超疏水表面在提高镁合金耐蚀性能上的研究进展
2017-01-06
(西南技术工程研究所,重庆 400039)
环境效应与防护
超疏水表面在提高镁合金耐蚀性能上的研究进展
佘祖新,牟献良,王玲,何建新
(西南技术工程研究所,重庆 400039)
总结和讨论了超疏水表面提高镁合金耐蚀性能的制备方法。采用电化学测试方法研究了超疏水表面的抗腐蚀行为。总结了在镁合金上制备抗腐蚀性超疏水表面的最新进展,提出了超疏水表面提高镁合金耐蚀性能的机理。镁合金上制备超疏水表面的方法较多,但是能进行大规模生产的较少,且制备的超疏水表面耐久性、机械稳定性较差。
镁合金;超疏水;腐蚀
镁及镁合金作为最轻的结构金属材料,具有多种优良性能,如低密度、高比强度、高尺寸稳定性、良好的加工性能及高回收率等[1—7]。由于镁元素具有极低的标准电极电位,这导致镁合金极易遭受腐蚀,尤其在溶液介质和潮湿环境中。较差的耐蚀能力严重地限制了镁合金的广泛应用,因此,如何提高镁合金的耐蚀性能力,并进而扩大其工程应用范围一直备受关注。
超疏水表面是指水接触角大于 150°,滚动角小于 10°的一类表面[8—9],因其独特的性能,如自清洁[10—11]、防污[12]、抗腐蚀[13—14]及油水分离[15—16]等,引了广大研究者的兴趣。通常,制备超疏水表面需要两个步骤:一是创建分级的微纳米复合结构;二是降低材料表面能[17—18]。目前制备超疏水表面主要有:化学刻蚀法[19]、溶胶凝胶技术[20]、溶液浸泡法[21]、水热法[22]、胶体模板法[23]、等离子体刻蚀[24]、化学气相沉积[25]和电沉积[26—28]等。
自2007年Liang等[29]第一次报道利用微弧氧化和化学修饰结合的方法在镁合金上制备超疏水表面以来,利用超疏水表面来改善镁合金耐蚀性能的研究引起了研究者的浓厚兴趣。目前,国内外在该领域已开展了大量工作,并取得了显著的进展。为此,文中总结了近年来在镁合金表面制备具有耐蚀性能的超疏水表面的研究成果,对超疏水表面的抗腐蚀机理进行了总结、分析与讨论,并对该研究方向进行了展望。
1 镁合金上抗腐蚀超疏水表面制备
由于镁及镁合金因其标准电极电位低,化学活性很高,因此相对于其他相对惰性的基体而言,在镁合金上制备超疏水表面通常有两种途径:一是利用其高活性直接在镁合金表面制备超疏水表面;二是先构建中间过渡保护层,再在镁合金上制备超疏水表面。
1.1 溶液浸泡法
溶液浸泡法是一种简单、高效和易实施的超疏水制备方法,主要是利用镁合金的高化学活性,直接浸泡到含特定溶质的溶液中,通过自发的化学/电化学反应制备超疏水表面。Ishizaki团队[30]利用溶液浸泡法在AZ31镁合金上制备了具有微纳米复合结构的氧化铈膜层,再采用含氟硅烷修饰得到超疏水膜层,其静态接触角为155°±2°,滚动角小于10°,并使用电化学方法研究了该膜层在5.0% NaCl溶液中的腐蚀行为。结果表明,具有超疏水膜层的镁合金自腐蚀电位发生正移(-1.50 V→-1.30 V),腐蚀电流密度降低了一个数量级,说明该超疏水膜层能显著提高镁合金的耐蚀能力。在随后的研究[31]中发现,利用含氟硅烷涂覆氧化铈膜层获得的超疏水膜层具有更好的抗腐蚀性能,在NaCl溶液中浸泡24 h后,具有超疏水膜层的镁合金低频区阻抗模量比基体镁合金高出5个数量级,说明超疏水膜层能为镁合金提供良好的保护,如图1所示。
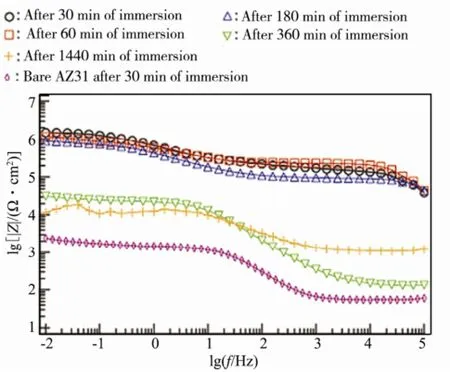
图1 AZ31基体和超疏水表面在5% NaCl溶液里浸泡不同时间的Bode图谱Fig.1 Bode plots of the bare AZ31 substrate and the superhydrophobic surface after immersion in 5% NaCl for different times
1.2 水热法
水热法是指利用高温高压,使试样与水溶液发生特定的水热反应,因其特殊的反应环境,可以得到许多常规环境下无法获取的结构。Wang等[32]采用水热法在AZ31镁合金上制备了具有微纳米花状结构的超疏水表面。制备过程为:先将镁合金基体浸泡在含有一定比例的 Mg(NO3)2·6H2O 和Al(NO3)3·9H2O溶液的反应釜中,在398 K下反应12 h原位生成MgO粗糙结构,再用长链硅烷疏水化处理制得超疏水表面。交流阻抗谱显示,该表面能对在 3.5% NaCl溶液中的镁合金起到良好的腐蚀保护作用,如图2所示。

