中药草豆蔻抗肿瘤化学成分和作用机制研究进展
2017-05-10石海莲吴晓俊
王 萍,石海莲,吴晓俊
(上海市复方中药重点实验室,教育部中药标准化重点实验室,上海中医药大学中药研究所,上海 201203)
中药草豆蔻(Semen Alpinia Katsumadai)为姜科山姜属植物草豆蔻(Alpinia katsumadaiHayata)的干燥近成熟种子,主产于广东和广西等地,自然资源丰富。草豆蔻最早以豆蔻之名载于《名医别录》,其“主温中,心腹痛,呕吐,去口气”,味辛,性温,无毒,归脾胃经,具有良好的燥湿化浊、温中散寒、行气消胀之功;多用于寒湿中阻、脾胃气滞之脘腹胀满冷痛、嗳气呕逆、痰饮积、不思饮食、脾胃虚寒夹湿之久泻等证[1]。现代药理学研究表明,草豆蔻具有保护胃黏膜、抗胃溃疡、促进肠胃功能[2]、镇吐[3]、抑菌[4]、抗氧化[5]、抗炎和抗肿瘤[6]等多种药理作用。随着对草豆蔻化学成分及药理作用研究的深入,特别是草豆蔻良好的抗肿瘤作用及其作用机制的研究,引起了国内外学者越来越多的关注。本文主要就近年来国内外对草豆蔻抗肿瘤活性成分、抗肿瘤作用及其机制的研究进展进行综述,以期为后续研究和应用提供参考。
1 草豆蔻抗肿瘤作用的主要化学成分
自20世纪以来,国内外学者已从草豆蔻中提取、分离、鉴定出上百种化学成分,目前已发现的化学成分主要有黄酮类、二苯庚烷类和挥发油类化合物,其次为微量元素等。近年来,对草豆蔻的抗肿瘤作用研究较多。据报道,草豆蔻对肝癌、胃癌、结肠癌、胶质瘤、肺癌、乳腺癌、骨髓瘤、宫颈癌、胰腺癌、食管鳞状细胞癌、卵巢癌、黑色素瘤和前列腺癌等均有一定的抑制作用,其抗肿瘤活性成分主要为黄酮类和萜类化合物。草豆蔻黄酮类化合物主要有山姜素、乔松素、球松素、柚皮素、小豆蔻明、蜡菊
2 草豆蔻抗肿瘤作用机制
草豆蔻有效成分具有广谱的抗肿瘤作用,其机制与抑制肿瘤细胞增殖、诱导肿瘤细胞凋亡、抑制肿瘤侵袭和转移、调节肿瘤细胞能量代谢和抗炎等密切相关。
2.1 抑制肿瘤细胞增殖
恶性肿瘤细胞区别于正常细胞最突出的特征之一是肿瘤细胞不受正常生长调控系统的控制。草豆蔻黄酮类和萜类活性成分能通过调控细胞内信号转导通路如磷脂酰肌醇3激酶(phosphatidylinositol 3 kinase,PI3K)/蛋白激酶 B(protein kinase B,Akt)、丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)、哺乳动物雷帕霉素靶蛋白(mammalian target of Rapamycin,mTOR)和阻滞细
胞周期等影响肿瘤细胞增殖。
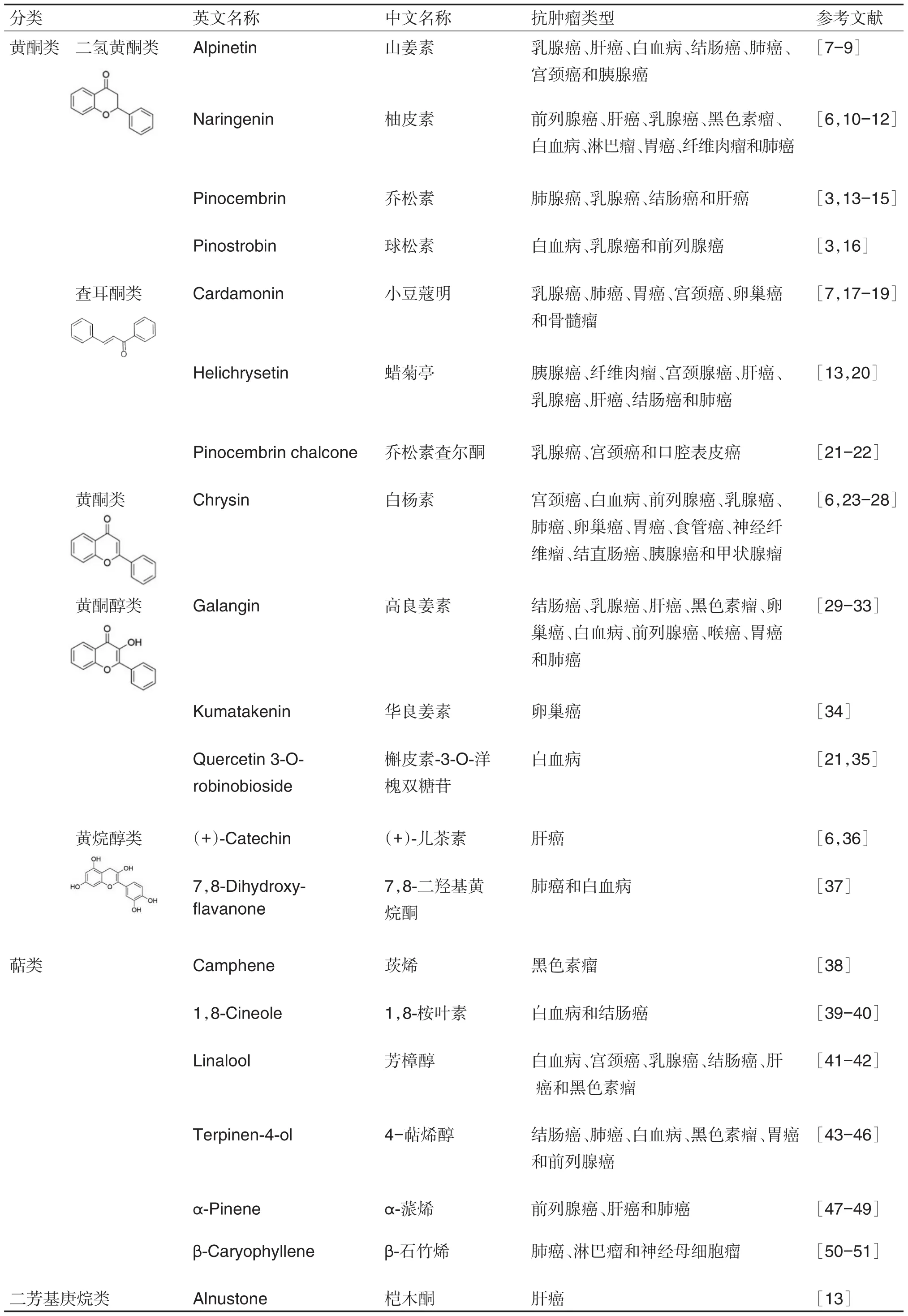
表1 草豆蔻的主要化学成分及其抗肿瘤作用
PI3K/Akt通路是癌发生发展的关键影响通路。研究表明,草豆蔻抗肿瘤活性成分山姜素、柚皮素[11]、小豆蔻明、1,8-桉叶油素[39]和高良姜素[30]能够通过调控PI3K/Akt通路抑制肿瘤细胞增殖。MAPK信号通路在调节细胞增殖、分化、迁移和细胞凋亡等方面也具有重要作用。Totta等[12]通过动态时间点进行台盼蓝染色计数活细胞发现,柚皮素能抑制肿瘤细胞增殖,但依赖于雌激素受体介导的细胞外调节蛋白激酶(extracellular regulated protein kinase,ERK)/MAPK信号通路;同时,柚皮素还能通过激活P38/MAPK信号通路,诱导肿瘤细胞凋亡。Chen等[52]通过细胞形态和密度的观察及MTT实验发现,小豆蔻明通过mTOR/NF-κB/白细胞介素6(interleukin-6,IL-6)途径调控肿瘤炎症,从而抑制正常和脂多糖诱导的卵巢癌细胞增殖。
