饮酒与心脑血管风险新解读
2016-12-21陈涵
饮酒与心脑血管风险新解读
大量证据表明,适量饮酒有益健康,而长期大量饮酒可增加心脑血管事件的发病率。然而生理学研究显示,酒精对于心血管系统的作用具有双面性:在急性期,中到大量饮酒后数小时可增加心率、导致电-机械延迟、引起纤溶亢进;而在远期,摄入酒精可降低血压并改善凝血功能。饮酒何谓“适量”是个饱受争议的话题,根据美国心脏病协会(AHA)的指南,适量饮酒被定义为饮酒1标准量(啤酒12液盎司约360ml,白酒5液盎司约150ml,烈酒1.5液盎司约45ml,换算为酒精量约15g),女性每天不超过1标准量,男性每天不超过2标准量。然而,最新的研究显示,这一标准略显苛刻了。
2016年3月1日至4日在美国亚利桑那州首府凤凰城召开的AHA心血管流行病学与预防医学2016年会(Epidemiology and Prevention,Lifestyle and Cardiometabolic Health 2016 Scientific Sessions,EPI-Lifestyle 2016)上,来自美国哈佛大学的流行病学专家通过荟萃分析,揭示了中等量饮酒与大量饮酒的人群,在饮酒后急性期与远期的心脑血管事件风险。研究结果发现,中等量饮酒的人群,饮酒后短时间内发生心肌梗死或卒中的风险增高,而饮酒后1d或1周出现心肌梗死或卒中的风险却降低了。大量饮酒的人群则无论是饮酒或数小时、1d或1周,均增加心肌梗死与卒中的风险[1]。
研究人员通过搜索各大数据库,选取了1987年至2015年间共23项饮酒后1周内心肌梗死与脑卒中的研究,其中有16项为病例对照研究,7项为病例交叉研究。研究中12项来自欧洲,6项来自澳大利亚与新西兰,4项来自美国,1项为多国参与。研究共入选29 457例患者,其中包括17 966例心肌梗死患者,2599例缺血性脑卒中患者和1 262例出血性脑卒中患者。研究显示,饮酒对于心血管的保护作用只适用于中等量饮酒的患者,而大量饮酒的患者无论在急性期还是远期均不会有任何保护作用。
例如,每天饮酒达2个标准量的人群较非饮酒者发生心肌梗死的风险是0.67,而每天饮酒达9个标准量的人群,发生心肌梗死的风险为非饮酒者的1.59倍。每天饮酒达2~4个标准量的人群,饮酒24h后心肌梗死和出血性卒中的风险下降30%。习惯性饮酒每周达6个标准量的人群,1周内发生缺血性卒中的风险下降19%。而且,饮酒24h后这一心脑血管保护作用呈现剂量依赖性。
与此相比,每天饮酒达6~9个标准量的人群饮酒后1d发生心肌梗死和卒中的风险是非饮酒者的1.3~2.3倍;每周饮酒9~30个标准量的人群发生心肌梗死和卒中的风险是非饮酒者的2.25~6.2倍。不论饮酒24h内还是24h后,大量饮酒均不能降低心血管事件的发生率。
研究显示,适量饮酒后最初1h发生心肌梗死或脑卒中的风险会短暂升高,但在适量饮酒后1d,该风险不但下降,甚至出现了保护作用,适量饮酒后1d发生心肌梗死或出血性脑卒中的风险较不饮酒的人群均有下降,而适量饮酒后1周还进一步降低了发生缺血性卒中的风险。习惯性中等量饮酒的人群,尽管饮酒后短时间内的心脑血管风险升高,但在后期可起到心脑血管保护的作用。尽管本研究发现起到保护作用的饮酒量比既往的研究(1~2标准量/d×4d/周)要高,大量饮酒对于心脑血管的危害仍然是一致的。
本研究首次报道了饮酒与急性心脑血管事件的风险,告诉人们习惯性适量饮酒的心脑血管保护作用只在饮酒24h后才出现,在饮酒24h内仍可增加心脑血管事件,这可能为患者生活方式教育和急诊医生快速诊断提供新的证据。但遗憾的是,本研究入选的人群主要聚焦在欧美,亚洲、非洲的人群可能因基因不同、生活方式不同和酒的种类不同而有不同结果。我国的酿酒历史悠久,酒文化深入,期待我国的流行病学专家能公布基于较大样本的研究结果,为我们展现饮酒适量的“中国标准”。
[1]Mostofsky E,Chahal H S,Mukamal K J,etal.Alcohol andimmediate risk of cardiovascular events:A systematic review and dose-response meta-analysis[J].Circulation,2016,133(10):979-987.
