CRISPLD2蛋白对内毒素急性呼吸窘迫综合征小鼠的保护作用
2017-03-14刘雪峰瞿金龙李文放林兆奋
刘雪峰,瞿金龙,李文放,林兆奋
·临床医学·
·论著·
CRISPLD2蛋白对内毒素急性呼吸窘迫综合征小鼠的保护作用
刘雪峰,瞿金龙,李文放,林兆奋
目的探讨CRISPLD2蛋白对内毒素急性呼吸窘迫综合征(acute respiratory disease syndrome, ARDS)小鼠的保护作用。方法采用数字表法,将120只健康雄性昆明小鼠随机分为对照组(A组,40只):气道内滴入LPS(5 mg/kg);LPS预处理组(B组,40只):先予小鼠LPS(2 mg/kg)腹腔内注射,注射后第8天气道内滴入LPS(5 mg/kg);重组CRISPLD2处理组(C组,40只):先予小鼠尾静脉注射重组 CRISPLD2(50 mg/kg),6 h后气道内滴入LPS(5 mg/kg)。(1)采用数字表法将30只小鼠随机分成A1组、B1组、C1组(各组10只),气道滴入LPS 6 h后处死,测定肺湿干重比(W/D)、通透指数(LPI)。(2)采用数字表法将90只小鼠随机分成A2组、B2组、C2组(各组20只),观察5 d内小鼠死亡情况,描绘生存曲线。结果B、C两组W/D、LPI及5 d死亡率显著低于A组,差异有统计学意义(P<0.05);B组各项检测结果低于C组,差异有统计学意义(P>0.05)。结论提升CRISPLD2蛋白血浓度对内毒素所致ARDS具有保护作用,可降低肺损伤程度,改善肺毛细血管通透性,降低死亡率。
CRISPLD2蛋白;内毒素;急性呼吸窘迫综合征
急性呼吸窘迫综合征(acute respiratory disease syndrome, ARDS)多由感染、休克、创伤、大量输血等病因诱发,以顽固性低氧血症为临床主要表现的急性进行性呼吸衰竭[1],其本质是过度炎症反应及多器官功能障碍综合征在肺部的表现。尽管诊治技术不断进步,但ARDS死亡率仍然居高不下,上海市12家三甲医院ICU2001年3月至2002年3月期间ARDS病死率高达68.5%[2]。CRISPLD2(cysteine-rich secretory protein containing LCCL Domain 2)蛋白是一个保守的、半胱氨酸富集的、血清浓度较高的LPS结合蛋白,拥有2个与LPS结合的LCCL结构域,并具有较高亲和力与LPS[3]结合,与大鼠肺、肾脏的发育以及无症状的唇裂有关[4-6]。CRISPLD2是一种分泌型蛋白,人类、小鼠和大鼠的肺脏、小肠、胎盘以及免疫细胞包括粒细胞,单核细胞,T、B、NK细胞都表达CRISPLD2[7-8]。本实验以气道内注射LPS制备的ARDS小鼠为对象,研究CRISPLD2蛋白对小鼠模型的保护作用。
1 材料与方法
1.1 实验动物与模型制备
健康雄性昆明小鼠,体质量20~22 g,购自第二军医大学实验动物中心,给予标准饮食和饮水。采用气道内滴入LPS(5 mg/kg)制备ARDS小鼠模型,空白对照组气道内滴入等量生理盐水。
1.2 主要试剂
LPS购自Sigma公司。重组CRISPLD2蛋白购自上海南方基因中心。其余试剂为国产分析纯试剂。
1.3 实验分组及实施
