Microstructure and molten salt impregnation characteristics of a micro-fine grain graphite for use in molten salt reactors
2017-01-07ZHANGWentingZHANGBaoliangSONGJinliangQIWeiHEXiujieLIUZhanjunLIANPengfeiHEZhoutongGAOLinaXIAHuihaoLIUXiangdongZHOUXingtaiSUNLibinWUXinxin
ZHANG Wen-ting, ZHANG Bao-liang,, SONG Jin-liang, QI Wei,HE Xiu-jie,, LIU Zhan-jun, LIAN Peng-fei, HE Zhou-tong,GAO Li-na, XIA Hui-hao, LIU Xiang-dong, ZHOU Xing-tai, SUN Li-bin, WU Xin-xin
(1.Key Laboratory of Nuclear Radiation and Nuclear Energy Technology,Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai201800, China;2.School of Physics and State Key Laboratory of Crystal Materials, Shandong University, Jinan250100, China;3.Institute of Nuclear and New Energy Technology, Collaborative Innovation Center of Advanced Nuclear Energy Technology, Key Laboratory ofAdvanced Reactor Engineering and Safety of Ministry of Education, Tsinghua University, Beijing100084, China;4.Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan030001, China)
Microstructure and molten salt impregnation characteristics of a micro-fine grain graphite for use in molten salt reactors
ZHANG Wen-ting1,2, ZHANG Bao-liang1,2,3, SONG Jin-liang1, QI Wei1,HE Xiu-jie1,2,3, LIU Zhan-jun4, LIAN Peng-fei4, HE Zhou-tong1,GAO Li-na1, XIA Hui-hao1, LIU Xiang-dong2, ZHOU Xing-tai1, SUN Li-bin3, WU Xin-xin3
(1.KeyLaboratoryofNuclearRadiationandNuclearEnergyTechnology,ShanghaiInstituteofAppliedPhysics,ChineseAcademyofSciences,Shanghai201800,China;2.SchoolofPhysicsandStateKeyLaboratoryofCrystalMaterials,ShandongUniversity,Jinan250100,China;3.InstituteofNuclearandNewEnergyTechnology,CollaborativeInnovationCenterofAdvancedNuclearEnergyTechnology,KeyLaboratoryofAdvancedReactorEngineeringandSafetyofMinistryofEducation,TsinghuaUniversity,Beijing100084,China;4.KeyLaboratoryofCarbonMaterials,InstituteofCoalChemistry,ChineseAcademyofSciences,Taiyuan030001,China)
The microstructure and molten salt impregnation characteristics of a micro-fine grain isotropic graphite ZXF-5Q from Poco Inc. was investigated. The microstructural characteristics of the pores caused by gas evolution, calcination cracks, Mrozowski cracks, and the crystal structure were characterized by optical microscopy, mercury porosimetry, helium pycnometry, transmission electron microscopy, X-ray diffraction and Raman spectroscopy. Results show that the ZXF-5Q has uniformly-distributed pores caused by gas evolution with very small entrance diameters (~0.4 μm), and numerous lenticular Mrozowski cracks. Molten salt impregnation with a molten eutectic fluoride salt at 650 ℃ and 1, 3 and 5 atm, indicate that ZXF-5Q could not be infiltrated even at 5 atm due to its very small pore entrance diameter. Some scattered global salt particles found inside the ZXF-5Q are possibly formed by condensation of the fluoride salt steam during cooling.
Molten salt reactor; Graphite; Molten salt impregnation; Microstructure
1 Introduction
The first nuclear reactor, designated as CP-1, achieved a self-sustaining nuclear chain reaction in 1942[1]. The group of scientists led by Enrico Fermi selected graphite as the moderator material because it was the only suitable material available at that time[1]. Since then, graphite has been used in many reactors as a moderator and reflector owing to its excellent neutron-moderating ratio just behind heavy water, a high stability, high chemical compatibility and high-temperature strength[1-3]. Hence, although fast neutron irradiation significantly damages the graphite by changing its dimensions and physical properties[4-6], nuclear grade graphite is still widely applied. Today, it is mainly used in the high-temperature gas-cooled reactor (HTGR) and molten salt reactor (MSR).