草豆蔻挥发油成分α-蒎烯、1,8-桉叶油素和黄酮类成分山姜素、小豆蔻明、松属素、蜡菊亭、乔松素、柚皮素、高良姜素和白杨素等能阻滞细胞周期进而抑制肿瘤细胞增殖。细胞周期蛋白、细胞周期蛋白依赖性激酶(cyclin dependent kinase,CDK)和周期蛋白依赖性激酶抑制剂(cyclin dependent kinase inhibitor,CKI)是参与细胞周期调控的主要因子。α-蒎烯可激活一系列细胞周期相关蛋白(Chk1,Chk2,Cdc25A,ATM和ATR)中文名称,增加其磷酸化表达,诱导DNA损伤和激活细胞周期检查点,并呈浓度依赖性地阻滞细胞周期于G2/M期,抑制肝癌细胞生长[47]。山姜素可特异性靶向尿苷胞苷激酶2,干扰MDM2-P53信号通路,阻滞细胞周期,抑制结肠癌细胞增殖[7]。
此外,α-蒎烯能够诱导中国仓鼠卵巢癌细胞基因组不稳定性,通过产生活性氧(reactive oxygen species,ROS)而改变细胞有丝分裂和损伤DNA,影响遗传信息的稳定性,从而抑制卵巢癌细胞增殖,并诱导肿瘤细胞凋亡[53]。白杨素可激活Notch1和Hes1表达,进而诱导聚ADP-核糖聚合酶蛋白表达,在体内外抑制甲状腺瘤生长[54]。
2.2 诱导肿瘤细胞凋亡
草豆蔻活性成分主要通过外源性的死亡受体途径、内源性的线粒体途径、内质网应激和自噬等诱导肿瘤细胞凋亡。
2.2.1 死亡受体途径
外源性死亡受体途径开始于特异性死亡受体与配体结合,进而激活胱天蛋白酶8后引发胱天蛋白酶级联反应促进细胞死亡。目前,外源性死亡受体途径主要有肿瘤坏死因子受体途径、肿瘤坏死因子相关凋亡诱导配体途径和Fas/FasL途径等。(+)-儿茶素、短叶松素、高良姜素[55]、柚皮素[56]和小豆蔻明[17]等可通过肿瘤坏死因子相关凋亡诱导配体介导的死亡受体途径诱导肿瘤细胞凋亡;而乔松素[14]和高良姜素[33]则调控Fas/FasL信号转导途径,激活下游信号分子胱天蛋白酶3,8和9,进而诱导白血病细胞凋亡。
2.2.2 线粒体途径
线粒体途径是多细胞生物体发生调亡的主要途径,作为抗肿瘤作用机制的研究更为广泛。与内在凋亡途径相关的Bcl-2家族(促进凋亡作用的Bax,Bak和Bim等,抗凋亡作用的Bcl-2,Bcl-xL,Bcl-w和X连锁凋亡抑制蛋白等)通过胱天蛋白酶的活化或通过调节细胞色素c的释放对控制细胞凋亡起关键作用[57]。
山姜素可调控PI3K/Akt信号通路,抑制Bcl-2,Bcl-xL和XIAP表达,上调Bax表达,释放细胞色素c,活化胱天蛋白酶3,8和9,诱导胰腺癌和肺癌细胞的凋亡[8]。小豆蔻明下调Bcl-2基因的表达,上调Bax和胱天蛋白酶3基因表达,从而诱导人早幼粒白血病HL-60细胞和骨髓瘤细胞的凋亡[58-59]。柚皮素通过调控PI3K/Akt和MAPK信号通路介导内源性线粒体凋亡,抑制前列腺癌和白血病[60-61]。白杨素可通过PI3K/Akt/P70S6K/P90RSK和ERK1/2-JNK-P38 MAPK诱导线粒体途径介导的细胞凋亡[25]。
2.2.3 内质网应激途径
内质网稳态平衡对于维持正常的细胞功能至关重要。长时间的内质网应激(endoplasmic reticulum stress,ERS)将引起细胞凋亡。高良姜素能诱导ERS,抑制Ca2+泵重摄取细胞浆Ca2+,并引起线粒体Ca2+过载引发线粒体介导的细胞死亡[31]。白杨素通过激活p-ERK、真核起始因子2B、葡萄糖调节蛋白78和未折叠蛋白诱导ERS,诱导前列腺癌细胞凋亡[25]。芳樟醇可调控沉默信息调节因子3-SOD2-ROS途径诱导胶质瘤细胞凋亡[62]。
2.2.4 自噬
