N-异丙基丙烯酰胺嵌段共聚物自组装行为研究进展
2017-02-28赵小燕单国荣
赵小燕,单国荣
(化学工程联合国家重点实验室,浙江大学化学工程与生物工程学院,浙江 杭州 310027)
N-异丙基丙烯酰胺嵌段共聚物自组装行为研究进展
赵小燕,单国荣
(化学工程联合国家重点实验室,浙江大学化学工程与生物工程学院,浙江 杭州 310027)
具有两亲性和温敏性的嵌段共聚物在选择性溶剂中能够自组装形成独特的微米级甚至纳米级有序结构,具有广泛的潜在应用。聚N-异丙基丙烯酰胺(PNIPAM)是一种典型的温敏性高分子材料,其分子链表现出独特的温敏行为,关于其修饰改性以及自组装行为的研究一直广受关注。以PNIPAM为基点介绍其嵌段共聚物的自组装行为,详细综述影响其行为的诸多因素如组成、分子量、外界刺激条件等,介绍其在生物医药、催化反应等方面的应用,并对其未来的发展方向进行了展望。
聚合物;PNIPAM;两亲性;选择性;纳米粒子
引 言
分子自组装行为在自然界以及日常生活中广泛存在,如生物细胞膜中的磷脂质、洗涤剂中的小分子表面活性剂等[1]。它们都有一个共同点:分子链中都有亲水的端基和疏水的链段,即具有两亲性。两亲性共聚物的不同链节具有不同的热力学相容性,所以其在选择性溶剂(水或有机溶剂)中会发生微相分离[2-3],能够自组装形成具有不同形态的微米级甚至纳米级有序结构,如胶束、球状、囊泡状、棒状等[4-5]。自组装得到的独特结构使两亲性共聚物具有特殊的性能。智能性高分子材料是一类具有环境敏感性或刺激响应性的聚合物材料,可以对外界环境刺激具有响应能力[6-7]。其中,温度对聚合物的刺激条件比较容易控制,而且温敏性聚合物因为其优越的性能具有广阔的应用前景,所以成为刺激响应类聚合物中重要的研究对象之一。具有温敏性和两亲性的共聚物同时兼具两种不同性质,在生物、医药、催化、化妆品、涂料等诸多领域拥有广阔的应用前景[8-10]。
在诸多温敏性聚合物中,聚N-异丙基丙烯酰胺(PNIPAM)是一种研究较广泛的温敏性高分子材料,其分子链在水溶液中表现出独特的温敏行为[11-12]。1967年,Scarpa等[13]首先发现了PNIPAM的温敏性,在31℃时PNIPAM的水溶液具有可逆的溶解-沉淀现象。如图1所示[14],在其最低临界溶解温度(LCST)上下,PNIPAM经历一个可逆的线团-球体转变过程,当溶液温度高于其LCST时,PNIPAM由舒展的亲水线团转变为蜷缩的疏水球粒,宏观上导致其水溶液出现相分离,当温度降低到其LCST以下时沉淀的PNIPAM又能溶解。
PNIPAM特殊的温敏性使其在诸多领域有着广泛的应用,近年来关于PNIPAM疏水化修饰改性和自组装行为的研究一直广受关注[15-17]。本文对近年来N-异丙基丙烯酰胺(NIPAM)嵌段共聚物自组装行为的研究进展进行综述,重点阐述影响其自组装行为的诸多因素。