The MSR is one of the six Generation IV reactors[7]. It is a high-temperature reactor that features circulating molten fluoride salts as fuel and has been successfully operated at the Oak Ridge National Laboratory (ORNL) in the 1960s[7,8]. Graphite is mainly used as the neutron moderator to control the flow patterns of the fuel salt in the MSR[8]. The requirements for the graphite material are a high stability against radiation-induced distortion and non-penetrability by the fuel-bearing molten salt. Graphite is a porous material and its pores can be easily impregnated with the molten fuel salt in a high pressure environment[8-10]. A seepage of the fuel salt into the graphite leads to the formation of local hot spots, which significantly damage the graphite, thereby reducing the service life of the graphite components[8]. Studies at ORNL suggested that the impregnation is closely related to the pressure environment and the pore diameter[8,9]. If the entrance pore diameters of the graphite, i.e. the neck of the open pore, are sufficiently small, molten salt penetration into the graphite can be restricted. Historically, grade CGB graphite[11]has been successfully used in previous MSR experiments. The small entrance pores (<0.4 μm help preventing molten salt impregnation. However, the high irradiation anisotropy (extrusion molding method) makes it not suitable for reactors. Most of the commercially available nuclear graphite materials are highly isotropic and show a good radiation-resistance[12,13]. Unfortunately, the entrance pore diameters of these graphite candidates (e.g., NBG-18 and IG-110 graphite) cannot meet the requirements for the MSR[8]. Therefore, sealing the pores in the nuclear graphite material or selecting a new graphite material that can meet the requirements for our MSR project is particularly important.
Recently, we tried several methods to modify the graphite material, e.g., isotropic pyrolytic carbon coating[14-16]and binderless nanopore-isotropic graphite (NPIG)[17]research. In addition, experiments were performed to find commercially available graphite that could prevent the impregnation with molten salt. In general, graphite produced from finer grains exhibits finer pores. We have studied many grades of fine grained isotropic graphite commercially available, such as ETU-10/ETU-15 (Ibiden, Japan), DS4/E+40 (Mersen, France), and AXF-5Q/ZXF-5Q (Poco, USA). According to the differences in grain size, these graphites can be divided into super-fine (grain size below 50 μm, e.g., ETU-10), ultra-fine (< 10 μm, e.g., AXF-5Q/DS4/E+40), and micro-fine (< 2 μm, e.g., ZXF-5Q) grained graphite. We have found that only micro-fine grained graphite has the entrance pore diameters below 1 μm and, therefore, could prevent the molten salt impregnation in a relative high-pressure environment. As a consequence, micro-fine grained graphite is promising candidate for the MSR.
However, there is only little information available on micro-fine grained graphite, especially concerning its irradiation and impregnation behavior. As shown by previous studies, the irradiation and impregnation behavior of graphite materials is closely related to their microstructure[14-19], such as gas-evolved pores, calcination cracks, Mrozowski cracks, and the crystal structure of the materials. It was reported that calcination cracks and Mrozowski cracks in filler particles are highly connected to the initial volume shrinkage of nuclear graphite under high-temperature irradiation[2,5,20]. In addition, there is a strong correlation between the diameters of the neck of the pores and the impregnation behavior of the graphite[9]. A detailed investigation of the microstructure of the micro-fine grained graphite will be helpful to understand its irradiation and impregnation behavior. Furthermore, molten salt impregnation tests are essential to find a promising candidate graphite material for a future application in a MSR[9]. Therefore, we also investigated the molten salt impregnation characteristics at different pressures.
In this study, the microstructure of micro-fine grained isotropic graphite ZXF-5Q was characterized by optical microscopy (OM), mercury porosimetry, helium gas pycnometry, transmission electron microscopy (TEM), Raman spectrometry, and X-ray diffraction (XRD). The molten salt impregnation characteristics were investigated by impregnating the graphite samples with a molten fluoride salt at 650 ℃, and 1, 3, and 5 atm. Afterwards, the samples were studied by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS).
2 Experimental
2.1 Materials
The ZXF-5Q graphite was investigated in this study and compared with nuclear grade IG-110 graphite. ZXF-5Q is a typical micro-fine grained isotropic graphite while the IG-110 is a conventional super-fine grained isotropic nuclear graphite used in the HTGRs. The typical physical and mechanical properties of the ZXF-5Q and the IG-110 graphite are compared in Table 1. Both of them were manufactured by isostatic pressing method, which exhibit a low anisotropy ratio (below 1.1) and a high density. The difference between the two graphite materials is the particle size. The average grain size of ZXF-5Q graphite (~1 μm) is much smaller than that of IG-110 graphite (~20 μm), resulting in smaller gas-evolved pores (see Fig. 1). This suggests that molten salt impregnation could be more likely prevented by using ZXF-5Q. Furthermore, ZXF-5Q is mechanically stronger than IG-110 and can better meet the reactor requirements accordingly.
