及金属离子含量高,不合理利用,会造成环境二次污染[5-6]。微藻可以吸收沼液中的碳、氮、磷等营养物质,积累生物量的同时可净化沼液[7-10],而微藻经水热液化技术又可转化为生物原油[11-13]。
水热液化技术指高温(200~380 ℃)、高压(5~ 28 MPa)及无氧环境下,废弃生物质被转化成类似石油的物质——生物原油[11,14-16]。由于水热液化技术可直接转化湿生物质,且适用于各种原料,被国内外众多科学家认为是一种期望的热化学转化方法[14]。近年来,关于微藻水热转化的研究逐渐引起国内外研究者的关注。利用废水培养的微藻为原料,用于水热液化的研究极少。Chen等[17]利用污水处理系统中的污水进行微藻培养,探讨了不同反应温度(260~320 ℃)及时间(0~1.5 h)对微藻水热产油的影响。Fortier等[18]利用市政污水培养微藻,对微藻水热产油进行了生命周期评价的研究。目前利用鸡粪发酵液培养微藻,并用于水热产油的研究,国内外未有相关报道。
本文以鸡粪沼气发酵液培养的微藻为原料,在不同温度(250~290 ℃)、时间(30~90 min)及含固量(15%~25%)下,探讨了此类型微藻水热转化规律,并系统分析水热液化气、油、水等各相产物的元素回收率及特性,以期为沼液资源再利用提供新的理论基础。
1 材料和方法
1.1 微藻培养及水热液化过程
温室中,小球藻在含有350 L鸡粪发酵液的开放盆中进行培养。发酵液初始氨氮质量浓度为500 mg/L,培养温度为20~30 ℃,光照为2 500~10 000 lx。培养的小球藻经滤膜反渗透及脱水后,直接收获用于水热转化试验。小球藻理化性质如表1所示,小球藻有较低的脂肪(1.00%)及较高的灰分(55.06%)。低脂肪的产生是由于发酵液含有高浓度的氮。高灰分是由于:1)敞盆培养,尘埃进入培养盆中;2)收获后的小球藻依然含有85%的水分(发酵液),烘干后存在于发酵液中的灰分被折算到小球藻灰分体系中。
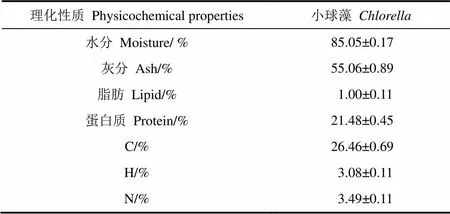
表1 小球藻理化性质
水热液化试验采用美国Parr4593高温高压100 mL反应釜[12]。将鲜质量40 g小球藻加入到反应釜中,密封反应釜,用N2吹扫3~5次排除体系中空气,并维持初始压强为1.5 MPa。加热反应釜至目标温度后开始计时,反应完成后,用电风扇进行快速降温。待反应釜降到常温后收集气相产物,釜中混合物经真空抽滤后获得水相产物及粗油,粗油通过丙酮进一步萃取,得到生物原油及固体残渣。
1.2 分析方法及计算公式
气相产物、水相产物的测试方法参照之前文献所 述[12]。利用热重分析仪(STA6000,Perkin Elmer,美国)及气质联用仪(GC-MS,Model QP2010,Shimadzu,日本)对生物原油进行组分分析[19]。热重分析仪采取高纯N2为载气,流速25 mL/min;初始温度为30 ℃,以10 ℃/min升至700 ℃;测试样品质量为5 mg。GC-MS采取DB-5色谱柱,以高纯氦气为载气,流速1.78 mL/min,分流比20∶1,进样量1L;接口温度、进样口温度及离子源温度分别是250、250及230 ℃,柱箱温度(40 ℃)保留5 min后,以10 ℃/min的升温速度升至150 ℃,保留2 min后再以5 ℃/min升到250 ℃,并保留1 min;EI源,电子能量70 eV,分子量扫描范围为50~500。主要指标计算详见公式(1)至(5)。

