药用植物双参的三萜类成分研究
2017-05-22张祖珍陈雅凤王福生
张祖珍,陈雅凤,王福生
(大理大学药学与化学学院,云南大理 671000)
药用植物双参的三萜类成分研究
张祖珍,陈雅凤,王福生*
(大理大学药学与化学学院,云南大理 671000)
目的:对药用植物双参的化学成分进行研究。方法:采用硅胶、Sephadex LH-20、ODS等多种柱层析方法对双参进行分离纯化,采用现代波谱学技术对分离得到的化合物进行结构鉴定。结果:从双参地下部分的乙酸乙酯部位分离得到4个三萜类成分,分别鉴定为3β,28-二羟基-乌苏烷(1)、3β,23-二羟基-12-烯-28-酸(2)、α-香树精(3)和3β-羟基-24-降-乌苏-4(23),12-二烯-28-酸(4)。结论:这4个三萜类成分首次从该药用植物中分离得到。
双参;地下部分;三萜类成分
双参(Triplostegia glandulifera)为川续断科双参属植物,又称萝卜参,对对参,童子参,肚拉,土洋参和一支蒿等,主要产于云南、西藏、四川等地〔1-2〕。双参因有补肾,健脾益气,活血调经的功能,常用于久病体虚、补血补气等〔2〕。本课题组前期研究发现,云南民间药用植物双参的粗提物具有明显的降血糖功能以及显著的抗氧化应激作用〔3-5〕。目前未见文献报道其化学成分,为了解药用植物双参的活性成分,本实验对该植物地下部分的乙酸乙酯部位进行了系统研究,并从中得到4个三萜类化合物,分别为3β,28-二羟基-乌苏烷(1)、3β,23-二羟基-12-烯-28-酸(2)、α-香树精(3)和3β-羟基-24-降-乌苏-4(23),12-二烯-28-酸(4)。化合物的结构见图1。
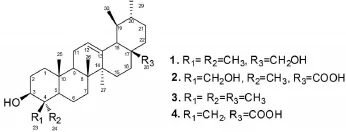
图1 化合物1~4的结构
1 仪器与材料
1.1材料本实验所用生药于2014年11月采自云南省大理州云龙县五宝山,由大理大学段宝忠副教授鉴定为川续断科双参属植物双参(Triplostegia glandulifera Wall.ex D C)的地下部分,植物标本(编号:WFS-20141128)存放于大理大学药学与化学学院王福生教授研究组。
1.2仪器与试剂NMR用Bruker AM-400型核磁共振仪测定;旋转蒸发仪RE-2000A(上海亚荣生化仪器厂);柱色谱硅胶与薄层色谱硅胶GF254(青岛海洋化工厂);Sephadex LH-20(Pharmacia);ODS(日本YMC公司);石油醚、氯仿、丙酮、乙酸乙酯和甲醇等试剂为工业级试剂,均需重蒸后使用。
2 提取与分离
取双参干燥地下部分3 kg,粉碎后依次用95%乙醇和50%乙醇水冷浸分别提取5次,将浓缩得到浸膏分散于水中,然后依次用石油醚、乙酸乙酯、正丁醇萃取。乙酸乙酯部分浓缩得24.6 g浸膏进行硅胶柱层析,用氯仿-甲醇(20:1,10:1,8:1,6:1,4:1,2:1,0:1)梯度洗脱,得到A-G 7个组分。Fr.C反复经硅胶柱层析(石油醚-乙酸乙酯)、凝胶柱层析,得化合物1(145 mg)和化合物2(15 mg)。Fr.G反复经硅胶柱层析(石油醚-乙酸乙酯)、凝胶和ODS柱层析,得化合物3(8 mg)和化合物4(12 mg)。
3 实验结果
化合物1:白色粉末。mp:208~210℃。1H NMR(400 MHz,Pyridine-d5)δ:1.60(m,1H,H-1a),1.09(m,1H,H-1b),1.98~1.89(m,2H,H-2),4.28~4.22(m,1H,H-3a),1.55(d,J=4.2 Hz,1H,H-5),1.46~1.41(m,1H,H-6a),1.70~1.62(m,1H,H-6b),1.36(m,1H,H-7a),1.47(m,1H,H-7b),1.79~1.69(m,1H,H-9),2.01~1.95(m,2H,H-11),5.51(t,J=3.7 Hz,1H,H-12),2.39~2.28(m,1H,H-15a),1.17~1.14(m,1H,H-15b),2.00(m,1H,H-16a),2.11(m,1H,H-16b),2.65(d,J= 11.3 Hz,1H,H-18),1.50~1.45(m,1H,H-19a),1.01(m,1H,H-20b),1.34~1.27(m,1H,H-21a),1.85~1.81(m,1H,H-21b),2.00~1.95(m,2H,H-22),1.24(s,3H,H-23),1.09(s,3H,H-24),0.98(s,3H,H-25),1.01(s,3H,H-26),1.19(s,3H,H-27),4.21(d,J=10.5 