脂蛋白酯酶在compound K调节血脂水平中的作用
2017-03-18苏晴邓小飞周人杰
苏晴+邓小飞++周人杰
[摘要] 目的 探讨脂蛋白酯酶在compound K调节血脂水平中的作用。 方法 36只雄性SD大鼠随机分为6组,除对照组外均给予高脂饮食,对照组和模型组给予生理盐水腹腔注射,compound K给药组分别给予compound K 1、3、9 mg/kg腹腔注射,GGPP组同时给予compound K 3、9 mg/kg GGPP。4周后处死动物,取样测定血脂、肝素后脂蛋白酯酶活性、脂蛋白酯酶表达水平等;培养大鼠肝细胞株BRL,分别给予compound K 3、10、30 μmol/L处理12 h后,检测脂蛋白酯酶表达水平。 结果 与模型组比较,compound K 3、9 mg/kg组的总胆固醇、低密度脂蛋白胆固醇、三酰甘油显著降低,高密度脂蛋白胆固醇、肝素后血清脂蛋白酯酶活性和脂蛋白酯酶表达量显著增高,差异有统计学意义(P < 0.05),且呈剂量依赖性。体外细胞试验结果与动物实验一致。 结论 compound K能够通过增加脂蛋白酯酶的表达和活性达到降低高脂饮食大鼠血清三酰甘油的作用,其机制与compound K激活肝X受体α相关。
[关键词] compound K;动脉粥样硬化;脂蛋白酯酶;肝X受体α
[中图分类号] R543 [文献标识码] A [文章编号] 1673-7210(2017)01(b)-0020-05
Role of lipoprotein lipase on serum lipid modulation of compound K
SU Qing DENG Xiaofei ZHOU Renjie
Department of Emergency, Xinqiao Hospital, the Third Military Medical University of PLA, Chongqing 400037, China
[Abstract] Objective To explore the role of lipoprotein lipase (LPL) on serum lipid modulation of compound K. Methods 36 male SD rats were divided into 6 groups. All animals were fed with high fat diet except control group. Control group and model group were given intraperitoneal injection of saline. compound K groups were treated with different doses of compound K (1, 3, 9 mg/kg). GGPP group was administrated with compound K 3 and 9 mg/kg GGPP simultaneously. After 4 weeks, animals were sacrificed. Serum lipid profile, LPL activity and the expression of LPL mRNA and protein were measured. BRL cells were treated with different doses of compound K (3, 10, 30 μmol/L) for 12 h, and the expression levels of LPL were measured. Results Compared with model group, total cholesterol, low-density lipoprotein cholesterol and triglycerides levels of compound K 3 and 9 mg/kg groups were significantly decreased, high density lipoprotein cholesterol, serum lipoprotein lipase activity after heparin and LPL expression of compound K 3 and 9 mg/kg groups were significantly increased, with statistical differences (P < 0.05), and the action depended on its dose. The results of in vitro cell experiments were consistent with animal experiments. Conclusion compound K can reduce the expression of serum triglyceride via up-regulation of LPL expression and activity, and the mechanism is associated with compound K activated liver X receptor α.
