饲料糖水平对大黄鱼生长和糖代谢的影响
2017-04-12邢淑娟孙瑞健马俊韦海明徐玮周慧慧张彦娇张文兵麦康森
邢淑娟孙瑞健马 俊韦海明徐 玮周慧慧张彦娇张文兵麦康森
(1. 中国海洋大学水产学院, 水产动物营养与饲料农业部重点实验室, 海水养殖教育部重点实验室, 青岛 266003; 2. 通威股份有限公司技术中心, 成都 610041)
饲料糖水平对大黄鱼生长和糖代谢的影响
邢淑娟1孙瑞健2马 俊1韦海明1徐 玮1周慧慧1张彦娇1张文兵1麦康森1
(1. 中国海洋大学水产学院, 水产动物营养与饲料农业部重点实验室, 海水养殖教育部重点实验室, 青岛 266003; 2. 通威股份有限公司技术中心, 成都 610041)
以初始体重为(137.5±0.4) g的大黄鱼Larimichthys crocea 为实验对象, 在海水浮式网箱中进行为期8周的摄食生长实验, 研究饲料中糖水平对其生长、饲料利用、血液生化指标和糖代谢酶活力等的影响, 以确定大黄鱼的饲料糖需求量。实验饲料按等氮(粗蛋白质45%)等能(18 kJ/g)设计, 糖含量分别为1.75%、6.67%、13.64%、21.15%、26.69%和32.25%。结果表明随着饲料糖水平的升高, 大黄鱼特定生长率(SGR)先升高后降低, 当糖含量为26.69%时, SGR达最大值, 显著高于糖含量为1.75%、6.67%、13.64%和32.25%处理组(P<0.05)。饲料效率(FER)和蛋白质效率(PER)均在糖含量为13.64%—21.15%时显著高于其他处理组(P<0.05)。随饲料中糖水平的升高, 全鱼粗脂肪含量显著降低, 在糖含量为32.25%时降至最低(10.56%), 显著低于其他处理组(P<0.05)。肝体比和肝糖原含量均随饲料糖水平的升高而显著升高(P<0.05), 在糖含量为32.25%时达到最大值, 显著高于糖含量为1.75%和6.67%处理组(P<0.05)。随饲料糖水平的升高, 血浆甘油三酯和胆固醇水平均显著降低(P<0.05), 而血糖水平不受饲料糖含量的影响(P>0.05)。大黄鱼血清溶菌酶、脂蛋白脂酶和肝脂酶活性均随饲料糖水平的升高显著降低(P<0.05), 而肠淀粉酶活性表现为先升高后降低, 在糖含量为26.69%时, 酶活力达到最大值。随饲料糖水平的升高, 大黄鱼肝脏己糖激酶活性先上升后下降, 在糖含量为21.15%时达到最大值, 显著高于糖含量为32.25%处理组(P<0.05), 而丙酮酸激酶活力在糖水平为32.25%时达到最大值, 显著高于糖含量为1.75%和6.67%处理组(P<0.05)。用二次多项回归模型拟合特定生长率和饲料糖水平的关系, 得到大黄鱼饲料中最适糖含量为22.7%。
大黄鱼; Larimichthys crocea; 糖; 营养; 饲料
在饲料中适量的糖和脂肪不仅可以节约饲料蛋白质, 提高饲料利用率, 还可以降低蛋白质代谢产物对养殖水体的污染[1—4]。然而, 饲料非蛋白源能量物质选择不当或不适宜的添加量都会对鱼类的生长、代谢和饲料效率产生不利影响[5,6]。
饲料脂肪除了提供鱼体必需脂肪酸外, 还为鱼体生理活动提供能量。摄入过量的脂肪不利于鱼类的生长和代谢[6—8], 多余的脂肪还将以体脂肪的形式储存, 影响鱼肉品质[9]。与脂肪相比, 糖类具有价格低廉, 易储存, 不易被氧化等优点。在饲料中以适宜的糖类物质替代脂肪, 可以直接为鱼体提供能量, 减少摄入多余脂肪, 提高鱼肉品质[10]。过高或过低的饲料糖含量, 会导致鱼类生长缓慢和代谢紊乱[11—16]。适量的饲料糖水平可促进鱼类生长, 提高饲料效率, 增强鱼体免疫力, 并降低养殖成本, 提高养殖效益[6,17—21]。
大黄鱼(Larimichthys crocea)是我国黄海南部、东海、台湾海峡以及南海北部海水养殖的名贵经济鱼类。到目前为止, 有关大黄鱼营养生理的研究已有相关报道[22—24]。并且, 在我们先前的研究中确定了较小规格(7.6—26.0 g)大黄鱼幼鱼对饲料糖的需求量为19%—21%, 但较大规格(体重>100 g)大黄鱼的饲料糖需求量尚未见报道。本研究拟通过分析饲料中不同糖水平对大黄鱼生长、消化、代谢和免疫反应等的影响, 确定较大规格大黄鱼幼鱼配合饲料中适宜的糖含量, 为大黄鱼不同生长阶段的精准饲料配方的设计提供基础数据。
1 材料与方法
1.1 实验饲料
以鱼肉浓缩蛋白(来源于鳕)、酪蛋白和明胶为蛋白源, 鱼油为脂肪源, 并以小麦淀粉梯度替代饲料中的鱼油, 使饲料糖水平依次为1.75%、6.67%、13.64%、21.15%、26.69%和32.25%, 分别记为Diet 1—Diet 6 (表 1)。原料经过粉碎后过320 μm筛网,将粉碎好的原料按饲料配方逐级放大充分混匀后,加入鱼油并手工搓匀, 随后加入30%的水混合揉匀成糜团状, 经F(II)-26 型双螺杆挤条机(华南理工大学, 广州)加工制成直径为2.5 mm×3.0 mm的颗粒饲料。饲料在恒温60℃的烘箱中烘干12h后, 再放于-20℃的冰箱保存待用。
1.2 实验鱼和养殖实验
养殖实验在浙江省宁波市象山县凤凰礁育苗场养殖基地进行, 实验用大黄鱼为当年人工培育的同一批苗种(由宁波市象山县凤凰礁育苗场提供)。正式实验前, 实验鱼暂养于3 m×3 m ×3 m的浮式网箱中, 以实验对照组饲料 (Diet 1) 饱食投喂2周, 使之逐渐适应实验饲料和养殖环境。
实验开始前, 停止投喂24h后挑选出体格健壮、规格一致的大黄鱼进行称重分组[初始体重: (137.5±0.35) g], 每个网箱 (1.5 m×1.5 m×2.0 m) 放养45尾, 每种实验饲料随机投喂3个网箱的实验鱼。每天饱食投喂2次(05:00和17:00), 养殖实验持续8周, 每天记录投饵量, 如有死亡则计数并称重。实验期间水温为27—30℃, 盐度为25‰—28‰, 溶氧约为7 mg/L。

表 1 实验饲料配方及成分分析(%干重)Tab. 1 Formulation and proximate composition of the experimental diets (% dry matter)
1.3 样品收集
8周养殖实验结束, 实验鱼饥饿24h后以丁香酚(1∶10000)麻醉, 然后逐尾称重和计数。每个网箱随机取5尾实验鱼保存于-20℃冰箱, 用于常规分析。
每个网箱中随机取5尾实验鱼, 分别测量体长、肝脏重、内脏重; 最后每个网箱随机取8尾实验鱼, 麻醉后由尾静脉取血, 并于4000×g离心10min (4℃)分离血清后立即置于-80℃保存待用。对完成取血的实验鱼, 迅速解剖取其肝脏、肠道和肌肉,液氮速冻后保存于-80℃冰箱用于酶活性的测定。
1.4 样品分析测定方法
常规分析鱼体水分、粗蛋白、粗脂肪及灰分测定参照AOAC (1995)方法。原料、饲料和鱼体样品在105℃烘箱中烘干至恒重, 得到各自水分含量; 用凯氏定氮仪(Kjeltec TM8400, FOSS, Sweden)测定粗蛋白; 索氏抽提仪(Buchi 36680, Switzerland) 测定粗脂肪; 在马弗炉中灼烧(550℃, 16h)测定灰分; 饲料能量采用氧弹仪(Par 6100, Moline, IL, USA)测定; 还原糖含量采用3, 5-二硝基水杨酸法测定[25]。
糖脂代谢物相关指标血糖、血浆总胆固醇、血浆甘油三酯水平由青岛大学附属医院测定。肝糖原、肌糖原、血清高/低密度脂蛋白胆固醇含量(HLD-C和LDL-C)、血浆总脂酶(脂蛋白脂酶LPL和肝脂酶HL)和血清溶菌酶均采用比色法检测(试剂盒A043、A112-2、A113-2、A067和A050-1, 南京建成生物工程研究所, 中国)。其中, 血清总脂酶活性定义为: 每毫升每小时在反应系统中所产生的1微摩尔(μmol)的游离脂肪酸FFA为1个酶活性单位(FFAμmol/mL血清×小时)。溶菌酶活性定义为: 在25℃, pH 6.2条件下, 于450 nm处每分钟使吸光度值降低0.001为一个酶活力单位。
消化酶活性消化酶液的制备: 准确称取组织重量, 按样品重的9倍加入预冷的磷酸缓冲液(0.01 mol/L, pH 7.4, 稀释1/10), 高速组织匀浆机冰浴匀浆, 匀浆液在4℃下 10000×g离心30min, 取上清液进行消化酶活力测定。
肝脏和肠道的胰蛋白酶、淀粉酶活性均采用比色法检测(试剂盒A080-2和C016, 南京建成生物工程研究所, 中国)。其中, 胰蛋白酶活性定义为:在pH 8.0, 37℃条件下, 每毫克蛋白中含有的胰蛋白酶每分钟使吸光度变化0.003即一个酶活力单位。淀粉酶活力定义为: 组织中每毫克蛋白在37℃与底物作用30min, 水解10 mg淀粉即为一个淀粉酶活力单位。参考Bradford[26]的考马斯亮兰法测定肝胰脏匀浆液中蛋白质的含量, 以牛血清白蛋白作为标准蛋白。
糖代谢酶活性匀浆缓冲液的制备参照Polakof等[27]的方法, 其中含有50 mmol/L Tris (pH 7.6), 5 mmol/L EDTA, 2 mmol/L 1, 4-二硫苏糖醇以及1%蛋白酶抑制剂混合物(Sigma Chemical, P-2714)。匀浆液在4℃下9000×g离心10min取上清后再10000×g离心20min, -80℃保存备用。