Hz,1H,H-28a),3.75(d,J=10.8 Hz,1H,H-28b),1.07(d,J=2.7 Hz,3H,H-29),0.95(d,J=5.8 Hz,3H,H-30);13C NMR(100 MHz,Pyridine-d5)δ:39.12(C-1),27.92(C-2),73.54(C-3),43.10(C-4),48.70(C-5),18.77(C-6),31.27(C-7),43.11(C-8),48.24(C-9),37.33(C-10),23.87(C-11),125.87(C-12),139.47(C-13),42.73(C-14),28.91(C-15),25.11(C-16),40.18(C-17),53.76(C-18),39.67(C-19),39.61(C-20),33.47(C-21),37.66(C-22),26.37(C-23),13.41(C-24),16.32(C-25),17.72(C-26),24.11(C-27),68.00(C-28),17.70(C-29),21.61(C-30)。上述波谱数据与文献〔6〕报道的数据基本一致,故鉴定化合物1为3β,28-二羟基-乌苏烷。
化合物2:无色片状结晶(甲醇)。mp:271~273℃。1H NMR(400 MHz,DMSO-d6)δ:0.87(d,J=1.7 Hz,1H,H-1a),1.52~1.49(m,1H,H-1b),1.49~1.42(m,2H,H-2),3.48~3.40(m,1H,H-3a),1.50~1.48(m,1H,H-5),1.13~1.07(m,1H,H-6a),1.43~1.35(m,1H,H-6b),1.22~1.15(m,1H,H-7a),1.49~1.42(m,1H,H-7b),1.13~1.07(m,1H,H-9),1.89~1.77(m,2H,H-11),5.12(t,J=3.7 Hz,1H,H-12),0.98(dd,J=13.4,3.2 Hz,1H,H-15a),1.83~1.74(m,1H,H-15b),1.93(td,J=13.1,4.1 Hz, 1H,H-16a),1.54~1.48(m,1H,H-16b),2.10(d,J=11.2 Hz,1H,H-18),1.34~1.22(m,1H,H-19a),0.91(m,1H,H-20b),1.43~1.35(m,1H,H-21a),1.36~1.21(m,1H,H-21b),1.58~1.50(m,2H,H-22),3.32(d,J=10.3 Hz,1H,H-23a),3.07(d,J=10.4 Hz,1H,H-23b),0.53(s,3H,H-24),0.88(s,3H,H-25),0.74(s,3H,H-26),1.04(s,3H,H-27),0.81(d,J=6.5 Hz,3H,H-29),0.91(d,J= 2.3 Hz,3H,H-30);13C NMR(100 MHz,DMSO-d6)δ:38.13(C-1),26.60(C-2),70.33(C-3),41.71(C-4),47.04(C-5),17.50(C-6),32.29(C-7),39.00(C-8),46.41(C-9),36.36(C-10),22.90(C-11),124.62(C-12),138.24(C-13),41.86(C-14),27.56(C-15),23.84(C-16),46.84(C-17),52.41(C-18),38.53(C-19),38.46(C-20),30.21(C-21),36.24(22),64.48(C-23),12.72(C-24),15.64(C-25),17.08(C-26),23.35(C-27),178.31(C-28),16.99(C-29),21.11(C-30)。上述波谱数据与文献〔7〕报道的数据基本一致,故鉴定化合物2为3β,23-二羟基-12-烯-28-酸。