[Key words] compound K; Atherosclerosis; Lipoprotein lipase; Liver X receptor α
近年來,因动脉粥样硬化导致的心肌梗死、中风等多种严重心脑血管疾病引发的死亡人数居各类疾病之首[1]。在目前广泛采取防治措施的情况下,动脉粥样硬化的发病率并没有出现显著的下降,提示现有防治手段尚待改进,并亟需作用于新靶点的动脉粥样硬化防治药物。compound K是二醇型人参皂苷经过消化代谢后产生并吸收入血液循环的活性物[2-3],具有多种药理学活性。近期有研究证实,compound K能够通过激活肝X受体α(liver X receptor α,LXRα)达到调节血脂和抗炎的作用,从而延缓实验动物动脉粥样硬化的形成[4]。
compound K作用靶点与现有调节血脂药物不同,其机制也不相同。但目前的研究只关注了胆固醇逆转运通路的变化,解释了低密度脂蛋白胆固醇(low-density lipoprotein cholesterol,LDL-C)、高密度脂蛋白胆固醇(high-density lipoprotein cholesterol,HDL-C)水平变化的机制,但对于三酰甘油(triglyceride,TG)水平变化的机制尚缺乏探讨[4]。有研究证实,脂蛋白酯酶(lipoprotein lipase,LPL)在血脂代谢中也具有重要作用,血浆LPL是清除TG的限速酶,能够通过催化富含TG的乳糜微粒水解供能或转化为脂肪储存,另外,LPL还在HDL-C合成中起一定的促进作用。研究也表明,LPL单基因突变能够导致TG异常升高和HDL-C降低等变化,并且显著增加冠心病的危险性[5-6]。本实验拟利用高血脂大鼠模型,对LPL在compound K调节血脂中的作用进行探讨,以进一步深化对compound K防治动脉粥样硬化作用的机制研究。
1 材料与方法
1.1 实验动物与试剂
雄性SD大鼠:180~200 g,8~10周龄,购于第三军医大学实验动物中心,实验前为SPF级饲养,许可证号:SYXK(渝)2012-0002;高脂饲料配置:3%胆固醇、10%猪油、0.2%丙基硫氧嘧啶及86.8%基础饲料。compound K(上海同田生物技术股份有限公司,纯度> 98%);GGPP(Sigma-Aldrich,USA);血清生化AU-2700全自动生化分析仪标准品(Randox Laboratories Ltd,UK);LPL活性检测试剂盒(HY-60073,上海鈺博生物科技有限公司);反转录试剂盒(RR047,Takara,Japan);Real-time定量PCR试剂盒(RR820,Takara,Japan);LPL一抗(sc-73646,Santa Cruz,USA);HRP标记羊抗小鼠IgG抗体(ZDR-5117,北京中杉金桥生物技术有限公司)。
1.2 动物分组
雄性SD大鼠36只随机分为6组,每组6只,除对照组外均给予高脂饮食,compound K给药组分别给予compound K 1、3、9 mg/kg腹腔注射,1次/d;对照组和模型组均给予生理盐水腹腔注射;GGPP组同时给予compound K 3、9 mg/kg GGPP腹腔注射,共持续4周。
1.3 样本采集
每周测量体重一次,在实验第4周禁食12 h后采血获得血清后用于血脂水平的检测;尾静脉给予肝素321.5 U/kg,15 min后采集肝素后血清样本用于LPL活性检测;脱颈椎法处死动物后,取肝脏用于Real-time PCR、Western blot检测。
1.4 血脂水平测定
将血清置于AU-2700全自动生化分析仪中检测总胆固醇(total cholesterol,TC)、TG、HDL-C和LDL-C水平。
1.5 LPL活性及表达水平测定