肝脏磷酸果糖激酶PFK和磷酸烯醇式丙酮酸羧激酶PEPCK活性测定参照Polakof等[27]的方法, 果糖-1, 6-二磷酸酶FBPase活性测定参照Susana Sangiao-Alvarellos等[28]的方法。参考Bradford[26]的考马斯亮兰法测定肝胰脏匀浆液中蛋白质的含量, 以牛血清白蛋白作为标准蛋白。
肝脏己糖激酶HK、丙酮酸激酶PK采用比色法检测(试剂盒A077-1和A076-1, 南京建成生物工程研究所, 中国)。HK活性定义为: 在37℃, pH7.6的条件下, 每克组织蛋白在本反应体系中每分钟生成1 mmol/L的NADP定义为一个酶活力单位。PK活性定义为: 在37℃, pH7.6的条件下, 每克组织蛋白每分钟将1 μmol的PEP转变成丙酮酸为一个酶活力单位。
1.5 计算公式和统计分析
存活率(Survival rate, SR, %)=100×终末尾数/初始尾数
特定生长率(Specific growth rate, SGR, %/d)= 100×[Ln(鱼体终重)-Ln(鱼体初重)]/实验天数
饲料效率(Feed efficiency ratio, FER)=100×(鱼体终重-鱼体初重)/摄食量
蛋白质效率(Protein efficiency ratio, PER)= 100× (鱼体终重-鱼体初重)/饲料蛋白摄食量
肥满度(CF, %)=100×鱼体终体重/鱼体长3
脏体比(Viserosomatic index, VSI, %)=100×内脏重/体重
肝体比(Hepatosomatic index, HSI, %)=100×肝脏重/体重
数据用平均值±标准误(means±SEM)表示, 采用单因素方差分析(One-way ANOVA), 显著性水平确定为0.05。当方差分析显示处理间结果差异显著时(P<0.05), 使用Tukey多重检验进行比较。数据分析采用的软件为SPSS 17.0。以SGR为评价指标, 用二次曲线模型[29]来确定大黄鱼的饲料糖需求量。
每天最少5升液体饲料,最多6升/天;开食料对瘤胃上皮的发育有重要作用,喂6升奶比喂8升奶能促使犊牛多采食1磅/天的开食料。
2 结果
2.1 饲料糖水平对大黄鱼生长性能和饲料利用的影响
经过8周的养殖实验, 大黄鱼存活率均在95.56%以上, 且在各处理组间无显著差异(P>0.05) (表 2)。
随着饲料糖水平的升高, 各处理组特定生长率SGR 先升高后降低, 在糖含量为26.69%时达到最大(0.37±0.01) %/d, 显著高于糖含量为1.75%、6.67%、13.64%和32.25%处理组 (P<0.05)。用二次曲线回归模型拟合SGR和饲料糖水平的关系, 得到大黄鱼饲料中糖的最适含量为22.7% (图 1)。饲料效率FER和蛋白质效率PER与特定生长率SGR有相似的变化趋势, 两者均在糖含量为21.15%时达最大值,显著高于糖含量为1.75%、6.67%和32.25%处理组(P <0.05)。
2.2 饲料糖水平对大黄鱼体组成的影响
随饲料糖水平的升高, 全鱼粗脂肪含量显著降低(P<0.05), 在糖含量为32.25%时降至最低(10.56%),显著低于其他各处理组(P<0.05)。全鱼水分(66.84%—68.97%)、粗蛋白(17.82%—18.24%)和灰分(3.45%—3.58%)均未受饲料糖水平的显著影响 (P>0.05)(表3)。
2.3 饲料糖水平对大黄鱼形体指标、肝糖原和肌糖原含量的影响
饲料糖水平对大黄鱼肝体比HSI和肝糖原含量影响显著(P<0.05), 随饲料中糖含量的增加, 两者均显著升高且在糖含量为32.25%时达到最大值, 显著高于糖含量为1.75%和6.67%处理组。大黄鱼脏体比VSI (1.71%—2.36%)、肥满度CF (1.55%—1.67%)、肌糖原含量(0.39—0.62 mg/g)在各处理间均无显著性差异(P>0.05)(表 4)。
2.4 饲料糖水平对大黄鱼血液指标的影响
大黄鱼血浆总胆固醇、血浆甘油三酯水平均受饲料糖水平的显著影响(P<0.05)(表 5)。血浆总胆固醇和甘油三酯水平随饲料糖含量的增加均显著降低, 在糖含量为32.25%时降至最小值, 显著低于糖含量为1.75%处理组(P<0.05)。然而饲料糖水平对大黄鱼血糖(3.32—4.48 mmol/L)、血清高/低密度脂蛋白胆固醇(1.63—2.13 mmol/L和1.17—1.92 mmol/L)水平均未产生显著影响 (P>0.05)。血清脂蛋白脂酶、肝脂酶和溶菌酶活力均随饲料糖含量的升高显著降低(P<0.05), 且在糖含量为32.25%处均降至最低, 显著低于糖含量为1.75%处理组(表5)。
2.5 饲料糖水平对大黄鱼消化酶活力的影响
大黄鱼肠道淀粉酶活力随饲料糖水平的升高表现为先上升后下降的趋势, 在糖含量为26.69%时活力达到最大值(0.47 U/mg pro.), 显著高于糖含量为1.75%、6.67%、13.64%和32.25%处理组(P<0.05) (表 6)。然而, 饲料糖水平并未对鱼体肝脏淀粉酶和胰蛋白酶、肠道胰蛋白酶活力产生显著影响(P>0.05)。
2.6 饲料糖水平对大黄鱼肝脏糖代谢酶活力的影响
肝脏糖酵解酶: 己糖激酶HK和丙酮酸激酶PK活力随饲料糖含量的升高均显著上升(P<0.05)。其中, HK活力在糖含量为21.15%时达到最大值(8.11 U/g prot.), 之后逐渐降低, 在糖含量为32.25%时, HK活力降至最低(4.6 U/g prot.), 显著低于糖含量为21.15%处理组(P<0.05)。PK活性在糖含量为32.25%时达到最大值(30.28 U/g prot.), 显著高于糖含量为1.75%和6.67%处理组(P<0.05)。肝脏磷酸果糖激酶PFK (3.75—9.14 U/g prot.)活性不受饲料糖水平的影响, 各组间无显著差异(P>0.05)。

表 2 饲料糖水平对大黄鱼生长效应和饲料利用的影响Tab. 2 Effects of dietary carbohydrate on growth performance and feed utilization of large yellow croaker
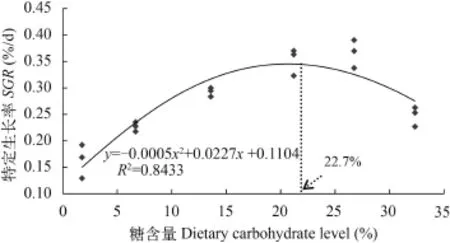
图 1 大黄鱼的特定生长率(SGR)与饲料糖水平的关系Fig. 1 Relationship between dietary carbohydrate levels and the specific growth rate (SGR) of large yellow croaker Larimichthys crocea fed with the experimental diets for 8 weeks
肝脏糖异生酶: 果糖-1, 6-二磷酸酶FBPase (11.92—17.68 U/g prot.)和磷酸烯醇式丙酮酸羧激酶PEPCK (9.95—12.23 U/g prot.)活性均不受饲料糖水平的显著影响(P>0.05)(表 7)。
3 讨论
本研究结果显示, 在饲料中适量的添加糖显著提高了大黄鱼的生长和饲料利用。在糖含量为21.15%和26.69%时(糖脂比分别为2.29和4.41), SGR达到最大值, 显著高于其他各处理组, 饲料糖含量的进一步增加反而显著降低了SGR, 表明饲料中糖含量过少或过量均导致鱼体生长受到抑制。同时, 当饲料中糖含量过高或过低时(糖脂比为9.92和0.10), 大黄鱼FER和PER显著低于其他处理组, 表明等氮等能饲料中, 可用适量的糖替代脂肪, 充分发挥鱼体内脂肪和糖代谢的协同效应[21], 从而有利于提高鱼类生长和饲料利用率[11]。饲料中适量糖的促生长和对蛋白质的节约效应可能是因为葡萄糖是组织中的优先氧化底物并且能抑制鱼体内糖异生途径, 从而有效地减少了氨基酸进入氧化途径[30]。同样, 在对许氏平鲉[13]、长吻[9]、尖齿胡子鲶[31]、蟾胡鲶[6]和眼斑拟石首鱼[32]等的研究中也有类似的发现。然而, 也有研究表明, 等氮等能饲料中糖水平不会对鱼体的生长产生显著影响[5,10,33—35]。不同的实验结果可能是由于实验鱼的种类、生长阶段、饲料糖源、糖脂比例、实验条件和周期等的差异造成。用二次多项回归模型拟合SGR和饲料糖水平的关系, 得到饲料中糖的最适含量为22.7%。这一结果与尖吻鲈20%[21]、长吻23.4%[9]、虹鳟18%[36]、军曹鱼21.1%[14]、许氏平鲉19.8%[13]等鱼类的饲料中糖的最适含量相似。
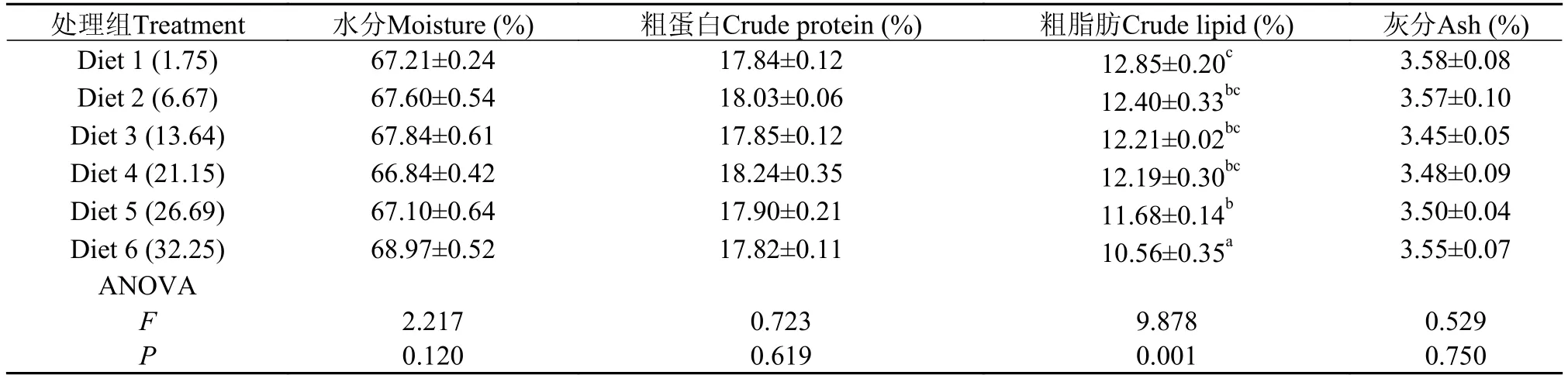
表 3 饲料糖水平对大黄鱼体组成的影响Tab. 3 Effects of dietary carbohydrate levels on the body compositions of large yellow croaker

表 4 饲料糖水平对大黄鱼形体指数、肝糖原和肌糖原含量的影响Tab. 4 Effects of dietary carbohydrate levels on body index, liver glycogen and muscle glycogen contents of large yellow croaker

