Large-scale synthesis of novel vertically-aligned helical carbon nanotube arrays
2017-01-07ZHANGJichengTANGYongjianYIYongMAKangfuZHOUMinjieWUWeidongWANGChaoyang
ZHANG Ji-cheng, TANG Yong-jian, YI Yong, MA Kang-fu,ZHOU Min-jie, WU Wei-dong, WANG Chao-yang
(1.Research Center of Laser Fusion, China Academy of Engineering Physics, Mianyang621900, China;2. College of Material Science and Engineering, Southwest University of Science and Technology, Mianyang621010, China)
Large-scale synthesis of novel vertically-aligned helical carbon nanotube arrays
ZHANG Ji-cheng1, TANG Yong-jian1, YI Yong2, MA Kang-fu1,ZHOU Min-jie1, WU Wei-dong1, WANG Chao-yang1
(1.ResearchCenterofLaserFusion,ChinaAcademyofEngineeringPhysics,Mianyang621900,China;2.CollegeofMaterialScienceandEngineering,SouthwestUniversityofScienceandTechnology,Mianyang621010,China)
The large-scale synthesis of vertically-aligned carbon nanotube arrays with different helical pitches and diameters was achieved using the floating catalyst method. Results indicate that they are aligned perpendicular to the substrate surface and have a well-graphitized structure and their growth is accompanied by the production of pentagonal, heptagonal and hexagonal carbon rings. The hexagonal carbon ring is the basic structure unit to form the graphite lattice. When paired pentagon-heptagon atomic rings arrange themselves periodically within the hexagonal carbon network, helical carbon nanotubes are formed. The growth rate of the helical?carbon nanotubes is about 4.5mg/cm2·h.
Helical carbon nanotube; Helical pitch; Hexagonal carbon ring
1 Introduction
As one of the most studied advanced functional materials, carbon nanotubes (CNTs) have potential applications in the fields of display, hydrogen storage, sensors, filler of composite, etc[1-9]. In particular, CNTs with different shapes such as toroid[10], coiled[11], helical and branched[12]other than the straight forms are widely used as high-performance electromagnetic wave absorbers, sensors, resonators, nanoscale mechanical springs, electrical inductors, and generators of magnetic beams, owing to their peculiar morphology and unique electrical, magnetic and mechanical properties[13-22]. The helical CNTs[11, 23-26]are the most promising ones as reinforcement fillers in nano-composites in advanced materials and nano-electronic devices in nano-circuit.
The production of straight CNTs is generally achieved via electric arc-discharge, laser evaporation, or chemical vapor deposition (CVD). For the coiled structure is believed to have exceptional properties and versatile applications, tremendous theoretical and experimental researches have been devoted to the studies of this intriguing carbon material. The helical carbon nanotubes was first predicted by Ihara and Dunlap in the early nineties[11,23-25]and the experimental observation was reported in 1994 by Zhang et al[26]. The first experimental production of multi-walled coiled carbon nanotubes sample was achieved by catalytic decomposition of acetylene over silica-supported Co catalyst at 700 °C with inner and outer diameter of 15-20 nm. After this, various techniques have been developed for the synthesis of helical CNTs, and helical CNTs can be produced at a high yield by catalytic CVD[27,28]. Vardhan Bajpai had also developed techniques for the synthesis of large-scale perpendicularly aligned helical CNT arrays on the quartz glass substrate using Fe(CO)5and pyridine as the catalyst and precursor, respectively[29].
Although both nonaligned and aligned helical CNTs have been reported, the synthesis of well aligned helical CNTs with various helical pitches and diameters is challenging and has not been successful until now. In this paper, large-scale perpendicularly aligned helical CNT arrays were synthesized by floating catalyst method using xylene and ferrocene as precursors and catalyst, respectively. The prepared CNTs have novel microstructure, both helical and straight nanotube exist in one CNT and helical CNTs with various helical pitches and diameters along growth direction.
2 Experimental
2.1 Sample synthesis
Similar to the successful preparation of large-scale, perpendicularly aligned straight CNTs, helical CNTs were synthesized in large scale by the floating catalyst (FC) method in a 400 mm horizontal tubular reactor made of quartz with a diameter of 60 mm using ferrocene and xylene as precursor and catalyst, respectively. The reactor was inserted into a furnace that provided controllable heating up to 850 ℃ with a non-gradient temperature zone (reaction zone) of 150 mm in length. The temperature distribution along the reactor was measured by K-type thermocouple. Three layers vertical aligned CNT arrays were grown on SiO2substrate in three times with an interval time between each layer for 10 min without breakdown the vacuum. The thickness of each layer was precisely controlled by the growth time (30 min for first layer, 60 min for the second and 90 min for the third). As a result, we found that three layers of perpendicularly aligned helical CNTs and straight CNTs were prepared onto the whole SiO2substrate surface. The sample area is 4 cm2, which is essentially limited by the size of the furnace diameter only. After synthesis, helical CNTs were cooled in an inert gas (Argon) flow of 500 mL/min.
