先心病中microRNA对DNA甲基化的调控及其应用前景*
2016-12-26桂永浩
杨 倩, 桂永浩, 李 强
(复旦大学附属儿科医院儿科研究所,上海 201102)
先心病中microRNA对DNA甲基化的调控及其应用前景*
杨 倩, 桂永浩△, 李 强△
(复旦大学附属儿科医院儿科研究所,上海 201102)
先天性心脏病; 微小RNA; DNA甲基化
先天性心脏病是最常见的出生缺陷,在活产婴儿中的总体发病率为0.8%[1],居非感染原因死亡率第1位。先心病发病机制至今仍未完全阐明,目前认为主要是由于遗产因素、环境因素单独或者共同作用导致的。近年来,表观遗传学在先心病中的作用越来越被人们所认识。表观遗传学是指在DNA序列不变的情况下,可决定基因表达与否并可稳定遗传下去的调控方式,包括 DNA甲基化、非编码RNA、基因组印记、染色质组蛋白修饰等。DNA甲基化和微小RNA(microRNA,miRNA)是最重要的表观遗传调控机制之一,不仅调节机体正常生长发育,而且可以介导环境因素影响疾病的发生发展。除此以外,miRNA还可作用于DNA甲基化,形成调控网络,影响先心病发生发展。
1 miRNA对心脏发育起着重要作用
miRNA是一类广泛存在于真核细胞中并且高度保守的约22个核苷酸组成的内源性非编码单链小分子RNA。成熟miRNA选择性整合入RNA诱导沉默复合物(RNA-induced silencing complex,RISC)后,靶向结合于mRNA,进而抑制翻译甚至引发mRNA降解。它们广泛参与器官发育、细胞增殖分化、肿瘤发生及心血管疾病等生理和病理过程,近年来其在心脏发育及致病中越来越得到重视。
许多miRNA在心脏发育中起着重要作用。miR-1靶向作用于组蛋白脱乙酰酶4(histone deacetylase 4,HDAC4)、心脏和神经嵴衍生物表达的蛋白2(heart- and neural crest derivatives-expressed protein 2,Hand2)、缝隙连接α1蛋白(gap junction alpha-1 protein, GJA1;又称connexin 43,Cx43)、钾电压门控通道亚家族J成员2(potassium voltage-gated channel subfamily J member 2,KCNJ2)等心脏发育相关基因,促进胚胎干细胞向心肌细胞分化,而miR-133起着相反作用。Zhao等[2]在胚胎鼠心脏过表达miR-1,胚胎于13.5 d死于心肌细胞缺失。Liu 等[3]联合敲除小鼠miR-133a-1 与miR-133a-2,约半数在胚胎期或出生早期发生致死性室间隔缺损(ventricular septal defect,VSD),存活至成年的小鼠也易进展为扩张型心肌病和心力衰竭,进一步实验证实其致病可能与miR-133a靶基因血清反应因子(serum response factor,SRF)和cyclin D2表达增加相关。Myo-miRNAs包括miR-208a、miR-208b和miR-499,它们分别位于Myh7、Myh7b和Myh6的内含子中。在心脏发育过程中,其表达水平与宿主基因表达水平相一致,出生后miR-208a/Myh7表达水平迅速降低,miR-208b/Myh7b和miR-499/Myh6表达水平增加。此外miR-17~92簇、miR-138、miR-218、miR-15家族等对心脏发育都有重要作用。
