病毒诱导的全新 tae-miR9663的沉默影响叶片发育和籽粒大小
2017-05-15朱妍峰赵惠贤
朱妍峰,王 倩,赵惠贤,2
(1.西北农林科技大学生命科学学院,陕西杨陵 712100; 2.西北农林科技大学旱区作物逆境生物学国家重点实验室,陕西杨陵 712100)
病毒诱导的全新 tae-miR9663的沉默影响叶片发育和籽粒大小
朱妍峰1,王 倩1,赵惠贤1,2
(1.西北农林科技大学生命科学学院,陕西杨陵 712100; 2.西北农林科技大学旱区作物逆境生物学国家重点实验室,陕西杨陵 712100)
MicroRNA(miRNA)是一类长度为18~24 nt的非编码小分子RNA,参与植物各种发育进程。 tae-miR9663是新发现的在小麦幼苗、旗叶和籽粒中高表达的miRNA,但其生物学功能未知。为了探索 tae-miR9663的功能,通过人工合成 tae-miR9663的小串联模拟靶标(short tandem target mimic, STTM),将其构建到大麦条斑花叶病毒(barley stripe mosaic virus, BSMV)载体上,利用病毒介导的基因沉默(virus-inducing gene silencing, VIGS)技术转染小麦宁春16五叶一心期的第5片叶,转染20 d后观察叶片表型并取旗叶进行实时定量PCR,成熟时观察种子大小。叶片表型观察结果表明,与BSMV:00相比,接种BSMV:STTM- tae-miR9663的9株幼苗中出现4种叶片表型,即第6片叶有白点或白条纹,旗叶(第7片叶)有白点或白条纹,第6片叶边缘有锯齿状,旗叶叶尖处有皱缩。实时定量PCR分析结果表明,STTM- tae-miR9663过表达植株的 tae-miR9663表达丰度下降,说明BSMV-VIGS技术可通过过表达STTM有效地沉默内源miRNA。成熟种子大小观察结果表明,与BSMV:00比较,接种BSMV:STTM- tae-miR9663的植株种子的长和宽均减小。
小麦; tae-miR9663;STTM;病毒诱导的基因沉默(VIGS)技术
miRNA是一类由18~24 nt核酸构成的非编码小分子RNA,通过切割降解靶基因或抑制靶基因的翻译起到调控作用[1]。miRNA参与植物各种发育进程,如种子萌发[2]、根发育[3]、叶的发育与极性[4]、以及花器官的发育[5]。目前,已从小麦多个组织中鉴定出大量miRNA,如豫麦18花后5、15、25、30 d籽粒的miRNA[6],根、成熟叶片、小穗的组织特异性表达的miRNA[7],叶片中参与非生物胁迫的miRNA[8]。通过对小麦品种小偃6号的幼苗、旗叶和花后5、10、20 d籽粒中的miRNA进行高通量深度测序和分析,鉴定出109个植物间保守的已知miRNA(分属于49个miRNA家族)和113个小麦中新发现的miRNA。 tae-miR9663是其中新发现的一个miRNA,其在幼苗、旗叶和发育的籽粒中都高表达,尤其在旗叶和籽粒中表达量最高[9-10],但 tae-miR9663在小麦旗叶和籽粒生长发育过程中的生物学功能尚不清楚。