2.2 Characterization
The microstructure was investigated using scanning electron microscopy(SEM; JSM6490, Jeol), high resolution transmission electron microscopy (HR-TEM; JEM3010, Jeol), thermogravimetric analysis (TGA; Labsys EVO TG,Setaram) and Raman spectroscopy (HORIBA, T6400). HR-TEM instruments were operated at an acceleration voltage of 200 kV and SEM instruments at a voltage of 20 kV. TGA was performed under oxygen atmosphere using a heating ramp of 10 ℃/min. Raman spectra were recorded at room temperature with the excitation wavelengths of 532 nm. The helical CNT array samples for SEM, TGA and Raman scattering analysis were as grown without treatment. The as-synthesized samples were dispersed in alcohol with ultrasonic apparatus, and then directly transferred to the TEM grid for HRTEM test operating at 200 kV.
3 Results and discussion
In order to examine the microstructures of CNTs, the grown films were peeled off from SiO2substrate. The aligned helical CNTs can be easily removed from substrate, and each layer can be detached from other layers (Fig.1(a) and Fig. 1(b)). An enlarged cross-section view of Fig. 1(a) along carbon nanotubes with various magnification under SEM are shown in Fig.1(c)-(g). It is clearly that the densely packed multilayer CNT films contain a large amount of well aligned helical CNTs with different helical pitches and diameters. One can find that most of the products have a helical structure, the growth rate of the helical CNTs is about 4.5 mg/cm2·h. When the helical pitch increases, the coil diameter decreases (became linear). The very interesting structure, the perpendicularly aligned helical structure at one end while straight at the other end, is also observed in Fig. 1. The periodicity of the coiling also varies along the helical structure, as shown in Fig. 1(e,f,g).
Fig. 2(a) shows TEM images of the helical CNTs. Fig. 2(b) and Fig. 2(c) show TEM images of individual helical CNT taken from the sample shown in Fig. 1(a) and after having been dispersed on a TEM grid, from which a helical nanotube with a pitch of ca. 150 nm and a hollow structure is clearly evident. Aligned helical CNT arrays with different helical structures have also been produced in synthesized experiment, as shown in Fig. 2(d) and Fig. 2(e) that the helical nanotube with different helical pitches of ca. 1 μm and diameter of ca. 0.4 μm. The helical structure and the coil shape are the two typical structures in the samples. Fig. 2(f) shows HR-TEM image of the clear hollow tube structure. It clearly demonstrates that our carbon products are mainly MWCNTs and well graphitized.

Fig. 1 (a,b) SEM images of vertically aligned three-layers helical CNT arrays at cross section view and plane view. (c,d,e,f,g) An enlarged cross section view of (a) at different regions, showing well aligned helical CNTs with various helical pitches and diameters.
TGA was conducted in oxygen atmosphere on a SETARAM TGA EVO instrument from 300 to 970 K at a heating rate of 10 K/min. Thermogravimetric data of the CNTs material is depicted in Fig. 3. The weight loss between 300 K and 735 K is due to the removal of physical absorbed water molecules and oxidation of amorphous carbon. The CNTs show a single monotonous fall in the weight loss of the sample in the range 735-795 K. This is the characteristic of CNT gasification. Above 795 K, it shows a 5% weight residue. This indicates that a small amount of Fe is present (Fig.2(f)) prior the TGA analysis and Fe2O3is formed as the analysis was performed in oxygen atmosphere. This also confirms the thermal stability of Fe2O3.
Raman spectrum of the helical CNTs is shown in Fig. 4. Raman peaks at 215, 1 325 and 1 590 cm-1, correspond to RBM band,D-band andG-band of CNTs, respectively. We can conclude that the helical CNTs have a well graphitized structure and a small amount of single walled or few walls (typically <5 layers) carbon nanotubes is also existed in the synthesized helical CNT arrays[30, 31]. Few walls carbon nanotubes can be seen from HR-TEM image, as shown in Fig. 5.