自噬是一个吞噬自身细胞质蛋白或细胞器,使其包被进入囊泡,并与溶酶体融合形成自噬溶酶体,降解其所包裹的内容物的过程,藉此实现细胞本身的代谢需要和某些细胞器的更新[63]。自噬调节涉及多种信号通路,其中以腺苷单磷酸活化蛋白激 酶(adenosine 5′-monophosphate activated protein kinase,AMPK)及mTOR信号通路为调控核心。在自噬过程中,微管相关蛋白1轻链3-β(microtubules associated protein 1 light chain 3-β,LC3)的前体形式被修饰为LC3-Ⅰ和LC3-Ⅱ。LC3-Ⅰ位于细胞溶质中,LC3-Ⅱ是膜相关的,也是自噬体形成的关键标志。高良姜素通过PI3K/Akt/mTOR通路上调喉癌细胞LC3-Ⅰ,LC3-Ⅱ和Beclin表达诱导自噬[30],并且可以通过TGF-β/Smad通路诱导HepG2肝癌细胞自噬[64]。小豆蔻明可调控P53/JNK通路,促进LC3-Ⅰ和LC3-Ⅱ表达,诱导结肠癌细胞自噬[65]。
2.3 抑制肿瘤侵袭和转移
肿瘤转移是临床化疗失败和癌症患者死亡的主要原因之一,控制肿瘤的侵袭和转移是癌症治疗的关键。目前研究表明,草豆蔻活性成分小豆蔻明、柚皮素、白杨素、高良姜素和β-石竹烯具有抑制肿瘤侵袭和转移的作用,主要通过多种生长因子及其受体、蛋白酶、E-钙黏蛋白和黏着斑激酶(focal adhesion kinase,FAK)及相关信号转导通路,影响侵袭和转移过程中破坏细胞外基质(extracellular matrix,ECM)屏障、上皮细胞间充质转化(epithelialmesenchymal transition,EMT)及肿瘤血管新生等关键环节。
ECM调节组织发育和体内平衡,ECM由基底膜和细胞间基质组成,基质金属蛋白酶(matrix metalloproteinases,MMP)家族是降解细胞间基质的重要酶类,能破坏ECM屏障,导致肿瘤细胞侵袭与转移。表皮生长因子受体(epidermal growth factor receptor,EGFR)信号通路与转移性质相关,包括细胞运动、黏附和侵袭。柚皮素通过抑制EGFR阻断MAPK/AP-1和PI3K/Akt/NF-κB信号通路,抑制苯二甲酸诱导的MMP-9基因表达,避免其破坏ECM屏障,抑制肝癌和胰腺癌的迁移和侵袭[11]。
EMT是指在发育过程中,上皮细胞的特征发生了巨大变化,上皮细胞的极性丧失,迁移和运动能力增强,同时上皮表型丢失而逐渐获得间质表型。细胞黏附能力的下降是肿瘤细胞转移过程的开端。mTOR及其下游的靶点S6K1可以通过影响细胞黏附来调整癌细胞的侵袭和转移。有研究报道,小豆蔻明能通过mTOR/S6K1[18]和转谷氨酰胺酶-2(Tgase-2)/TGF-β1[66]信号通路,抑制 EMT进而抑制肺癌细胞的侵袭和转移。另有研究表明,小豆蔻明通过Wnt/β-联蛋白信号通路,阻断EMT从而抑制体内外三阴性乳腺癌的转移[19]。白杨素通过抑制PI3K/Akt信号通路抑制MMP-10蛋白表达,抑制EMT〔表现为E-钙黏蛋白表达增加,波形蛋白(vimentin)和锌指转录因子表达下降〕,从而抑制人三阴性乳腺癌细胞的转移[26];而在胃癌细胞,白杨素通过抑制JNK/c-Jun和ERK/c-Fos信号通路降低MMP-9的表达而抑制肿瘤侵袭。FAK的活性,在肿瘤向恶性侵袭表型演进的过程中起着关键作用。高良姜素在体内外均可降低FAK表达而抑制B16F10黑素瘤转移[33]。
血管新生在肿瘤的侵袭和转移过程中具有重要作用,通过激活“血管生成转换”,从而导致通常静止的脉管系统持续地发芽新血管,以维持营养物质和氧气的供给,为肿瘤侵袭转移提供有利条件[67]。低氧不仅限于原发性肿瘤,而且也出现在转移部位,低氧诱导因子-1α(hypoxia inducible factor-1α,HIF-1α)是低氧反应的主要调节因子。血管内皮生长因子(vascular endothelial growth factor,VEGF)是最有效的血管生成因子之一,在缺氧细胞中表达上调,进一步诱导低氧组织中的血管发生。Xue等[67]通过体外细胞实验及体内鸡胚尿囊模型实验发现,小豆蔻明可通过抑制mTOR/HIF-1α/VEGF通路,抑制血管生成,发挥抗肿瘤作用。除了HIF-1α,其他调节因子,如信号转导与转录活化因子3(signal transducers and activators of transcription 3,STAT3),Akt,ERK和NF-kB也可被低氧激活,并参与低氧诱导的VEGF基因表达。白杨素抑制乳腺癌侵袭和转移除了通过抑制EMT外,还与抑制低氧诱导的STAT3磷酸化而抑制血管生成有关。此外,α-蒎烯抑制裸鼠体内肺癌生长也与通过降低VEGF表达、趋化因子释放而抑制血管新生相关[68]。