表 5 饲料糖水平对大黄鱼血液指标的影响Tab. 5 Effects of dietary carbohydrate levels on plasma parameters of large yellow croaker
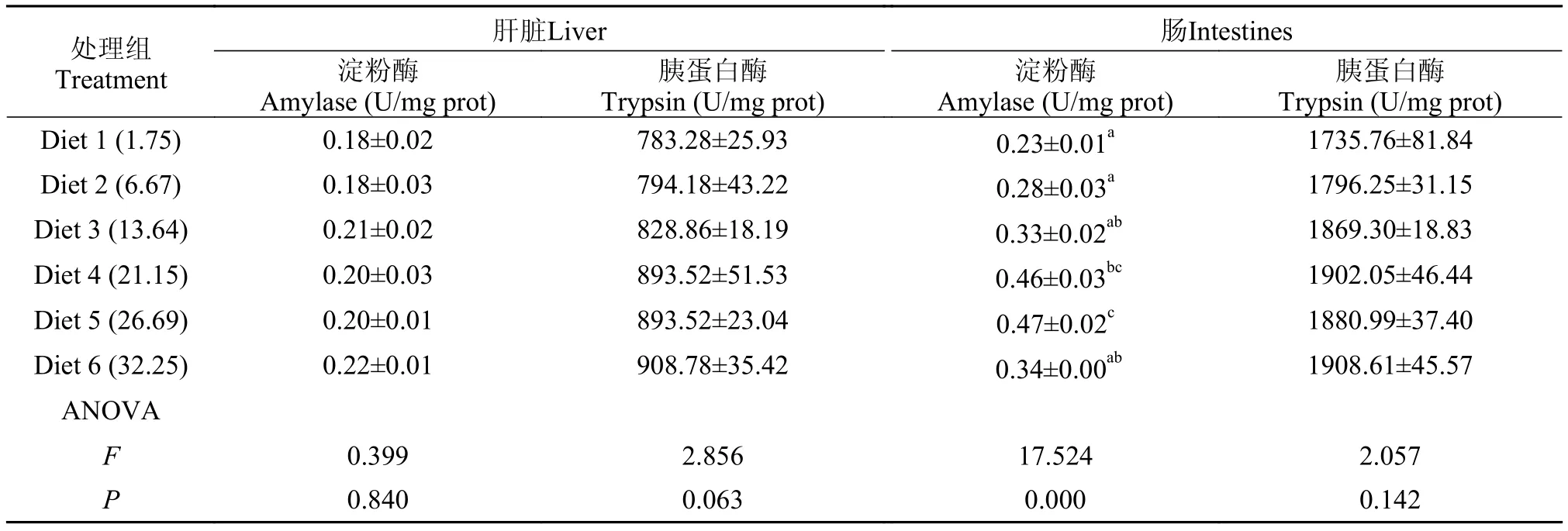
表 6 饲料糖水平对大黄鱼肝脏和肠消化酶活力的影响Tab. 6 Effects of dietary carbohydrate levels on digestive enzyme activities in liver and intestinal tract of large yellow croaker
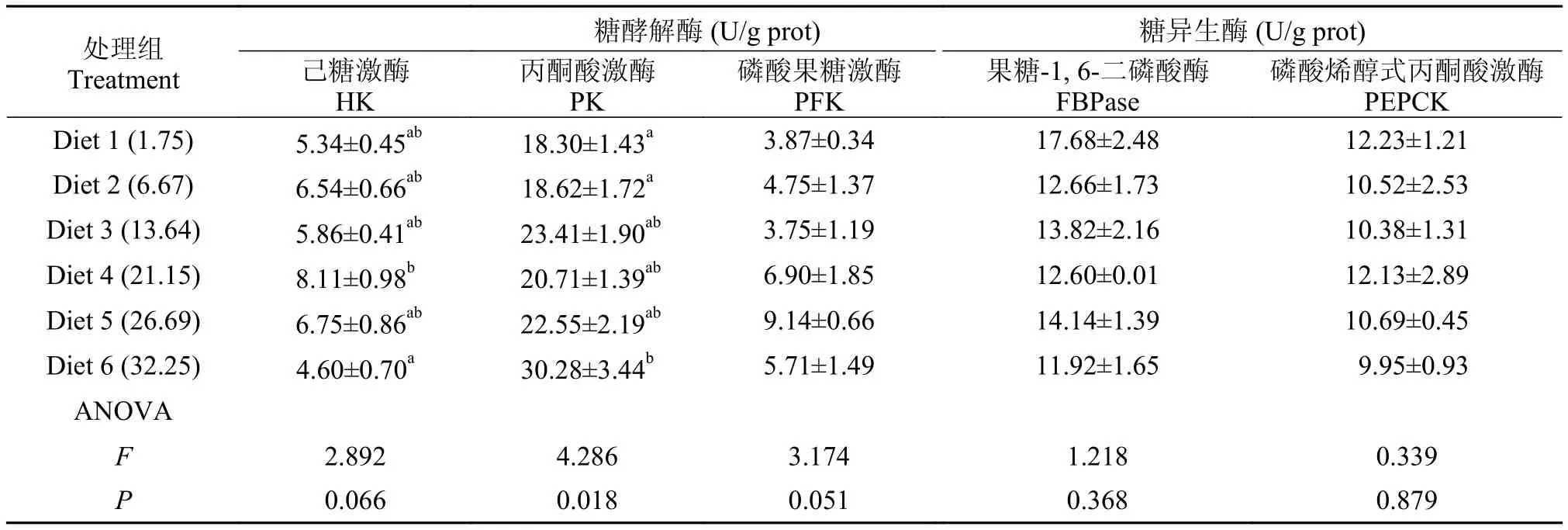
表 7 饲料糖水平对大黄鱼肝脏糖酵解和糖异生酶活性的影响Tab. 7 Effects of dietary carbohydrate levels on the activities of glycolysis and gluconeogenesis enzyme in the liver of large yellow croaker
在本研究中各处理组饲料能量相等, 为保证在等氮的前提下饲料中糖含量的梯度, 各实验饲料间脂肪和纤维素含量差异较大。有研究表明, 饲料中纤维素含量增加会导致鱼体对饲料中其他营养素的利用率降低[37]。然而, Wiesmann和Pfeffer[38]研究发现饲料中的纤维素并不影响虹鳟对饲料蛋白质和能量的利用, 并且在鱼类营养学研究中微晶纤维素作为填充剂被广泛采用[39,40]。此外, 饲料中微晶纤维素含量对鱼体产生的影响还与鱼的种类和食性有关[17]。在饲料中添加40%的微晶纤维素并没有对斑点叉尾(Ictalurus punctatus)和罗非鱼(Tilapia zillii)的生长产生显著性影响[5,41]。由此可见, 本研究中微晶纤维素对大黄鱼产生的影响还需要进一步研究确定。
在本研究中, 对于高脂低糖饲料组, 由于脂肪过高导致鱼摄食减少[31], 从而减少了蛋白质和其他营养素的摄入, 最终导致生长缓慢, 饲料效率和蛋白质效率显著降低。对于高糖低脂饲料组, 饲料提供的必需脂肪酸含量可能不足以满足鱼体的最佳生长需要, 同时高糖饲料导致鱼体血糖迅速升高,产生饱足感从而抑制摄食, 最终影响其生长[6,11]。已有研究表明, 鱼类特别是肉食性鱼类对高糖饲料耐受性较差, 高糖饲料的摄入将导致体内代谢紊乱,进而抑制其生长[42]。此外, 不论高脂低糖还是高糖低脂饲料组, 微晶纤维素含量过高或过低均会影响大黄鱼胃排空时间和实验饲料的适口性, 从而影响鱼类正常摄食。总之, 大黄鱼摄食高脂低糖或高糖低脂饲料均对其生长、代谢和饲料利用产生不利影响。在本研究中, 高糖低脂组(Diet 6)的SGR显著高于高脂低糖组(Diet 1), 由此推测在饲料蛋白质和能量含量一定的情况下, 相对于饲料脂肪, 大黄鱼能在一定范围内更好地利用饲料中的糖作为能量来源。当然, 该推测还需要进一步的研究。
饲料中任何非蛋白质能量的来源或含量的不平衡均会影响鱼类生长、营养物质利用和体脂肪沉积[43]。在本研究中, 无论是高脂低糖组(糖脂比为0.10)还是高糖低脂组(糖脂比为9.92), 大黄鱼SGR、PER和鱼体粗脂肪含量均显著降低。已有研究表明, 饲料糖脂比能显著影响糖类对蛋白质的节约效应, 适宜的饲料糖脂比CHO/L能够充分发挥糖和脂肪的协同效应[15], 从而促进鱼类生长和饲料利用[11]。在本研究中, 饲料中糖脂比为1.11和2.29时, 大黄鱼FER和PER均显著高于糖脂比为0.10和9.92时的值。本研究结果显示, 大黄鱼饲料最适糖脂比为2.29—4.41, 这与在虹鳟(CHO/L 2.45)、长吻(CHO/L 1.98)、蟾胡鲶(CHO/L 3.389)、尖齿胡子鲶(CO/L 1.7—3.4)上的研究结果相近[6,9,31,36]。
饲料中的脂肪含量是影响鱼体脂肪含量的最重要因素[44], 鱼体脂肪含量会随着摄入脂肪量的增加而上升[10]。Hemre和Kahrs[45]采用同位素标记葡萄糖研究大西洋鳕体内脂肪重新合成, 发现只有少量的的糖作为碳源合成脂肪, 饲料中的糖类促进机体脂肪重新合成主要通过增加细胞质内的还原型NADPH的量, 而不是通过为脂肪的合成提供碳架。在本研究中, 在饲料糖含量增加的同时, 饲料脂肪含量在降低(饲料糖脂比含量升高), 全鱼粗脂肪含量显著减少。这表明相对于糖类, 饲料脂肪更容易转化成鱼体脂肪并沉积。全鱼粗脂肪和饲料中脂肪含量的正相关性与在长吻[9]、星斑川鲽[33]、尖齿胡子鲶[31]、尖吻鲈[10]以及蟾胡鲶[6]等鱼类中的研究结果一致。
肝糖原是一种应急能源储备。当出现应激时,肝糖原很容易被合成存储或分解利用, 从而造成肝糖原的升高或降低[46]。Brauge等[28]研究表明, 随着饲料糖含量的增加, 由14C标记的葡萄糖转化成肝脏脂肪的含量也随之增加, 摄食高糖饲料会诱导虹鳟脂肪合成, 并增加肝脏脂肪的沉积。在本研究中,大黄鱼的肝体比HSI和肝糖原含量随饲料糖含量的增加而显著升高, 其中的原因可能是大黄鱼能将饲料中过量的糖转化成糖原储存在肝脏中, 进而导致肝体比的增大。在对长吻[9]、南方鲇[47]、欧州鲈[48,49]、星斑川鲽[33]、金头鲷[50,51]和虹鳟[52,53]等鱼类的研究中也得到了相似结论。然而在对尖齿胡子鲶[31]和杂交条纹鲈[54]的研究发现, 饲料糖含量并不影响鱼体肝糖原含量和肝体比。在对塞内加尔鳎[35]和许氏平鲉[13]的研究中发现饲料糖含量的增加反而使鱼体肝糖原含量和肝体比显著降低。不同的实验结果除了来自鱼种类的差异外, 不同的生长阶段、饲料配方、饲料糖水平设计范围、实验条件和周期等也造成了结果的差异。
肝脏肿大和肝糖原的过度累积可能导致肝脏解毒能力下降等免疫抑制反应[55]。在本研究中, 高糖饲料显著降低了大黄鱼血清溶菌酶的活性, 可能是由于肝糖原和肝脏脂肪的过度累积在一定程度上造成的免疫抑制反应。已有研究表明, 高血糖水平和高肝体比导致虹鳟对水中铜离子的耐受力降低[56]。然而, 肝糖原的累积可在一定程度上缓解鱼类摄食高糖饲料后导致的持续高血糖反应[57], 同时减少丙酮酸、乳酸和乙酰辅酶等血糖代谢中间产物对鱼体造成的影响[58,59]。在本研究中, 随着饲料糖水平的升高, 大黄鱼血糖水平并没有发生显著变化, 可能与大量的肝糖原累积有关, 在一定程度上缓解了高糖饲料对鱼体生理代谢平衡产生的影响。
血浆甘油三酯和胆固醇是鱼类血脂的重要组成成分, 具有供给能量、合成某些酶、维生素和激素等作用, 是维持正常生命活动所必需的物质[60]。血浆甘油三酯一般由饲料摄取的脂肪分解以及肝脏中转运出来的极低密度脂蛋白VLDL水解产生。在本研究中大黄鱼血浆甘油三酯水平随饲料脂肪含量的降低而显著下降, 可能是因为摄入的脂肪减少, 同时脂蛋白脂酶LPL活力的显著降低导致从肝脏转运出来的VLDL水解减弱, 最终导致了血浆甘油三酯水平的显著下降, 这也从一定程度上说明大黄鱼体内的脂肪沉积主要是来自饲料脂肪的摄入而不是脂肪合成。在高脂低糖实验组中, 鱼体为了应对高脂饲料而加强了对脂肪的运输, 表现为血浆胆固醇水平也显著升高。在对大菱鲆[61]、罗非鱼[62]、团头鲂[63]和长吻[9]等的研究中也发现了相似的结论。
大量研究表明, 鱼类能够根据摄食不同的饲料来调节自身消化酶的分泌, 但是这种调节能力随鱼种类不同而异[64]。一般来说, 杂食性和草食性鱼类的调节能力远高于肉食性鱼类[63]。在本研究中, 随饲料糖含量的升高, 大黄鱼肠道淀粉酶活力显著提高, 表明大黄鱼对饲料中的糖类具有一定的适应能力。这与在鲤[65]、胡鲶[66]和尖齿胡子鲶[67]的研究结果一致。然而, 饲料糖含量的进一步升高(26.69%—32.25%, 糖脂比由4.41升高至9.92)显著降低了大黄鱼肠道淀粉酶活性, 可能是由于当饲料糖含量为26.69%(糖脂比为4.41)时, 淀粉酶活性已接近饱和,过多糖的摄入反而抑制了其活性。此外, 可能还与饲料脂肪含量降低、脂肪酶活性变化有关, 这有待进一步研究。
本研究结果显示, 饲料糖含量的升高显著提高了大黄鱼肝脏丙酮酸激酶PK活力, 这在一定程度上促进了葡萄糖酵解, 从而缓解了鱼体高血糖症状。同样地, 有关欧洲鲈[68]、金头鲷[69]和河鲈[70]的研究表明高糖饲料能显著提高肝脏PK活力。然而,一些研究发现饲料糖水平对鱼类的PK活力没有产生显著影响[35,53,71,72]。Panserat等[73]用高糖饲料喂养虹鳟, 其PK的mRNA表达量无显著变化。因此,鱼类PK活力的调节可能是由基因转录后的修饰作用完成, 如PK酶原的磷酸化或去磷酸化作用。因此, 饲料糖水平对鱼类肝脏PK活力的影响仍需进一步研究。