在法洛四联症(tetralogy of Fallot,TOF)患儿中,Zhang等[4]发现47个表达显著变化的miRNAs,他们认为表达异常的miRNAs可能通过靶向结合有丝分裂活化蛋白激酶(mitogen-activated protein kinase,MAPK)通路基因,导致心室肥厚。在综合征性先心病中,21三体综合征中21号染色体上5种miRNAs在心脏中过表达[5];DiGeorge综合征中,22号染色体部分缺失导致RISC的组分表达缺失[6]。综上所述,散发性先心病和综合征性先心病的发生发展都与miRNAs异常表达有着密切关系。本文总结了与心肌细胞增殖、凋亡,心脏发育和致病相关miRNA,见表1[2,7-25]。
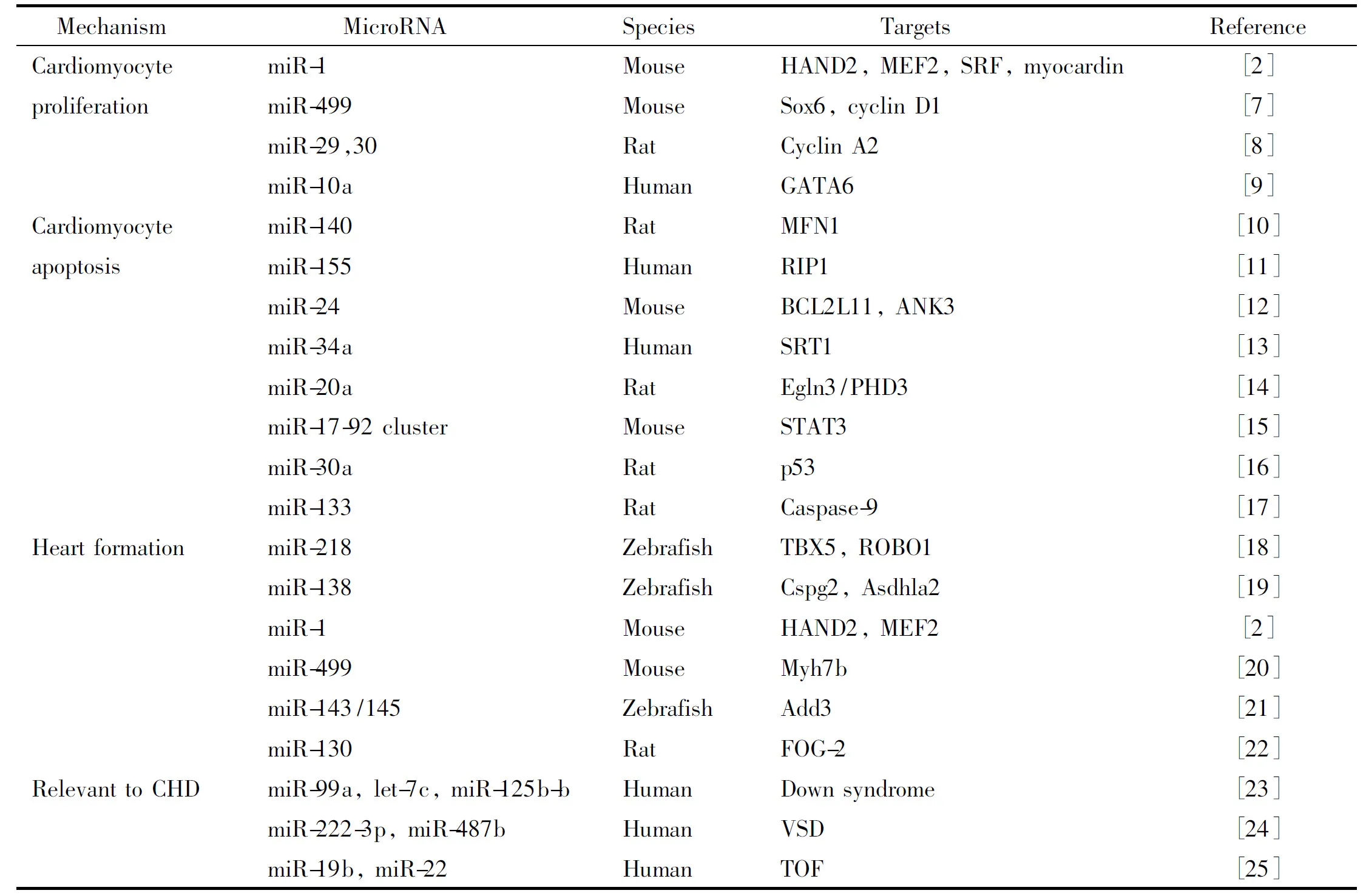
表1 心脏发育相关miRNA
目前研究表明血浆中 miRNAs水平与心脏疾病密切相关,根据其表达特异性,临床上可以将miRNAs作为分子标记物早期诊断或者鉴别诊断疾病[26]。通过拟miRNA技术和抗miRNA反义寡核苷酸(anti-miRNA antisense oligonucleotides,AMO)技术,外源性补充或者抑制miRNA,可在一定程度上发挥治疗作用[27]。
2 DNA甲基化异常导致先心病发生
DNA甲基化是在DNA甲基转移酶(DNA me-thyltransferase,DNMT)的催化下,以S-腺苷蛋氨酸为供体,将甲基转移到胞嘧啶的5位碳原子上。DNA甲基化修饰主要发生在富含CpG岛的启动子区,其高甲基化会阻碍转录激活因子与序列的结合,直接抑制基因表达,而去甲基化则使沉默的基因重新激活,出现高表达甚至基因组的不稳定。DNA甲基化参与动物胚胎发育、基因印迹和X染色体失活等生理过程,也在疾病发生中起重要作用。
许多研究表明,心脏发育与疾病和DNA甲基化密切相关。心肌细胞发育、成熟和疾病过程中DNA甲基化呈动态变化。大鼠出生后心肌细胞DNA甲基化水平开始增高,DNA甲基转移酶1(DNA methyltransferase 1,DNMT1)、甲基化CpG结合结构域蛋白1~3(methyl-CpG-binding domain 1~3,MBD1~3)和甲基化CpG结合蛋白2(methyl-CpG-binding protein 2,MeCP2)的表达也随之增高[28]。用5-氮杂胞苷处理胚胎干细胞,可诱导心肌细胞分化,并增加其DNA合成[29]。与成人相比,新生儿心肌细胞去甲基化程度更明显,其去甲基化区域主要位于心脏转录因子肌细胞增强因子2C(myocyte enhancer factor 2C,MEF2C)、GATA结合蛋白1~4(GATA-binding protein 1~4,GATA1~4)等的结合位点和心肌细胞基因体内。慢骨骼肌肌钙蛋白I(slow skeletal troponin I,ssTnI)基因启动子上游100 bp至2 000 bp间存在CpG岛,其甲基化水平调控ssTnI的表达[30]。Chamberlain等[31]发现DNA甲基转移酶3b调控透明质酸合成酶2(hyaluronan synthase 2,Has2)的表达,从而影响心脏瓣膜的形成。DNA甲基化水平随着心脏发育的阶段、细胞种类和基因的不同,呈现不同的表达模式,其在心脏发育中的作用还需要进一步探索。