图2 镁合金及超疏水表面在3.5% NaCl溶液中的EIS图谱Fig.2 EIS plots of Mg alloy and the superhydrophobic surface in 3.5% NaCl solution
Wang等[33]利用水热法在 AZ91D镁合金上制备了接触角为155°、滚动角约为2°的超疏水膜层,并利用电化学方法研究了超疏水膜层在 Hank’s溶液中的腐蚀行为。实验结果表明,经过超疏水化处理的镁合金,其自腐蚀电位从-1741 mV提高到了-1048 mV,腐蚀电流密度降低了2个数量级,显著地提高了镁合金的耐蚀能力,如图3所示。
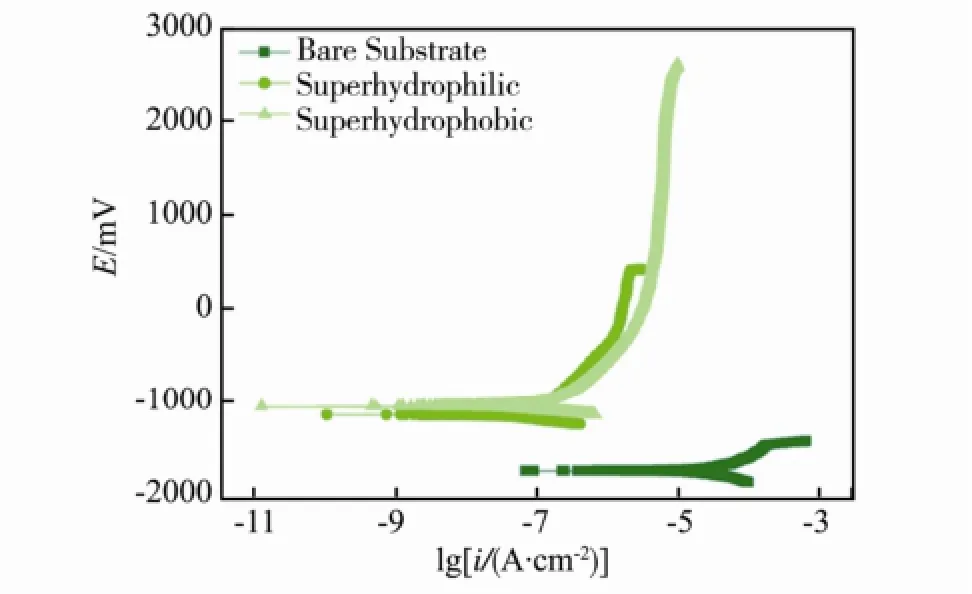
图3 镁合金基体与超疏水表面在Hank′s溶液中极化曲线Fig.3 Potentiodynamic curves of substrate and superhydrophobic surface in Hank′s solution
Li研究小组[34]采用一步水热法在 AZ31镁合金上制备了牡丹花状的超疏水 Ni(CH3(CH2)16-COO)2表面,将镁合金浸泡在含有硫酸镍的硬脂酸乙醇溶液的反应釜中,150 ℃下反应 8 h。最后获得的超疏水表面接触角为156.7°,并具有良好的化学稳定性和长期稳定性。在3.5% NaCl溶液中,该表面能为镁合金提供良好的腐蚀保护。
Zhang等[35]采用水热法在AZ31镁合金上构建Mg(OH)2粗糙结构再硬脂酸疏水化处理制备超疏水表面。通过超疏水表面在NaCl溶液中的电化学测试,该表面能为镁合金基体良好的腐蚀防护。
Wan和Hu[36]采用一步水热法在ZK60镁合金表面制备了具有康乃馨花瓣状粗糙结构的超疏水表面,如图4所示。其接触角为158.5°,滚动角为2.0°。动电位极化曲线测试结果表明,超疏水表面能较大程度提高镁合金在 3.5% NaCl溶液中的耐蚀性能。
Gao和Liu等[37]以水热法在AZ31镁合金上构建出微纳米的分级结构,再 FAS疏水化处理制备超疏水膜层。该膜层能够在3.5% NaCl溶液中为镁合金基体提供良好的腐蚀保护。

图4 超疏水表面微观形貌及接触角、滚动角Fig.4 Surface morphologies, contact angle and sliding angle of superhydrophobic surface
1.3 电沉积法
电沉积法是指利用电化学反应,在基体表面沉积各类物质的材料制备方法。Xu等[38]报道了一种利用电解加工技术侵蚀镁合金表面,以获得具有粗糙结构的腐蚀产物膜层,再使用含氟硅烷修饰,最终制得具有抗腐蚀效应的超疏水表面。Wang和Li等[39]采用电沉积方法在AZ91D镁合金表面制备了微纳米结构的超疏水表面,其制备流程如图 5所示。制得的超疏水表面具有圆柱状的微纳米结构,其接触角为 154°,如图 6所示,同时,该超疏水表面还具有显著的自清洁效应和良好的抗耐蚀性能,如图7和图8所示。
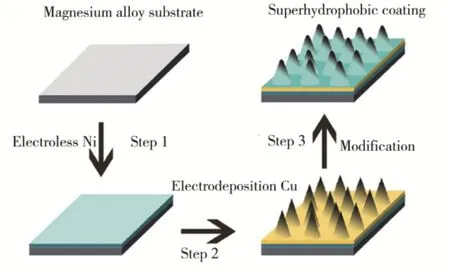
图5 电沉积法制备超疏水表面的流程Fig.5 Schematic illustration for fabricating the superhydrophobic surface by electrodeposition

图6 电沉积法制备超疏水表面的微观形貌Fig. 6 Surface morphologies of the superhydrophobic surface prepared by electrodeposition

图7 电沉积法制备的超疏水表面的在不同腐蚀介质中的极化曲线Fig.7 Self-cleaning effect experiments and potentiodynamic polarization curves of the superhydrophobic coatings prepared by electrodeposition in different corrosive media

图8 电沉积法制备的超疏水表面的自清洁效应Fig.8 Self-cleaning effect of the superhydrophobic surface prepared by electrodeposition
She和Li等[40]采用电沉积方法,在AZ91D镁合金表面制备了具有羽毛状结构的超疏水 CuO表面。该表面对水的接触角高达 155°±1.3°,滚动角低于3°。电化学测试结果表明,超疏水CuO表面能为 3.5% NaCl溶液中的镁合金提供良好的腐蚀保护作用。附着力测试显示,超疏水表面具有良好的机械稳定性(如图9所示),说明超疏水镁合金具有足够的稳定性,能够在更多的环境被使用。