(2)
(3)

(5)
2 结果与分析
2.1 产油率及液化率
小球藻在不同温度(250~330 ℃)、时间(30~ 90 min)及含固量(15%~25%)下进行水热反应,生物原油产率为13.23%~23.83%(图1),表明反应条件影响生物原油的转化。本研究的最高生物原油产率低于其他研究者(>30%)[20-23],主要是其他研究者所用小球藻是人工纯净环境培养,有机成分高(>90%),尤其是利于生物原油转化的脂肪含量高。本研究所用小球藻培养于鸡粪发酵液,培养环境导致小球藻有较高的灰分(55.06%)及较低的脂肪(1.00%)。过高的灰分会附着在有机物表面,阻碍有机物转化,导致生物原油产率偏低[11]。此外,多数研究表明微藻3大组分(脂肪、蛋白质及碳水化合物),脂肪是转化生物原油最有效的化合物[11,21]。尽管在不同反应条件下,生物原油产率不到24%,但经水热反应后,小球藻液化率达到72.77%~80.73%(图1),暗示灰分在水热过程中被重新分配。生物原油有机组分高达99%,而残渣中灰分为59%~73%,表明部分灰分被迁移到水相中。鸡粪发酵液培养的小球藻富含钾、钠等离子,这些离子在水热过程中会优先进入到水相中。其他研究者也得到类似结果,Anastasakis等[24]的研究表明生物质中金属离子在水热条件下被分配进入到水相。
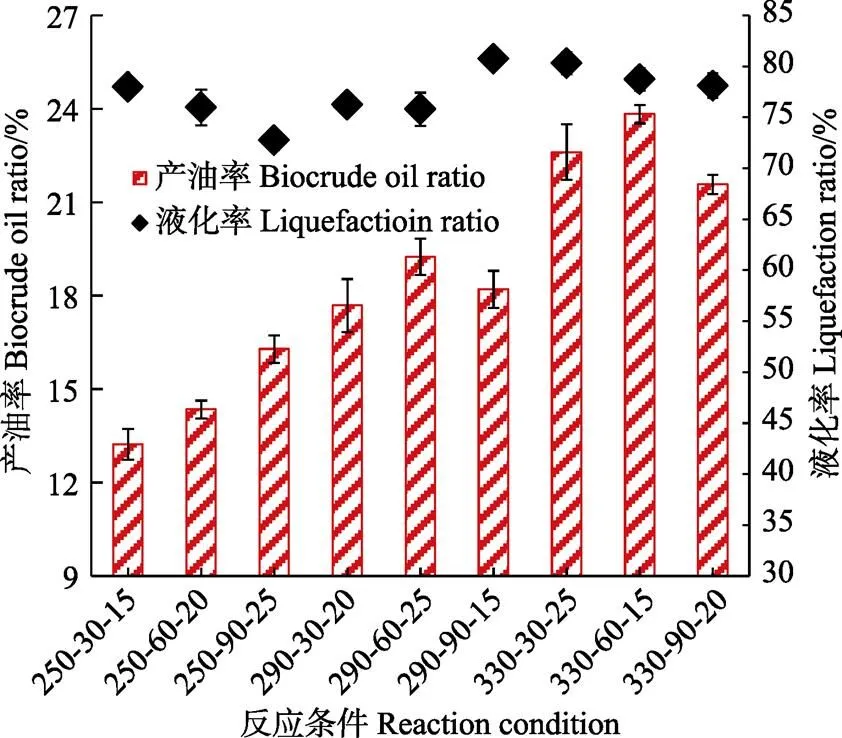
注:250-30-15代表反应温度、时间及含固量分别为250 ℃、30 min及15%,下同。
2.2 有机元素回收
生物原油中氢及碳回收率分别为19.18%~34.89%及16.13%~31.14%(图2),最高生物原油碳回收率为31.14%,在330 ℃、60 min、含固量15%时获得。显然,在不同反应条件下,生物原油中氢质量分数近似于10%(表2),变化趋势不明显,表明生物原油中氢回收率主要是由产油率决定。生物原油中碳含量在不同反应条件下差异明显,暗示碳回收率主要是由产油率及生物原油中碳含量共同决定。生物原油中氮回收率为5.97%~14.32%,最低氮回收率(5.97%)在250 ℃、30 min、15%时获得。过高的氮对生物原油品质造成不利影响,需进一步对生物原油进行脱氮提质。相较于生物原油,气相产物中碳及氢回收率较低,分别为1.47%~3.51%及0~3.72%。在温和反应条件下(如250 ℃),碳及氢回收率偏低,尤其是氢回收率基本趋于0。随着反应剧烈程度加强(如330 ℃),碳及氢回收率显著提升,表明剧烈的反应条件会促进气体的转化。此外,水相中碳、氢及氮回收率在所有反应条件下,均占主导地位,分别是48.74%~60.43%、46.81%~62.13%及74.84%~82.67%。高碳及氢回收率的产生主要是碳水化合物被降解,形成糖、酸及醛等易溶性化合物进入到水相,被Sasaki等[25]研究所支持,其研究表明纤维素等碳水化合物在水热条件下可被降解成各种糖、酸及醛。水相中高氮回收率会间接减少生物原油中氮回收率,影响生物原油品质。高氮回收率的产生主要由于原料中蛋白质在水热条件下进行脱氮反应,形成氨氮进入水相,Yu等[26]研究结果也表明氨基酸在水热条件下易被降解进入到水相。