使用LPL活性检测试剂盒测定肝素后血清中LPL活性。Real-time PCR法测定肝脏组织中LPL mRNA表达水平,引物为5′-TCCCAACCATACAAGACTCC-3′,5′-ACGTCGTCCATTGCTTTTGC-3′;内参为GAPDH,引物为5′-TGAAGGTCGGTGTGAACGGATTTGG-3′,5′-ACGACATACTCAGCACCAGCATCAC-3′。Western blot法测定肝脏组织中LPL蛋白表达水平,LPL一抗浓度为1∶500,二抗浓度1∶5000。
1.6 细胞培养及处理
常规培养BRL大鼠肝细胞于含10%胎牛血清的DMEM培养基中,置37℃、5%CO2恒温培养箱。对照组给予空溶剂处理,药物处理组分别给予compound K 3、10、30 μmol/L处理12 h,GGPP组在给予compound K 10 μmol/L的基础上给予GGPP 10 mmol/L。Real-time PCR法及Western blot方法同上。
1.7 统计学方法
采用SPSS 13.0统计软件对数据进行分析和处理,计量资料以均数±标准差(x±s)表示,两组间比较采用t检验,多组间比较采用单因素方差分析,以P < 0.05为差异有统计学意义。
2 结果
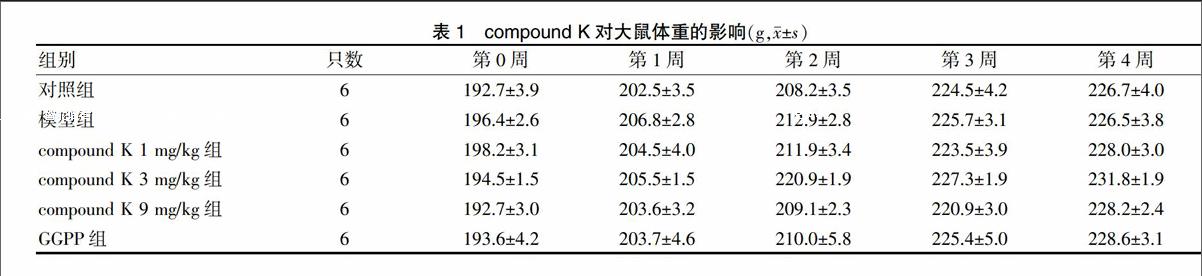
2.1 compound K对大鼠体重的影响
各组大鼠体重在试验期间正常增长,各组大鼠体重比较,差异无统计学意义(P > 0.05)。见表1。
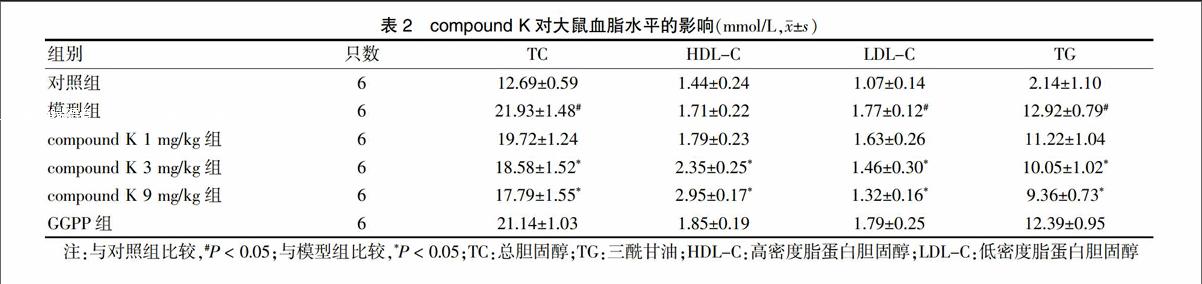
2.2 compound K对大鼠血脂水平的影响
模型组与对照组TC、LDL-C和TG比较,差异有统计学意义(P < 0.05),提示高血脂动物模型造模成功。与模型组比较,compound K 3 mg/kg组、compound K 9 mg/kg组的TC、LDL-C和TG降低,HDL-C升高,差异有统计学意义(P < 0.05),且具有剂量依赖性。GGPP组与模型组TC、HDL-C、LDL-C和TG比较,差异无统计学意义(P > 0.05)。见表2。
2.3 compound K对大鼠肝素后血清LPL活性的影响
与对照组比较,模型组肝素后LPL活性降低,差异有统计学意义(P < 0.05);与模型组比较compound K 3 mg/kg组、compound K 9 mg/kg组的LPL活性升高,差异有统计学意义(P < 0.05),且各给药组导致的LPL活性增高呈剂量依赖性。GGPP组与模型组的LPL活性比较,差异无统计学意义(P > 0.05)。见表3。
2.4 compound K对大鼠肝脏中LPL表达水平的影响
与对照组比较,模型组肝脏中LPL mRNA表达水平显著降低,差异有统计学意义(P < 0.05);模型组与对照组肝脏中LPL蛋白表达水平比较,差异无统计学意义(P > 0.05);与模型组比较,compound K各剂量组的LPL mRNA表达水平呈剂量依赖性升高,分别为306%、557%和618%,差异有统计学意义(P < 0.05);LPL蛋白表达水平与mRNA表达水平变化规律一致,各组差异有统计学意义(P < 0.05)。GGPP组与模型组LPL mRNA和蛋白表达水平比较,差异无统计学意义(P > 0.05)。见图1。
2.5 compound K对体外培养大鼠肝细胞LPL表达水平的影响
与动物实验结果一致,与对照组比较,compound K 3、10、30 μmol/L组肝细胞中LPL mRNA表达水平呈剂量依赖性升高,分别为252%、317%、408%,差异均有统计学意义(P < 0.05);LPL蛋白表达水平变化与mRNA表达水平变化规律一致,且各组差异有统计学意义(P < 0.05)。GGPP组LPL mRNA和蛋白表达水平与compound K 3、10、30 μmol/L组比较,差异有统计学意义(P < 0.05),提示阻断LXR途径能够抑制compound K导致的LPL蛋白表达水平增高。见图2。