在本研究中大黄鱼肝脏已糖激酶HK活力在饲料糖含量为21.15% (糖脂比为2.29)时达到最大值(8.11 U/g prot), 之后随糖含量的进一步升高, HK活性反而显著降低, 在糖含量为32.25% (糖脂比为9.92)时降至最低。HK活力的降低可能是因为其活性受到其产物(葡萄糖-6-磷酸)的负反馈抑制[74], 这在一定程度上可视为大黄鱼对高糖饲料的一种负反馈调节。在对鲈的研究中也发现饲料糖水平能显著提高肝脏HK活力[70]。然而, 在对虹鳟[75,76]、金头鲷[71]和欧洲鲈[68,77]的研究中并未发现饲料糖水平对其肝脏HK活力产生显著影响。
4 结论
在本研究中, 饲料糖含量在21.15%—26.67% (糖脂比2.29—4.41)时, 大黄鱼取得了最高的饲料效率、最佳的SGR和生理状态。用二次曲线回归模型拟合特定生长率和饲料糖水平的关系, 得到大黄鱼(130 g)饲料中糖的最适含量为22.7%。
[1]Fernández F, Miquel A G, Córdoba M, et al. Effects of diets with distinct protein-to-carbohydrate ratios on nutrient digestibility, growth performance, body composition and liver intermediary enzyme activities in gilthead sea bream (Sparus aurata, L. ) fingerlings [J]. Journal of Experimental Marine Biology and Ecology, 2007, 343(1): 1—10
[2]Stone D, Allan G L, Anderson A J. Carbohydrate utilization by juvenile silver perch, Bidyanus bidyanus (Mitchell). III. The protein-sparing effect of wheat starchbased carbohydrates [J]. Aquaculture Research, 2003, 34(2): 123—134
[3]Dias J, Alvarez M J, Diez A, et al. Regulation of hepatic lipogenesis by dietary protein/energy in juvenile European seabass (Dicentrarchus labrax) [J]. Aquaculture, 1998, 161(1): 169—186
[4]Peres H, Goncalves P, Oliva-Teles A. Glucose tolerance in gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) [J]. Aquaculture, 1999, 179(1): 415—423
[5]El-Sayed A M, Garling Jr D L. Carbohydrate-to-lipid ratios in diets for Tilapia zillii fingerlings [J]. Aquaculture, 1988, 73(1): 157—163
[6]Jafri A K. Effect of dietary carbohydrate-to-lipid ratio on growth and body composition of walking catfish (Clarias batrachus) [J]. Aquaculture, 1998, 161(1): 159—168
[7]Kjær M A, Todorčević M, Torstensen B E, et al. Dietary n-3 HUFA affects mitochondrial fatty acid β-oxidation capacity and susceptibility to oxidative stress in Atlantic salmon [J]. Lipids, 2008, 43(9): 813—827
[8]Tan Q, Xie S, Zhu X, et al. Effect of dietary carbohydrate-to-lipid ratios on growth and feed utilization in Chinese longsnout catfish (Leiocassis longirostris Günther) [J]. Journal of Applied Ichthyology, 2007, 23(5): 605—610
[9]Hanley F. Effects of feeding supplementary diets containing varying levels of lipid on growth, food conversion, and body composition of Nile tilapia, Oreochromis niloticus (L. ) [J]. Aquaculture, 1991, 93(4): 323—334
[10]Nankervis L, Matthews S J, Appleford P. Effect of dietary non-protein energy source on growth, nutrient retention and circulating insulin-like growth factor I and triiodothyronine levels in juvenile barramundi, Lates calcarifer [J]. Aquaculture, 2000, 191(4): 323—335
[11]Hemre G I, Mommsen T P, Krogdahl Å. Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes [J]. Aquaculture Nutrition, 2002, 8(3): 175—194
[12]Cowey C B, Walton M J. Intermediary metabolism [J]. Fish Nutrition, 1989, 2: 259—329
[13]Moon T W. Glucose intolerance in teleost fish: fact or fic-tion [J]? Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2001, 129(2): 243—249
[14]Wilson R P. Utilization of dietary carbohydrate by fish [J]. Aquaculture, 1994, 124(1): 67—80
[15]Brauge C, Corraze G, Médale F. Effects of dietary levels of carbohydrate and lipid on glucose oxidation and lipogenesis from glucose in rainbow trout, Oncorhynchus mykiss, reared in freshwater or in seawater [J]. Comparative Biochemistry and Physiology Part A: Physiology, 1995, 111(1): 117—124
[16]Catacutan M R, Coloso R M. Growth of juvenile Asian seabass, Lates calcarifer, fed varying carbohydrate and lipid levels [J]. Aquaculture, 1997, 149(1): 137—144
[17]Gao W, Liu Y J, Tian L X, et al. Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngodon idella) [J]. Aquaculture Nutrition, 2010, 16(3): 327—333
[18]Vielma J, Koskela J, Ruohonen K, et al. Optimal diet composition for European whitefish (Coregonus lavaretus): carbohydrate stress and immune parameter responses [J]. Aquaculture, 2003, 225(1): 3—16
[19]Lee S M, Kim K D. Effects of dietary carbohydrate to lipid ratios on growth and body composition of juvenile and grower rockfish, Sebastes schlegeli [J]. Aquaculture Research, 2009, 40(16): 1830—1837