图9 超疏水CuO表面胶带测试的数码照片Fig.9 The digital images of tape test of the CuO superhydrophobic surface
She等[41]采用电沉积Ni镀层的方法在AZ91D镁合金上制备了一种耐磨、抗腐蚀的自清洁超疏水镍表面。该超疏水表面具有高达163.3°±0.7°的接触角和极低的滚动角(1.2°±0.9°),制备过程操作简单,易于实施。超疏水Ni表面具有良好的长期稳定性和良好的抗腐蚀效应。此外,Li和她的团队[42]采用电沉积方法在AZ91D镁合金上制备的超疏水Ni-Co表面,不仅具有高度的抗腐蚀性能,且具有良好的耐磨擦性能和自清洁效应,其接触角高达167.3°±1.3°,滚动角低于 1°。Kang等[43]以Ce(NO3)3·6H2O和肉豆蔻酸乙醇溶液作为电镀液,在MB8镁合金上电沉积Ce(CH3(CH2)12COO)3制备了接触角为159.8o、滚动角为1o的超疏水表面,其能为NaCl溶液中的镁合金提供良好的腐蚀保护。该团队[44]还采用电沉积法在 AZ31镁合金上构建了耐蚀性能优异、机械稳定性良好的超疏水表面。Kang团队[45]采用电沉积法在 Mg-Mn-Ce镁合金上制备了接触角高达159.8°,滚动角低于 2°的超疏水表面,并采用电化学方法研究了超疏水表面在3.5% NaCl,Na2SO4,NaClO3,NaNO3溶液中的腐蚀行为。电化学测试结果显示,超疏水表面具有良好的抗腐蚀效应,能为镁合金提供良好的腐蚀保护。
1.4 微弧氧化法
康志新等[46]采用微弧氧化法在 Mg-Mn-Ce 镁合金上构筑蜂窝状微弧氧化膜层,在有机镀膜制得接触角高达 173.3°的超疏水复合膜层。该膜层在3.5% NaCl 溶液中,与基体相比,动电位极化腐蚀电流密度降低了3个数量级,电化学阻抗提高了3个数量级(如图10所示),耐腐蚀性能明显改善,说明超疏水复合膜层能够显著提高镁合金的耐腐蚀性能。

图10 经不同处理后的Mg-Mn-Ce镁合金在NaCl溶液中的动电位极化曲线和Bode图谱Fig.10 Polarization curves and Bode plots of different treated Mg-Mn-Ce alloys in NaCl solution
1.5 自组装法
王燕华等[47]采用自组装方法,在镁基体上制备了一层超疏水的硬脂酸表面。该表面能在一定程度上抑制镁的局部腐蚀。
1.6 其他
黄艳萍[48]等人以AZ31镁合金为基体,在硫酸中刻蚀以构建出微米级及亚微米级的网纹沟槽粗糙结构,再电沉积十四烷酸获得接触角高达 160°的超疏水表面。该超疏水表面在 3.5%的 NaCl 溶液中,自腐蚀电位提高了95 mV,腐蚀电流密度和腐蚀速率则大幅度减小,表现出良好的耐腐蚀性。
Yang等[49]采用相分离法成功地在AZ91D镁合金上制备了超疏水的PVC膜层。具体制备过程为:在30 ℃将一定量的PVC溶解在THF中获得均匀的 PVC-THF溶液;采用浸渍提拉法将 PVC-THF溶液涂到经阳极氧化处理的镁合金表面制得超疏水膜层。探讨了超疏水膜层在3.5% NaCl,0.6 mol/L Na2SO4,pH=13的NaOH溶液和pH=3的H2SO4溶液中的腐蚀行为。电化学测试结果显示,超疏水PVC膜层对镁合金具有良好的防腐作用。
2 超疏水表面的抗腐蚀效应机理
2.1 Wenzel模型
在1936年,Wenzel[50—51]研究了表面粗糙度对表面润湿性的影响,提出当液体与固体表面接触时,液滴将完全充满粗糙表面的缝隙的Wenzel模型,如图11a所示,用式(1)表示:

式中:θr为粗糙表面的平衡接触角;r为粗糙度常数,指实际的固-液界面接触面积与表观固-液界面接触面积之比,也即固体的真实表面积与表观表面积之比。根据Wenzel方程可知,对于亲水表面(θ< 90°),当表面的粗糙度增加后表面将变得更亲水,而疏水表面(θ> 90°)则更疏水,甚至达到超疏水状态(θ> 150°)。

图11 水滴在固体表面的接触模型Fig.11 Contact models of water drops on solid surface
2.2 Cassie-Baxter模型