病毒诱导的基因沉默(virus induced gene silencing, VIGS)是指携带目的基因的重组病毒载体侵染植物后,可诱导植物内源基因发生沉默而引起表型变化,从而有助于研究目的基因的功能[11]。研究表明,大麦条纹花叶病毒(barley stripe mosaic virus, BSMV)可作为VIGS的有效载体,它的基因组包含α、β、γ三个部分,其中经修饰的γ分子是最常用的载体[12]。与传统的基因功能分析方法相比,VIGS可以在植物当代对目标基因进行沉默,具有简单、有效、高通量等优点[13],已被广泛用于小麦基因功能分析[12, 14-16],但是利用VIGS技术进行小麦miRNA研究的报道较少[17]。
鉴于此,本研究首先人工合成新发现的小麦 tae-miR9663的小串联模拟靶标(short tandem target mimic, STTM),然后插入BSMV-γ载体上获得BSMV:STTM- tae-miR9663,体外转录后转染小麦叶片,实施BSMV诱导的通过过表达STTM而沉默小麦内源 tae-miR9663,以快速分析 tae-miR9663对小麦旗叶和籽粒生长发育的作用,为小麦超高产分子育种奠定理论基础。
1 材料与方法
1.1 材 料
本研究所用材料为春小麦品种宁春16,将发芽一周的小麦幼苗在4 ℃春化2周后,转移至温室培养。温室条件为24 ℃(昼)/20 ℃(夜),光照16 h·d-1。
BSMV基因组由a、β、γ三条RNA组成,BSMV病毒载体α、β、γ质粒由美国蒙塔那州立大学Huang L博士惠赠。
1.2 目的基因片段的克隆
根据miRBase(http://www.mirbase.org/)中小麦 tae-miR9663的成熟序列和Yan等[18]的方法设计 tae-miR9663 STTM序列,并由上海生工生物工程股份有限公司合成。以合成的质粒为模板,利用引物STTM- tae-miR9663-F/STTM- tae-miR9663-R(STTM- tae-miR9663-F:5′-GTTGTG TGGAATGTATGGAGC-3′,STTM- tae-miR9663-R:5′-GCTGTAATCACACTGGCTCA-3′,均由上海英俊生物技术有限公司合成。)进行目的片段STTM- tae-miR9663的扩增。扩增产物经1%琼脂糖凝胶电泳分离后,利用胶回收试剂盒(天根)回收目的片段。
1.3 BSMV载体的构建
Ma等[19]将Huang L.博士惠赠的γ载体改造后,使其序列含有两个XcmⅠ酶切位点,被XcmⅠ(NEB)切割后使其线性化,然后通过TA克隆方法连接胶回收的STTM- tae-miR9663,转化DH5α感受态细胞(天根),菌落PCR检测,引物为γ-F:5′-GTGATCAACTGCCAATCGTG-3′,γ-R:5′-GTTTCCAATTCAGGCATCGT-3′。测序正确的重组载体命名为γ-STTM- tae-miR9663。
1.4 BSMV转染小麦