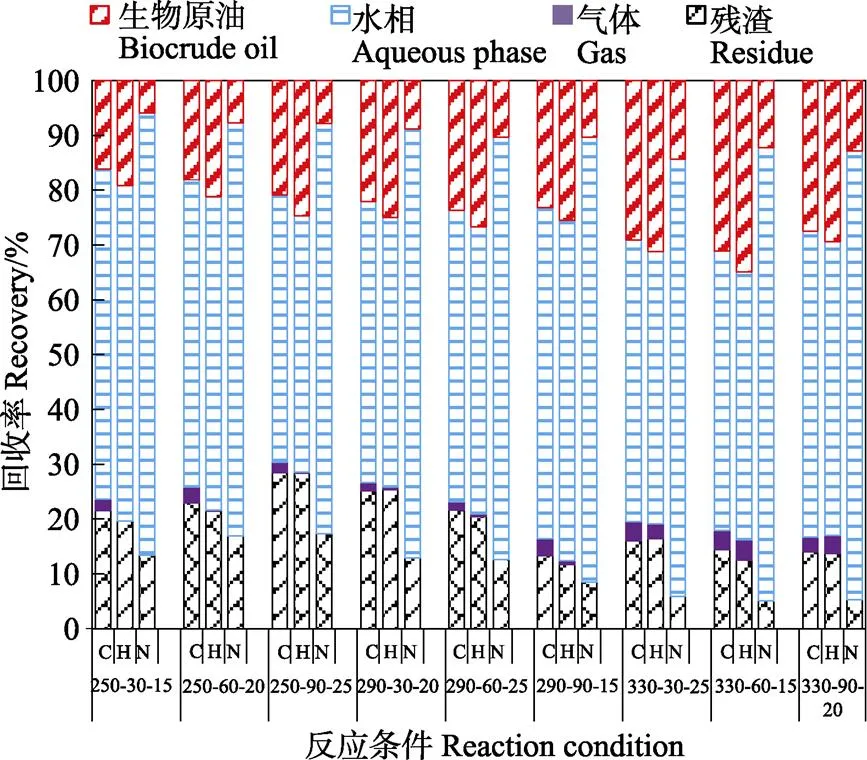
图2 水热产物元素回收率
2.3 生物油特性
小球藻含有26.46%碳、3.08%氢及3.49%氮。水热反应后,生物原油中碳及氢质量分数分别提升至71.77%~76.95%及9.36%~10.38%,H/C比也由原料中1.40提升至1.50~1.66(表2)。生物原油中氮含量有轻微提升,但N/C比由原料中0.11降至0.04~0.06,接近石油N/C比[11]。
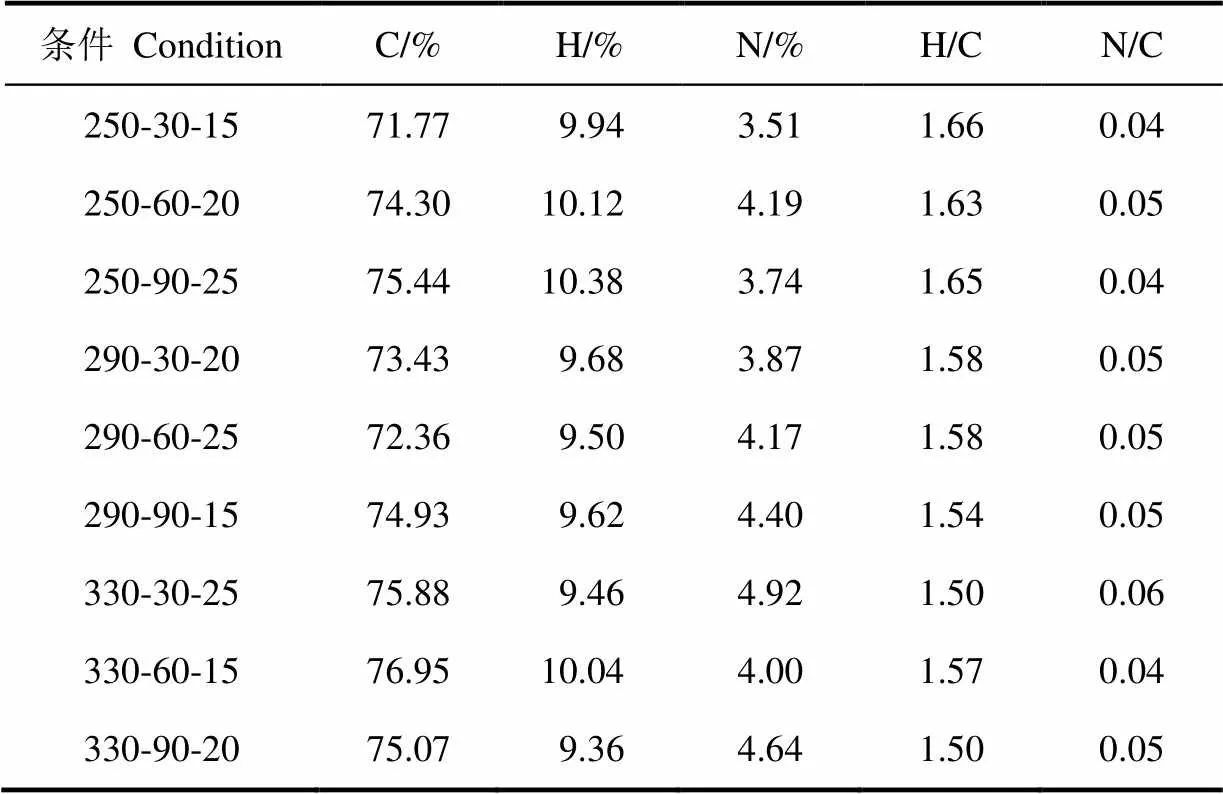
表2 生物原油元素分布
生物原油成分复杂,含有烃类、酮类、醛类等物质[11]。生物原油热重反应试验中,这些化合物由于沸点和热稳定性不同,会在不同温度区间内挥发。在低温条件下(<300 ℃),生物原油中挥发的是轻组分,主要是低碳链的烃类、醇类等,重油组分在更高的温度区间内挥发。Anastasakis等[24]的研究也表明生物原油中轻组分在 250 ℃以下挥发,重组分在高温挥发。这些轻组分可能来源于小球藻中碳水化合物或蛋白质水解,重组分主要是由于蛋白质或碳水化合物的水解产物重新发生缩合聚合等反应,或者蛋白质与碳水化合物的美拉德反应[14]。热重分析仪被选择用于模拟生物原油沸点分布[24]。100~200 ℃,生物原油被蒸馏的质量分数为0.94%~5.42% (图3),Chen等[27]的研究表明,石油经过蒸馏,汽油的温度区间为100~200 ℃;柴油的温度区间为200~300 ℃,此温度内,生物原油被蒸馏的质量分数为17.53%~32.31%;300~400 ℃,生物原油被蒸馏的质量分数为17.90%~29.86%,可用于发动机润滑油;400~550 ℃,轮船润滑油或燃料可经石油蒸馏得到,此时,生物原油被蒸馏的质量分数为17.91%~30.20%;550~700 ℃,用于工厂及集中供热的燃料可经石油炼制而获得,而生物原油被蒸馏的质量分数为3.63%~5.35%。这些结果表明本研究所获得的生物原油可能适合润滑油生产,而汽油或柴油的生产,需进一步对生物原油进行提制。
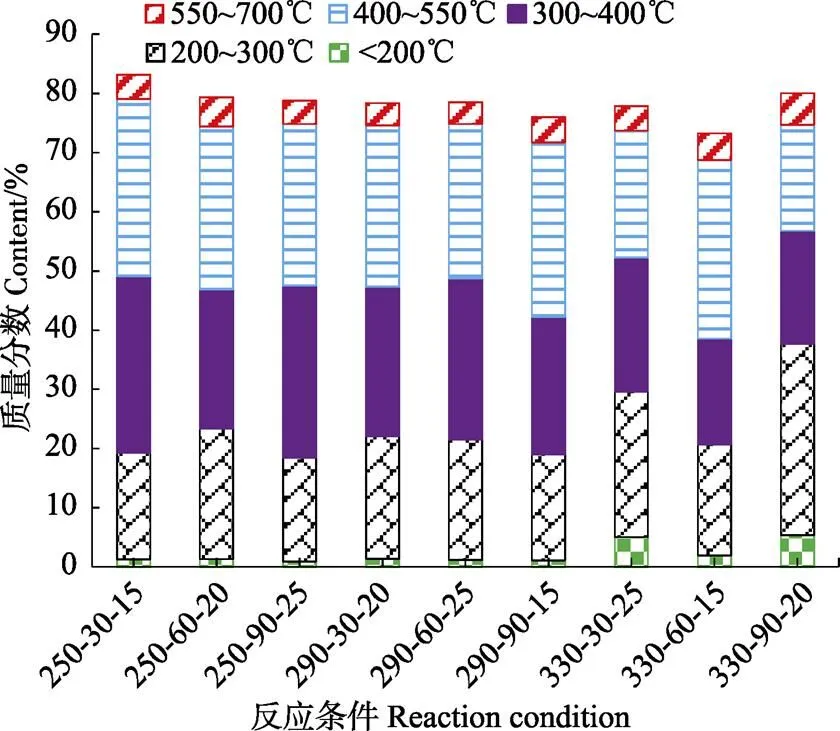
图3 生物原油失质量率
根据C、N、O,生物原油中化合物被分成4类,烃类(CH)、碳氮杂合物(CN)、碳氧化合物(CO)及碳氮氧杂合物(CNO)。由GC-MS分析可知(图4),生物原油中烃类物质达到16.14%~24.91%,这些化合物主要以低碳链烃类为主,如甲苯、枯烯及二氢茚等。而生物原油中的氮化合物(0.72%~3.75%)及氮氧化合物(0%~2.60%)较少,与前文生物原油中氮含量分析结果趋于一致。生物原油中主要以氧化合物为主(>69%),这些氧化合物主要是酯类、酮类及醛类物质,如2-戊酮及邻苯二甲酸二甲酯等。