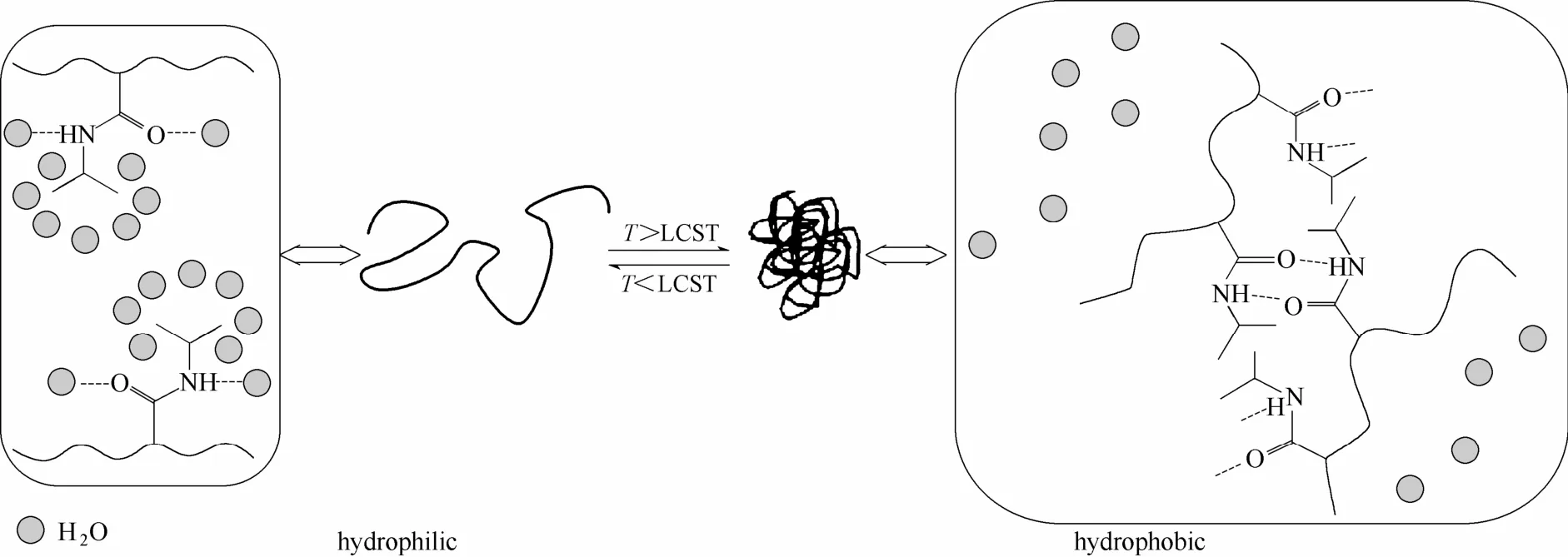
图1 温度诱发的PNIPAM相转变行为[14]Fig. 1 Temperature induced PNIPAM phase transition[14]
1 不同组成NIPAM嵌段共聚物自组装
含有NIPAM的两亲性嵌段共聚物大体上可以分为两种类型[18]:一种结构在温度低于其LCST时比较稳定,一般与不溶于水的聚合物嵌段,如聚苯乙烯、聚甲基丙烯酸丁酯(PBMA)、聚D,L-丙交酯等;另一种结构在温度高于其LCST时比较稳定,一般与亲水性的聚合物嵌段,如聚环氧乙烷(PEO)等。这两种不同结构的NIPAM嵌段共聚物在自组装时,前者PNIPAM为壳,后者PNIPAM为核。这两种具有不同组成的嵌段共聚物具有不同的LCST值,能够在不同的温度下进行自组装,适用于不同的环境条件。
PBMA和PNIPAM的嵌段共聚物在溶液中可以形成具有温敏性的核-壳结构纳米粒子,如图2所示,PBMA为核,PNIPAM为壳[19]。改变温度,所形成的纳米粒子壳的尺寸发生明显的变化,这由PNIPAM的相转变引起。
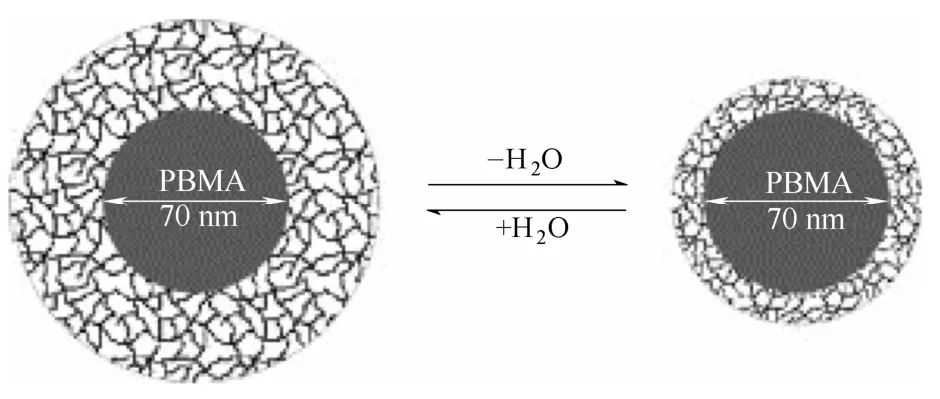
图2 温度变化引起的PBMA(核)/PNIPAM(壳)纳米粒子体积变化[19]Fig. 2 Temperature-induced volume transition of PBMA (core)/PNIPAM (shell) nanoparticles[19]
Jochum等[20]合成了包含偶氮苯的PEO和PNIPAM的嵌段共聚物(图3),其自组装行为具有温敏性和光敏性,温度升高或改变光照条件时形成具有核-壳结构的胶束,PNIPAM为核,PEO为壳。
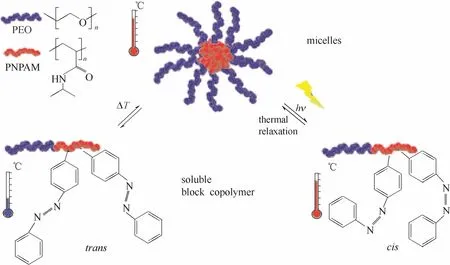
图3 水溶液中热引发的PEO-b-PNIPAM胶束化行为和光控制下的胶束分解/再形成过程[20]Fig. 3 Temperature induced micellation and light controlled micelle disruption/thermal reformation of PEO-b-PNIPAM in water[20]
2 不同分子链长度的NIPAM嵌段共聚物自组装
对于相同组成的NIPAM嵌段共聚物,改变其分子量和分子链长度也会对形成胶束的尺寸具有重要的影响。分子链的长度增加,每个嵌段的表面积增加,导致分子链间的排斥力减小,驱使更多的分子链聚集,胶束尺寸增大。
艾长军等[21]通过原子转移自由基聚合(ATRP)法合成分子量可控、分布较窄的PtBMA-b-PNIPAM嵌段共聚物,设计不同的嵌段比例,研究其胶束化行为。通过动态光散射(DLS)和透射电镜(TEM),发现胶束核嵌段部分(PtBMA)长度不变,随着壳嵌段部分(PNIPAM)的长度逐渐增加,相同温度下,分子链越长,聚集得到的胶束粒径越大;而当控制胶束的壳嵌段不变,依次减小核嵌段部分,相同温度下粒径也依次减小。