叶酸代谢关键酶甲硫氨酸合成酶(methionine synthase,MTR)和胱硫醚β-合酶(cystathionine beta-synthase,CBS)的基因多态性与先心病易感性有关,它们的转录受到甲基化调控,其本身又在甲基代谢中起着关键作用[32]。在TOF患者心脏组织中,心脏相关转录因子NKX2.5和HAND1甲基化状态异常,全基因组甲基化水平相对正常心脏组织下降,DNMT1/3B表达降低,这可能是TOF发病机制之一[33]。在另一项研究中,Yuan等[34]发现TOF患者VANGEL2基因启动子区域甲基化水平显著高于健康对照组,VANGEL2的mRNA表达下降。Serra-Juhe等[35]在2015年发现先心病患者不同组织DNA甲基化有差异,在血标本中,其异常富集于免疫反应相关通路;在心脏组织中,异常富集于肌肉收缩与心肌疾病相关通路。以上研究表明DNA甲基化模式在先心病发病中起着重要作用,异常甲基化可能导致心脏发育畸形。
3 内源性miRNA表达异常影响DNA甲基化水平,导致疾病发生
随着研究的不断深入,人们发现miRNA作为表观遗传的重要内容,参与了DNA甲基化的调控。2007年,Fabbri等[36]首次在肿瘤中发现miR-29a和miR-29b表达下降,相反地,DNMT3a和DNMT3b表达增高,经证实,miR-29与DNMT3a/b的3’-UTR端高度互补,直接导致其表达减少。Garzon等[37]进一步证实在急性髓细胞白血病(acute myelocytic leukemia,AML)细胞系中miR-29b表达增加,直接抑制DNMT3a和DNMT3b,间接抑制DNMT1,诱导基因组DNA低甲基化及抑癌基因p15和雌激素受体1(estrogen receptor 1,ESR1)的表达。
Qin等[38]在系统性红斑狼疮(systemic lupus erythematosus,SLE)患者CD4+T细胞中也证实了miR-29b可通过作用于转录因子Sp1,间接抑制DNMT1的表达。SLE患者CD4+T细胞miR-126表达也升高。miR-148和miR-126都作用于DNMT1,其抑制剂可恢复CD4+T细胞DNMT表达。在SLE患者中miR-126启动子区域低甲基化,表达升高,说明miR-126与DNMT1之间存在反馈机制[39]。
不仅如此,miRNA对DNA甲基化的调控在心血管疾病中也得到证实。在高同型半胱氨酸血症中,miR-133a和miR-499表达下降。用同型半胱氨酸处理心肌细胞HL-1,过表达miR-133a时,DNMT1表达下降;抑制miR-133a时,DNMT1表达升高[40]。Chavali等[41]在糖尿病性心肌病中发现,miR-133a表达减少,DNMT1和DNMT3b表达增加,DNMT3a表达降低。在正常心肌细胞中过表达miR-133a,DNMT1/3a/3b表达都降低。在25 mmol/L葡萄糖培养的心肌细胞中,DNMT1表达增高;转染miR-133a进入细胞,DNMT1表达趋于正常,表明在糖尿病性心肌病中,miR-133调节DNA甲基化的作用。影响心脏发育的miRNA很多,通过TargetScanHuman、PicTar等网站进行预测,发现数种可调控DNMTs表达的miRNA,详见表2。
表2 调控DNMTs的心脏发育相关miRNA
Table 2.DNMTs-modulating miRNAs with relevance to heart development

miRNADNMT1DNMT3ADNMT3BmiR-133a+miR-30+miR-19b+miR-22+miR-34a+miR-101+miR-125b+miR-206+miR-487-3P+miR-29++