鸡粪沼气发酵液培养的小球藻制备生物原油,实现了沼液净化及能源再生的双赢局面。但相比于原油,生物原油的氮氧含量仍偏高。通过添加催化剂(Co/Mo/ Al2O3、KOH、Na2CO3等)及引入还原气体(CO及H2)等辅助方法,可降低生物原油氮氧含量,有效改善油 品[28-30]。此外,寻找适合生物原油脱氧脱氮的炼制方法也是必须的。
2.4 气相及水相特性
水热反应产生的气相产物质量分数为1.36%~3.33%,在所有反应条件下,CO2均占主导地位(>93%)(表3),暗示水热反应中,形成CO2是脱氧反应的一个主要途径。相对于CO2,其他气体产物含量较低,尤其是在温和的反应条件下,如250℃时,CH4及H2的质量分数仅为0~1.14%及0.00%~0.06%。随着反应强度的加强,CH4及H2的含量得到提升,在330℃时,CH4及H2的质量分数分别提升至2.24%~3.05%及2.22%~3.55%。
水相产物的pH值是8.53~9.15(表3),呈碱性,表明小球藻水热反应后,可能形成了碱性或弱碱性的化合物。此外,水相中氨氮质量浓度为1 218~3 629 mg/L,过高的氨氮可能也会导致pH值呈碱性。水相总磷及总有机碳分别为107~270 mg/L及24 360~47 760 mg/L,表明水相含有丰富的有机物,可被进一步资源化利用。
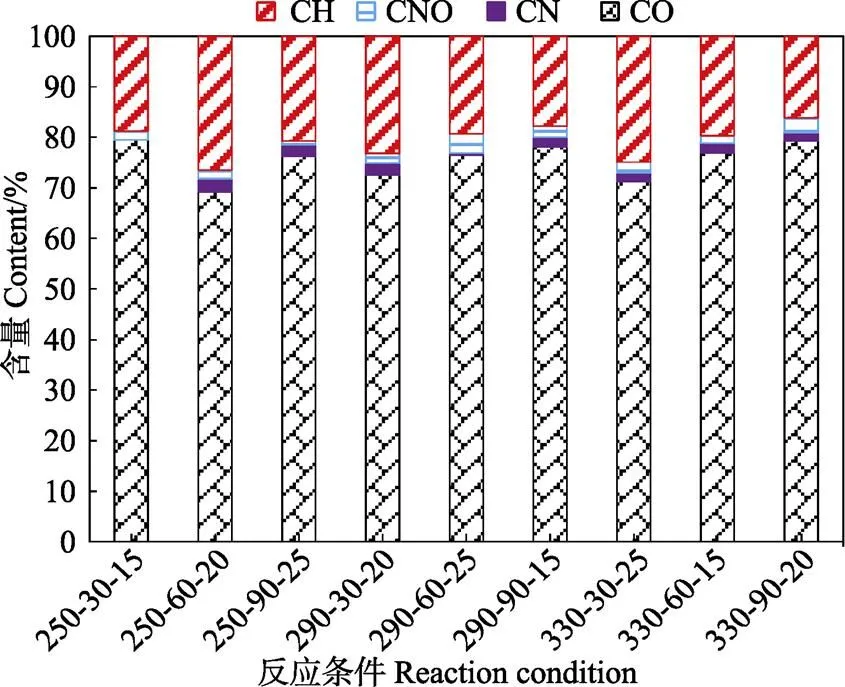
注:CH、CN、CO和CNO分别指烃类、碳氮杂合物、碳氧化合物、碳氮氧杂合物。
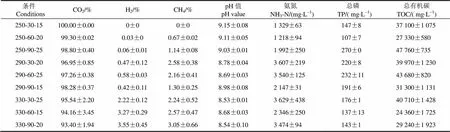
表3 气相及水相产物分析
3 结 论
1)鸡粪沼气发酵液培养的小球藻经水热液化技术可被转化为生物原油,最大产油率为23.83%,在330 ℃、60 min、含固量15%时取得,原料高灰分、低脂肪含量制约着生物原油的转化。
2)生物原油中最高碳回收率及最低氮回收率分别是31.14%(330 ℃、60 min、含固量15%)及5.97%(250 ℃、 30 min、含固量15%),碳及氮回收率主要由生物原油产率决定。水相中氮回收率超过74%,主要是原料中蛋白质形成氨氮进入水相。
3)相比于原油,水热液化技术所得到的生物原油氮氧含量偏高,需进一步对生物原油进行脱氧脱氮处理。
[1] 耿维,胡林,崔建宇,等. 中国区域畜禽粪便能源潜力及总量控制研究[J]. 农业工程学报,2013,29(1):171-179. Geng Wei, Hu Lin, Cui Jianyu, et al. Biogas energy potential for livestock manure and gross control of animal feeding in region level of China[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2013, 29(1): 171-179. (in Chinese with English abstract)