用MluⅠ线性化BSMV-α、γ质粒,用SpeⅠ线性化BSMV-β质粒,用BssHⅡ线性化γ-STTM- tae-miR9663质粒。参照试剂盒说明书进行体外转录(NEB),按a、β、γ各10 μL转录物等量混合,共30 μL,命名为BSMV:00;按a、β、γ-STTM- tae-miR9663各10 μL转录物等量混合,共30 μL,命名为BSMV:STTM- tae-miR9663。将配好的病毒混合液置于冰上,及时接种。将要接种前再向30 μL混合病毒液中加220 μL的GKPbuffer(50 mmol·L-1Gly、30 mmol·L-1磷酸氢二钾、1%膨润土、1%硅藻土),制得250 μL的接种病毒混合液。转染时用刚开封的乳胶手套,摩擦接种于宁春16小麦五叶一心期的第5片叶。病毒转染小麦后,每天观察、记录转染植株叶片和穗部的表型变化。
1.5 BSMV转染小麦后的qRT-PCR分析
采用茎环法[20]设计 tae-miR9663的反转录及后续qRT-PCR分析所用引物(表1)。利用RNAiso Plus(TAKARA)提取小麦叶片总RNA,经DNA酶消化后,通过cDNA第一链合成试剂盒(康为世纪)合成cDNA。以看家基因GADPH为内参,采用BioRad-IQ5型实时荧光定量PCR仪进行扩增。经过3次生物学重复和3次技术重复,得到 tae-miR9663的Ct值,然后对数据进行2-ΔΔCt相对定量分析,检测目的基因的表达水平。
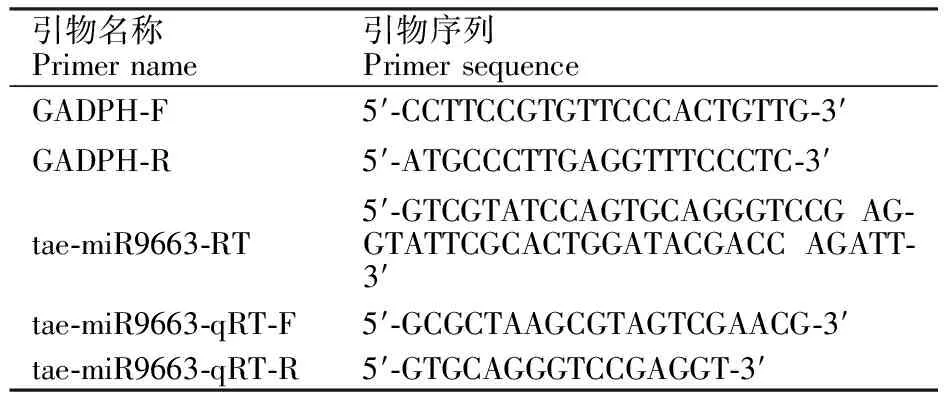
表1 反转录和qRT-PCR分析所用引物
2 结果与分析
2.1 重组BSMV:STTM- tae-miR9663载体的构建
以上海生工生物工程股份有限公司合成的含有 tae-miR9663 STTM序列的质粒为模板,采用引物STTM- tae-miR9663-F/STTM- tae-miR9663-R进行 PCR扩增,得到长度为96 bp的STTM- tae-miR9663序列,经纯化、回收后连接到γ载体的TA克隆位点(TACS)上,转化DH5α(图1)。用引物γ-F和γ-R进行菌落PCR,鉴定重组转化子。测序证实获得正确的重组载体γ-STTM- tae-miR9663。
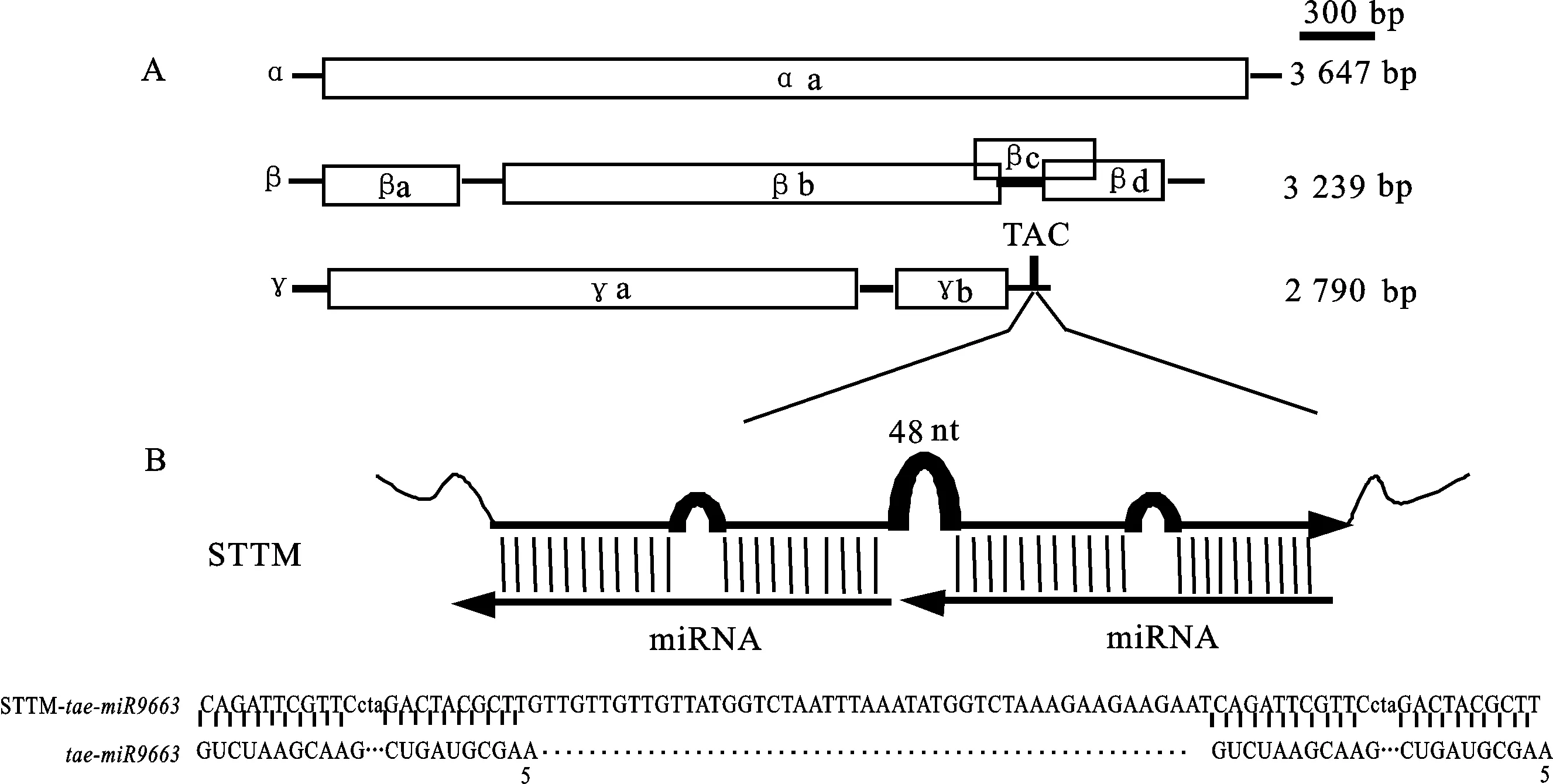
A:BSMV由a、β、γ三条链组成,用长方框表示其开放阅读框,标尺为300 bp;TACS为连接插入片段的TA克隆位点。B:STTM包含2个重复的模拟靶标和一段48 nt的间隔序列。
A:Genomic organization of three BSMV components, including α, β and γ, and open reading frames are indicated by boxes with scale of 300 bp; TACS is TA cloning site designed for direct cloning of inserts. B:STTM structure contains two tandem target mimics separated by a 48 nt stem-loop linker.