Chang等[22]将开环聚合和RAFT两种聚合方法相结合合成了聚己内酯-b-聚N-异丙基丙烯酰胺-b-聚己内酯三嵌段共聚物(PCL-b-PNIPAM-b-PCL),其在水溶液中自组装形成了核-壳结构的胶团。通过TEM观察胶团的形貌以及利用DLS测试其粒径,分别改变核、壳的分子链长度,发现胶团的尺寸及尺寸分布有所变化。如图4所示,保持PCL链长不变,增加PNIPAM链长,胶团尺寸变小、分布变窄。
Pramanik等[23]合成了不同NIPAM链长的PEO-b-PNIPAM两嵌段共聚物,并研究了其在水溶液中的自组装行为。变温DLS研究表明,PEO链长一定时,改变PNIPAM链长,不同温度下聚集体的直径都有所不同。
3 不同外界刺激下的NIPAM嵌段共聚物自组装
聚N-异丙基丙烯酰胺是典型的智能高分子聚合物,外界刺激如温度、pH、光照等对其嵌段共聚物的自组装行为具有重大的影响。
Mäkinen等[24]利用一种非离子大分子RAFT试剂合成了温敏性的N-丙烯酰甘氨酰胺(NAGA)、EO和NIPAM的两种三嵌段共聚物PEO-b-PNAGA-b-PNIPAM和PEO-b-PNIPAM-b-PNAGA。变温1H NMR研究发现,这两种嵌段共聚物都表现出了最高临界溶解温度(UCST)和LCST这两种类型的相转变(图5)。对于PEO-b-PNIPAM-b-PNAGA,当加热到50℃时,PNIPAM的峰几乎完全消失,PNAGA的信号则有所增强;当冷却到1℃时,则会观察到相反的现象——PNAGA的信号消失而PNIPAM的信号变强。对于PEO-b-PNAGA-b-PNIPAM也有类似的现象。
Klaikherd等[25]研究了聚甲基丙烯酸羟乙酯(HEMA)和PNIPAM的嵌段共聚物(PHEMA-b-PNIPAM)的自组装行为及其对外界刺激的多重响应性。从图6可以明显看到,当升高温度时,会有白色的沉淀形成,并且这个沉淀-溶解的过程是热力学可逆的,这表明该嵌段共聚物在自组装过程中表现出了LCST相转变行为。
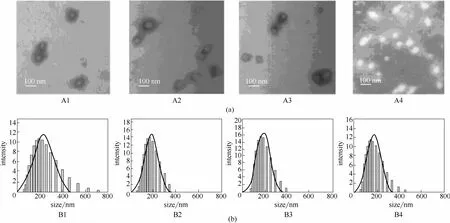
图4 蒸馏水中胶团的形貌结构(a)和粒径分布(b)[22]Fig. 4 TEM micro pictures (a) and size distributions (b) of micelles in distilled water[22]
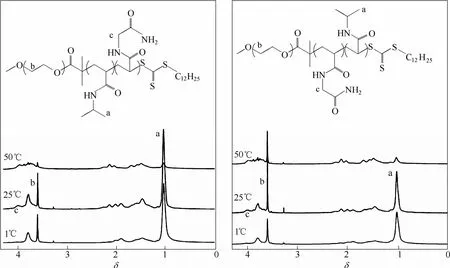