3 讨论
compound K是二醇型人参皂苷(如Rb1等)在体内的代谢产物,早期研究发现,口服Rb1后血清中只能检测出compound K成分,而无Rb1[7],且给肠道无微生物小鼠口服Rb1(200 mg/kg)后,血液中未检测出Rb1及其代谢产物。提示compound K可能是口服二醇型人参皂苷后在体内发挥药理作用的活性代谢产物。研究发现,compound K具有多种药理学活性,包括抑制多种肿瘤细胞生长[8-9]、增强顺铂化疗效果[10]、抑制平滑肌细胞增殖[11]、激动糖皮质激素受体[12]、缓解吗啡成瘾[13]和抗炎作用[14]等,但对于compound K对心血管系统和血脂代谢方面的药理学研究还比较少见。本研究通过体内外试验探索了compound K对血脂代谢关键酶LPL的可能作用及机制。
本研究结果中compound K能够显著降低LDL-C和升高HDL-C的活性,与Zhou等[4]对胆固醇逆转运活性方面的研究结果一致。本研究还发现,compound K能够导致高脂饮食大鼠血清中TG显著下降。TG是动脉粥样硬化形成的独立危险因素,目前临床上广泛使用的苯氧乙酸类(贝特类)降血脂药就是以降低TG水平为靶点的。本研究结果提示,compound K除了能夠通过增强胆固醇逆转运的途径达到防治动脉粥样硬化的目的外,还具有显著降低TG的作用。为探索compound K降低TG水平的机制,我们对在TG代谢中起重要作用的LPL活性进行了检测。结果显示,compound K能够导致肝素后血清中LPL活性的显著升高,其变化规律与TG水平的变化规律一致。且该活性升高的机制与LPL表达水平增高有关,其上调作用发生在转录环节。体外细胞实验也得到相同的结果。结合关于compound K具有LXRα激动剂作用的结果,以及LXRα具有调节LPL转录表达作用的报道,我们推测,compound K上调LPL表达与激活LXRα有关。故利用GGPP阻断LXRα的活性进行验证,结果显示,GGPP在体内外试验中均能够显著抑制compound K导致的LPL表达水平上调,提示compound K上调LPL表达水平确与其激活LXRα相关。
血管内皮上的LPL能够有效地脂解富含TG脂蛋白,使其转变为不易致动脉粥样硬化的脂蛋白谱[15],并在HDL-C的形成中具有重要作用[16],故LPL活性的增高对动脉粥样硬化病变具有一定的保护作用,还能改善高脂饮食导致的代谢功能紊乱[17-18]。尽管有研究发现特异性地增加动脉壁巨噬细胞LPL的表达可能通过促进LDL在内皮下基质的潴留而促进动脉粥样硬化进程[19],但研究认为,LPL对动脉粥样硬化的具体作用与其表达部位关系更密切[20],本试验发现的肝脏中LPL表达增高与乳糜微粒在肝脏中的摄取和代谢水平增高有关,提示compound K调节血脂从而抑制动脉粥样硬化形成的作用可能与其增加LPL活性有关。故深入探索compound K上调LPL表达及活性的机制,进一步明确其构效关系,可能为将来研发更有效的动脉粥样硬化防治药物提供新的策略和靶点。
[参考文献]
[1] Logue J,Murray HM,Welsh P,et al. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation [J]. Heart,2011,97(7):564-568.
[2] Akao T,Kida H,Hatori M,et al. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseg [J]. J Pharm Pharmacol,1998,50(10):1155-1160.