1.3.1 分组 采用数字表法将120只雄性昆明小鼠随机分为以下3组:(1)对照组(A组,40只):气道内滴入LPS(5 mg/kg);(2)LPS预处理组(B组,40只):先予实验小鼠LPS(2 mg/kg)腹腔内注射,注射后第8天气道内滴入LPS(5 mg/kg);(3)重组CRISPLD2处理组(C组,40只):首先给予小鼠尾静脉注射重组 CRISPLD2(50 mg/kg),6 h后气道内滴入LPS(5 mg/kg)。
1.3.2 具体实施 (1)取A、B、C组小鼠各10只,分别作为A1、B1、C1组,往这3组小鼠气道滴入LPS 6 h断尾取血测定血浆蛋白含量后处死实验小鼠,取右肺计算湿干重比(W/D),取左肺行支气管肺泡灌洗,测量肺通透指数(LPI)(肺泡灌洗液总蛋白含量/血浆总蛋白含量)。(2)A、B、C组每组剩下的30只小鼠分别作为A2、B2、C2组,观察各组96 h内小鼠死亡情况,描绘生存曲线。
1.4 统计学处理
检测数据采用均数±标准差(x±s)表示,以SPSS 18.0统计软件进行分析。2组之间的比较采用t检验或秩和检验;多组之间采用方差分析或者秩检验。P<0.05为差异具有统计学意义。
2 结果
2.1 W/D比值、LPI指数
实验结果表明LPS预处理组与重组CRISPLD2蛋白处理组的W/D比值和LPI明显低于LPS组,差异均有统计学意义(P<0.05)。LPS预处理组的W/D和LPI值低于重组CRISPLD2蛋白处理组,但差异无统计学意义(P>0.05)。见表1。
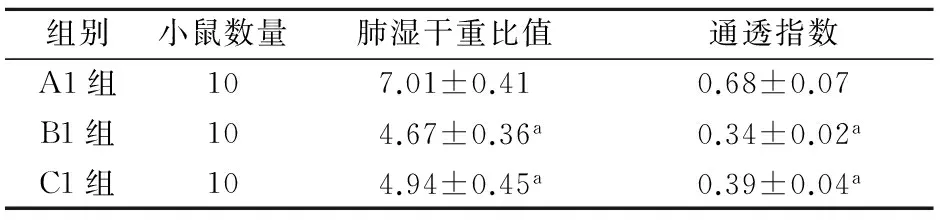
表1 CRISLPD2蛋白对内毒素急性呼吸窘迫综合征小鼠肺水肿的影响(x±s)
注:ARDS为急性呼吸窘迫综合征;与A1组比较aP<0.05
2.2 实验小鼠5 d生存率
数据分析表明LPS预处理组与重组CRISPLD2蛋白处理组的实验小鼠5 d生存率明显高于LPS组,差异均有统计学意义(P<0.05)。LPS预处理组的5 d生存率高于重组CRISPLD2蛋白处理组,但差异无统计学意义(P>0.05)。见图1。
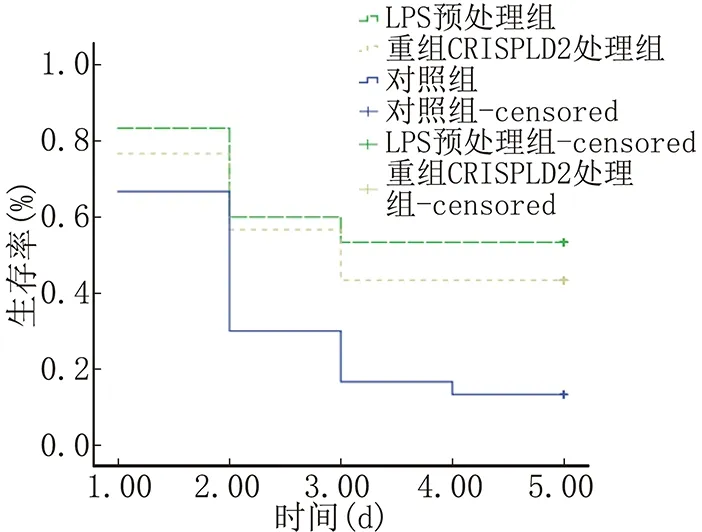
注:与重组CRISPLD2蛋白组比较P<0.05
3 讨论
ARDS实质是肺毛细血管通透性损伤,其发病诱因中全身性感染及肺部感染约占50%以上,因肺部感染直接损伤所引起的ARDS约占70%[9-10]。因此笔者选用气道滴入LPS(5 mg/kg)直接引起肺部损伤制备ARDS小鼠模型,结果表明小鼠肺W/D、LPI符合ARDS表现,与文献报道一致[11],提示动物模型制备成功。
目前认为CRISPLD2蛋白是一种保守的与肺、肾发育相关的分泌型蛋白,可由肺、肠、免疫细胞等多种组织细胞分泌。现有研究发现LPS可刺激NK细胞、单核细胞、PBMC以及粒细胞等多种免疫细胞增加CRISPLD2分泌;同时与其他应急反应蛋白相比,小鼠腹腔注射小剂量非毒性剂量LPS以后,12 h内血清CRISPLD2浓度快速下降,24 h后其浓度又大幅反弹,此时可高于正常血清1~2倍以上,并维持2周之久[12],因此实验分组中的LPS预处理组选择实验小鼠小剂量LPS腹腔内注射后第8天行气道内滴入LPS。实验研究还发现CRISPLD2可与LPS结合,影响LPS与TLR4受体结合,从而发挥反馈调控机制,减少炎症介质的释放,降低全身性炎症反应[12]。