[20]Ren M, Ai Q, Mai K, et al. Effect of dietary carbohydrate level on growth performance, body composition, apparent digestibility coefficient and digestive enzyme activities of juvenile cobia, Rachycentron canadum L [J]. Aquaculture Research, 2011, 42(10): 1467—1475
[21]Jafri A K. Growth rate, feed conversion, and body composition of Catla catla, Labeo rohita, and Cirrhinus mrigala fry fed diets of various carbohydrate-to-lipid ratios [J]. Journal of the World Aquaculture Society, 1998, 29(1): 84—91
[22]Duan Q, Mai K, Zhong H, et al. Studies on the nutrition of the large yellow croaker, Pseudosciaena crocea R. I: growth response to graded levels of dietary protein and lipid [J]. Aquaculture Research, 2001, 32(s1): 46—52
[23]Ma H, Cahu C, Zambonino J, et al. Activities of selected digestive enzymes during larval development of large yellow croaker (Pseudosciaena crocea) [J]. Aquaculture, 2005, 245(1): 239—248
[24]Mai K, Zhang C, Ai Q, et al. Dietary phosphorus requirement of large yellow croaker, Pseudosciaena crocea R [J]. Aquaculture, 2006, 251(2): 346—353
[25]Yu S, Olsen C E, Marcussen J. Methods for the assay of 1, 5-anhydro-D-fructose and α-1, 4-glucan lyase [J]. Carbohydrate Research, 1997, 305(1): 73—82
[26]Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [J]. Analytical Biochemistry, 1976, 72(1): 248—254
[27]Polakof S, Miguez J M, Soengas J L. Dietary carbohydrates induce changes in glucosensing capacity and food intake of rainbow trout [J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2008, 295(2): R478-R489
[28]Sangiao-Alvarellos S, Laiz-Carrión R, Guzmán J M, et al. Acclimation of S. aurata to various salinities alters energy metabolism of osmoregulatory and nonosmoregulatory organs [J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2003, 285(4): R897-R907
[29]Zeitoun I H, Ullrey D E, Magee W T, et al. Quantifying nutrient requirements of fish [J]. Journal of the Fisheries Board of Canada, 1976, 33(1): 167—172
[30]Sánchez muros M J, García rejón L, Lupianez J A, et al. Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout (Oncorhynchus mykiss). II. Adaptive response of glucose 6-phosphate dehydrogenase activity to high-carbohydrate/low-protein and high-fat/non-carbohydrate diets [J]. Aquaculture Nutrition, 1996, 2(4): 193—200
[31]Ali M Z, Jauncey K. Optimal dietary carbohydrate to lipid ratio in African catfish Clarias gariepinus (Burchell 1822) [J]. Aquaculture International, 2004, 12(2) : 169—180
[32]Ellis S C, Reigh R C. Effects of dietary lipid and carbohydrate levels on growth and body composition of juvenile red drum, Sciaenops ocellatus [J]. Aquaculture, 1991, 97(4): 383—394
[33]LEE S M, Lee J H. Effect of dietary glucose, dextrin and starch on growth and body composition of juvenile starry flounder Platichthys stellatus [J]. Fisheries Science, 2004, 70(1): 53—58
[34]Mohapatra M, Sahu N P, Chaudhari A. Utilization of gelatinized carbohydrate in diets of Labeo rohita fry [J]. Aquaculture Nutrition, 2003, 9(3): 189—196
[35]Dias J, Rueda Jasso R, Panserat S, et al. Effect of dietary carbohydrate-to-lipid ratios on growth, lipid deposition and metabolic hepatic enzymes in juvenile Senegalese sole (Solea senegalensis, Kaup) [J]. Aquaculture Research, 2004, 35(12): 1122—1130
[36]Yamamoto T, Konishi K, Shima T, et al. Influence of dietary fat and carbohydrate levels on growth and body composition of rainbow trout Oncorhynchus mykiss under self-feeding conditions [J]. Fisheries Science, 2001, 67(2): 221—227
[37]Kirchgessener M, Kürzinger H, Schwarz F J. Digestibility of crude nutrients in different feeds and estimation of their energy content for carp (Cyprinus carpio L. ) [J]. Aquaculture, 1986, 58(3—4): 185—194
[38]Wiesmann D, Pfeffer E. Influence of indigestible carbo-hydrates on the efficiency of utilization of dietary energy and protein in growing rainbow trout (Salmo gairdnerii, R) [J]. Archives of Animal Nutrition, 1986, 36(12): 1145—1149