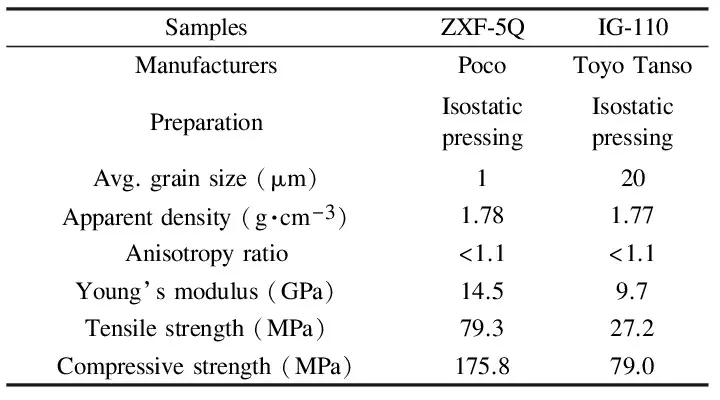
Table 1 A comparison of the typical physical and mechanical properties of ZXF-5Q and IG-110 graphite.
2.2 Characterization
The analysis of the surface morphology was performed by optical microscopy (OM) (Zeiss, Germany). The OM specimens were hand-polished using 1 200 grit sand paper and then placed into a vibratory polisher filled with a 0.05 μm Al2O3water-based suspension for 5 h. Mercury porosimetry was performed using a mercury intrusion porosimeter (AutoPore IV 9500) to obtain the entrance pore diameter distribution. The total and open porosity were then calculated based on the apparent and the pycnometric density. The pycnometric density was measured using a helium gas pycnometer. The size of the Mrozowski cracks in the filler particles was compared by TEM (Tecnai G2F20, USA) at an acceleration voltage of 200 kV. Specimens for the TEM investigations were prepared by cutting disks with a diameter of 3 mm, which were then sand milled to a thickness of approx. 50 μm, followed by ion-beam thinning (without dimpling) in a Gatan PIPS ion mill (USA) using Ar ions (4.5 keV). The surface structure was analyzed using a micro-Raman spectrometer (LabRAM HR800, France) with an excitation wavelength of 532 nm and an effective penetration depth of ~ 50 nm. The diameter of the laser spot focused on the specimen was about 2 μm and 3 different positions were selected to determine the average, considering the inhomogeneity of graphite. The crystal structure of the samples was analyzed by XRD using a Bruker-AXS D8 Advance verticalθ/2θgoniometer (Bruker AXC GmbH, Karlsruhe, Germany).
2.3 Molten salt impregnation tests
The specimens used for the molten salt impregnation experiments were prepared by cutting samples to a diameter of 12.7 mm and a thickness of 20 mm, which were then successively cleaned by ultrasonication in acetone and deionized water to remove potential contaminants, and finally heated in a vacuum oven at 120 ℃ to remove the residual moisture. The specimens were impregnated with molten salt at 650 ℃ and 1, 3, and 5 atm for 12 h in a pressure vessel. The fluoride salt used for the experiments was a molten eutectic FLiNaK consisting of LiF, NaF and KF salt with a ratio of 46.5/11.5/42 mol%. The fluoride salt was handled in an argon-filled glove box to prevent contamination with oxygen and water. Before heating the pressure vessel, it was evacuated for 5 h with a molecular vacuum pump to ensure a low oxygen and water concentration. The weight gain of the specimens was measured directly in the argon-filled glove box. After impregnation, the specimens were studied by SEM (LEO 1530 VP, Germany) and EDS.
3 Results and discussion
3.1 A comparison of the microstructure of ZXF-5Q and IG-110 graphite
3.1.1 Surface morphology
Graphite materials are generally manufactured from a mixture of a petroleum/coal coke filler and a coal-tar-pitch binder, which results in a polygranular graphite with a porous structure after a high-temperature treatment[1]. Fig. 1 is the optical micrographs of the polished graphite materials, showing the surface morphologies of ZXF-5Q and IG-110. Fig. 1c shows a typical polished surface of IG-110 graphite with numerous acicular filler particles (marked by F, anisotropic) and globular pores (marked by P). The anisotropic filler particles are randomly oriented, resulting in the high isotropy ratio of the graphite components. The cracks (marked by C in Fig. 1c) within the filler particles are formed during calcination of the filler material before it is incorporated in the formed particles[1,21]. Previous studies suggested that the calcination cracks and Mrozowski cracks (in section 3.1.3) are highly related to the initial volume shrinkage of nuclear graphite under high-temperature irradiation[2,5]. The calcination cracks in Fig. 1c are aligned parallel to the axial direction of the filler particles, with a length comparable to the length of the filler particles. The globular pores are caused by escaping volatile gases, generated by the pyrolysis of the binder material (i.e., coal-tar pitch) during the baking process[1], which were accessible. It can be clearly seen that the size of these pores could reach more than ten micrometers. It is worth noting that the entrance pore diameter, correlated to the impregnation, refers to the neck of these gas-evolved open pores[18].