Wenzel方程只适用于液滴与固体表面完全接触的情况,当固体表面粗糙度很高时,液体不仅与固体发生接触,还会部分与气体接触的情况时则不适用。Cassie和Baxter[52]进一步拓展了Wenzel方程,提出复合接触的概念,即将粗糙度不均匀的固体表面设想为一个复合平面,液滴在粗糙表面的接触是一种复合接触,如图11b所示。
Cassie和 Baxter从热力学角度分析得到了适合任何复合表面接触的Cassie-Baxter方程:

式中:θr为复合表面的接触角;θ1,θ2分别为两种介质上的本征接触角;f1,f2分别为这两种介质在表面的面积分数,f1+f2= 1。当液体与空气的接触角为180°时,式(2)可进一步简化为:

相对于 Wenzel模型,Cassie-Baxter模型更能真实地反映液体与粗糙表面的接触情况。这两种模型都可以是超疏水状态的一种形式。处于 Wenzel模型的超疏水表面,其疏水性较低,水滴易于渗透到表面的粗糙结构中,而处于 Cassie-Baxter模型的超疏水表面,其疏水性高,水滴难以渗入到表面的粗糙结构中将空气挤压出去。
2.3 抗腐蚀机理分析
对于超疏水表面的抗腐蚀效应机理已经得到了广泛的研究,目前普遍认为超疏水表面与腐蚀介质界面间存在的空气是其具有优良的耐蚀能力的关键。与其他膜层或镀层相同,超疏水表面也是通过充当传质和电荷转移的壁垒来抑制腐蚀反应的发生。在溶液介质中,超疏水表面的凹槽中的捕获空气可以防止溶液与膜层直接接触,充当绝佳的传质和电荷转移壁垒[53—54],可以有效提高膜层的耐腐蚀能力。在大气环境中,超疏水化的表面使得表面难以形成连续的电解质膜,可以有效抑制微区电化学腐蚀反应的发生,从而提高基体的耐蚀能力。
3 抗腐蚀性超疏水表面的发展方向
3.1 提高抗腐蚀性超疏水表面的机械稳定性及化学稳定性
材料在使用过程中,会不可避免地受到外界环境的冲击或摩擦等机械作用。目前,已有的超疏水表面大多机械强度不高,导致机械稳定性很差,在超疏水表面受到外力作用时,表面结构会遭受损伤,引起表面疏水性下降,进而降低防护能力[55—59]。此外,这些超疏水表面的化学稳定性较差,在紫外光照、酸、碱等苛刻环境下易被破坏。因此,提高机械稳定性和化学稳定性成为发展耐久性抗腐蚀性超疏水表面的关键。She等[40—41]在镁合金上采用电沉积Ni镀层和Ni-Co镀层的方法制备的超疏水表面,采用摩擦试验的方法探讨了超疏水表面的机械稳定性,如图12所示。以800目的SiC砂纸作为摩擦底面,1200 Pa的承载下以 5 mm/s的速度拖动超疏水表面在砂纸上前进。图13为超疏水表面的接触角、滚动角与摩擦距离的关系,可以看到,在摩擦距离为700 mm时,超疏水表面的滚动角从1.2°±0.9°增加到52.7°±1.4°,接触角仍高于 150°。说明制得的超疏水表面具有良好的机械稳定性,这对扩大镁合金的广泛应用具有重要意义。

图12 摩擦试验Fig.12 Schematic illustration of the scratch test
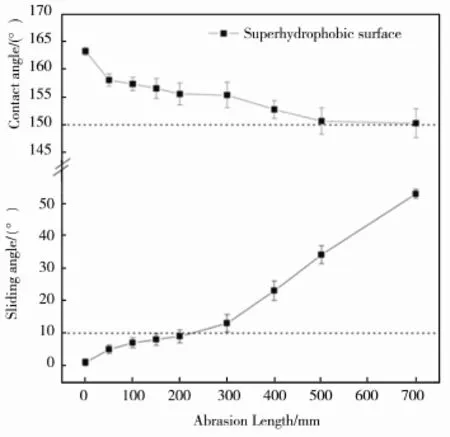
图13 摩擦距离与接触角和滚动角的关系Fig.13 The CA and SA of the superhydrophobic surface as a function of the abrasion length
3.2 开发工艺简单、经济环保的超疏水表面制备方法
目前,已有的在镁合金上制备超疏水表面的报道,绝大多数采用氟代硅烷或者长链硅烷来降低表面能。这些试剂成本较高,并因吸附作用形成的单分子层而具有较差的结合力,以及含氟试剂在生产和使用过程中会在一定程度上对自然环境和人体健康造成危害,而且这类低表面能试剂的修饰过程也较耗时。此外,制备超疏水表面的过程中有的需要特殊的一起设备,难以实现大面积生产。因此,急需开发简单快速、经济环保的超疏水表面制备方法。
4 结语
超疏水表面可以有效提高镁合金耐腐蚀性能,对于扩大镁合金应用范围具有较大应用价值。目前虽然制备超疏水表面的方法较多,但是主要停留在实验室制备,真正能用于镁合金上并进行大规模生产的较少。另一方面,目前制备的超疏水表面的耐久性较差,机械稳定性未获得足够重视。因此,未来的相关工作可以从以下两个方向开展:一是开发工艺简单、经济、环保的制备方法,特别是适合大规模生产的;二是制备的超疏水表面不但要有良好的耐腐蚀性能,还应该具备一定的机械稳定性和化学稳定性,以便真正应用到实际环境中来。