[39]Mishra K, Samantaray K. Interacting effects of dietary lipid level and temperature on growth, body composition and fatty acidprofile of rohu, Labeo rohita (Hamilton) [J]. Aquaculture Nutrition, 2004, 10(6): 359—369
[40]Raven P A, Devlin R H, Higgs D A. Influence of dietary digestible energy content on growth, protein and energy utilization and body composition of growth hormone transgenic and nontransgenic coho salmon (Oncorhynchus kisutch) [J]. Aquaculture, 2006, 254(1—4): 730—747
[41]Garling Jr D L, Wilson R P. Effects of dietary carbohydrate-to-lipid ratios on growth and body composition of fingerling channel catfish [J]. The Progressive Fish-Culturist, 1977, 39(1—4): 43—47
[42]Masayuki furuichi, Yasuo yone. Change of blood sugar and plasma insulin levels of fishes in glucose tolerance test [J]. Bulletin of the Japanese Society of Scientific Fisheries, 1981, 47(6): 761—764
[43]Garling Jr D L, Wilson R P. Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus [J]. The Journal of Nutrition, 1976, 106(9): 1368—1375
[44]Sargent J, Henderson R J, Tocher D R. The lipids [A]. In: Fish Nutrition. In: Halver J E (Eds. ), Fish Nutrition [M]. New York: Academic Press. 1989, 154—209
[45]Hemre G I, Kahrs F.14C-glucose injection in Atlantic cod, Gadus morhua, metabolic responses and excretion via the gill membrane [J]. Aquaculture Nutrition, 1997, 3(1): 3—8
[46]Soengas J L, Aldegunde M, Andrés M D. Gradual transfer to sea water of rainbow trout: effects on liver carbohydrate metabolism [J]. Journal of Fish Biology, 1995, 47(3): 466—478
[47]Luo Y, Xie X. Effects of body lipid content on the resting metabolic rate and postprandial metabolic response in the southern catfish Silurus meridionalis [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2009, 154(4): 547—550
[48]Moreira I S, Peres H, Couto A, et al. Temperature and dietary carbohydrate level effects on performance and metabolic utilisation of diets in European sea bass (Dicentrarchus labrax) juveniles [J]. Aquaculture, 2008, 274(1): 153—160
[49]Peres H, Oliva-Teles A. Utilization of raw and gelatinized starch by European sea bass (Dicentrarchus labrax) juveniles [J]. Aquaculture, 2002, 205(3): 287—299
[50]Bou M, Todorčević M, Fontanillas R, et al. Adipose tissue and liver metabolic responses to different levels of dietary carbohydrates in gilthead sea bream (Sparus aurata) [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2014, 175(1): 72—81
[51]Couto A, Enes P, Peres H, et al. Effect of water temperature and dietary starch on growth and metabolic utilization of diets in gilthead sea bream (Sparus aurata) juveniles [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2008, 151(1): 45—50
[52]Capilla E, Médale F, Navarro I, et al. Muscle insulin binding and plasma levels in relation to liver glucokinase activity, glucose metabolism and dietary carbohydrates in rainbow trout [J]. Regulatory Peptides, 2003, 110(2): 123—132
[53]Hilton J W, Atkinson J L. Response of rainbow trout (Salmo gairdneri) to increased levels of available carbohydrate in practical trout diets [J]. British Journal of Nutrition, 1982, 47(3): 597—607
[54]Nematipour G R, Brown M L, Gatlin D M. Effects of dietary carbohydrate: lipid ratio on growth and body composition of hybrid striped bass [J]. Journal of the World Aquaculture Society, 1992, 23(2): 128—132
[55]Cai C F. Study on the utilization of dietary carbohydrate by Myloparyngodon piecus Richrdson and Carassius auratus and their mechanism of metabolism [D]. Thesis for Doctor of Science. East China Normal University, Shanghai. 2004 [蔡春芳. 青鱼和鲫对饲料糖的利用及其代谢机制的研究. 博士学位论文, 华东师范大学, 上海. 2004]
[56]Dixon D G, Hilton J W. Influence of available dietary carbohydrate content on tolerance of waterborne copper by rainbow trout, Salmo gairdneri Richardson [J]. Journal of Fish Biology, 1981, 19(5): 509—518