图5 PEO-b-PNIPAM-b-PNAGA和PEO-b-PNAGA-b-PNIPAM变温1H NMR谱图[24]Fig. 5 Variable temperature1H NMR spectra for PEO-b-PNIPAM-b-PNAGA and PEO-b-PNAGA-b-PNIPAM in D2O[24]
4 PNIPAM共聚物自组装的应用
嵌段共聚物自组装形成的胶束、囊泡等,在生物、医药、催化、化妆品、涂料等诸多领域拥有广阔的应用前景[26-29]。
Wei等[30]通过PNIPAM-b-聚甲基丙烯酸甲酯(PNIPAM-b-PMMA)的自组装制得了具有温敏性的组装体,并用DLS验证了组装体随温度变化而呈现的可逆的溶解聚集性能,并且利用组装体装载药物,发现温度在LCST上下变化时药物的释放量有明显的变化。如图7(a)所示,当温度低于LCST(27℃)时只有少量的药物被释放出来,但是当温度高于LCST(40℃)时药物可以快速地从囊泡中释放出去。同时还研究了振荡温度变化下囊泡结构变化的可逆性。如图7(b)所示,24℃时3个小时内药物只释放了22%,而38℃时释放了45%。也就是说38℃时的药物释放速率约是24℃时的2倍。
Qin等[18]利用制得的PEO-b-PNIPAM嵌段聚合物囊泡装载抗癌药物阿霉素(Dox),通过温度控制其释放速度。如图8所示,在37℃时Dox基本没有释放,而在27℃时Dox的释放速度大大加快。
嵌段共聚物自组装形成的纳米级胶束还可以作为纳米反应器,利用自组装过程以及胶束的可控性制备尺寸大小可控的纳米材料。Urbani等[31]利用P(DMA68-b-NIPAM73)-SCSSC4H9作为纳米反应器成功地制备出了分子量分布较窄且大小可控的聚苯乙烯纳米微球;Valade等[32]也曾研究将P(DMA69-b-NIPAM60)-SCSSC12H25用作纳米反应器。两者的研究结果都表明PNIPAM嵌段共聚物胶束作为纳米反应器具有聚合速率高、分子量分布与粒径分布窄的特点。
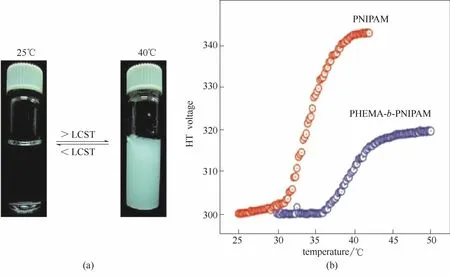
图6 PHEMA-b-PNIPAM的温度敏感性[(a)左图:室温下的水溶液;右图:加热到40℃的水溶液]以及PHEMA-b-PNIPAM和PNIPAM的浊度实验:HT电压随温度的变化关系(b)[25]Fig.6 Temperature sensitivity of PHEMA-b-PNIPAM[(a) photograph showing aqueous solution of PHEMA-b-PNIPAM; left—at room temperature, right—after heating to 40℃] and turbidity experiment showing change in HT voltage with temperature of PHEMA-b-PNIPAM and PNIPAM(b)[25]