2.4 调控肿瘤细胞的能量代谢
柚皮素、小豆蔻明、白杨素、高良姜素和(+)-儿茶素等均能改善胰岛素抵抗,调节糖代谢。与正常细胞不同,肿瘤细胞即便是在有氧环境中,仍然偏好通过糖酵解途径提供能量,大量消耗葡萄糖,经糖酵解途径生成大量中间代谢产物和乳酸,满足肿瘤细胞快速增殖物质合成所需,酸化肿瘤微环境,促进免疫逃逸和肿瘤细胞转移。研究表明,白杨素通过抑制己糖激酶2抑制肝癌细胞糖酵解[27]。柚皮素抑制PI3K/Akt信号通路的活化进而抑制葡萄糖转运蛋白,同时还能抑制ERK/MAPK信号通路的磷酸化进而降低胰岛素刺激的葡萄糖摄取,最终抑制乳腺癌细胞MCF-7增殖[10]。上述结果提示,草豆蔻活性成分抗肿瘤作用机制也可能与其调控肿瘤能量代谢有关。
2.5 抗炎作用
肿瘤相关炎症能够通过促进肿瘤血管新生、促进肿瘤侵袭和转移、影响肿瘤免疫应答及改变肿瘤细胞对化疗药的敏感性等,促进肿瘤的生长和发展。白杨素可抑制肾鸟氨酸脱羧酶活性和增殖细胞核抗原、诱导型一氧化氮合酶、诱导环氧化酶2和前列腺素E2表达,及促炎细胞因子IL-6和肿瘤坏死因子α分泌,改善炎症反应,抑制N-亚硝基二乙胺和次氮基三乙酸铁诱导的肾癌发生[69]。
3 草豆蔻协同化疗药物抗肿瘤作用及机制
肿瘤患者的预后在很大程度上与肿瘤细胞对化疗药物的敏感性有关,而大部分患者在后续的化疗中逐渐产生继发性耐药,伴有严重的肾毒性,导致化疗结果不理想。顺铂作为一线高效广谱抗肿瘤药物,具有较严重的胃肠道、肾和中枢神经系统毒性等不良反应。小豆蔻明能显著降低顺铂诱导的肾损伤,同时有效增强顺铂抑制SKOV3细胞增殖的作用。小豆蔻明联合顺铂能够诱导卵巢癌SKOV3细胞自噬,阻滞细胞周期,对耐药基因谷胱甘肽巯基转移酶-π、多药耐药相关蛋白基因和抗凋亡基因Bcl-2、X连锁凋亡抑制蛋白及存活蛋白表达有抑制作用,协同作用显著优于顺铂单用[70]。山姜素通过抑制多药耐药相关蛋白1和5及P-糖蛋白的表达,增强肿瘤细胞对顺铂的敏感性[8]。
4 结语
综上所述,草豆蔻的多种化学成分,主要通过抑制肿瘤细胞增殖、诱导肿瘤细胞凋亡、抑制血管生成与组织侵袭转移、调节能量代谢,抗炎和协同化疗药物等发挥抗肿瘤和转移的作用。但是,目前的研究具有显著的局限性。①目前临床上抗肿瘤药物的主要给药方式为静脉、口服和腹膜内给药等,而草豆蔻抗肿瘤的主要药效物质为黄酮类和萜类化合物,这2类化合物按上述给药方式的生物利用度均不高。因此,后续应加强草豆蔻抗肿瘤活性成分药物制剂改善、或作为先导化合物进行结构修饰以增效减毒并增加生物利用度的研究。②考虑到草豆蔻临床上应用广泛,历史悠久,且多以汤剂服药,而胃肠道是草豆蔻进入机体的首过之处。因此,胃肠道可能是草豆蔻各活性成分转换转化代谢入血的重要场所,而肠道菌群可能在其中扮演重要角色。加强探讨肠道菌群参与草豆蔻有效成分在肠道中的分解、代谢、生物转化和活性成分的入血作用,可提高其生物利用度甚至可以获得经代谢或转化途径产生的具有更高生物活性的新化合物。③目前较多文献报道表明,一些药物成分通过调控肠道菌群(影响肠道菌群的构成和多样性)发挥抗癌作用,而草豆蔻有效成分是否也具有类似作用途径尚不清楚,后期可加强此方向研究。④目前草豆蔻抗肿瘤作用的化学成分研究主要集中在黄酮类和萜类化合物,而对二芳基庚烷类化合物的抗肿瘤作用知之甚少,也值得进一步挖掘其活性。通过对草豆蔻抗肿瘤作用的化学成分进行深入的机制探讨,期待更多的活性化合物可以应用于临床。