[2] 李轶,刘雨秋,张镇,等. 玉米秸秆与猪粪混合厌氧发酵产沼气工艺优化[J]. 农业工程学报,2014,30(5):185-192. Li Yi, Liu Yuqiu, Zhang Zhen, et al. Optimization of anaerobic fermentation with mixed materials of corn straw and pig manure[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2014, 30(5): 185-192. (in Chinese with English abstract)
[3] 李金平,周丹丹,张庆芳,等. 温度对高浓度恒温厌氧发酵产沼气成分的影响[J]. 兰州理工大学学报,2012,38(6):44-48. Li Jinping, Zhou Dandan, Zhang Qingfang, et al. Influence of temperature on composition of biogas fermented in high-concentrated thermostatic anaerobic environment[J]. Journal of Lanzhou University of Technology, 2012, 38(6): 44-48. (in Chinese with English abstract)
[4] 秦文弟,蒋湖波,黄凌志,等. 牲畜粪便沼气发酵过程中微生物丰度变化分析[J]. 广西林业科学,2015,44(4): 416-420. Qin Wendi, Jiang Hubo, Huang Lingzhi, et al. Analysis on abundance changes of microorganisms during biogas fermentation of livestock dung[J]. Guangxi Forestry Science, 2015, 44(4): 416-420. (in Chinese with English abstract)
[5] 靳红梅,常志州,叶小梅,等. 江苏省大型沼气工程沼液理化特性分析[J]. 农业工程学报,2011,27(1):291-296. Jin Hongmei, Chang Zhizhou, Ye Xiaomei, et al. Physical and chemical characteristics of anaerobically digested slurry from large-scale biogas project in Jiangsu Province[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2011, 27(1): 291-296. (in Chinese with English abstract)
[6] 陈玉成,杨志敏,陈庆华,等. 大中型沼气工程厌氧发酵液的后处置技术[J]. 中国沼气,2010,28(1):14-20. Chen Yucheng, Yang Zhimin, Chen Qinghua, et al. An overview on disposal of anaerobic digestate for large scale biogas engineering[J]. China Biogas, 2010, 28(1): 14-20. (in Chinese with English abstract)