[1] 杨小奎,张涛,张世艳,等. 环氧富锌涂层对 AZ91D镁合金的腐蚀防护能力研究[J]. 装备环境工程,2014, 11(1): 18—23. YANG Xiao-kui, ZHANG Tao, ZHANG Shi-yan, et al. Research on the Corrosion Protection Properties of Zn-rich Epoxy Coating Provided for AZ91D Magnesium Alloy[J]. Equipment Environmental Engineering, 2014, 11(1): 18—23.
[2] 李锦妍, 郝建民, 陈永楠, 等. 镁合金微弧氧化膜层退除工艺[J]. 表面技术, 2015, 44(10):27—32. LI Jin-yan, HAO Jian-min, CHEN Yong-nan, et al. Stripping Process of the Magnesium Alloy Micro-arc Oxidation Coating[J]. Surface Technology, 2015, 44(10):27—32.
[3] 朱青, 朱明, 余勇, 等. AZ91D 镁合金 Mo-Mn 无铬转化膜的制备与耐蚀性[J]. 表面技术, 2015, 44(8): 9—14. ZHU Qing, ZHU Ming, YU Yong, et al. Preparation and Corrosion Resistance of Mo-Mn Chromium-free Conversion Coating Formed on AZ91D Magnesium Alloys[J]. Surface Technology, 2015, 44(8): 9—14.
[4] 贾平平, 马捷, 王晓光, 等. 镁合金表面沉积铜钨复合涂层工艺及涂层性能研究[J].表面技术, 2015, 44(5): 43—47. JIA Ping-ping, MA Jie, WANG Xiao-guang, et al. Process Preparation and Properties of Cu/ W Composite Coatings on the Surface of Mg Alloy[J]. Surface Technology, 2015, 44(5): 43—47.
[5] 董凯辉, 宋影伟, 单大勇, 等. 镁合金微弧氧化技术的研究进展[J]. 表面技术, 2015, 44(3): 74—80. DONG Kai-hui, SONG Ying-wei, SHAN Da-yong, et al. Research Progress of Micro-arc Oxidation Technology on Magnesium Alloys[J]. Surface Technology, 2015, 44(3): 74—80.
[6] 宋辉, 赵明, 何广平, 等. 镁合金磷酸盐 /氮化硅双层复合膜结构及耐蚀性能研究[J]. 表面技术, 2014, 43(5): 71—74. SONG Hui, ZHAO Ming, HE Guang-ping, et al. Study on the Structure and Corrosion-resistant Performance of Phosphate/Silicon Nitride Double Composite Coating on Magnesium Alloy[J]. Surface Technology, 2014, 43(5): 71—74.
[7] 尚伟, 温玉清, 司延举, 等. 镁合金表面磷化/溶胶凝胶复合膜的制备及其耐蚀性[J]. 表面技术, 2014, 43(2): 95—99. SHANG Wei, WEN Yu-qing, SI Ting-jv, et al. Preparation and Corrosion Resistance of Phosphating Sol-Gel Composite Coatings on Magnesium Alloy[J]. Surface Technology, 2014, 43(2): 95—99.
[8] YUAN Z Q, CHEN H, TANG J X, et al. Facile Method to Fabricate Stable Superhydrophobic Polystyrene Surface by Adding Ethanol[J]. Surface and Coatings Technology, 2007, 201: 7138—7142.