参考文献:
[1] Chinese Pharmacopoeia Commission.Chinese Pharmacopoeia(中华人民共和国药典)[M].Beijing:China Medical Science Press,2015:238-239.
[2] Wu Z,Chen YS,Du SM ,Wang QB,Tong Q.Effect of volatile oil ofAlpinia katsumadaion gastric ulcer induced by acetic acid in rats[J].Chin Hosp Pharm J(中国医院药学杂志),2010,30(7):560-563.
[3] Yang Y,Kinoshita K,Koyama K,Takahashi K,Tai T,Nunoura Y,et al.Anti-emetic principles ofAlpinia katsumadaiHayata[J].Nat Prod Sci,1999,5(1):20-24.
[4] Huang WZ,Dai XJ,Liu YQ,Zhang CF,Zhang M,Wang ZT.Studies on antibacterial activity of flavonoids and diarylheptanoids fromAlpinia katsumadai[J].J Plant Resour Environ(植物资源与环境学报), 2006,15(1):37-40.
[5] Lee SE,Shin HT,Hwang HJ,Kim JH.Antioxidant activity of extracts fromAlpinia katsumadaiseed[J].Phytother Res,2003,17(9):1041-1047.
[6] Tang J.Studies on chemical constituents ofAlpinia katsumadaiand Curcuma longa(草豆蔻和姜黄的化学成分研究)[D].Hefei:Anhui University(安徽大学),2010.
[7] Wang XQ,Yang XJ,Li JS.Studies on chemical constituents ofAlpinia katsumada[iJ].J Chin Med Mater(中药材),2008,31(6):853-855.
[8] Malami I,Abdul AB,Abdullah R,Kassim NK,Rosli R,Yeap SK,et al.Crude extracts,flavokawain B and alpinetin compounds from the rhizome ofAlpinia muticainduce cell death via UCK2 enzyme inhibition and in turn reduce 18S rRNA biosynthesis in HT-29 cells[J/OL].PLoS One,2017,12(1):e0170233(2017-01-19).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5245823/
[9] Tang B,Du J,Wang J,Tan G,Gao Z,Wang Z,et al.Alpinetin suppresses proliferation of human hepatoma cells by the activation of MKK7 and elevates sensitization to cis-diammined dichloridoplatium[J].Oncol Rep,2012,27(4):1090-1096.