图1 BSMV基因组结构及插入片段STTM- tae-miR9663的示意图
Fig.1 Schematic organization of the BSMV genomes and STTM- tae-miR9663
2.2 重组BSMV:STTM- tae-miR9663转染后小麦叶片表型及 tae-miR9663表达丰度的变化
待宁春16小麦生长至五叶一心期时,第五片叶分别接种空病毒BSMV:00和含有外源目的片段的重组病毒BSMV:STTM- tae-miR9663,各接种9株。接种BSMV:STTM- tae-miR9663的9个植株分别编号为9663-1、9663-2、9663-3、9663-4、9663-5、9663-6、9663-7、9663-8和9663-9。接种20 d后,将接种BSMV:STTM- tae-miR9663的9个植株与接种BSMV:00的植株对比,产生四种不同的表型,即第6片叶有白点或白条纹,第6片叶边缘有锯齿状,旗叶(即第7片叶)有白点或白条纹,旗叶叶尖处有皱缩(图2、表2)。同时,取接种小麦植株的旗叶,以GADPH基因作为内参,通过实时定量PCR检测 tae-miR9663的表达丰度,发现与对照小麦植株相比, tae-miR9663的表达丰度均下降(表2)。
2.3 重组BSMV:STTM- tae-miR9663转染后小麦籽粒的变化
与BSMV:00相比,BSMV:STTM- tae-miR9663转染后的小麦成熟籽粒变小(图3),籽粒的平均长度、宽度极显著降低,平均粒重显著降低(表3、图4)。
A:第6片叶有白点或白条纹;B:第6片叶边缘有锯齿状;C:旗叶有白点或白条纹;D:旗叶叶尖处有皱缩。
A:White dots or white stripes on the sixth leaf; B:Serrate at the sixth leaf margins; C:White dots or white stripes on flag leaf; D:Shrinking on flag leaf blade tips.

图2 BSMV:STTM- tae-miR9663转染后小麦叶片出现的四种表型
“+”表示该植株出现此表型;“-”表示该植株未出现此表型。
“+”represents the phenotypes occurred on the plant; “-”represents that the phenotypes did not occur on the plant.

图3 BSMV:00和BSMV:STTM- tae-miR9663转染后的小麦成熟籽粒
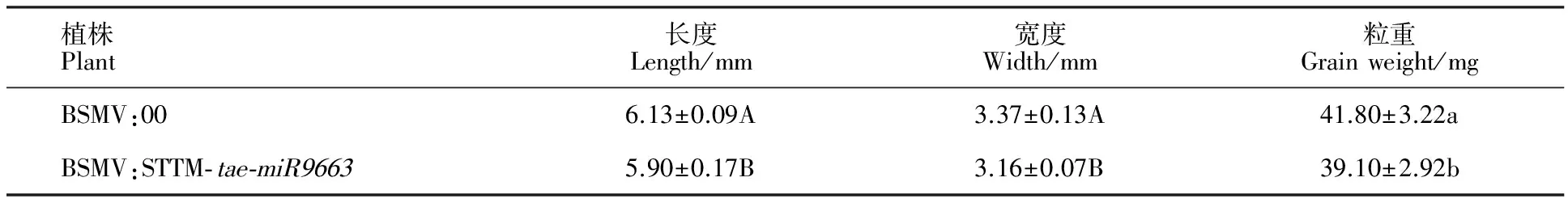
植株Plant长度Length/mm宽度Width/mm粒重Grainweight/mgBSMV:006.13±0.09A3.37±0.13A41.80±3.22aBSMV:STTM⁃tae⁃miR96635.90±0.17B3.16±0.07B39.10±2.92b
表中数据为从3个重复中的3个植株中随机挑选的10粒种子的平均值;数据后不同大、小写字母表示差异在0.01和0.05水平上显著。
The data in the table was obtained by measuring ten seeds randomly from three plants in triplicates;Different capital or low-case letters following values indicated significant difference at 0.01 and 0.05 levels,respectively.