Fig. 1 Optical micrographs showing the polished graphite surface: (a) ZXF-5Q, (b) magnified region of (a), and (c) IG-110. P-Gas-evolved pore, F-Filler particle, C-Calcination shrinkage crack.
Fig. 1a shows a corresponding micrograph of ZXF-5Q graphite using the same magnification. The filler particles and pores are difficult to distinguish. Fig. 1b shows a magnified micrograph of the sample section shown in Fig. 1a. Still, the filler particles are too small to identify. Due to the small filler particle size, calcination cracks may not exist within the filler particles. The pores of ZXF-5Q graphite could only reach a few micrometers in diameter, which are about only one-tenth smaller than that for IG-110 graphite and more uniform than IG-110 due to their small sizes.
3.1.2 Porosity
The results of the mercury porosimetry experiments are shown in Fig.2. Fig. 2a shows the change in the cumulative mercury intrusion with pressure for ZXF-5Q and IG-110 graphite. The mercury intrusion curves reveal the impregnation mechanism of a graphite for a liquid with a contact angle larger than 90°. At the beginning, the pressure is relatively low, the mercury could not infiltrate both graphite materials. However, once the pressure exceeds a certain value , the detected mercury infiltration sharply increases in both cases, indicating that the graphite pores are quickly filled after the pressure have reached the threshold value. The obtained threshold pressures for ZXF-5Q and IG-110 are approx. 4.2 and 0.4 MPa, respectively. This indicates that it is harder for a liquid with a contact angle larger than 90° to infiltrate the ZXF-5Q graphite than IG-110. Once the pressure exceeds the threshold value, the mercury injection process starts to slow down, indicating that the entrance pore diameter for both ZXF-5Q and IG-110 graphite is rather narrowly distributed, especially for ZXF-5Q. The entrance pore diameters are calculated using the Washburn relation.
(1)
where Δp,γ,δ, andθdenote the pressure difference, the surface tension (γ= 485 dynes cm-1in this study), the entrance diameter of the penetrated pores (i.e., the entrance pore diameter), and the contact angle (θ= 152° used here, respectively. The results of the calculation of the entrance pore diameter distribution for both ZXF-5Q and IG-110 graphite are shown in Fig. 3b. The entrance pore diameters are mainly in the range from 0.41 to 0.36 μm and 4.24 to 3.51 μm for ZXF-5Q and IG-110, respectively. The maximum values obtained for the entrance pore diameter, corresponding to the threshold pressures, are about 4.24 and 0.41 μm for IG-110 and ZXF-5Q, respectively. This indicates that the threshold pressure for ZXF-5Q would also be ten times more than that for IG-110, suggesting that ZXF-5Q graphite exhibits a good molten salt penetration inhibition ability.

Fig. 2 Results of the mercury porosimetry experiments: (a) cumulative mercury intrusion plotted as a function of pressure and (b) the distribution of the entrance pore diameters for ZXF-5Q and IG-110 graphite. Table 2 A comparison of the porous properties of ZXF-5Q and IG-110 graphite.
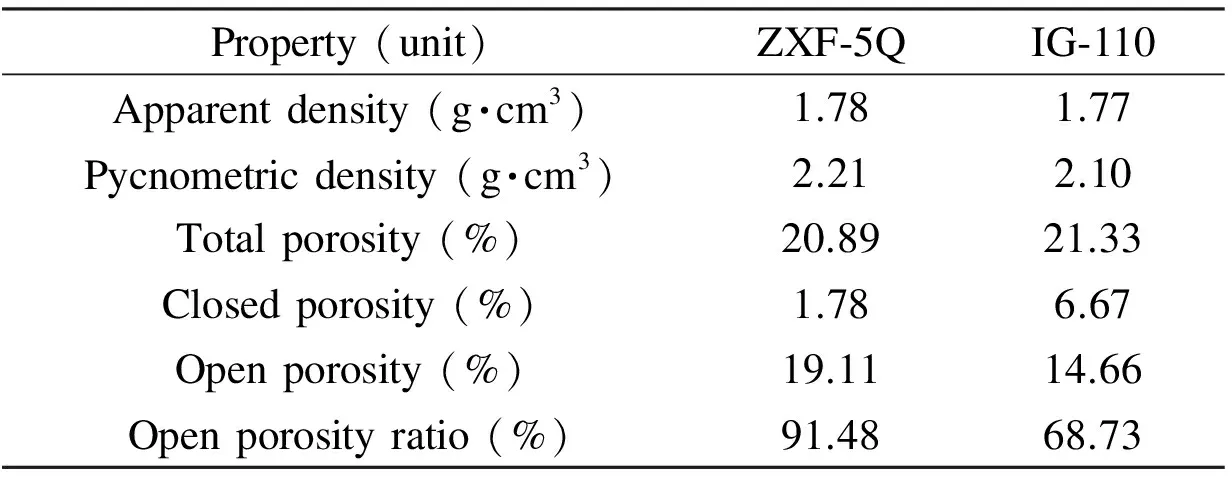
Property(unit)ZXF⁃5QIG⁃110Apparentdensity(g·cm3)1.781.77Pycnometricdensity(g·cm3)2.212.10Totalporosity(%)20.8921.33Closedporosity(%)1.786.67Openporosity(%)19.1114.66Openporosityratio(%)91.4868.73
The porous properties of ZXF-5Q and IG-110 graphite are compared in Table 2. Both contain a large number of pores. The total porosity (ε), closed porosity (εc), and open porosity (εo) are calculated based on the apparent density (dv) and the He-pycnometric density (dp) according to the following equations:
(2)
(3)
where 2.25 g·cm-3is the theoretical density of the graphite crystal. The total porosity of ZXF-5Q and IG-110 is 20.89% and 21.33%, respectively and the open pore ratio is 91.48% and 68.73% for ZXF-5Q and IG-110, respectively. The high open porosity of ZXF-5Q indicates that most of its pores are accessible. Although 91.48% of the ZXF-5Q pores are accessible, unlike those in the coating (mainly closed porosity), the molten salt still could not penetrate into them due to their small entrance pore diameters (Fig. 2b). Therefore, a micro-fine grained isotropic graphite, such as ZXF-5Q, can successfully prevent molten salt infiltration.
3.1.3 Mrozowski cracks
Mrozowski cracks[22]are believed to be generated during cooling after graphitization and are thought to have been formed to relieve the internal stress caused by the interaction between the restraining effect of the strong, inter-crystalline C—C bonds and the anisotropic contraction of the crystal lattice[23]. The cracks exist in parallel array and have been observed in the shape of lenticular by TEM[20,23]. These cracks are believed to accommodate for the thermal-[20]and initial irradiation-induced[2,20]expansion by absorbing the c-axis crystal growth. Representative TEM images of the Mrozowski cracks (marked M) in the filler component of ZXF-5Q and IG-110 graphite are shown in Fig. 3. Although the average ZXF-5Q coke particle diameter is only about 1 μm, numerous lenticular cracks in ZXF-5Q, similar to those found in IG-110, could be observed. The cracks in ZXF-5Q (Fig. 3a) are only about 200 nm long, which is shorter than the cracks with a length of several microns found in IG-110 (Fig. 3b) because the ZXF-5Q coke particles are much smaller than the IG-110 particles. This indicates that the filler particles of ZXF-5Q could also accommodate for the initial irradiation-induced crystal expansion just as the filler particles of IG-110. Hence, ZXF-5Q may exhibit an irradiation-induced volume change similar to IG-110, once again suggesting that ZXF-5Q may be a promising candidate for application in nuclear reactors.
3.1.4 Crystal structure

(4)
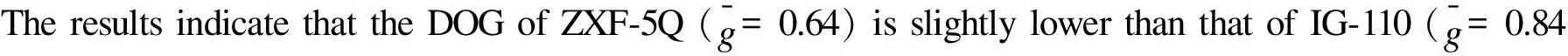

Fig. 3 Representative TEM images revealing the Mrozowski cracks in the filler component of (a) ZXF-5Q and (b) IG-110 graphite. M - Mrozowski crack.

Fig. 4 XRD patterns of the ZXF-5Q and IG-110 graphite.
3.1.5 Raman spectroscopy
Raman spectroscopy is an useful tool for the surface structural characterization of graphitic materials[25-28]. The Raman spectra of ZXF-5Q and IG-110 are shown in Fig. 5. The first-order Raman spectra consist of three distinct peaks, marked as theG,D, andD′ band, appearing at 1 582 (graphite), ~1 355 (disorder) and ~1 620 cm-1, respectively. TheG' band is Raman active for the sp2-carbon networks[25]. TheDband and theD′ band are attributed to two scattering processes, i.e. one elastic scattering event due to crystal defects and one inelastic scattering event due to phonon emission or absorption[28]. The main contribution to theDband is the crystallite boundaries[18,28]. The intensity ratiosID/IGfor ZXF-5Q and IG-110 are 0.45 and 0.12, respectively. The in-plane “crystallite size” (La) is generally calculated using an empirical Tuinstra-Koenig equation[29,30](valid for graphitic materials ifLa> 2 nm) as follows:
(5)
with C ~ 4.4 nm here. The results indicate that theLaof ZXF-5Q (~ 9.8 nm) is smaller than that of IG-110 (~ 36.7 nm), which is consistent with the XRD results, implying that ZXF-5Q graphite has a lower DOG than IG-110.