大量体内外实验表明,miRNA可通过3种方式调节DNA甲基化状态。(1) miRNA直接结合于DNMTs的3’-UTR影响DNMTs的表达,如miR-29[42]、miR-152[43]等。(2) miRNA直接结合于DNMTs的编码区,影响DNA甲基化水平。在HeLa细胞中,miR-148结合于DNMT3B编码区,而非通常的3’-UTR。表明miR-148在调节DNMT3B剪接变异体的多样性起着调控作用。(3) miRNAs还可通过调节与DNMTs相关的转录因子,间接影响DNA甲基化水平。Dicer缺陷小鼠胚胎干细胞DNMT1表达下降,造成DNA甲基化水平降低。其可能机制为DNMT抑制物RBL2上调,而RBL2为miR-290的靶点。用miR-290转染Dicer敲除的胚胎干细胞,DNMT1表达恢复正常,DNA甲基化水平亦恢复正常,表明在胚胎干细胞miR-290通过间接调节DNMT1表达来调控DNA甲基化[44]。
4 外源性调控miRNA表达,使靶基因异常甲基化状态恢复正常,可干预先心病发生发展
miRNAs与许多疾病的发生发展具有密切联系,它不仅可以直接作用于靶基因,还可以通过影响DNA甲基化水平间接改变靶基因表达水平。miRNA作为药物研发的重要靶点,可设计相应的药物,通过上调或者下调miRNA使靶基因的表达恢复正常。
目前,基于microRNA的分子药物设计尚处于起步阶段,研究主要集中于模拟miRNA(如miRNA mimics)增强其对靶基因的作用效能和拮抗miRNA的小分子物质(如AMO和antagomir)。microRNAs参与DNMTs转录后修饰,也可能通过调节与DNMTs相关的转录因子直接或间接影响DNMTs的表达,从而影响疾病发生发展。在先心病动物模型与病人中已经发现DNMTs表达紊乱导致心脏发育关键转录因子GATA-4、NKX2.5和HAND1甲基化状态异常[33],可通过寻找特异性作用于DNMTs的miRNA,外源性过表达或者低表达miRNA,降低DNMTs导致的甲基化效应,使DNA甲基化维持于正常水平,促使其下游基因表达恢复正常,可能达到治疗疾病和改善预后的目的。
miRNA广泛表达于全身,其靶基因有多个,且其靶基因可受到多个miRNA的调控,miRNA如何靶向改变DNMTs的表达还存在困难。此外,miRNA在体内容易降解,外源性给药后很难维持较好的血药浓度,如何富集于靶器官也需要解决,其有效性和安全性均需进一步证实。
5 miRNA作为药物治疗先心病具有广阔前景,需深入研究探索
综上所述,miRNA对DNA甲基化的调节在先心病致病中起着重要作用。通过外源性调节miRNAs,改变体内致病基因甲基化状态,探索治疗先心病的新路径将成为未来研究的热点。基于miRNA的治疗方法还处在探索阶段,深入研究 miRNA 在先心病中复杂的调控机制,显得尤为重要。随着干预 miRNA 表达技术不断进步,基于 miRNA 的治疗策略将成为先心病治疗的一个重要方向,将其应用于临床具有广阔的前景。
[1] Reller MD, Strickland MJ, Riehle-Colarusso T, et al. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005[J]. J Pediatr, 2008, 153(6):807-813.
[2] Zhao Y, Ransom JF, Li A, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2[J]. Cell, 2007, 129(2):303-317.