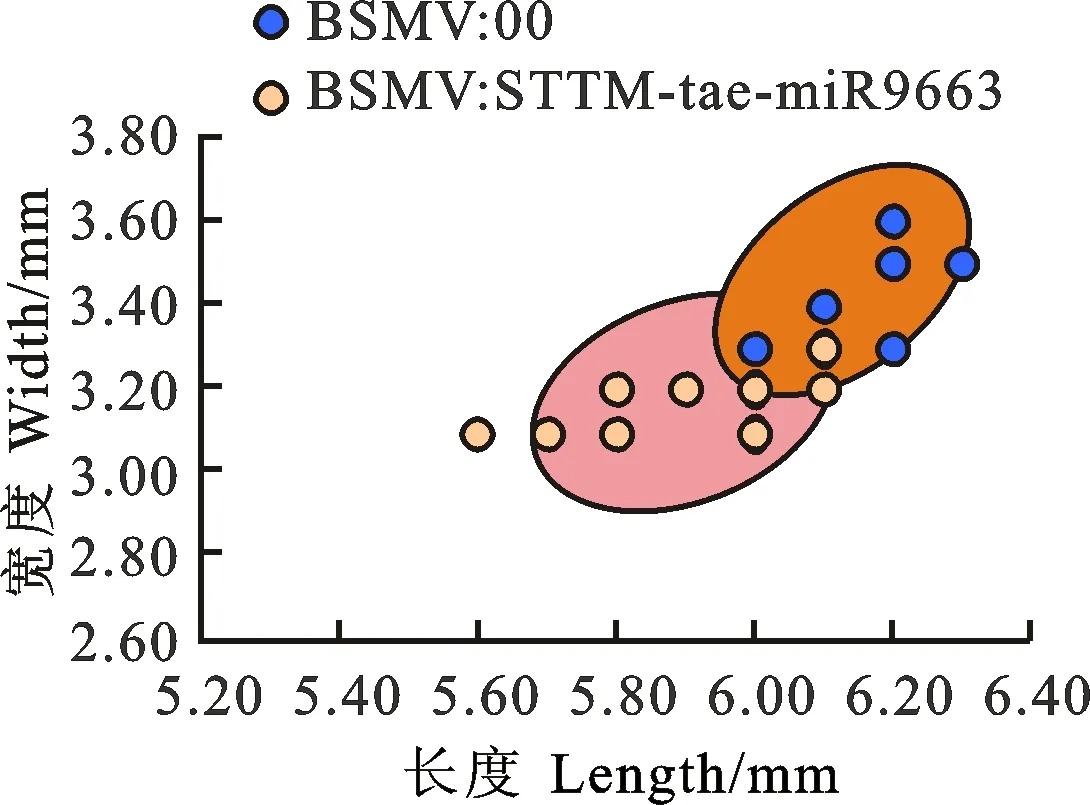
图中的点代表从3个重复中的3个植株中随机挑选的成熟籽粒。
The dots in the figure represent mature seeds chosen randomly from three plants in triplicates.
图4 比较BSMV:00和BSMV:STTM- tae-miR9663转染后的小麦成熟籽粒的长和宽
Fig.4 Comparison of length and width of mature seeds from wheat inoculated by BSMV:00 and BSMV:STTM- tae-miR9663
3 讨 论
miRNA调控植物生长、发育,参与生物或非生物胁迫[21-23]。通过高通量测序已鉴定出大量的小麦miRNA[10, 24-25],其中新发现的一部分miRNA的表达量很高,如 tae-miR9663在小麦幼苗、旗叶和发育的籽粒中的表达量分别为1 634、5 562和10 441 RPM[10],但它们的功能尚不清楚。高表达量表明它们可能在小麦生长发育过程中起到重要作用,因此鉴定它们的功能就显得非常重要。然而稳定的转基因小麦的获得费力且效率低,而BSMV-VIGS技术具有操作简单、周期短、不需要遗传转化等优点,被广泛应用于小麦基因功能的快速分析[26-27],为后期转基因小麦的筛选奠定基础。
近年来,STTM的应用为植物miRNA功能研究提供了一种高效的沉默机制[17],但利用VIGS技术,通过过表达STTM而有效沉默植物内源miRNA的报道较少,而在烟草、棉花和小麦中用病毒载体分别将miR171c、miR165/166和小麦内源miRNA的STTM序列过表达后均获得良好的沉默效果[28-29, 17]。本研究通过构建BSMV:STTM- tae-miR9663,利用VIGS技术转染小麦五叶一心期的第5片叶,转染20 d后取旗叶进行实时定量PCR检测,结果发现,与BSMV:00相比,STTM- tae-miR9663过表达植株 tae-miR9663的表达量下降45%~97%。这表明利用STTM沉默小麦miRNA的方法很有效,可被推广应用。STTM序列包含两个TM和一段间隔序列。研究表明,48 nt间隔序列的STTM效果较好且应用较广泛[17-18, 29-31]。而张 力等利用间隔序列为31 nt的STTM也有效地阻止烟草microRNA171c对其靶基因的切割而沉默内源miRNA[28]。本研究利用VIGS技术,通过过表达48 nt间隔序列的STTM有效沉默小麦内源 tae-miR9663。除STTM外,还可使用MIMICS和SPONGES等方法获得miRNA缺失功能的植物。研究表明,MIMICS和STTM的沉默效率会变化。不同的miRNA家族对这3种不同的方法的反应不同,没有一个方法对所有的检测的miRNA工作得都好[32]。因此用STTM沉默植物内源miRNA的效率不理想时,可采取尝试其他两种方法。
tae-miR9663在小麦旗叶中高表达[10],而且通过过表达其STTM沉默后引起叶片出现白点或白条纹、叶边缘锯齿状和叶尖处皱缩的表型。miRNA是通过抑制其靶基因的表达来行使其生物学功能的。我们的降解组数据里没有 tae-miR9663的靶基因的相关信息(数据未发表)。利用patmatch软件预测 tae-miR9663的靶基因为MCTP2(Multiple C2 and transmembrane domain-containing protein 2)。MCTP是在进化上保守的Ca2+依赖型C2结构域蛋白的一个新家族,由一个可变的N端序列、三个C2结构域、两个跨膜域和一个短的C端序列组成。C2结构域蛋白通过两个紧密间隔的跨膜域被锚定在膜上,是仅次于EF-手型结构的第二大类Ca2+感受器[33]。然而该蛋白在植物的生长发育过程中的生物学功能尚未报道。