[3] Kim HK. Pharmacokinetics of ginsenoside Rb1 and its met-abolite compound K after oral administration of Korean Red Ginseng extract [J]. J Ginseng Res,2013,37(4):451-456.
[4] Zhou L,Zheng Y,Li ZY,et al. Compound K attenuates the development of atherosclerosis in ApoE-/- mice via LXRα activation [J]. Int J Mol Sci,2016,17(7):1054.
[5] Nordestgaard BG,Wittrup HH,Tybjrg-Hansen A. Lipoprotein lipase mutations,plasma lipids and lipoproteins and risk of ischemic heart disease. A meta-analysis [J]. Circulation,1999,99(22):2901-2907.
[6] Soto AG,McIntyre A,Agrawal S,et al. Severe hypertriglyceridemia due to a novel p.Q240H mutation in the Lipoprotein Lipase gene [J]. Lipids Health Dis,2015,14:102.
[7] Paek IB,Moon Y,Kim J,et al. Pharmacokinetics of a ginseng saponin metabolite compound K in rats [J]. Biopharm Drug Dispos,2006,27(1):39-45.
[8] Hu C,Song G,Zhang B,et al. Intestinal metabolite compound K of panaxoside inhibits the growth of gastric carcinoma by augmenting apoptosis via bid-mediated mitochondrial pathway [J]. J Cell Mol Med,2012,16(1):96-106.
[9] Zheng ZZ,Ming YL,Chen LH,et al. Compound K-induced apoptosis of human hepatocellular carcinoma MHCC97-H cells in vitro [J]. Oncol Rep,2014,32(1):325-331.
[10] Li Y,Zhou T,Ma C,et al. Ginsenoside metabolite compound K enhances the efficacy of cisplatin in lung cancer cells [J]. J Thorac Dis,2015,7(3):400-406.
[11] Park ES,Lee KP,Jung SH,et al. Compound K,an intestinal metabolite of ginsenosides,inhibits PDGF-BB-induced VSMC proliferation and migration through G1 arrest and attenuates neointimal hyperplasia after arterial injury [J]. Atherosclerosis,2013,228(1):53-60.
[12] Yang CS,Ko SR,Cho BG,et al. The ginsenoside metabolite compound K,a novel agonist of glucocorticoid receptor,induces tolerance to endotoxin-induced lethal shock [J]. J Cell Mol Med,2008,12(5A):1739-1753.
[13] Yayeh T,Yun K,Jang S,et al. Morphine dependence is attenuated by red ginseng extract and ginsenosides Rh2,Rg3,and compound K [J]. J Ginseng Res,2016,40(4):445-452.
[14] Chen J,Wang Q,Wu H,et al. The ginsenoside metabolite compound K exerts its anti-inflammatory activity by down regulating memory B cell in adjuvant-induced arthritis [J]. Pharm Biol,2016,54(7):1280-1288.
[15] He D,Huang L,Xu Y,et al. Computational analysis and enzyme assay of inhibitor response to disease single nucleotide polymorphisms(SNPs)in lipoprotein lipase [J]. J Bioinform Comput Biol,2016,14(5):1650028.
[16] Tani M,Horvath KV,Lamarche B,et al. High-density lipoprotein subpopulation profiles in lipoprotein lipase and hepatic lipase deficiency [J]. Atherosclerosis,2016, 253:7-14.
[17] Walton RG,Zhu BB,Unal R,et al. Increasing adipocyte lipoprotein lipase improves glucose metabolism in high fat diet-induced obesity [J]. J Biol Chem,2015,290(18):11547-11556.
[18] Vishram JK,Hansen TW,Torp-Pedersen C,et al. Relationship between two common lipoprotein lipase variants and the metabolic syndrome and its individual components [J]. Metab Syndr Relat Disord,2016,14(9):442-448.
[19] Mead JR,Cryer A,Ramji DP. Lipoprotein lipase,a key role in atherosclerosis? [J]. FEBS Lett,2000,462(1-2):1-6.
[20] Savonen R,Hiden M,Hultin M,et al. The tissue distribution of lipoprotein lipase determines where chylomicrons bind [J]. J Lipid Res,2015,56(3):588-598.
(收稿日期:2016-10-02 本文編辑:李亚聪)