[3] Liu N, Bezprozvannaya S, Williams AH, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart[J]. Genes Dev, 2008, 22(23):3242-3254.
[4] Zhang HS, Wu QY, Xu M, et al. Mitogen-activated protein kinase signal pathways play an important role in right ventricular hypertrophy of tetralogy of Fallot[J]. Chin Med J (Engl), 2012, 125(13):2243-2249.
[5] Kuhn DE, Nuovo GJ, Martin MM, et al. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and hearts[J]. Biochem Biophys Res Commun, 2008, 370(3):473-477.
[6] Latronico MV, Catalucci D, Condorelli G. MicroRNA and cardiac pathologies[J]. Physiol Genomics, 2008, 34(3):239-242.
[7] Li X, Wang J, Jia Z, et al. MiR-499 regulates cell proliferation and apoptosis during late-stage cardiac differentiation via Sox6 and cyclin D1[J]. PLoS One, 2013, 8(9):e74504.
[8] Zhang Y, Matsushita N, Eigler T, et al. Targeted microRNA interference promotes postnatal cardiac cell cycle re-entry[J]. J Regen Med, 2013, 2:2.
[9] Mollova M, Bersell K, Walsh S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans[J]. Proc Natl Acad Sci U S A, 2013, 110(4):1446-1451.
[10]Li J, Li Y, Jiao J, et al. Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis[J]. Mol Cell Biol, 2014, 34(10):1788-1799.
[11]Liu J, van Mil A, Vrijsen K, et al. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1[J]. J Cell Mol Med, 2011, 15(7):1474-1482.
[12]王 珏, 黄伟聪, 郑亮承,等. MicroRNA-24对心肌梗死后心肌细胞凋亡的调控作用[J].中国病理生理杂志,2013,29(4):590-596.
[13]Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis[J]. Proc Natl Acad Sci U S A, 2008, 105(36):13421-13426.
[14]Frank D, Gantenberg J, Boomgaarden I, et al. Micro-RNA-20a inhibits stress-induced cardiomyocyte apoptosis involving its novel target Egln3/PHD3[J]. J Mol Cell Cardiol, 2012, 52(3):711-717.
[15]Du W, Pan Z, Chen X, et al. By targeting Stat3 microRNA-17-5p promotes cardiomyocyte apoptosis in response to ischemia followed by reperfusion[J]. Cell Physiol Biochem, 2014, 34(3):955-965.
[16]Forini F, Kusmic C, Nicolini G, et al. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis[J]. Endocrinology, 2014, 155(11):4581-4590.
[17]Xu C, Hu Y, Hou L, et al. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression[J]. J Mol Cell Cardiol, 2014, 75:111-121.
[18]Chiavacci E, Dolfi L, Verduci L, et al. MicroRNA 218 mediates the effects of Tbx5a over-expression on zebrafish heart development[J]. PLoS One, 2012, 7(11):e50536.
[19]Morton SU, Scherz PJ, Cordes KR, et al. microRNA-138 modulates cardiac patterning during embryonic development[J]. Proc Natl Acad Sci U S A, 2008, 105(46):17830-17835.
[20]Wilson KD, Hu S, Venkatasubrahmanyam S, et al. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499[J]. Circ Cardiovasc Genet, 2010, 3(5):426-435.
[21]Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury[J]. Genes Dev, 2009, 23(18):2166-2178.
[22]Kim GH, Samant SA, Earley JU, et al. Translational control of FOG-2 expression in cardiomyocytes by microRNA-130a[J]. PLoS One, 2009, 4(7):e6161.
[23]Latronico MV, Catalucci D, Condorelli G. MicroRNA and cardiac pathologies[J]. Physiol Genomics, 2008, 34(3):239-242.
[24]Li D, Ji L, Liu L, et al. Characterization of circulating microRNA expression in patients with a ventricular septal defect[J]. PLoS One, 2014, 9(8):e106318.
[25]Zhu S, Cao L, Zhu J, et al. Identification of maternal serum microRNAs as novel non-invasive biomarkers for prenatal detection of fetal congenital heart defects[J]. Clin Chim Acta, 2013, 424:66-72.
[26]Fang L, Ellims AH, Moore XL, et al. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy[J]. J Transl Med, 2015, 13:314.
[27]郭 敏, 李育敏, 费 嘉, 等.以microRNA-21为靶标的反义寡核苷酸对人白血病K562细胞的抑制作用[J].中国病理生理杂志,2009,25(6):1127-1131.