叶片出现白点或白条纹可能是接种病毒后出现的比对照更明显的病斑,也可能是过表达 tae-miR9663的STTM后,Ca2+结合蛋白钙调素(calmodulin, CaM)增多,而CaM与光合作用有关[34],可能抑制了光合色素的产生。叶片出现锯齿状和皱缩的表型可能是由于靶基因增多使得植物为了防御刺激而产生的结构。通过比较BSMV:00和BSMV:STTM- tae-miR9663接种植株的成熟种子,我们发现BSMV:STTM- tae-miR9663接种植株种子的长和宽均减小。从接种BSMV开始30 d内病毒发挥作用,本研究接种BSMV 25 d植株开始孕穗,35 d开始抽穗,因此BSMV:STTM- tae-miR9663接种植株的种子变小可能是由于上述原因影响了旗叶发育,而旗叶为籽粒提供营养,进而影响了籽粒发育,也可能是 tae-miR9663 STTM的过表达直接影响籽粒发育。综上所述, tae-miR9663对小麦旗叶和籽粒发育起重要调控作用,它的发现具有重要意义,但还有许多尚未解决的问题,如 tae-miR9663 STTM过表达的转基因小麦的表型和 tae-miR9663靶基因的生物学功能的鉴定等,值得进一步深入研究。
[1] YANG X J,ZHAO Y M,XIE D Y,etal.Identification and functional analysis of microRNAs involved in the anther development in cotton genic male sterile line Yu98-8A [J].InternationalJournalofMolecularSciences,2016,17(10):1677.
[2] WANG L W,LIU H H,LI D T,etal.Identification and characterization of maize microRNAs involved in the very early stage of seed germination [J].BMCGenomics,2011,12:154.
[3] HEWEZI T,MAIER T R,NETTLETON D,etal.TheArabidopsismicroRNA396-GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection [J].PlantPhysiology,2012,159(1):321.
[4] LIU Q L,YAO X Z,PI L M,etal.The ARGONAUTE10 gene modulates shoot apical meristem maintenance and establishment of leaf polarity by repressing miR165/166 inArabidopsis[J].ThePlantJournal,2009,58(1):27.
[5] ZHU Q H,UPADHYAYA N M,GUBLER F,etal.Over-expression of miR172 causes loss of spikelet determinacy and floral organ abnormalities in rice(Oryzasativa) [J].BMCPlantBiology,2009,9:149.
[6] MENG F R,LIU H,WANG K T,etal.Development-associated microRNAs in grains of wheat(TriticumaestivumL.) [J].BMCPlantBiology,2013,13:140.
[7] PANDEY R,JOSHI G,BHARDWAJ A R,etal.A comprehensive genome-wide study on tissue-specific and abiotic stress-specific miRNAs inTriticumaestivum[J].PLoSOne,2014,9(4):e95800.