[9] PAN Q M, CHENG Y X. Superhydrophobic Surfaces Based on Dandelion-like ZnO Microspheres[J]. Applied Surface Science, 2009, 255: 3904—3907.
[10] PARK Y B, IM H, IM M, et al. Self-cleaning Effect of Highly Water-repellent Microshell Structures for Solar Cell Applications[J]. Journal of Materials Chemistry, 2011, 21: 633—636.
[11] BHUSHAN B, JUNG Y C, KOCH K. Self-cleaning Efficiency of Artificial Superhydrophobic Surfaces[J]. Langmuir, 2009, 25: 3240—3248.
[12] ZHOU Y L, LI M, SU B, et al. Superhydrophobic Surface Created by the Silver Mirror Reaction and Its Drag-reduction Effect on Water[J]. Journal of Materials Chemistry, 2009, 19: 3301—3306.
[13] WANG Z W, LI Q, SHE Z X, et al. Low-cost and Large-scale Fabrication Method for an Environmentally-friendly Superhydrophobic Coating on Magnesium Alloy[J]. Journal of Materials Chemistry, 2012, 22: 4097—4105.
[14] WANG P, ZHANG D, QIU R, et al. Super-hydrophobic Film Prepared on Zinc as Corrosion Barrier[J]. Corrosion Science, 2011, 53: 2080—2086.
[15] YU Q B, TAO Y L, HUANG Y P, et al. Preparation of Porous Polysulfone Microspheres and Their Application in Removal of Oil from Water[J]. Industrial & Engineering Chemistry Research, 2012, 51: 8117—8122.
[16] ZHU Q, PAN Q M, LIU F T. Facile Removal and Collec-tion of Oils from Water Surfaces Through Superhydrophobic and Superoleophilic Sponges[J]. The Journal of Physical Chemistry C, 2011, 115: 17464—17470.
[17] FENG X J, FENG L, JIN M H, et al. Reversible Superhydrophobicity to Superhydrophilicity Transition of Aligned ZnO Nanorod Films[J]. Journal of the American Chemical Society, 2004, 126: 62—63.
[18] SUN T L, WANG G J, FENG L, et al. Reversible Switching between Superhydrophilicity and Superhydrophobicity[J]. Angewandte Chemie-Interntional Edition, 2004, 43: 357—360.
[19] QIAN B T, SHEN Z Q. Fabrication of Superhydrophobic Surfaces by Dislocation-selective Chemical Etching on Aluminum, Copper, and Zinc Substrates[J]. Langmuir, 2005, 21: 9007—9009.
[20] HAN J T, LEE D H, RYU C Y, et al. Fabrication of Superhydrophobic Surface from a Supramolecular Organosilane with Quadruple Hydrogen Bonding[J]. Journal of the American Chemical Society, 2004, 126: 4796—4797.
[21] SONG J L, XU W J, LIU X, et al. Ultrafast Fabrication of Rough Structures Required by Superhydrophobic Surfaces on Al Substrates Using an Immersion Method[J]. Chemical Engineering Journal, 2012, 211—212: 143—152.
[22] WANG S L, WANG C Y, LIU C Y, et al. Fabrication of Superhydrophobic Spherical-like α-FeOOH Films on the Wood Surface by a Hydrothermal Method[J]. Colloids and Surfaces A, 2012, 403: 29—34.
[23] PERNITES R B, SANTOS C M, MALDONADO M, et al. Tunable Protein and Bacterial Cell Adsorption on Colloidally Templated Superhydrophobic Polythiophene Films[J]. Chemistry Materials, 2012, 24: 870—880.
[24] FERNÁNDEZ-BLÁZQUEZ J P, FELL D, BONACCURSO E, et al. Superhydrophilic and Superhydrophobic Nanostructured Surfaces Via Plasma Treatment[J]. Journal of Colloid and Interface Science, 2011, 357: 234—238.
[25] YANG R, ASATEKIN A, GLEASON K K. Design of Conformal, Substrate-independent Surface Modification for Controlled Protein Adsorption by Chemical Vapor Deposition (CVD)[J]. Soft Matter, 2012(8): 31—43.
[26] YE W C, YAN J F, YE Q, et al. Template-free and Direct Electrochemical Deposition of Hierarchical Dendritic Gold Microstructures: Growth and Their Multiple Applications[J]. The Journal of Physical Chemistry C, 2010, 114: 15617—15624.
[27] WANG L, GUO S J, HU X G, et al. Facile Electrochemical Approach To Fabricate Hierarchical Flowerlike Gold Microstructures: Electrodeposited Superhydrophobic Surface[J]. Electrochemistry Communications, 2008, 10: 95—99.
[28] HANG T, HU A M, LING H Q, et al. Super-hydrophobic Nickel Films with Micro-nano Hierarchical Structure Prepared by Electrodeposition[J]. Applied Surface Science, 2010, 256: 2400—2404.
[29] LIANG J, GUO Z G, FANG J, et al. Fabrication of Superhydrophobic Surface on Magnesium Alloy[J]. Chemistry Letters, 2007, 36: 416—417.