[7] 霍书豪,陈玉碧,刘宇鹏,等. 添加沼液的BG11营养液微藻培养试验[J]. 农业工程学报,2012,28(8):241-246. Huo Shuhao, Chen Yubi, Liu Yupeng, et al. Experiment on microalgae cultivation in BG11 nutrient solution adding biogas slurry[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2012, 28(8): 241-246. (in Chinese with English abstract)
[8] 巫小丹,刘伟,阮榕生,等. 不同预处理方法对沼液养殖微藻的影响[J]. 环境污染与防治, 2014,36(1):9-12. Wu Xiaodan, Liu Wei, Ruan Rongsheng, et al. Impacts of different pretreatment methods on microalgae cultivation in biogas slurry[J]. Environmental Pollution & Control, 2014, 36(1): 9-12. (in Chinese with English abstract)
[9] 李攀荣,邹长伟,万金保,等. 微藻在废水处理中的应用研究[J]. 工业水处理,2016,36(5):5-9. Li Panrong, Zou Zhangwei, Wan Jinbao, et al. Research of micro algae processing wastewater[J]. Industrial Water Treatment, 2016, 36(5): 5-9.(in Chinese with English abstract)
[10] Zhang Li, Lu Haifeng, Zhang Yuanhui, et al. Nutrient recovery and biomass production by cultivating Chlorella vulgaris 1067 from four types of post-hydrothermal liquefaction wastewater[J]. Journal of Applied Phycology, 2016, 28(2): 1031-1039.
[11] Tian Chunyan, Li Baoming, Liu Zhidan, et al. Hydrothermal liquefaction for algal biorefinery: A critical review[J]. Renewable and Sustainable Energy Reviews, 2014, 38: 933-950.