[8] MA X L,ZIN Z Y,YANG Q H,etal.Identification and comparative analysis of differentially expressed miRNAs in leaves of two wheat(TriticumaestivumL.) genotypes during dehydration stress [J].BMCPlantBiology,2015,15:21.
[9] 闫 妍,韩 冉,赵惠贤.4条小麦保守microRNAs的表达谱分析及其靶基因预测[J].麦类作物学报,2012,32(6):1041.
YAN Y,HAN R,ZHAO H X.Expression profile analysis and target gene prediction of four conserved microRNAs in wheat [J].JournalofTriticeaeCrops,2012,32(6):1041.
[10] HAN R,JIAN C,LV J Y,etal.Identification and characterization of microRNAs in the flag leaf and developing seed of wheat(TriticumaestivumL.)[J].BMCGenomics,2014,15:289.
[11] UNVER T,BUDAK H.Virus-induced gene silencing,a post transcriptional gene silencing method [J].InternationalJournalofPlantGenomics,2009,2009:198680.
[12] SCOFIELD S R,HUANG L,BRANDT A S,etal.Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway [J].PlantPhysiology,2005,138(4):2166.
[13] BENNYPAUL H S,MUTTI J S,RUSTGI S,etal.Virus-induced gene silencing(VIGS) of genes expressed in root,leaf,and meiotic tissues of wheat [J].Functional&IntegrativeGenomics,2012,12(1):144.
[14] 赵 丹,赵继荣,黄 茜,等.利用BSMV-VIGS技术快速分析小麦 TNBL1基因的抗黄矮病功能[J].作物学报,2011,37(11):2106.
ZHAO D,ZHAO J R,HUANG X,etal.Functional analysis of TNBL1 gene in wheat defense response to barley yellow dwarf virus using BSMV-VIGS technique [J].ActaAgronomicaSinica,2011,37(11):2106.
[15] CHEN K M,LI H W,CHEN Y F,etal. TaSCL14,a novel wheat(TriticumaestivumL.) GRAS gene,regulates plant growth,photosynthesis,tolerance to photooxidative stress,and senescence [J].JournalofGeneticsandGenomics,2015,42(1):21.
[16] BUHROW L M,CLARK S M,LOEWEN M C.Identification of an attenuated barley stripe mosaic virus for the virus-induced gene silencing of pathogenesis-related wheat genes [J].PlantMethods,2016,12:12.
[17] JIAO J,WANG Y C,SELVARAJ J N,etal.Barley stripe mosaic virus(BSMV) induced microRNA silencing in common wheat(TriticumaestivumL.) [J].PLoSOne,2015,10(5):e0126621.
[18] YAN J,GU Y Y,JIA X Y,etal.Effective small RNA destruction by the expression of a short tandem target mimic inArabidopsis[J].PlantCell,2012,24(2):415.
[19] MA M,YAN Y,HUANG L,etal.Virus-induced gene-silencing in wheat spikes and grains and its application in functional analysis of HMW-GS-encoding genes [J].BMCPlantBiology,2012,12:141.
[20] CHEN C F,RIDZON D A,BROOMER A J,etal.Real-time quantification of microRNAs by stem-loop RT-PCR [J].NucleicAcidsResearch,2005,33(20):e179.
[21] FENG H,WANG B,ZHANG Q,etal.Exploration of microRNAs and their targets engaging in the resistance interaction between wheat and stripe rust [J].FrontiersinPlantScience,2015,6:469.
[22] KAUR A,GUPTA O P,MEENA N L,etal.Comparative temporal expression analysis of micrornas and their target genes in contrasting wheat genotypes during osmotic stress [J].AppliedBiochemistryandBiotechnology,2017,181(2):613.
[23] DENG P C,BIAN J X,YUE H,etal.Characterization of microRNAs and their targets in wild barley(Hordeumvulgaresubsp.spontaneum)using deep sequencing [J].Genome,2016,59(5):339.
[24] CHEN F,ZHANG X F,ZHANG N,etal.Combined small RNA and degradome sequencing reveals novel MiRNAs and their targets in the high-yield mutant wheat strain Yunong 3114 [J].PLoSOne,2015,10(9):1.
[25] LIU H P,SEARLE L R,WATSON-HAIGH N S,etal.Genome-wide identification of microRNAs in leaves and the developing head of four durum genotypes during water deficit stress [J].PLoSOne,2015,10(11):1.
[26] 李建嫄,张立荣,张 娜,等.利用VIGS技术分析TcLr19中 TaSTKC8基因的抗叶锈性功能[J].河北农业大学学报,2015,38(5):12.