Fig. 5 Raman spectra of the ZXF-5Q and IG-110 graphite.
3.2 Molten salt impregnation characteristics
The weight increment plotted as a function of pressure for ZXF-5Q and IG-110 after impregnation with the molten salt at a pressure of 1, 3, and 5 atm is shown in Fig. 6. The corresponding cross-sectional SEM images are shown in Fig. 7. IG-110 impregnated at a pressure of 1 atm only shows a ~1% increase in weight, and no obvious molten salt particles are found (Fig.7b), indicating that the IG-110 has actually not been infiltrated with the molten salt. The slight increment in weight can be attributed to the salt film on the graphite surface (Fig. 8b). However, the weight increment sharply increases to ~16% after the pressure is increased to 3 atm, indicating that the IG-110 graphite has been infiltrated obviously. These results are consistent with results previously reported by Song et al.[17]and He et al.[15]. The corresponding cross-sectional SEM image is shown in Fig. 7d, which clearly reveals that globular salt particles spread over the graphite. The gas-evolved pores are now clearly filled with the molten salt, which confirms the results of the weight increment experiments. The weight does not increase further when the pressure is increased to 5 atm, indicating that most of the open pores has already been filled, which is consistent with the results of the mercury intrusion results.

Fig. 6 Weight increment plotted as a function of the applied pressure for IG-110 and ZXF-5Q impregnated with the molten salt.

Fig. 7 Cross-sectional SEM micrographs of the impregnated graphite: (a-e) show ZXF-5Q impregnated with molten salt at a pressure of 1, 3, and 5 atm, respectively; (b-f) show IG-110 impregnated with molten salt at a pressure of 1, 3, and 5 atm, respectively.
On the other hand, ZXF-5Q only shows a slight increase in weight (~ 0.5%) over the entire pressure range, indicating that ZXF-5Q is difficult to be infiltrated, similar to pyrolytic carbon coating[15]and NPIG[17]. The weight increment can mainly be attributed to the salt film on the graphite surface (Fig. 8a). The corresponding cross-sectional SEM images of ZXF-5Q impregnated at 1, 3, and 5 atm are shown in Figs. 7a, c, and e, respectively. No obvious difference is found. The molten salt impregnation mechanism is the same as that for mercury intrusion. According to the Washburn relation (in section 3.1.2), the threshold pressure of ZXF-5Q for molten salt impregnation should be ten times larger than the that of IG-110. Therefore, it is inferred that ZXF-5Q graphite can not be infiltrated even if the pressure is increased to 10 atm.

Fig. 8 Backscattered SEM micrographs of the graphite materials impregnated at 1 atm: (a) ZXF-5Q and (b) IG-110.
In order to better distinguish graphite and salt, backscattered SEM micrographs were also recorded[17,19]. Fig. 8 shows cross-sectional backscattered SEM micrographs of ZXF-5Q and IG-110 graphite impregnated at 1 atm. For both graphite materials, a salt film is found adhesive to the graphite surface. The thickness of the salt film on ZXF-5Q (Fig. 8a) is thinner than that found on IG-110 (Fig. 8b) because its surface pores are much smaller than that of IG-110 (Fig.1). This can explain the small weight increment (Fig. 6) for ZXF-5Q. In addition, some scattered salt particles (highlighted by the red circles) inside the graphite matrix of both materials are also observed. Some of them might be attributed to surface salt relocated into the matrix when the graphite is cut off. However, there is another possible source for the other salt particles. Fluoride salt steam might enter the open porosity and condense into small salt particles during the cooling process.
Fig. 9a shows the SEM micrograph and EDS results obtained for the selected region of ZXF-5Q containing a salt particle.
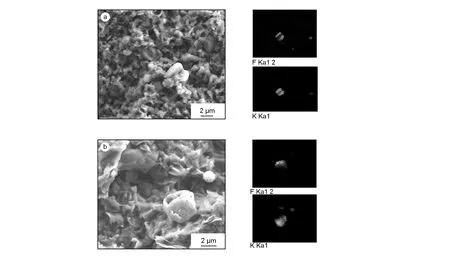
Fig. 9 SEM and EDS micrographs of the graphite materials impregnated at 1 atm: (a) ZXF-5Q and (b) IG-110.