Fig. 2 (a) Low magnification TEM image of the general morphology of the synthesized CNTs. (b) A TEM image of individual helical CNT. (c) An enlarged view of the individual helical CNT shown in (b). (d, e) An enlarged view of the helical nanotubes different from that represented by Fig. 2(b), showing different morphologies, pitches and diameters along the helical nanotube. (f) HR-TEM image of iron particle encapsulated in a CNT.

Fig. 3 Thermogravimetric plot of the synthesized CNTs performed in an oxygen atmosphere. The heating ramp is 10 K/min.
From the inspection by SEM and TEM (Fig. 1 and Fig. 2), it is observed that the straight CNTs could be gradually changed to the helical carbon nanotubes. This implies that there is considerable room for tailoring the nanotube structures by controlling the growth conditions. The nucleation and growth of the helical CNTs on the basis of previously reported models are proposed as follows. First, under the Ar atmosphere flow, it is likely that ferrocene [Fe(C5H5)2] first decomposed into atomic iron, hydrocarbon species and carbon, while xylene [C8H10] molecules decomposed into hydrocarbon species of different carbon numbers. The newly produced iron atoms then segregated on the substrate surface to form carbon-surrounded Fe nanoparticles. Once a Fe particle reached its optimal size for carbon nucleation, the surrounding carbon transformed into a graphite tube. A high surface packing density of Fe particles facilitates the growing nanotubes to align along their normal direction, as previously demonstrated for the growth of perpendicularly aligned straight CNTs[32-34]. Second, A further supply of carbon source to the contact region between the Fe particle and the growing nanotube segment allowed a continuous growth of the CNT in the direction normal to the substrate surface. This growth process is accompanied by producing of pentagonal, heptagonal and hexagonal carbon rings. The hexagonal carbon rings are the basic structural units to form the graphite lattice. The pentagonal carbon ring is required to force a hexagonal network to curve inward, forming a surface with a positive curvature. The heptagonal carbon ring, on the other hand, makes the hexagonal network curve outward, forming a negative curvature. Both pentagonal and heptagonal carbon rings are required to accommodate the change of surface curvature, making it possible to form any geometrical surface, such as straight structure and helical structure. The periodicity and the coiling diameter of the helix are determined by the twist angle and the distance between the adjacent pentagonal-hexagonal carbon rings, which can be varies in practice by changing experimental conditions, such as temperature and gas-flow rate, resulting in the change in coiling periodicity and the diameters of the helix. The growth of the helical structure is continuous if the pentagonal-hexagonal carbon rings are continuously produced; otherwise, a straight section of the tube can be grown if the pentagonal-hexagonal carbon rings are absent, in agreement with the result shown in Fig. 1(a) and Fig. 2(a). In practice, the fluctuation in the creation rates of the pentagonal-hexagonal carbon rings can produce a complex shape. This may be the reason that the structures of CNTs are very versatile.
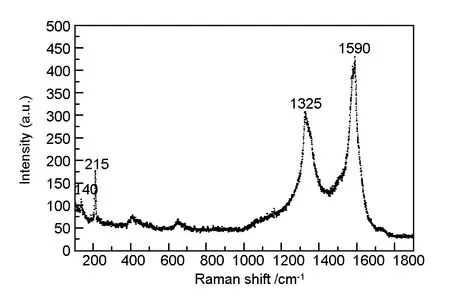
Fig. 4 Raman spectrum of the as-grown helical CNT arrays taken from its surface. The Raman spectrum of the aligned helical CNTs shows an intense peak at 1 590 cm-1, attributable to the E2g mode of the multi-walled nanotube, with a shoulder centered at 1 325 cm-1 arising from the amorphous carbon, at the same time there is also a peak at 215 cm-1, which is attributed to the RBM mode of the single walled or few walls CNTs.

Fig. 5 HR-TEM image of few walls CNT with a diameter about 4 nm.
4 Conclusions
Large-scale aligned CNT arrays perpendicular to the substrate surface were successfully synthesized through co-pyrolysis of xylene and ferrocene in the furnace at 850C under the flow of Ar. The produced CNT arrays include a large amount of helical CNTs. The growth mechanism of the CNTs was discussed in detail. If the growth process can be further optimized to produce well aligned CNT with a predetermined straight length, helical pitch and helical diameter based on the growth mechanism, the CNT array can be effectively used in electronic devices for practical applications, for examples, in high-performance sensors, resonators, electromagnetic transformers, antennas and inductions at nanoscale.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Granted No. 60908023, 11075143/A050609) and the Key Laboratory of Ultra-Precision Machining Technology Foundation of CAEP(Granted No. ZZ15003). We thank Dr.Jiangfeng WANG, who is now studying at CNRS/CEMS in France, for his interest in our work and help with analysis of CNT Raman spectra.