LI J Y,ZHANG L R,ZHANG N,etal.Functional analysis of TaSTKC8 gene on wheat leaf rust resistance in TcLr19 by virus induced gene silencing(VIGS) [J].JournalofAgriculturalUniversityofHebei,2015,38(5):12.
[27] LEE W-S,RUDD J J,KANYUKA K.Virus induced gene silencing(VIGS) for functional analysis of wheat genes involved inZymoseptoriatriticisusceptibility and resistance [J].FungalGeneticBiology,2015,79:84.
[28] 张 力,沙爱华.烟草microRNA171c的功能分析[J].植物科学学报,2016,34(5):775.
ZHANG L,SHA A H.Functional analysis of microRNA171c in tobacco [J].PlantScienceJournal,2016,34(5):775.
[29] GU Z H,HUANG C J,LI F F,etal.A versatile system for functional analysis of genes and microRNAs in cotton [J].PlantBiotechnologyJournal,2014,12(5):638.
[30] SHA A H,ZHAO J P,YIN K Q,etal.Virus-based microRNA silencing in plants [J].PlantPhysiology,2014,164(1):37.
[31] TANG G L,YAN J,GU Y Y,etal.Construction of short tandem target mimic(STTM) to block the functions of plant and animal microRNAs [J].Methods,2012,58(2):124.
[32] REICHEL M,LI Y J,LI J Y,etal.Inhibiting plant microRNA activity:molecular SPONGEs,target MIMICs and STTMs all display variable effcacies against target microRNAs [J].PlantBiotechnologyJournal,2015,13(7):915.
[33] SHIN O H,HAN W P,WANG Y,etal.Evolutionarily conserved multiple C2 domain proteins with two transmembrane regions(MCTPs) and unusual Ca2+binding properties [J].TheJournalofBiologicalChemistry,2005,280(2):1641.
[34] 周 卫,汪 洪.植物钙吸收、转运及代谢的生理和分子机制[J].植物学通报,2007,24(6):771.
ZHOU W,WANG H.The physiological and molecular mechanisms of calcium uptake,transport,and metabolism in plants [J].ChineseBulletinofBotany,2007,24(6):771.
Silencing of tae-miR9663 in Wheat by VIGS Affects Leaf Development and Seed Size
ZHU Yanfeng1, WANG Qian1, ZHAO Huixian1, 2
(1.College of Life Science, Northwest A&F University, Yangling, Shaanxi 712100, China; 2.Northwest A&F University, State Key Laboratory of Crop Stress Biology for Arid Areas, Yangling, Shaanxi 712100, China)
MicroRNA (miRNA), which is a class of non-coding small RNA, with 18 to 24 nt in length, regulates various developmental processes in plants. tae-miR9663 is a novel miRNA highly expressing in seedling, flag leaf and seed in wheat, but its biological function is unknown. In order to explore the function of tae-miR9663, STTM (short tandem target mimic) of tae-miR9663 was synthesized, which was connected to BSMV (barley stripe mosaic virus) vector. The fifth leaves were inoculated by VIGS (virus-inducing gene silencing) in Ningchun 16. The phenotypes of leaves were observed at 20 d post-inoculation. tae-miR9663 expression in flag leaves was detected by real-time quantitative PCR and size of mature seeds was observed. Phenotypic observation results on leaves indicated that compared with BSMV:00,there are four phenotypes including dots or white stripes appeared on the sixth leaf,dots or white stripes appeared on flag leaf(the seventh leaf),serrate margins appeared in the sixth leaf,and shrinking existed on flag leaf blade tips.Real-time quantitative PCR results indicated that the expression of tae-miR9663 was decreased, showing that BSMV-VIGS could silence endogenous miRNA by overexpression of STTM effectively. Comparison of seeds from BSMV:STTM- tae-miR9663 with those from control plants, the lengths and widths of seeds from BSMV:STTM- tae-miR9663 inoculated plants were decreased.
Wheat; tae-miR9663; STTM; Virus-induced gene silencing (VIGS) technology
时间:2017-04-07
2016-12-06
2017-02-16 基金项目:国家自然科学基金青年基金项目(31501295);国家自然科学基金面上项目(31471482) 第一作者E-mail:xnzyf2014@163.com 通讯作者:王 倩(E-mail:ramese1021@126.com);赵惠贤(E-mail:hxzhao212@nwsuaf.edu.cn)
S512.1;S336
A
1009-1041(2017)04-0465-07
网络出版地址:http://kns.cnki.net/kcms/detail/61.1359.S.20170407.1020.012.html