[30] ISHIZAKI T, SAITO N. Rapid Formation of a Superhydrophobic Surface on a Magnesium Alloy Coated with a Cerium Oxide Film by a Simple Immersion Process at Room Temperature and Its Chemical Stability[J]. Langmuir, 2010, 26: 9749—9755.
[31] ISHIZAKI T, MASUDA Y, SAKAMOTO M. Corrosion Resistance and Durability of Superhydrophobic Surface Formed on Magnesium Alloy Coated with Nanostructured Cerium Oxide Film and Fluoroalkylsilane Molecules in Corrosive NaCl Aqueous Solution[J]. Langmuir, 2011, 27: 4780—4788.
[32] WANG J, LI D D, LIU Q, et al. Fabrication of Hydrophobic Surface with Hierarchical Structure on Mg Alloy and Its Corrosion Resistance[J]. Electrochimica Acta, 2010, 55: 6897—6906.
[33] WANG Z W, SU Y L, LI Q, et al. Researching a Highly Anti-corrosion Superhydrophobic Film Fabricated on AZ91D Magnesium Alloy and Its Anti-bacteria Adhesion Effect[J]. Materials Characterization, 2015, 99: 200—209.
[34] LI J H, LIU Q, WANG Y L, et al. Formation of a Corrosion-resistant and Anti-icing Superhydrophobic Surface on Magnesium Alloy Via a Single-step Method[J]. Journal of The Electrochemical Society, 2016, 163(5): C213—C220.
[35] ZHANG F, ZHANG C L, SONG L, et al. Fabrication of the Superhydrophobic Surface on Magnesium Alloy and Its Corrosion Resistance[J]. Journal of Materials Science & Technology, 2015, 31: 1139—1143.
[36] WAN H G, HU X F. One-step Solve-thermal Process for the Construction of Anticorrosion Bionic Superhydrophobic Surfaces on Magnesium Alloy[J]. Materials Letters, 2016, 174: 209—212.
[37] GAO R, LIU Q, WANG J, et al. Fabrication of Fibrous Szaibelyite with Hierarchical Structure Superhydrophobic Coating on AZ31 Magnesium Alloy for Corrosion Protection[J]. Chemical Engineering Journal, 2014, 241: 352—359.
[38] XU W J, SONG J L, SUN J, et al. Rapid fabrication of Large-area, Corrosion-resistant Superhydrophobic Mg Alloy Surfaces[J]. ACS Applied Materials & Interfaces, 2011(3): 4404—4414.
[39] WANG Z W, LI Q, SHE Z X, et al. Low-cost and Large-scale Fabrication Method for an Environmentally-friendly Superhydrophobic Coating on Magnesium Alloy[J]. Journal of Material Chemistry, 2012, 22: 4097—4105.
[40] SHE Z X, LI Q, WANG Z W, et al. Novel Method for Controllable Fabrication of a Superhydrophobic CuO Surface on AZ91D Magnesium Alloy[J]. ACS Applied Materials & Interfaces, 2012(4): 4348—4356.
[41] SHE Z X, LI Q, WANG Z W, et al. Researching the Fa-brication of Anticorrosion Superhydrophobic Surface on Magnesium Alloy and Its Mechanical Stability and Durability[J]. Chemical Engineering Journal, 2013, 228: 415—424.
[42] SHE Z X, LI Q, WANG Z W, et al. Highly Anticorrosion, Self-cleaning Superhydrophobic Ni-Co Surface Fabricated on AZ91D Magnesium Alloy[J]. Surface Coatings and Technology, 2014, 251: 7—14.
[43] LIU Q, KANG Z X. One-step Electrodeposition Process to Fabricate Superhydrophobic Surface with Improved Anticorrosion Property on Magnesium Alloy[J]. Materials Letters, 2014, 137: 210—213.
[44] LI W, KANG Z X. Fabrication of Corrosion Resistant Superhydrophobic Surface with Self-cleaning Property on Magnesium Alloy and Its Mechanical Stability[J]. Surface & Coatings Technology, 2014, 253: 205—213.
[45] LIU Q, CHEN D X, KANG Z X. One-Step Electrodeposition Process To Fabricate Corrosion-Resistant Superhydrophobic Surface on Magnesium Alloy[J]. ACS Appl. Mater. Interfaces, 2015(7): 1859—1867.
[46] 康志新, 赖晓明, 王芬, 等. Mg-Mn-Ce 镁合金表面超疏水复合膜层的制备及耐腐蚀性能[J]. 中国有色金属学报, 2011, 21(2): 283—289. KANG Zhi-xin, LAI Xiao-ming, WANG Fen, et al. Preparation of super-hydrophobic duplex-treated film on surface of Mg-Mn-Ce magnesium alloy and its corrosion resistance[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(2): 283—289.
[47] 王燕华, 钟莲, 刘圆圆, 等. 镁基体超疏水表面制备及表征.腐蚀科学与防护技术[J]. 腐蚀科学与防护技术, 2010, 22(4): 329—332. WANG Yan-hua, ZHONG Lian, LIU Yuan-yuan, et al. Preparation and Properties of Superhydrophobic Surface Film on Magnesium Substrate[J]. Corrosion Science and Protection Technology, 2010, 22(4): 329—332.