[12] 屈埴,刘志丹,朱张兵,等. 厨余垃圾水热液化成油特性研究[J]. 太阳能学报,2016,37(5):1327-1333. Qu Zhi, Liu Zhidan, Zhu Zhangbing, et al. Bio-crude production from kitchen waste through hydrothermal liquefaction[J]. Acta Energiae Solaris Sinica, 2016, 37(5): 1327-1333. (in Chinese with English abstract)
[13] 徐玉福,俞辉强,朱利华,等. 小球藻粉水热催化液化制备生物油[J]. 农业工程学报, 2012,28(19):194-199. Xu Yufu, Yu Huiqiang, Zhu Lihua, et al. Preparation of bio-fuel fromby hydrothermal catalytic liquefaction[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2012, 28(19): 194-199. (in Chinese with English abstract)
[14] López Barreiro Diego, Prins Wolter, Ronsse Frederik, et al. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects[J]. Biomass and Bioenergy, 2013, 53:113-127.
[15] Lu Jianwen, Zhang Jiaren, Zhu Zhangbing, et al. Simultaneous production of biocrude oil and recovery of nutrients and metals from human feces via hydrothermal liquefaction[J]. Energy Conversion and Management, 2017, 134: 340-346.
[16] Tian Chunyan, Liu Zhidan, Zhang Yuanhui, et al. Hydrothermal liquefaction of harvested high-ash low-lipid algal biomass from Dianchi Lake: Effects of operational parameters and relations of products[J]. Bioresource Technology, 2015, 184: 336-343.
[17] Chen Wanting, Zhang Yuanhui, Zhang Jixiang, et al. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil[J]. Bioresource Technology, 2014, 152: 130-139.
[18] Fortier Marie-Odile P, Roberts Griffin W, Stagg-Williams Susan M, et al. Life cycle assessment of bio-jet fuel from hydrothermal liquefaction of microalgae[J]. Applied Energy, 2014, 122: 73-82.
[19] Zhu Zhangbing, Liu Zhidan, Zhang Yuanhui, et al. Recovery of reducing sugars and volatile fatty acids from cornstalk at different hydrothermal treatment severity[J]. Bioresource Technology, 2016, 199: 220-227.
[20] Li Hao, Liu Zhidan, Zhang Yuanhui, et al. Conversion efficiency and oil quality of low-lipid high-protein and high- lipid low-protein microalgae via hydrothermal liquefaction[J]. Bioresource Technology, 2014, 154: 322-329.
[21] Biller P, Ross A B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content[J]. Bioresource Technology, 2011, 102(1): 215-225.
[22] Gai Chao, Zhang Yuanhui, Chen Wanting, et al. An investigation of reaction pathways of hydrothermal liquefaction using Chlorella pyrenoidosa and Spirulina platensis[J]. Energy Conversion and Management, 2015, 96: 330-339.
[23] Xu Yufu, Zheng Xiaojing, Yu Huiqiang, et al. Hydrothermal liquefaction of Chlorella pyrenoidosa for bio-oil production over Ce/HZSM-5[J]. Bioresource Technology, 2014, 156: 1-5.
[24] Anastasakis K, Ross A B. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition[J]. Bioresource Technology, 2011, 102(7): 4876-4883.
[25] Sasaki Mitsuru, Fang Zhen, Fukushima Yoshiko, et al. Dissolution and hydrolysis of cellulose in subcritical and supercritical water[J]. Industrial & Engineering Chemistry Research, 2000, 39(8): 2883-2890.
[26] Yu Guo, Zhang Yuanhui, Schideman Lance, et al. Distributions of carbon and nitrogen in the products from hydrothermal liquefaction of low-lipid microalgae[J]. Energy & Environmental Science, 2011, 4: 4587-4595.
[27] Chen Wanting, Zhang Yuanhui, Zhang Jixiang, et al. Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil[J]. Applied Energy, 2014, 128: 209-216.
[28] Akhtar Javaid, Amin Nor Aishah Saidina. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass[J]. Renewable and Sustainable Energy Reviews, 2011, 15(3): 1615-1624.
[29] Toor Saqib Sohail, Rosendahl Lasse, Rudolf Andreas. Hydrothermal liquefaction of biomass: A review of subcritical water technologies[J]. Energy, 2011, 36(5): 2328-2342.
[30] Xiu Shuangning, Shahbazi Abolghasem. Bio-oil production and upgrading research: A review[J]. Renewable and Sustainable Energy Reviews, 2012, 16(7): 4406-4414.