本实验通过内毒素直接引起肺部损伤的小鼠模型为基础,分别通过LPS预处理、重组CRISPLD2蛋白预注射进行提前干预,发现通过LPS预先腹腔内注射或重组CRISPLD2蛋白直接静脉注射提高小鼠血清CRISPLD2蛋白浓度能够减轻内毒素所致肺损伤程度,改善预后。
本实验结果中LPS预处理组W/D、LPI、5 d死亡率低于重组CRISPLD2处理组,原因考虑与2种干预方式所升高的小鼠血清CRISPLD2蛋白浓度差异相关。通过增加实验制模前重组CRISPLD2预注射剂量能否再次降低内毒素所致ARDS小鼠的死亡率以及将重组CRISPLD2蛋白由预注射改为实验制模后的不同时间点注射是否能起到同样改善预后的作用尚需进一步研究。
[1] Atabai K,Matthay MA.The pulmonary physician in critical care.5: acute lung injury and the acute respiratory distress syndrome:definitions and epidemiology[J].Tborax,2002, 57(5):452-458. DOI: 10.1136/thorax.57.5.452.
[2] Lu Y,Song Z,Zhou X,et a1.A 12-month clinical survey of incidence and outcome of acute respiratory distress syndrome in Shanghai intensive care units[J].Intensive Care Med,2004,30(12):2197-2203.DOI:10.1007/s00134-004-2479-y.
[3] Oyewumi L,Kaplan F,Sweezey NB. LGL1,a mesenchymal modulator of early lung branching morphogenesis, is a secreted glycoprotein imported by late gestation lung epithelial cells[J]. Biochem J, 2003,376(pt 1): 61-69. DOI:10.1042/BJ20030591.
[4] Oyewumi L,Kaplan F,Gagnon S, et al. Antisense oligodeoxynucleotides decrease LGL1 mRNA and protein levels and inhibit branching morphogenesis in fetal rat lung[J]. Am J Respir Cell Mol Biol, 2003, 28(2): 232-240. DOI:10.1165/rcmb.4877.
[5] Nadeau K,Jankov RP,Tanswell AK, et al. Lgl1 is suppressed in oxygen toxicity animal models of bronchopulmonary dysplasia and normalizes during recovery in air[J]. Pediatr Res, 2006,59(3): 389-395. DOI: 10.1203/01.pdr.0000198819.81785.f1.