化合物3:白色粉末。mp:187~190℃。1HNMR(400 MHz,CD3OD)δ:1.66(m,1H,H-1a),1.59(m,1H,H-1b),1.59~1.54(m,2H,H-2),3.11(dd,J=11.1,5.0 Hz,1H,H-3a),0.72~0.69(m,1H,H-5),1.52(m,1H,H-6a),1.39(m,1H,H-6b),1.32~1.22(m,1H,H-7a),1.49~1.46(m,1H,H-7b),1.53(m,1H,H-9),1.92~1.87(m,2H,H-11),5.22~5.17(m,1H,H-12),1.89~1.82(m,1H,H-15a),1.09~1.01(m,1H,H-15b),2.06~1.97(m,1H,H-16a),1.66~1.60(m,1H,H-16b),2.16(d,J=11.4 Hz,1H,H-18),1.32(q,J=3.1 Hz,1H,H-19a),0.94~0.93(m,1H,H-20b),1.32~1.22(m,1H,H-21a),1.50~1.46(m,1H,H-21b),1.66~1.60(m,2H,H-22),0.74(s,3H,H-23),0.92(s,3H,H-24),0.81(s,3H,H-25),0.81(s,3H,H-26),1.08(s,3H,H-27),0.94(s,3H,H-28),0.85(d,J=6.5 Hz,3H,H-29),0.93(d,J=2.8 Hz,3H,H-30);13C NMR(100 MHz,CD3OD)δ:38.12(C-1),27.89(C-2),79.68(C-3),39.84(C-4),56.73(C-5),19.48(C-6),34.33(C-7),40.77(C-8),48.99(C-9),40.54(C-10),24.36(C-11),126.86(C-12),139.64(C-13),43.24(C-14),29.21(C-15),25.33(C-16),31.63(C-17),54.36(C-18),40.42(C-19),40.43(C-20),31.78(C-21),38.10(C-22),16.40(C-23),28.78(C-24),16.04(C-25),17.81(C-26),24.11(C-27),28.73(C-28),17.67(C-29),21.60(C-30)。上述波谱数据与文献〔8〕报道的数据基本一致,故鉴定化合物3为a-香树精。
化合物4:白色粉末。1H NMR(400 MHz,CD3OD)δ:1.16(m,1H,H-1a),1.75~1.69(m,1H,H-1b),1.46~1.37(m,1H,H-2a),1.60~1.50(m,1H,H-2b),3.90(dd,J=11.6,5.7 Hz,1H,H-3a),1.59~1.55(m,1H,H-5),1.52~1.48(m,2H,H-6),1.50~1.44(m,1H,H-7a),1.34(dd,J=11.4,3.5 Hz,1H,H-7b),1.72~1.67(m,1H,H-9),1.97(m,1H,H-11a),1.64(m,1H,H-11b),5.22(t,J=3.7 Hz,1H,H-12),1.96~1.89(m,1H,H-15a),1.12~1.03(m,1H,H-15b),1.67(m,1H,H-16a),2.03(m,1H,H-16b),2.18(d,J=11.5 Hz,1H,H-18),1.39~1.27(m,1H,H-19a),0.98~0.94(m,1H,H-20b),1.89~1.80(m,1H,H-21a),1.39(m,1H,H-21b),1.76~1.62(m,2H,H-22),5.00(s,1H,H-23a),4.58(s,1H,H-23b),0.73(s,3H,H-25),0.86(s,3H,H-26),1.11(s,3H,H-27),0.93(d,J=2.0 Hz,3H,H-29),0.84(d,J=6.4 Hz,3H,H-30);13C NMR(100 MHz,CD3OD)δ:39.92(C-1),32.89(C-2),73.72(C-3),154.32(C-4),51.56(C-5),22.32(C-6),31.79(C-7),40.95(C-8),46.42(C-9),39.71(C-10),25.28(C-11),127.05(C-12),139.88(C-13),43.48(C-14),29.16(C-15),25.34(C-16),49.07(C-17),54.51(C-18),40.42(C-19),40.39(C-20),33.13(C-21),38.10(C-22),102.91(C-23),14.18(C-25),17.69(C-26),24.07(C-27),181.68(C-28),21.59(C-29),17.82(C-30)。上述波谱数据与文献〔9〕报道的波谱数据基本一致,故鉴定化合物4为3β-羟基-24-降-乌苏-4(23),12-二烯-28-酸。