Biocrude oil preparation by hydrothermal liquefaction ofcultivated in biogas digestate from chicken manure and its characteristic
Zhu Zhangbing1, Wang Meng1, Zhang Yuanhui1,2, Li Baoming1, Zhang Tingting1, Dong Taili3, Liu Zhidan1※
(1.()100083; 2.61801;3.265600)
Improper treatment of biogas slurry results in serious environmental pollution. Cultivating algae using the biogas slurry is a promising strategy. By doing this, we can realize the reuse of nutrients, the further treatment of wastewater and the biomass production. In this study, the producedcultivated in the biogas slurry of chicken manure was used as feedstock for biocrude oil production through hydrothermal liquefaction (HTL). An orthogonal design was applied to investigate the effects of operational parameters on biocrude oil production, including the holding temperature (250, 290 and 330 ℃), the retention time (30, 60 and 90 min) and the total solid content (15%, 20% and 25%). The characteristics of products and element migration during HTL were analyzed. The highest biocrude oil yield reached up to 23.83% under a temperature of 330 ℃, a retention time of 60 min and a total solid content of 15%. The low yield of biocrude oil in this study may result from the low content of lipid (1.00%) and high content of ash (55.06%). The reaction conditions significantly affected the biocrude oil yields and chemical distribution of HTL products. The carbon recovery, hydrogen recovery and nitrogen recovery of the biocrude oil were 16.13%-31.14%, 19.18%-34.89% and 5.97%-14.32%, respectively. The highest carbon recovery was achieved under the condition of 330 ℃, 60 min and 15%, and the lowest nitrogen recovery was achieved at the condition of 250 ℃, 30 min and 15%. The increased carbon and hydrogen recovery of biocrude oil were mainly due to the increase of the biocrude oil yield. Carbon (48.74%-60.43%), hydrogen (46.81%-62.13%) and nitrogen (74.84%-82.67%) were effectively recovered in the aqueous phase. The high nitrogen recovery in the aqueous phase was mainly due to the promotion of the denitrification during the HTL process. The high nitrogen distribution in the aqueous phase had a harmful effect to biocrude oil, nitrogen content of which needed to be further decreased. Gas chromatograph-mass spectrometer (GC-MS) was chosen to analyze the organic groups in the biocrude oil. The hydrocarbons content in the biocrue oil was 16.14%-24.91%. The highest hydrocarbon content was obtained under the condition of 330 ℃, 30 min and 25%. However, the high content of oxygenates and nitrogen containing compounds in the biocrude oil decreased the quality of biocrude oil. Hence, the further deoxygenation and denitrogenation of the biocrude oil were maybe required before its application to the transport fuel. A thermogravimetric analyzer (TGA) was used to simulate the distribution of boiling points in the biocrude oil. The results indicated that the biocrude oil contained a lot of high molecular weight compounds. Based on the analysis, the biocrude oil seemed suitable for the production of lubricating oil. The concentration of total organic carbon, the total phosphorous and the ammonia nitrogen in the aqueous phase were 24 360-47 760, 107-270 and 1 218-3 629 mg/L, respectively, and the pH value was 8.53-9.15. The aqueous phase rich in nutrients could be recycled for algae cultivation. In addition, the main gas products CO2(>93%) could be used as carbon asset for algae cultivation. This study provides a potential approach for the biofuel production fromcultivated in biogas slurry.
recovery; biogas; carbon; microalgae; thermochemistry; hydrothermal liquefaction; biocrude oil; biogas digestate of chicken manure
10.11975/j.issn.1002-6819.2017.08.026
S216.2
A
1002-6819(2017)-08-0191-06
2016-09-24
2017-04-11
国家自然科学基金(U1562107, 51576206);北京市科技计划项目(Z161100001316009);大北农教育基金会(1091-2415001)
朱张兵,男,安徽安庆,博士生,研究方向为生物质水热液化技术研究。北京 中国农业大学水利与土木工程学院,100083。 Email:zhuzhangbing@163.com
刘志丹,男,河南安阳,博士,副教授,博士生导师,主要从事环境增值能源与水热液化技术研究。北京 中国农业大学水利与土木工程学院,100083。Email:zdliu@cau.edu.cn
中国农业工程学会高级会员:刘志丹(E041200655S)
朱张兵,王 猛,张源辉,李保明,张婷婷,董泰丽,刘志丹. 鸡粪发酵液培养的小球藻水热液化制备生物原油及其特性[J]. 农业工程学报,2017,33(8):191-196. doi:10.11975/j.issn.1002-6819.2017.08.026 http://www.tcsae.org
Zhu Zhangbing, Wang Meng, Zhang Yuanhui, Li Baoming, Zhang Tingting, Dong Taili, Liu Zhidan. Biocrude oil preparation by hydrothermal liquefaction ofcultivated in biogas digestate from chicken manure and its characteristic[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(8): 191-196. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2017.08.026 http://www.tcsae.org