[57]Lin X Z, Luo Y P, Xie X J. Effect of dietary carbohydrate level on glycolytic enzymes and serum glucose concentrations in the juvenile southern catfish after feeding [J]. Acta Hydrobiologica Sinica, 2006, 30(3): 304—310 [林小植, 罗毅平, 谢小军. 饲料碳水化合物水平对南方鲇幼鱼餐后糖酵解酶活性及血糖浓度的影响.水生生物学报, 2006, 30(3): 304—310]
[58]Nakano K, Tagawa M, Takemura A, et al. Temporal changes in liver carbohydrate metabolism associated with seawater transfer in Oreochromis mossambicus [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 1998, 119(4): 721—728
[59]Luo Y P. Ecophysiological responses in a carnivorous fish, the southern catfish (Silurus meridionalis Chen) to nutritional stress of dietary carbohydrate [D]. Thesis for Doctor of Science. Southwest University, Chongqing. 2007 [罗毅平. 肉食性鱼类南方鲇对饲料碳水化合物营养胁迫的生理生态学反应. 博士学位论文, 西南大学,重庆. 2007]
[60]Likimani T A, Wilson R P. Effects of diet on lipogenicenzyme activities in channel catfish hepatic and adipose tissue [J]. The Journal of Nutrition, 1982, 112(1): 112—117
[61]Regost C, Arzel J, Cardinal M, et al. Dietary lipid level, hepatic lipogenesis and flesh quality in turbot (Psetta maxima) [J]. Aquaculture, 2001, 193(3): 291—309
[62]Shimeno S, Ming D, Takeda M. Metabolic response to dietary carbohydrate to lipid ratios in Oreochromis niloticus [J]. Nippon Suisan Gakkaishi, 1993, 59(5): 827—833
[63]Li X, Jiang Y, Liu W, et al. Protein-sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingerlings: effects on digestive and metabolic responses [J]. Fish Physiology and Biochemistry, 2012, 38(2): 529—541
[64]Moro G V, Camilo R Y, Moraes G, et al. Dietary nonprotein energy sources: growth, digestive enzyme activities and nutrient utilization by the catfish jundiá, Rhamdia quelen [J]. Aquaculture Research, 2010, 41(3): 394—400
[65]Kawai S, Ikeda S. Studies on digestive enzymes of fishes. 2. Effect of dietary change on activities of digestive enzymes in carp intestine [J]. Bulletin of the Japanese Society of Scientific Fisheries, 1972, 38(3): 265
[66]Mukhopadhyay P K. Studies on the enzymatic activities related to varied pattern of diets in the air-breathing cat fish, Clarias batrachus (Linn. ) [J]. Hydrobiologia, 1977, 52(2): 235—237
[67]Ali M Z, Jauncey K. Effect of dietary lipid to carbohydrate ratios on body composition, digestive enzyme activities and blood plasma components in African catfish Clarias gariepinus (Burchell, 1822) [J]. Journal of Aquaculture in the Tropics, 2005, 20: 57—70
[68]Enes P, Panserat S, Kaushik S, et al. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2006, 143(1): 89—96
[69]Fernández F, Miquel A G, Córdoba M, et al. Effects of diets with distinct protein-to-carbohydrate ratios on nutrient digestibility, growth performance, body composition and liver intermediary enzyme activities in gilthead sea bream (Sparus aurata, L. ) fingerlings [J]. Journal of Experimental Marine Biology and Ecology, 2007, 343(1): 1—10
[70]Borrebaek B, Christophersen B. Hepatic glucose phosphorylating activities in perch (Perca fluviatilis) after different dietary treatments [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2000, 125(3): 387—393
[71]Enes P, Panserat S, Kaushik S, et al. Hepatic glucokinase and glucose-6-phosphatase responses to dietary glucose and starch in gilthead sea bream (Sparus aurata) juveniles reared at two temperatures [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2008, 149(1): 80—86
[72]Suárez M D, Sanz A, Bazoco J, et al. Metabolic effects of changes in the dietary protein: carbohydrate ratio in eel (Angilla anguilla) and trout (Oncorhynchus mykiss) [J]. Aquaculture International, 2002, 10(2): 143—156
[73]Panserat S, Plagnes-Juan E, Kaushik S. Nutritional regulation and tissue specificity of gene expression for proteins involved in hepatic glucose metabolism in rainbow trout (Oncorhynchus mykiss) [J]. Journal of Experimental Biology, 2001, 204(13): 2351—2360