[10] Harmon AW,Patel YM.Naringenin inhibits glucose uptake in MCF-7 breast cancer cells:a mechanism for impaired cellular proliferation[J].Breast Cancer Res Treat,2004,85(2):103-110.
[11] Yen HR,Liu CJ,Yeh CC.Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells[J].Chem Biol Interact,2015,235:1-9.
[12] Totta P,Acconcia F,Leone S,Cardillo I,Marino M.Mechanisms of naringenin-induced apoptotic cascade in cancer cells:involvement of estrogen receptor alpha and beta signaling[J].IUBMB Life,2004,56(8):491-599.
[13] Li YY,Yang L,Wang CH,Chou GX,Wang ZT.Chemical constituents fromAlpinia katsumadaiHayata and their anti-tumor activity[J].Acta Univ Tradit Med Sin Pharmacol Shanghai(上海中医药大学学报),2010,24(1):72-75.
[14] Kumar MA, Nair M, Hema PS, Mohan J,Santhoshkumar TR.Pinocembrin triggers Bax-dependent mitochondrial apoptosis in colon cancer cells[J].Mol Carcinog,2007,46(3):231-241.
[15] Rasul A,Millimouno FM,Ali Eltayb W,Ali M,Li J,Li X.Pinocembrin:a novel natural compound with versatile pharmacological and biological activities[J/OL].Biomed Res Int,2013,2013:379850(2013-08-05).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3747598/
[16] Patel NK,Jaiswal G,Bhutani KK.A review on biological sources,chemistry and pharmacological activities of pinostrobin[J].Nat Prod Res,2016,30(18):2017-2027.
[17] Yadav VR,Prasad S,Aggarwal BB.Cardamonin sensitizes tumour cells to TRAIL through ROS-and CHOP-mediated up-regulation of death receptors and down-regulation of survival proteins[J].Br J Pharmacol,2012,165(3):741-753.
[18] Niu PG,Zhang YX,Shi DH,Liu Y,Chen YY,Deng J.Cardamonin inhibits metastasis of Lewis lung carcinoma cells by decreasing mTOR activity[J/OL].PLoS One,2015,10(5):e0127778(2015-03-21).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4440626/
[19] Shrivastava S,Jeengar MK,Thummuri D,Koval A,Katanaev VL,Marepally S,et al.Cardamonin,a chalcone,inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition[J].Biofactors,2017,43(2):152-169.
[20] Ho YF,Karsani SA,Yong WK,Abd Malek SN.Induction of apoptosis and cell cycle blockade by helichrysetin in a549 human lung adenocarcinoma cells[J/OL].Evid Based Complement Alternat Med,2013,2013:857257(2013-03-03).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3603683/
[21] Xiao XH,Si XX,Tong X,Li GK.Preparation of flavonoids and diarylheptanoid fromAlpinia katsumadaiHayata by microwave-assisted extraction and highspeed counter-current chromatography[J].Sep Purif Technol,2011,81:265-269.
[22] Malek SN,Phang CW,Ibrahim H,Norhanom AW,Sim KS.Phytochemical and cytotoxic investigations ofAlpinia muticarhizomes[J].Molecules,2011,16(1):583-589.
[23] Liu G,Xie W,He AD,Da XW,Liang ML,Yao GQ,et al.Antiplatelet activity of chrysin via inhibiting platelet αIIbβ3-mediated signaling pathway[J].Mol Nutr Food Res,2016,60(9):1984-1993.
[24] Kasala ER,Bodduluru LN,Madana RM,V AK,Gogoi R,Barua CC.Chemopreventive and therapeutic potential of chrysin in cancer:mechanistic perspectives[J].Toxicol Lett,2015,233(2):214-225.
[25] Ryu S,Lim W,Bazer FW,Song G.Chrysin induces death of prostate cancer cells by inducing ROS and ER stress[J].J Cell Physiol,2017,232(12):3786-3797.
[26] Yang B,Huang J,Xiang T,Yin X,Luo X,Huang J,et al.Chrysin inhibits metastatic potential of human triple-negative breast cancer cells by modulating matrix metalloproteinase-10,epithelial to mesenchymal transition,and PI3K/Akt signaling pathway[J].J Appl Toxicol,2014,34(1):105-112.
[27] Xu D,Jin J,Yu H,Zhao Z,Ma D,Zhang C,et al.Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2[J].J Exp Clin Cancer Res,2017,36(1):44(2017-03-20).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5359903/
[28] Lirdprapamongkol K,Sakurai H,Abdelhamed S,Yokoyama S,Maruyama T,Athikomkulchai S,et al.A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells[J].Oncol Rep,2013,30(5):2357-2364.
[29] Xin BR,Ren SJ,Li J.A new flavonone from seeds ofAlpinia katsumadaiand its neuroprotective effect on PC12 cells[J].China J Chin Mater Med(中国中药杂志),2014,39(14):2674-2678.