[28]Kou CY, Lau SL, Au KW, et al. Epigenetic regulation of neonatal cardiomyocytes differentiation[J]. Biochem Biophys Res Commun, 2010, 400(2):278-283.
[29]Abbey D, Seshagiri PB. Aza-induced cardiomyocyte differentiation of P19 EC-cells by epigenetic co-regulation and ERK signaling[J]. Gene, 2013, 526(2):364-373.
[30]Xu Y, Liu L, Pan B, et al. DNA methylation regulates mouse cardiac myofibril gene expression during heart development[J]. J Biomed Sci, 2015, 22:88.
[31]Chamberlain AA, Lin M, Lister RL, et al. DNA methylation is developmentally regulated for genes essential for cardiogenesis[J]. J Am Heart Assoc, 2014, 3(3):e000976.
[32]Zhao JY, Qiao B, Duan WY, et al. Genetic variants reducingMTRgene expression increase the risk of congenital heart disease in Han Chinese populations[J]. Eur Heart J, 2014, 35(11):733-742.
[33]Sheng W, Qian Y, Wang H, et al. DNA methylation status of NKX2-5, GATA4 and HAND1 in patients with tetralogy of Fallot[J]. BMC Med Genomics, 2013, 6:46.
[34]Yuan Y, Gao Y, Wang H, et al. Promoter methylation and expression of theVANGL2 gene in the myocardium of pediatric patients with tetralogy of Fallot[J]. Birth Defects Res A Clin Mol Teratol, 2014, 100(12):973-984.
[35]Serra-Juhe C, Cusco I, Homs A, et al. DNA methylation abnormalities in congenital heart disease[J]. Epigene-tics, 2015, 10(2):167-177.
[36]Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B[J]. Proc Natl Acad Sci U S A, 2007, 104(40):15805-15810.
[37]Garzon R, Liu S, Fabbri M, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1[J]. Blood, 2009, 113(25):6411-6418.
[38]Qin H, Zhu X, Liang J, et al. MicroRNA-29b contributes to DNA hypomethylation of CD4+T cells in systemic lupus erythematosus by indirectly targeting DNA methyltransferase 1[J]. J Dermatol Sci, 2013, 69(1):61-67.
[39]Zhao S, Wang Y, Liang Y, et al. MicroRNA-126 regulates DNA methylation in CD4+T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1[J]. Arthritis Rheum, 2011, 63(5):1376-1386.
[40]Chaturvedi P, Kalani A, Givvimani S, et al. Differential regulation of DNA methylation versus histone acetylation in cardiomyocytes during HHcyinvitroandinvivo: an epigenetic mechanism[J]. Physiol Genomics, 2014, 46(7):245-255.
[41]Chavali V, Tyagi SC, Mishra PK. MicroRNA-133a regulates DNA methylation in diabetic cardiomyocytes[J]. Biochem Biophys Res Commun, 2012, 425(3):668-672.
[42]Pandi G, Nakka VP, Dharap A, et al. MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage[J]. PLoS One, 2013, 8(3):e58039.
[43]Miao CG, Yang YY, He X, et al. MicroRNA-152 modulates the canonical Wnt pathway activation by targeting DNA methyltransferase 1 in arthritic rat model[J]. Biochimie,2014,106:149-156.
[44]Benetti R, Gonzalo S, Jaco I, et al. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases[J]. Nat Struct Mol Biol,2008,15(9):998.
(责任编辑: 陈妙玲, 罗 森)
Advances in modulation of microRNA and DNA methylation in congenital heart diseases
YANG Qian, GUI Yong-hao, LI Qiang
(InstituteofPediatrics,Children’sHospitalofFudanUniversity,Shanghai201102,China.E-mail:liq@fudan.edu.cn)
Aberrations in microRNA (miRNA) expression and DNA methylation are causal factors for congenital heart diseases (CHD), which belongs to epigenetic mechanisms. Complex modulation of miRNA on DNA methylation is a critical regulator of gene expression, leading to disease development. The aim of this review is to provide recent progress in the regulation of miRNA and DNA methylation in CHD.
Congenital heart diseases; MicroRNA; DNA methylation
1000- 4718(2016)11- 2101- 06
2016- 04- 18
2016- 08- 16
国家自然科学基金资助项目(No. 81470442)
R363
A
10.3969/j.issn.1000- 4718.2016.11.032
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 021-64931066; E-mail: liq@fudan.edu.cn