[1] Zhang M, Fang S L, Zakhidov A A, et al. Strong, transparent, multifunctional, carbon nanotube sheets[J]. Science, 2005, 309(5738): 1215-1219.
[2] Michael F L, De Volder, Sameh H, et al. Carbon nanotubes: Present and future commercial applications[J]. Science, 2013, 339: 535-539.
[3] Pan J Y, Chen C Y, Gao Y L, et al. Improved field emission characteristics of screen-printed CNT-FED cathode by interfusing Fe/Ni nano-grains[J]. Displays, 2009, (30): 114-118.
[4] XU Yao, ZHAN Liang, WANG Yun, et al. Fluorinated graphene as a cathode material for high performance primary lithium ion batteries[J]. New Carbon Materials, 2015, 30(1): 79-85.
[5] Phaedon Avouris. Carbon nanotube electronics[J]. Chemical Physics, 2002, 281(2-3): 429-445.
[6] ZHENG Wei, QI Tao, ZHANG Yong-chao, et al. Fabrication and characterization of a multi-walled carbon nanotube-based counter electrode for dye-sensitized solar cells[J]. New Carbon Materials, 2015, 30(5): 391-396.
[7] Nicole Grobert. Carbon nanotubes-becoming clean[J]. Materials Today, 2007, 10 (1-2): 28-35.
[8] Qin Y, Kim Y, Zhang L B. Preparation and elastic properties of helical nanotubes obtained by atomic layer deposition with carbon nanocoils as templates[J], Small, 2010, 6(8): 910-914.
[9] Iijima S. Helical microtubules of graphitic carbon[J]. Nature, 1991, (354): 56-58.
[10] Sigeo Ihara, Satoshi Itoh, Jun-ichi Kitakami. Toroidal forms of graphitic carbon[J]. Phys ReV B 1993, 47(19): 12908-12911.
[11] Dunlap B I. Connecting carbon tubules[J]. Phys Rev B, 1992, 46(2): 1933-1936.
[12] Zhang M, Li J. Carbon nanotube in different shapes[J]. Materials Today, 2009, 12(6): 12-18.
[13] Ahmed Shaikjee, Neil J Coville. The synthesis, properties and uses of carbon materials with helical morphology[J]. Journal of Advanced Research, 2012, 3: 195-223.
[14] Szabó A, Fonseca A, Nagy J B . Synthesis, properties and applications of helical carbon nanotubes[J]. Fullerenes, Nanotubes and Carbon Nanostructures, 2005, 13(S1): 139-146.
[15] Tang N J, Wen J F, Zhang Y. Helical carbon nanotubes: Catalytic particle size-dependent growth and magnetic properties[J]. Acs Nano, 2010, 4(1): 241-250.
[16] Prabhakar R B. Electrical properties and applications of carbon nanotube structures[J]. Journal of Nanoscience and Nanotechnology, 2007, 7: 1-29.
[17] Wen J F, Zhang Y, Tang N J, et al. Synthesis, photoluminescence, and magnetic properties of nitrogen doping helical carbon nanotubes[J]. J Phys Chem C, 2011, 115: 12329-12334.
[18] Qi X S, Zhong W, Deng Y, et al. Characterization and magnetic properties of helical carbon nanotubes and carbon nanobelts synthesized in acetylene decomposition over Fe-Cu nanoparticles at 450oC[J]. J Phys Chem C, 2009, 113: 15934-15940.
[19] R Byron Pipes, Pascal Hubert. Helical carbon nanotube arrays: mechanical properties[J]. Composites Science and Technology, 2002, 62(3): 419-428.
[20] Philip G C, Phaedon A. Nanotubes for Electronics[J]. Scientific American, 2000: 62-69.
[21] Kong J, Zhou C, Morpurgo A. Synthesis, integration, and electrical properties of individual single-walled carbon nanotubes[J]. Appl Phys A, 1999, 69: 305-308.
[22] Moretadha J K, Jaber S A, Fyath R S. Performance investigation of loop and helical carbon nanotube antennas[J]. Journal of Emerging Trends in Computing and Information Sciences, 2012, 3(12): 1606-1613.
[23] Itoh S, Ihara S, Kitakami J. Toroidal form of carbon C360[J]. Phys ReV B, 1993, 47(3): 1703-1704.