[48] 黄艳萍, 张友法, 余新泉, 等. 镁合金表面超疏水性的构建及耐腐蚀性分析[J]. 东南大学学报(自然科学版), 2012, 42(5): 915—920. HUANG Yan-Ping, ZHANG You-fa, YU Xin-quan, et al. Fabrication of Superhydrophobic Surface on Magnesium Alloy and Corrosion Resistance Analysis[J]. Journal of Southeast University: Natural Science Edition, 2012, 42(5): 915—920.
[49] YANG N, LI J C, BAI N N, et al. One Step Phase Separation Process to Fabricate Superhydrophobic PVC Films and Its Corrosion Prevention for AZ91D Magnesium Alloy[J]. Materials Science and Engineering B, 2016, 209: 1—9.
[50] WENZEL R N. Resistance of Solid Surfaces to Wetting by Water[J]. Industrial & Engineering Chemistry, 1936, 28: 988—994.
[51] WENZEL R N. Surface Roughness and Contact Angle[J]. The Journal of Physical Chemistry, 1949, 53: 1466 —1467.
[52] CASSIE A B D, BAXTER S. Wettability of Porous Surfaces[J]. Transactions of the Faraday Society, 1944, 40: 546—551.
[53] LIU L J, ZHAO J S, ZHANG Y, et al. Fabrication of Superhydrophobic Surface by Hierarchical Growth of Lotus-leaf-like Boehmite on Aluminum Foil[J]. Journal of Colloid Interface and Science, 2011, 358: 277—283.
[54] HONG Y C, CHO S C, SHIN D H, et al. A Facile Method for the Fabrication of Super-hydrophobic Surfaces and Their Resulting Wettability[J]. Scripta Materialia, 2008, 59: 776—779.
[55] 殷波, 方亮, 唐安琼, 等. 镁合金超疏水表面制备的研究进展[J]. 材料导报, 2011, 25(4): 89—93. YIN Bo, FANG Liang, TANG An-qiong, et al. Research Progress of Superhydrophobic Surface on Magnesium Alloy [J]. Materials Review, 2011, 25(4): 89—93.
[56] 钱鸿昌, 李海扬, 张达威. 超疏水表面技术在腐蚀防护领域中的研究进展[J]. 表面技术, 2015, 44(3): 15—24. QIAN Hong-chang, LI Hai-yang, ZHANG Da-wei. Research Progress of Superhydrophobic Surface Technologies in the Field of Corrosion Protection[J]. Surface Technology, 2015, 44(3): 15-24.
[57] 赵欢, 吕晓璇, 周圣文, 等. 金属防护用超疏水表面主要制备方法及应用研究进展[J]. 表面技术, 2015, 44(12): 49—55. ZHAO Huan, LYU Xiao-xuan, ZHOU Sheng-wen, et al. Research Progress in the Preparation Methods and Applications of the Superhydrophobic Surface for Metal Protection[J]. Surface Technology, 2015, 44(12): 49—55.
[58] 陈小娇, 武卫东, 汪德龙. 超疏水表面抑制结霜研究进展[J]. 表面技术, 2015, 44(2): 87—92. Chen xiao-jiao, wu wei-dong, wang de-long. Research Progress in Anti-frosting Performance of Super-hydrophobic Surfaces[J]. Surface Technology, 2015, 44(2): 87—92.
[59] 丁云飞, 伍彬, 吴会军. 柱状微结构超疏水表面制备及其结霜性能研究[J]. 表面技术, 2015, 44(1): 106—111. DING Yun-fei, WU Bin, WU Hui-jun. Preparation of Super-hydrophobic Surface with Cylindrical Microstructure and the Research of the Surface Frost Characteristics[J]. Surface Technology, 2015, 44(1): 106—111.
Research Progress of Superhydrophobic Surfaces in Improving Corrosion Resistance of Magnesium Alloy
SHE Zu-xin,MOU Xian-liang,WANG Ling,HE Jian-xin
(Southwest Research Institute of Technology and Engineering, Chongqing 400039, China)
The paper aims to summarize and discuss methods for preparing superhydrophobic surfaces on magnesium alloys to enhance their corrosion resistance. Corrosion resistant behaviors on superhydrophobic surfaces were investigated by the electrochemical method. The research progress of fabricating superhydrophobic surfaces with anti-corrosion function on magnesium alloys was summarized and the action mechanism of superhydrophobic surfaces in improving the corrosion resistance was discussed in this paper. Though there are a lot of methods for preparing superhydrophobic surfaces on magnesium alloys, most of them cannot be used for larger-scale production, and as-prepared superhydrophobic surfaces are lack of durability and mechanical stability.
magnesium alloy; superhydrophobic; corrosion
10.7643/ issn.1672-9242.2016.06.021
TJ01;TG174.4
A
1672-9242(2016)06-0120-10
2016-07-18;
2016-08-07
Received:2016-07-18;Revised:2016-08-07
佘祖新(1988—),女,贵州人,硕士,工程师,主要从事环境试验研究工作。
Biography:SHE Zu-xin(1988—), Female, Guizhou, Master, Engineer, Research focus: environmental test.