The salt particle in the central zone is clearly embedded in the graphite matrix, indicating that there exist globular salt particles probably formed by condensation of fluoride salt steam. It seems that the size of the steam-condensed salt particles is below a few micrometers. The same phenomena are observed for IG-110, as shown in Fig. 9b. This indicates that, although no infiltration occurs at a low pressure, some salt are condensed inside the graphite matrix due to cooling of salt steam.
4 Conclusions
The microstructure and molten salt impregnation characteristics of the micro-fine grained isotropic graphite ZXF-5Q were investigated. ZXF-5Q shows uniformly-distributed gas-evolved pores. No calcination cracks are observed in the filler particles of ZXF-5Q, probably due to their small size. The entrance pore diameter distributions of both IG-110 and ZXF-5Q are rather narrow, with the entrance pore diameter of ZXF-5Q being much smaller than that of IG-110. Numerous lenticular cracks (i.e., Mrozowski cracks) are found in the filler particles of ZXF-5Q, indicating that the ZXF-5Q filler particles could accommodate for thermal and initial irradiation-induced expansion. ZXF-5Q exhibits a good crystal structure. ZXF-5Q could not be infiltrated even when the pressure is increased to 5 atm due to its very small entrance pore diameters.
[1] Nightingale RE. Nuclear Graphite[M]. Academic Press, 1962.
[2] Burchell TD. Carbon Materials for Advanced Technologies[M]. Pergamon, 1999.
[3] Virgil'ev YS, Kalyagina IP. Reactor Graphite[M]. Inorg Mater 2003, 39: S46-S58.
[4] Simmons JHW. Radiation Damage in Graphite[M]. Pergamon Press, 1965.
[5] Haag G, Reaktortechnik FJIfSu. Properties of ATR-2E Graphite and Property Changes Due to Fast Neutron Irradiation[M]. Forschungszentrum Jülich GmbH, Zentralbibliothek, 2005.
[6] Telling RH, Heggie MI. Radiation defects in graphite[J]. Philos Mag, 2007, 87(31): 4797-4846.
[7] A technology roadmap for generation IV nuclear energy systems[R]. USDOE, GIF-002-00, 2002.
[8] Rosenthal MW, Haubenreich PN, Briggs RB. The development status of motten-salt dreeder reactor[R]. ORNL-4812, 1972.
[9] Briggs RB. Program semi annual progress report[R]. MSRE, ORNL-3708, 1964.
[10] Ketsten PR. Graphite behavior and its effects on MSBR perform[R]. ORNL-TM-2136, 1969.
[11] McCoy HE, Weir JR. Materials development for motten salts breeder reactor[R]. ORNL-TM-1854, 1967: 46-56.
[12] Ishiyama S, Burchell TD, Strizak JP, et al. The effect of high fluence neutron irradiation on the properties of a fine-grained isotropic nuclear graphite[J]. J Nucl Mater, 1996, 230(1): 1-7.
[13] Burchell TD, Snead LL. The effect of neutron irradiation damage on the properties of grade NBG-10 graphite[J]. J Nucl Mater, 2007, 371(1-3): 18-27.
[14] Feng SL, Xu L, Li L, et al. Sealing nuclear graphite with pyrolytic carbon[J]. J Nucl Mater, 2013, 441(1-3): 449-454.
[15] He XJ, Song JL, Xu L, et al. Protection of nuclear graphite toward liquid fluoride salt by isotropic pyrolytic carbon coating[J]. J Nucl Mater, 2013, 442(1-3): 306-308.
[16] He XJ, Song JL, Tan J, et al. SiC coating: An alternative for the protection of nuclear graphite from liquid fluoride salt[J]. J Nucl Mater, 2014, 448(1-3): 1-3.
[17] Song JL, Zhao YL, Zhang JP, et al. Preparation of binderless nanopore-isotropic graphite for inhibiting the liquid fluoride salt and Xe135 penetration for molten salt nuclear reactor[J]. Carbon, 2014, 79(0): 36-45.
[18] Zhang BL, Xia HH, He XJ, et al. Characterization of the effects of 3-MeV proton irradiation on fine-grained isotropic nuclear graphite[J]. Carbon, 2014, 77: 311-318.
[19] Bernardet V, Gomes S, Delpeux S, et al. Protection of nuclear graphite toward fluoride molten salt by glassy carbon deposit[J]. J Nucl Mater, 2009, 384(3): 292-302.
[20] Wen KY, Marrow J, Marsden B. Microcracks in nuclear graphite and highly oriented pyrolytic graphite (HOPG)[J]. J Nucl Mater, 2008, 381(1-2): 199-203.
[21] Kane J, Karthik C, Butt DP, et al. Microstructural characterization and pore structure analysis of nuclear graphite[J]. J Nucl Mater, 2011, 415(2): 189-197.