[6] Quinlan J,Kaplan F,Sweezey N,et al. LGL1, a novel branching morphogen in developing kidney, is induced by retinoic acid[J]. Am J Physiol Renal Physiol, 2007,293(4): F987-F993. DOI:10.1152/ajprenal.00098.2007.
[7] Chiquet BT, Lidral AC,Stal S, et al. CRISPLD2: a novel NSCLP candidate gene[J]. Hum Mol Genet, 2007, 16(18): 2241-2248.DOI:10.1093/hmg/ddm176.
[8] Warren HS, Suffredini AF, Robert S. Risks and Benefits of Activated Protein C Treatment for Severe Sepsis[J]. N Engl J Med,2002,347(13):1027-1030.DOI:10.1056/NEJMsb020574.
[9] Bernard GR,Artigas A,Brigham KL,et al.Report of the American-European Consensus conference on acute respiratory distress syndromes:defimitions,mechanisms, relevant outcomes,and clinical trial coordination.Consensus Committee[J].Intensive Care Med,1994,20:225-232.DOI:10.1007/BF01704707.
[10] Ball J, Venn R. The 21st International Symposium Intensive Care and Emergency Medicine,Brussels, Belgium, 20-23 March 2001[J]. Crit Care, 2001,5(3): 138-141.DOI:10.1186/cc1013.
[11] Rojas M,Woods CR,Mora AL,et a1.Endotoxin-induced lung injury in mice:Structural,functional,and biochemical responses[J]. Am J Physiol Lung Cell Mol Physiol, 2005,288(2):333-341.DOI:10.1152/ajplung.00334.2004.
[12] Wang ZQ,Xing WM,Fan HH,et al. The Novel Lipopolysaccharide-Binding Protein CRISPLD2 Is a Critical Serum Protein to Regulate Endotoxin Function[J]. J Immunol, 2009,183(10):6646-6656. DOI: 10.4049/jimmunol.0802348.
(本文编辑:张阵阵)
Protective effect of CRISPLD2 protein on acute respiratory disease syndrome induced by lipopolysaecharide in mice
LiuXuefeng,QuJinlong,LiWenfang,LinZhaofen
Objective To investigate the protective effect of CRISPLD2 protein on acute respiratory disease syndrome (ARDS) induced by lipopolysaecharide (LPS) in mice.Methods Healthy male Kunming mice were randomly divided into 3 groups: the control group (or group A) which received lipopolysaecharide via the intratracheal instillation at a dosage of 5 mg/kg, the LPS preconditioning group (or group B) which had intraperitoneal injection of LPS at a dosage of 2mg/kg and at day 8 received LPS via intratracheal instillation also at a dosage of 5mg/kg. The recombinant CRISPLD2 preconditioning group (or group C) which was injected with recombinant CRISPLD2 at a dosage of 50mg/kg via cauda vein, and then at hour 6 received LPS via intratracheal instillation at the same dosage. (1)Thirty mice were randomly divided into group A1, group B1 and group C1, each consisting of 10 animals. The mice were sacrificed 6 hours after LPS intratracheal instillation. Then, W/D and LPI were measured. (2) Ninety mice were randomly divided into group A2, group B2 and group C2, each consisting of 30 animals. The death of the animals in each group was closely observed and the survival curve was described.Results W/D, lung index and mortality after 5 days in group B and C were significantly lower, as compared with those of group A (P<0.05). The detected data of various indexes in group B were lower than those in group C (P>0.05).Conclusion Increased serum concentration of CRISPLD2 could obviously produce protective effect on ARDS induced by LPS, lighten injury on the lungs and improve permeability of pulmonary capillary and decrease mortality.
CRISPLD2; Endotoxin; Lipopolysaecharide; Acute respiratory disease syndrome
[参考文献]
上海市卫生和计划生育委员会项目(2012210)
200003 上海,第二军医大学附属长征医院急救科
R563
A
10.3969/j.issn.1009-0754.2017.01.008
(DepartmentofCriticalCare,ChangzhengHospital,Shanghai200003,China)
2016-12-30)