〔1〕何银堂,胡作亮.本草名释与传说〔M〕.北京:中国中医药出版社,1998.
〔2〕大理白族自治州人民政府.大理中药资源志〔M〕.昆明:云南民族出版社,1991.
〔3〕刘晓波,郭美仙,李龙星,等.双参降血糖作用的研究〔J〕.云南中医中药杂志,2008,29(5):49-50.
〔4〕刘晓波,郭美仙,施贵荣,等.双参降血糖作用机制研究〔J〕.安徽农业科学,2012,40(33):16111-16112.
〔5〕刘晓波,郭美仙,施贵荣.鸡肉参对小鼠抗应激作用的实验研究〔J〕.中国药事,2008,22(8):659-660.
〔6〕王福东,丁兰,汪汉卿.蓝萼香茶菜三萜成分的研究〔J〕.中国中药杂志,2005,30(24):1929-1932.
〔7〕宋家玲,杨永建,戚欢阳,等.栀子花化学成分研究〔J〕.中药材,2013,36(5):752-755.
〔8〕COSTA H N R D,SANTOS M C D,ALCÂNTARA A F D C,et al.Constituintes químicos e atividade antiedema⁃togênica de Peltodon radicans(Lamiaceae)〔J〕.Química Nova,2008,31(4):744-750.
〔9〕王强,刘二伟,韩立峰,等.中药川续断化学成分的研究〔J〕.药学学报,2013,48(7):1124-1127.
Study on Trierpenoid Compositions of MedicinalPlants Triplostegia glandulifera
Zhang Zuzhen,Chen Yafeng,Wang Fusheng*
(College of Pharmacy and Chemistry,Dali University,Dali,Yunnan 671000,China)
Objective:To study the chemical compositions of the Medicinal Plants Triplostegia glandulifera.Methods:The compounds were isolated and purified by silica gel,Sephadex LH-20 and ODS column chromatography methods,and their structures were identified by modern spectroscopy techniques.Results:Four trierpenoid compositions were isolated from ethylacetate fraction of the underground part of T.glandulifera and identified as 3β,28-dihydroxy-ursane(1),3β,23-Dihydroxyurs-12-en-28-oic-acid(2),α-Amirina(3),and 3β-hydroxy-24-nor-urs-4(23),12-dien-28-oic acid(4).Conclusion:Four trierpenoid compositions were isolated from this genus for the firsttime.
Triplostegia glandulifera;underground part;trierpenoid compositions
R932
A
2096-2266(2017)04-0006-03
10.3969/j.issn.2096-2266.2017.04.002
(责任编辑 李 杨)
国家自然科学基金资助项目(31360082)
2016-08-16
2016-12-23
张祖珍,硕士研究生,主要从事天然药物化学研究.
*通信作者:王福生,教授,博士.