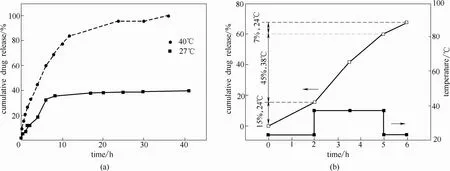
图7 PNIPAM-b-PMMA装载、释放prednisone药物(a)以及24℃和38℃之间PNIPAM-b-PMMA装载、释放prednisone药物的振荡变化(b)[30]Fig. 7 Drug release from thermoresponsive PNIPAM-b-PMMA micelles containing prednisone acetate (a) and drug release from PNIPAM-b-PMMA micelles loaded with prednisone acetate in response to temperature switching between 24 and 38℃(b)[30]
5 结 语
综述了PNIPAM嵌段共聚物的自组装行为,重点阐述了影响其自组装行为的诸多因素及其应用。PNIPAM是一种典型的温敏性智能高分子材料,可与多种物质形成嵌段共聚物,进行自组装。嵌段共聚物的组成、分子量以及分子链长度、温度等外界刺激等因素都对其自组装行为具有重要影响。其在生物、医药、催化等领域具有广泛的潜在的应用前景。目前,关于其应用的研究有很多,但是相关方面的研究还不够完善,如对其应用的研究还停留在实验室阶段,生物医学方面的应用还没有进行生物相容性的研究等。随着研究的不断深入,这些方面的探索也将会成为研究的重点。
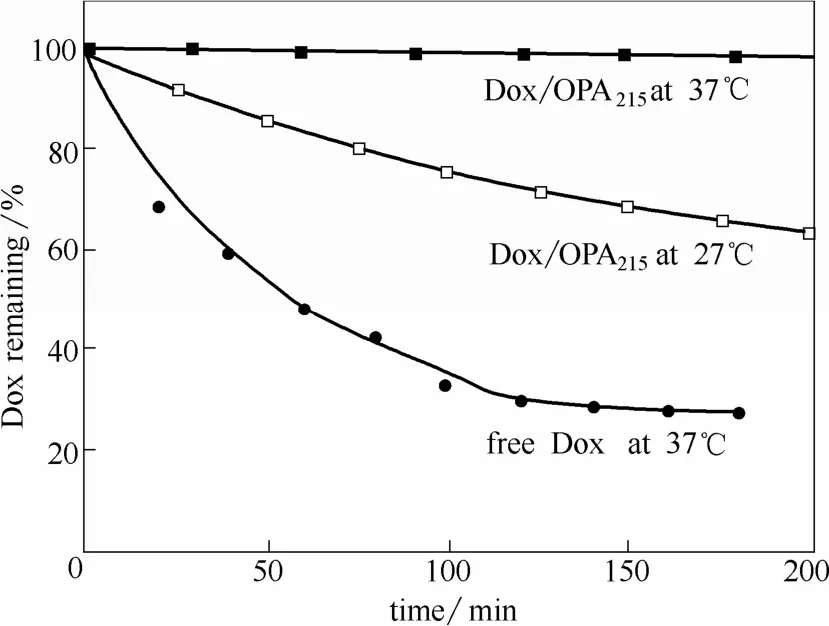
图8 37℃和27℃下PEO-b-PNIPAM嵌段聚合物囊泡控制释放抗癌药物阿霉素[18]Fig. 8 Release of Dox from PEO-b-PNIPAM vesicles at 37℃ and 27℃[18]
[1] LI X Y, GAO Y, BOOTT C E,et al.“Cross” supermicellesviathe hierarchical assembly of amphiphilic cylindrical triblock comicelles[J]. Journal of the American Chemical Society, 2016, 138(12): 4087-4095.
[2] THOMAS E L, ANDERSON D M, HENKEE C S,et al.Periodic area-minimizing surfaces in block copolymers[J]. Nature, 1988, 334(6183): 598-601.
[3] BATES F S. Polymer-polymer phase-behavior[J]. Science, 1991, 251(4996): 898-905.