[74]Pen-Hsing Tung, Shi-Yen Shiau. Effects of meal frequency on growth performance of hybrid tilapia, Oreochromis niloticus×O. aureus, fed different carbohydrate diets [J]. Aquaculture, 1991, 92: 343—350
[75]Cowey C B, Knox D, Walton M J, et al. The regulation of gluconeogenesis by diet and insulin in rainbow trout (Salmo gairdneri) [J]. British Journal of Nutrition, 1977, 38(3): 463—470
[76]Kirchner S, Seixas P, Kaushik S, et al. Effects of low protein intake on extra-hepatic gluconeogenic enzyme expression and peripheral glucose phosphorylation in rainbow trout (Oncorhynchus mykiss) [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2005, 140(2): 333—340
[77]Enes P, Panserat S, Kaushik S, et al. Rapid metabolic adaptation in European sea bass (Dicentrarchus labrax) juveniles fed different carbohydrate sources after heat shock stress [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2006, 145(1): 73—81
EFFECTS OF DIETARY CARBOHYDRATE ON GROWTH PERFORMANCE AND GLYCOMETABOLISM OF LARGE YELLOW CROAKER LARIMICHTHYS CROCEA
XING Shu-Juan1, SUN Rui-Jian2, MA Jun1, WEI Hai-Ming1, XU Wei1, ZHOU Hui-Hui1, ZHANG Yan-Jiao1, ZHANG Wen-Bing1and MAI Kang-Sen1
(1. Key Laboratory of Mariculture (Ministry of Education), Key Laboratory of Aquaculture Nutrition and Feeds (Ministry of Agriculture), Ocean University of China, Qingdao 266003, China; 2. Tongwei Technology Center Limited by Share Ltd., Chengdu 610041, China)
To evaluate the effects of dietary carbohydrate on growth performance, feed utilization, plasma parameters, and glycometabolism enzyme activities of large yellow croaker Larimichthys crocea, triplicate groups of fish [the initial body weight: (137.5±0.35) g] were randomly fed six isonitrogenous (45% crude protein) and isoenergetic (18 kJ/g gross energy) diets containing graded levels of carbohydrate (1.75%, 6.67%, 13.64%, 21.15%, 26.69% and 32.25%) in floating sea cages for an 8-week feeding trial. Results showed that the highest specific growth rate (SGR) was by 26.69% of dietary carbohydrate. The highest dietary carbohydrate content (32.25%) significantly decreased the SGR (P<0.05). Both feed efficiency ratio (FER) and protein efficiency ratio (PER) of large yellow croaker fed diets with 13.64% and 21.15% of carbohydrate were significantly higher than those of other groups (P<0.05). The whole-body lipid content significantly decreased the increased dietary carbohydrate contents with the lowest lipid content 32.25% of carbohydrate group (P<0.05). Hepatosomatic index (HSI) and liver glycogen content significantly increased with the increased dietary carbohydrate contents (P<0.05). Plasma total cholesterol and triglyceride levels significantly decreased with the increased dietary carbohydrate level (P<0.05), but plasma glucose did not impact by dietary carbohydrate level (P>0.05). The increased dietary carbohydrate significantly depressed activities of serum lysozyme, lipoprotein lipase and hepatic lipase (P<0.05). The activities of intestinal tract amylase and hepatic hexokinase (HK) increased significantly and then decreased with the highest value at 26.69% and 21.15% dietary carbohydrate contents, respectively (P<0.05). Dietary carbohydrate significantly up-regulated activities of pyruvate kinase (PK) with the peak level by 32.25% carbohydrate group (P<0.05). These results indicated the requirement of dietary carbohydrate for large yellow croaker was 22.7%.
Large yellow croaker; Larimichthys crocea; Carbohydrate; Nutrition; Feed
S963
A
1000-3207(2017)02-0265-12
10.7541/2017.33
2016-03-02;
2016-08-14
国家重点基础研究发展计划(“973”计划)项目(2014CB138600); 广东恒兴水产动物营养与饲料院士工作站项目(2014B09090514)资助 [Supported by the National Basic Research Program (“973” Program, 2014CB138600); Academican Workstation for Aquatic Animal Nutrition and Feed in Guangdong Evergreen (2014B09090514)]
邢淑娟(1989—), 女, 贵州铜仁人; 硕士; 研究方向为水产动物营养与饲料。E-mail: shujuanxing0504@163.com
张文兵, 教授; E-mail: wzhang@ouc.edu.cn