[30] Wang HX,Tang C.Galangin suppresses human laryngeal carcinoma via modulation of caspase-3 and AKT signaling pathways[J].Oncol Rep,2017,38(2):703-714.
[31] Su L,Chen X,Wu J,Lin B,Zhang H,Lan L,et al.Galangin inhibits proliferation ofhepatocellular carcinoma cells by inducing endoplasmic reticulum stress[J].Food Chem Toxicol,2013,62:810-816.
[32] Song W,Yan CY,Zhou QQ,Zhen LL.Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK[J].Biomed Pharmacother,2017,89:845-856.
[33] Zhang W,Tang B,Huang Q,Hua Z.Galangin inhibits tumor growth and metastasis of B16F10 melanoma[J].J Cell Biochem,2013,114(1):152-161.
[34] Woo JH,Ahn JH,Jang DS,Lee KT,Choi JH.Effect of kumatakenin isolated from cloves on the apoptosis of cancer cells and the alternative activation of tumor-associated macrophages[J].J Agric Food Chem,2017,65(36):7893-7899.
[35] Cong Y,Guo JG,Wang TX,Li M,Li K,Wang JH,et al.Chemical constituents and antitumor activity on leukemia K562 cell ofLeonurus heterophyllus[J].China J Chin Mater Med(中国中药杂志),2009,34(14):1816-1818.
[36] Jain P,Kumar N,Josyula VR,Jagani HV,Udupa N,Mallikarjuna Rao C,et al.A study on the role of(+)-catechin in suppression of HepG2 proliferation via caspase dependent pathway and enhancement of itsin vitroandin vivocytotoxic potential through liposomal formulation[J].Eur J Pharm Sci,2013,50(3-4):353-365.
[37] Hahm ER,Park S,Yang CH.7,8-Dihydroxyflavanone as an inhibitor for Jun-Fos-DNA complex formation and its cytotoxic effecton cultured human cancer cells[J].Nat Prod Res,2003,17(6):431-436.
[38] Girola N,Figueiredo CR,Farias CF,Azevedo RA,Ferreira AK,Teixeira SF,et al.Camphene isolated from essential oil ofPiper cernuum(Piperaceae)induces intrinsic apoptosis in melanoma cells and displays antitumor activityin vivo[J].Biochem Biophys Res Commun,2015,467(4):928-934.
[39] Murata S,Shiragami R,Kosugi C,Tezuka T,Yamazaki M,Hirano A,et al.Antitumor effect of 1,8-cineole against colon cancer[J].Oncol Rep,2013,30(6):2647-2652.
[40] Moteki H,Hibasami H,Yamada Y,Katsuzaki H,Imai K,Komiya T.Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines,but not a in human stomach cancer cell line[J].Oncol Rep,2002,9(4):757-760.
[41] Jiang DM,Zhu Y,Yu JN,Xu XM.Advances in research of pharmacological effects and formulation studies of linaloo[lJ].China J Chin Mater Med(中国中药杂志),2015,40(18):3530-3533.
[42] Chang MY,Shen YL.Linalool exhibits cytotoxic effects by activating antitumor immunity[J].Mole⁃cules,2014,19(5):6694-6706.
[43] Nakayama K,Murata S,Ito H,Iwasaki K,Villareal MO,Zheng YW,et al.Terpinen-4-ol inhibits colorectal cancer growth via reactive oxygen species[J].Oncol Lett,2017,14(2):2015-2024.
[44] Khaw-on P,Banjerdpongchai R.Induction of intrinsic and extrinsic apoptosis pathways in the human leukemic MOLT-4 cell line by terpinen-4-ol[J].Asian Pac J Cancer Prev,2012,13(7):3073-3076.
[45] Banjerdpongchai R,Khaw-On P.Terpinen-4-ol induces autophagic and apoptotic cell death in human leukemic HL-60 cells[J].Asian Pac J Cancer Prev,2013,14(12):7537-7542.
[46] Shapira S,Pleban S,Kazanov D,Tirosh P,Arber N.Terpinen-4-ol:a novel and promising therapeutic agent for human gastrointestinal cancers[J/OL].PLoS One,2016,11(6):e0156540(2016-06-08).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4898785/
[47] Chen W,Liu Y,Li M,Mao J,Zhang L,Huang R,et al.Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest[J].J Pharmacol Sci,2015,127(3):332-338.
[48] Zhang Z,Guo S,Liu X,Gao X.Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma(NSCLC)[J].Drug Res(Stuttg),2015,65(4):214-218.
[49] Zhao Y,Chen R,Wang Y,Yang Y.α-Pinene inhibits human prostate cancer growth in a mouse xenograft model[J].Chemotherapy,2018,63(1):1-7.
[50] Sain S,Naoghare PK,Devi SS,Daiwile A,Krishnamurthi K,Arrigo P,et al.Beta caryophyllene and caryophyllene oxide,isolated fromAegle marmelos,as the potent anti-inflammatory agents against lymphoma and neuroblastoma cells[J].Antiinflamm Antiallergy Agents Med Chem,2014,13(1):45-55.