[22] Mrozowski S. Anisotropy of thermal expansion and internal stresses in polycrystalline graphite and carbons[J]. Physical Review, 1952, 86(4): 622.
[23] Sutton AL, Howard VC. The role of porosity in the accommodation of thermal expansion in graphite[J]. J Nucl Mater, 1962, 7(1): 58-71.
[24] Chi S-H, Kim G-C. Comparison of 3 MeV C(+) ion-irradiation effects between the nuclear graphites made of pitch and petroleum cokes[J]. J Nucl Mater, 2008, 381(1-2): 98-105.
[25] Tuinstra F, Koenig JL. Raman spectrum of graphite[J]. J Chem Phys, 1970, 53(3): 1126-1130.
[26] Nikiel L, Jagodzinski PW. Raman-spectroscopic characterization of graphites - a reevaluation of spectra/structure correlation[J]. Carbon, 1993, 31(8): 1313-1317.
[27] Jawhari T, Roid A, Casado J. Raman-spectroscopic characterization of some commercially available carbon-black materials[J]. Carbon, 1995, 33(11): 1561-1565.
[28] Pimenta MA, Dresselhaus G, Dresselhaus MS, et al. Studying disorder in graphite-based systems by Raman spectroscopy[J]. Physical Chemistry Chemical Physics, 2007, 9(11): 1276-1291.
[29] Knight DS, White WB. Characterization of diamond films by raman-spectroscopy [J]. Journal of Materials Research, 1989, 4(2): 385-393.
[30] Ferrari AC, Robertson J. Interpretation of Raman spectra of disordered and amorphous carbon[J]. Phys Rev B, 2000, 61(20): 14095-14107.
1007-8827(2016)06-0585-09
熔盐堆用超细颗粒石墨结构和熔盐浸渗研究
张文婷1,2, 张宝亮1,2,3, 宋金亮1, 戚 威1, 贺秀杰1,2,3, 刘占军4,连鹏飞4, 贺周同1, 高丽娜1, 夏汇浩1, 刘向东2, 周兴泰1, 孙立斌3, 吴莘馨3
(1.中国科学院上海应用物理研究所 核辐射与核能技术重点实验室,上海201800;2.山东大学物理学院,山东 济南250100;3.清华大学 核能与新能源技术研究院,先进核能技术协同创新中心,先进反应堆工程与安全教育部重点实验室,北京100084;4.中国科学院山西煤炭化学研究所 炭材料重点实验室,山西 太原030001)
研究各向同性微细颗粒石墨ZXF-5Q的微结构及熔盐浸渗特性。使用光学显微镜、压汞仪、真密度仪、透射电子显微镜、X射线衍射仪以及拉曼光谱仪,对其气孔、煅烧裂纹、Mrozowski裂纹及晶体结构进行表征。结果表明,ZXF-5Q拥有均匀分布的气孔,非常小的入孔孔径(0.4 μm),数量众多的透镜状Mrozowski裂纹以及良好的晶体结构。熔盐浸渗实验在不同压力(分别为1、3、5个大气压)650 ℃环境下进行。利用扫描电子显微镜和X射线能谱仪对其浸渗特性进行了观察分析。研究表明,由于其非常小的入口孔径,ZXF-5Q在5个大气压外加压强环境下依然可以很好阻止熔盐浸渗。尽管没有浸渗发生,在ZXF-5Q内部可以发现球形熔盐颗粒,可能是熔盐蒸汽凝结造成的。
熔盐堆; 石墨; 熔盐浸渗; 微结构
TB333
A
国家自然科学基金 (51602336);上海市自然科学基金(16ZR1443400);国家自然科学基金 (51572274, 11305240, 11075097, 11375108);中国科学院战略性先导科技专项“未来先进核裂变能-钍基熔盐堆核能系统”(XDA02004220).
宋金亮,副研究员. E-mail: jlsong1982@yeah.net; 张宝亮,博士研究生. E-mail: zhangbaoliang@sinap.ac.cn
张文婷,硕士研究生. E-mail: zhangwenting@sinap.ac.cn
Foundationitem: National Natural Science Foundation of China (51602336); Natural Science Foundation of Shanghai (16ZR1443400); National Natural Science Foundation of China (51572274, 11305240, 11075097, 11375108); “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA02004220).
SONG Jin-liang, Associate Professor. E-mail: jlsong1982@yeah.net.; ZHANG Bao-liang, Ph. D Candidate. E-mail: zhangbaoliang@sinap.ac.cn
Authorintroduction: ZHANG Wen-ting, Master Student. E-mail: zhangwenting@sinap.ac.cn
10.1016/S1872-5805(16)60034-3
Receiveddate: 2016-09-10;Reviseddata: 2016-11-26
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