[4] MAI Y Y, EISENBERG A. Self-assembly of block copolymers[J]. Chemical Society Reviews, 2012, 41(18): 5969-5985.
[5] FONG C, Le T, DRUMMOND C J. Lyotropic liquid crystal engineering-ordered nanostructured small molecule amphiphile self-assembly materials by design[J]. Chemical Society Reviews, 2012, 41(3): 1297-1322.
[6] YAO C, LIU Z, YANG C,et al.Poly(N-isopropylacrylamide)-clay nanocomposite hydrogels with responsive bending property as temperature-controlled manipulators[J]. Advanced Functional Materials, 2015, 25(20): 2980-2991.
[7] WANG W, GAO C Q, QU Y Q,et al.In situsynthesis of thermoresponsive polystyrene-b-poly(N-isopropylacrylamide)-bpolystyrene nanospheres and comparative study of the looped and linear poly(N-isopropylacrylamide)s[J]. Macromolecules, 2016, 49(7): 2772-2781.
[8] CAO Z H, ZIENER U, LANDFESTER K. Synthesis of narrowly size-distributed thermosensitive poly(N-isopropylacrylamide) nanocapsules in inverse miniemulsion[J]. Macromolecules, 2010, 43(15): 6353-6360.
[9] LI S Y, LIN D L, ZHOU J F,et al.Preparation of silver nanoparticles loaded photoresponsive composite microgels and their lightcontrollable catalytic activity[J]. Journal of Physical Chemistry C, 2016, 120(9): 4902-4908.
[10] BAJPAI A K, SHUKLA S K, BHANU S,et al.Responsive polymers in controlled drug delivery[J]. Progress in Polymer Science, 2008, 33(11): 1088-1118.
[11] WU C, WANG X H. Globule-to-coil transition of a single homopolymer chain in solution[J]. Physical Review Letters, 1998, 80(18): 4092-4094.
[12] RZAEV Z M O, DINCER S, PISKIN E. Functional copolymers ofN-isopropylacrylamide for bioengineering applications[J]. Progress in Polymer Science, 2007, 32(5): 534-595.
[13] SCARPA J S, MUELLER D D, KLOTZ I M. Slow hydrogendeuterium exchange in a non-alpha-helical polyamide[J]. Journal of the American Chemical Society, 1967, 89(24): 6024-6030.
[14] DIMITROV I, TRZEBICKA B, MULLER A H E,et al.Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities[J]. Progress in Polymer Science, 2007, 32(11): 1275-1343.
[15] SERPE M J, JONES C D, LYON L A. Layer-by-layer deposition of thermoresponsive microgel thin films[J]. Langmuir, 2003, 19(21): 8759-8764.
[16] GE Z S, LUO S Z, LIU S Y. Syntheses and self-assembly of poly(benzyl ether)-b-poly(N-isopropylacrylamide) dendritic-linear diblock copolymers[J]. Journal of Polymer Science Part A—Polymer Chemistry, 2006, 44(4): 1357-1371.
[17] NYKANEN A, NUOPPONEN M, LAUKKANEN A,et al.Phase behavior and temperature-responsive molecular filters based on self-assembly of polystyrene-block-poly(N-isopropylacrylamide)-block-polystyrene[J]. Macromolecules, 2007, 40(16): 5827-5834.
[18] QIN S H, GENG Y, DISCHER D E,et al.Temperature-controlled assembly and release from polymer vesicles of poly(ethylene oxide)-block-poly(N-isopropylacrylamide)[J]. Advanced Materials, 2006, 18(21): 2905-2909.
[19] GAN D J, LYON L A. Fluorescence nonradiative energy transfer analysis of crosslinker heterogeneity in core-shell hydrogel nanoparticles[J]. Analytica Chimica Acta, 2003, 496(1-2): 53-63.
[20] JOCHUM F D, THEATO P. Thermo- and light responsive micellation of azobenzene containing block copolymers[J]. Chemical Communications, 2010, 46(36): 6717-6719.
[21] 艾长军, 唐燕春, 张莉,等.聚(甲基)丙烯酸叔丁酯-b-聚(N-异丙基丙烯酰胺)嵌段共聚物的ATRP法合成及其自组装行为[J]. 材料导报, 2010, 25(S1): 408-411. AI C J, TANG Y C, ZHANG L,et al. Synthesis of poly(tert-butyl(meth)acrylate)-b-poly(N-isopropyl acrylamide) by atom transfer radical polymerization and their self-assembly behavior[J]. Materials Review, 2010, 25(S1): 408-411.