[51] Fidyt K,Fiedorowicz A,Strzadała L,Szumny A.β-Caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties[J].Cancer Med,2016,5(10):3007-3017.
[52] Chen HJ.Antiproliferative effects of cardamonin mediated by anti-inflammation via mTOR on ovarian cancer cells(mTOR介导小豆蔻明抗炎作用抑制卵巢癌细胞增殖的研究)[D].Fuzhou:Fujian Medical University(福建医科大学),2014.
[53] Catanzaro I,Caradonna F,Barbata G,Saverini M,Mauro M,Sciandrello G.Genomic instability induced by α-pinene in Chinese hamster cell line[J].Muta⁃genesis,2012,27(4):463-469.
[54] Yu XM,Phan T,Patel PN,Jaskula-Sztul R,Chen H.Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinomain vitroandin vivo[J].Cancer,2013,119(4):774-781.
[55] Szliszka E,Sokół-Łętowska A,Kucharska AZ,Jaworska D,Czuba ZP,Król W.Ethanolic extract ofpolish propolis:chemical composition and TRAIL-R2 death receptor targeting apoptotic activity against prostate cancer cells[J/OL].Evid Based Complement Alternat Med,2013,2013:757628(2013-11-12).https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3845518/
[56] Jin CY,Park C,Hwang HJ,Kim GY,Choi BT,Kim WJ,et al.Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells[J].Mol Nutr Food Res,2011,55(2):300-309.
[57] Lopez J,Tait SW.Mitochondrial apoptosis:killing cancer using the enemy within[J].Br J Cancer,2015,112(6):957-962.
[58] Yang F.The study of cardamomin inducing HL-60 cell apoptosis and its mechanism(小豆蔻明诱导HL-60细胞凋亡及其机制的研究)[D].Hangzhou:Zhejiang Chinese Medical University(浙江中医药大学),2013.
[59] Shu XR.Cardamonin induces caspase-dependent apoptosis in human multiple myeloma cells in bone marrow microenvironmen(t小豆蔻明诱导骨髓微环境中多发性骨髓瘤细胞caspase依赖性凋亡)[D].Wuhan:Huazhong University of Science and Technology(华中科技大学),2013.
[60] Park JH,Jin CY,Lee BK,Kim GY,Choi YH,Jeong YK.Naringenin induces apoptosis through downregulation of Akt and caspase-3 activation in human leukemia THP-1 cells[J].Food Chem Toxicol,2008,46(12):3684-3690.
[61] Lim W,Park S,Bazer FW,Song G.Naringenininduced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways[J].J Cell Biochem,2017,118(5):1118-1131.
[62] Cheng Y,Dai C,Zhang J.SIRT3-SOD2-ROS pathway is involved in linalool-induced glioma cell apoptotic death[J].Acta Biochim Pol,2017,64(2):343-350.
[63] Zhao GX,Pan H,Ouyang DY,He XH.The critical molecular interconnections in regulating apoptosis and autophagy[J].Ann Med,2015,47(4):305-315.
[64] Wang Y,Wu J,Lin B,Li X,Zhang H,Ding H,et al.Galangin suppresses HepG2 cell proliferation by activating the TGF-β receptor/Smad pathway[J].Toxicology,2014,326:9-17.
[65] Kim YJ,Kang KS,Choi KC,Ko H.Cardamonin induces autophagy and an antiproliferative effect through JNK activation in human colorectal carcinoma HCT116 cells[J].Bioorg Med Chem Lett,2015,25(12):2559-2564.
[66] Park MK,Jo SH,Lee HJ,Kang JH,Kim YR,Kim HJ,et al.Novel suppressive effects of cardamonin on the activity and expression of transglutaminase-2 lead to blocking the migration and invasion of cancer cells[J].Life Sci,2013,92(2):154-160.
[67] Xue ZG,Niu PG,Shi DH,Liu Y,Deng J,Chen YY.Cardamonin inhibits angiogenesis by mTOR downregulation in SKOV3 Cells[J].Planta Med,2016,82(1-2):70-75.
[68] Magkouta S, Stathopoulos GT, Psallidas I,Papapetropoulos A,Kolisis FN,Roussos C,et al.Protective effects of mastic oil fromPistacia Lentiscusvariationchiaagainst experimental growth of lewis lung carcinoma[J].Nutr Cancer,2009,61(5):640-648.
[69] Rehman MU,Tahir M,Khan AQ,Khan R,Lateef A,Oday-O-Hamiza,et al.Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation,oxidative stress and inflammation:plausible role of NF-κB[J].Toxicol Lett,2013,216(2-3):146-158.
[70] Zhang XS.Investigation on the antiproliferation effect and its mechanism of cardamonin combined with cisplatin on SKOV3 cells(小豆蔻明联合顺铂抑制卵巢癌SKOV3细胞增殖及其作用机制研究)[D].Fuzhou:Fujian Medical University(福建医科大学),2014.