[24] Itoh S, Ihara S, Kitakami J. Helically coiled cage forms of graphitic carbon[J]. Phys Rev B, 1993, 48(8): 5643-5647.
[25] Itoh S, Ihara S. Toroidal forms of graphitic carbon II Elongated tori[J]. Phys Rev B, 1993, 48(11): 8323-8328.
[26] Zhang X B, Zhang X F, Bernaerts D, et al. The texture of catalytically grown coil-shaped carbon nanotubules[J]. Europhys Lett, 1994, 27: 141-146.
[27] Qin Y H, Zhang Y H, Sun X. Synthesis of helical and straight carbon nanofibers by chemical vapor deposition using alkali chloride catalysts[J]. Microchim Acta, 2009, 164: 425-430.
[28] Zhang Q, Zhao M Q, Tang D M. Carbon-nanotube-array double helices[J]. Angew Chem Int Ed, 2010, 49: 3642-3645.
[29] Bajpai V, Dai L M, Ohashi T. Large-scale synthesis of perpendicularly aligned helical carbon nanotubes[J]. J Am Chem Soc, 2004, 126: 5070-5071.
[30] Rao A M, Richter E, Bandow S, et al. Diameter-selective raman scattering from vibrational modes in carbon nanotubes[J]. Science, 1997, (275): 187-191.
[31] H Kuzmany, W Plank, M Hulman, et al. Determination of SWCNT diameters from the Raman response of the radial breathing mode[J]. The European Physical Journal B, 2001,22: 307-320.
[32] Devin Conroy, Anna Moisala, Silvana Cardoso, et al. Carbon nanotube reactor: Ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up[J]. Chemical Engineering Science, 2010, (65): 2965-2977.
[33] Kalpana Awasthi, Rajesh Kumar, Himanshu Raghubanshi. Synthesis of nano-carbon (nanotubes, nanofibres, graphene) materials[J]. Bull Mater Sci, 2011, 34(4): 607-614.
[34] Li X S, Cao A Y, Jung Y J, et al. Bottom-up growth of carbon nanotube multilayers: unprecedented growth[J]. Nano Lett, 2005, 5(10): 1997- 2000.
1007-8827(2016)06-0568-06
垂直定向螺旋碳纳米管阵列的大量合成
张继成1, 唐永建1, 易 勇2, 马康夫1, 周民杰1, 吴卫东1, 王朝阳1
(1.中国工程物理研究院 激光聚变研究中心,四川 绵阳621900;2.西南科技大学 材料科学与工程学院,四川 绵阳621010)
以二甲苯作为碳源、二茂铁作为催化剂前驱体,采用催化裂解法大规模合成了具有不同螺距和螺旋直径、垂直于基底生长的碳纳米管阵列。通过拉曼光谱和高分辨透射电镜测试分析,结果表明,所制备的碳纳米管阵列分布均匀、石墨化程度高,且沿其长度方向具有不同的螺距和螺旋直径。由于在碳纳米管的生长过程中,会伴随着碳五环、碳七环与碳六环的生成,而碳六环是形成石墨晶格的基本结构单元。当碳六环网络结构中出现碳五环和碳七环时,螺旋形的碳纳米管就会形成。实验中螺旋形碳纳米管的产率约为4.5 mg/cm2·h。螺旋形碳纳米管在高性能传感器、谐振器、纳米机械弹簧、电感等纳米电子器件中具有潜在的应用价值。
螺旋形碳纳米管; 螺距; 碳六环
TQ127.1+1
A
国家自然科学基金(60908023, 11075143/A050609);中国工程物理研究院超精密加工技术重点实验室基金(ZZ15003).
张继成,博士,副研究员.E-mail: zhangjccaep@126.com
Foundationitem: National Natural Science Foundation of China (60908023, 11075143/A050609); Key Laboratory of Ultra-Precision Machining Technology Foundation of CAEP (ZZ15003).
ZHANG Ji-cheng, Ph. D. E-mail: zhangjccaep@126.com English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
10.1016/S1872-5805(16)60032-X
猜你喜欢
杂志排行
新型炭材料的其它文章
- 石墨烯/聚合物复合材料的研究进展及其应用前景
- Preparation and electrochemical performance of a polyaniline-carbon microsphere hybrid as a supercapacitor electrode
- 反相乳液法无乳化剂制备炭微米球及其电化学性能
- 球形“花”状结构MoS2/石墨烯锂离子电池负极材料及其电化学行为
- 纳米MnO2/膨胀石墨复合材料的制备及其电化学性能
- Microstructures of carbon nanoscrolls characterized by polarized micro-Raman spectroscopy