[22] CHANG C, WEI H, QUAN C Y, et al.Fabrication of thermosensitive PCL-PNIPAAm-PCL triblock copolymeric micelles for drugdelivery[J]. Journal of Polymer Science Part A—Polymer Chemistry, 2008, 46(9): 3048-3057.
[23] PRAMANIK P, GHOSH S. Thermoresponsive polymersome from a double hydrophilic block copolymer[J]. Journal of Polymer Science Part A—Polymer Chemistry, 2015, 53(21): 2444-2451.
[24] MÄKINEN L, VARADHARAJAN D, TENHU H,et al.Triple hydrophilic UCST-LCST block copolymers[J]. Macromolecules, 2016, 49(3): 986-993.
[25] KLAIKHERD A, NAGAMANI C, THAYUMANAVAN S. Multi-stimuli sensitive amphiphilic block copolymer assemblies[J]. Journal of the American Chemical Society, 2009, 131(13): 4830-4838.
[26] AHMED F, PAKUNLU R I, BRANNAN A,et al.Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug[J]. Journal of Controlled Release, 2006, 116(2): 150-158.
[27] BROZ P, DRIAMOV S, ZIEGLER J,et al.Toward intelligent nanosize bioreactors: a pH-switchable, channel-equipped, functional polymer nanocontainer[J]. Nano Letters, 2006, 6(10): 2349-2353.
[28] LOMAS H, CANTON I, MACNEIL S,et al.Biomimetic pH sensitive polymersomes for efficient DNA encapsulation and delivery[J]. Advanced Materials, 2007, 19(23): 4238-4243.
[29] MAI Y, EISENBERG A. Controlled incorporation of particles into the central portion of vesicle walls[J]. Journal of the American Chemical Society, 2010, 132(29): 10078-10084.
[30] WEI H, ZHANG X Z, ZHOU Y,et al.Self-assembled thermoresponsive micelles of poly(N-isopropylacrylamide-b-methyl methacrylate)[J]. Biomaterials, 2006, 27(9): 2028-2034.
[31] URBANI C N, MONTEIRO M J. RAFT-mediated emulsion polymerization of styrene in water using a reactive polymer nanoreactor[J]. Australian Journal of Chemistry, 2009, 62(11): 1528-1532.
[32] VALADE D, JEON Y, KESSEL S,et al.Influence of the Z-group on the RAFT-mediated polymerizations in nanoreactors[J]. Journal of Polymer Science Part A—Polymer Chemistry, 2012, 50(22): 4762-4771.
Research progress on self-assembly of block copolymers ofN-isopropyl acrylamide
ZHAO Xiaoyan, SHAN Guorong
(State Key Laboratory of Chemical Engineering,College of Chemical and Biological Engineering,Zhejiang University,Hangzhou310027,Zhejiang,China)
The amphiphilic and thermosensitive block copolymers can self-assemble and turn into special micron level or nanoscale morphologies in selective solvents, and they have extensive potential applications. Poly(N-isopropyl acrylamide) (PNIPAM) is a typical kind of thermal-sensitive macromolecular material. It can undergo a special “coil to globule” volume phase transition near the lower critical solution temperature in solution. The study of self-assembly of its block copolymers has been drawn great interests. In this paper, self-assembly of PNIPAM block copolymers is introduced. The influence of several aspects including composition, molecular weight, external stimulus conditions and so on is systematically reviewed. Some of its applications on biological medicine, catalysis reaction,etc. are also introduced. The direction of its future development is also forecasted.
polymers; PNIPAM; amphiphilic; selectivity; nanoparticles
Prof. SHAN Guorong, shangr@zju.edu.cn
TQ 326.4
:A
:0438—1157(2017)02—0535—07
10.11949/j.issn.0438-1157.20160755
2016-05-31收到初稿,2016-08-18收到修改稿。
联系人:单国荣。
:赵小燕(1992—),女,博士研究生。
化学工程联合国家重点实验室开放课题项目(SKL-ChE-15D02)。
Received date: 2016-05-31.
Foundation item: supported by the State Key Laboratory of Chemical Engineering (SKL-ChE-15D02).