(陈涵编译)
A 57-year-old man with a structurally normal heart and normal baseline ECG underwent pulmonary vein isolation for atrial fibrillation.An electrophysiology study was then undertaken with an octapolar catheter positioned at the His bundle and an ablation catheter at the mid-right atrium.
At baseline,the sinus cycle length was 890 ms,the AH interval 48 ms,the HV interval 80 ms,and the QRS duration 80 ms.During extrastimulus atrial pacing,the HV interval shortened and the QRS complex widened with a left bundle branch block morphology(Figure 1). Atrial burst pacing at cycle length 330 ms demonstrates progressive shortening of the HV interval and widening of the QRS complex with left bundle branch block morphology(Figure 2A and 2B).On the final 3 beats in the figure,a His bundle electrogram seems after the QRS complex.On termination of pacing,a wide QRS complex tachycardia of identical morphology is noted (Figure 3).What is the mechanism of the tachycardia?
Discussion
Although the baseline ECG is normal,the presence of a slowly conducting accessory pathway is apparent from atrial extrastimulus pacing demonstrating HV interval shortening,QRS widening,and a long A-V interval(Figure 1).Further inferences of the nature of the accessory pathway may also be made.The retrograde right bundle(RB)and His activation preceding the preexcited ventricular complex with left bundle branch block morphology are most consistent with an accessory pathway connection directly into the right bundle branch (atriofascicular pathway or nodofascicular pathway).
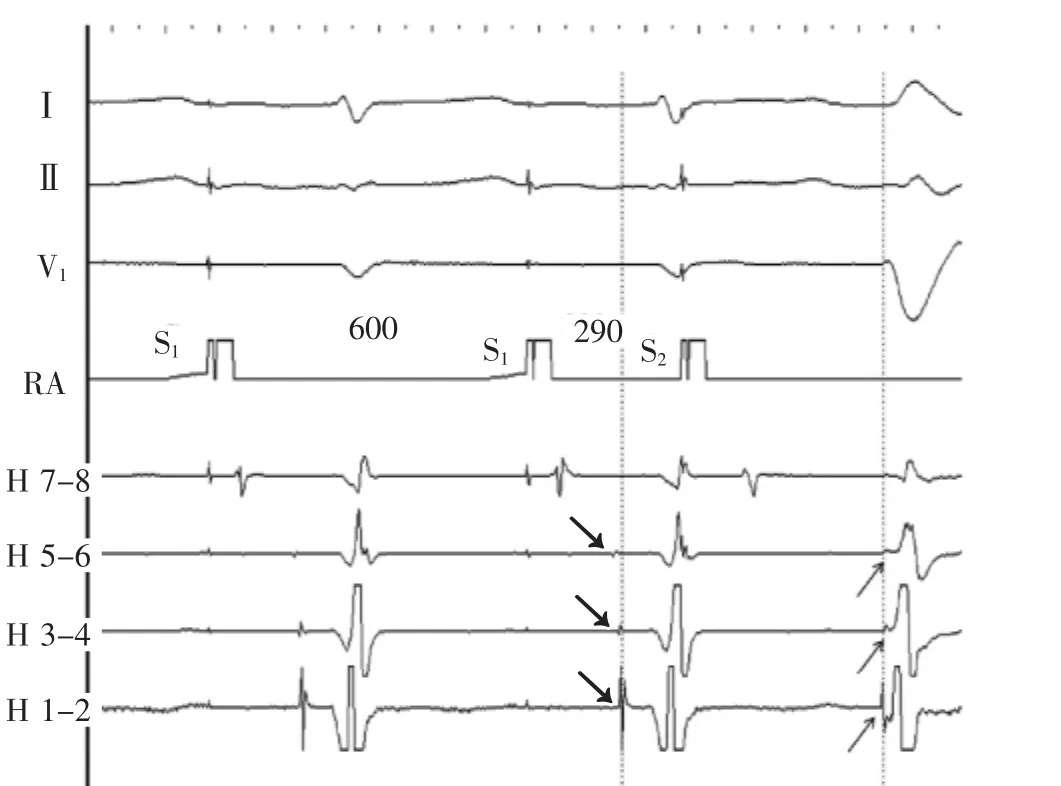
Figure 1Atrial extrastimulus pacing.Following S2,the QRS widens,the HV interval shortens and the His and right bundle activation sequence reverses.Dotted line and arrows provide reference for the anterograde to retrograde activation change.Preexcitation with a long AV interval,left bundle branch block morphology, and right bundle activation preceding ventricular activation suggest an atriofascicular accessory pathway.His 7 to 8 through 1 to 2 His bundle catheter recordings are proximal to distal;pacing intervals in milliseconds are marked.H indicates His;RA,right atrium;S1,drive train stimulus;and S2,extrastimulus.
Similar changes are noted at the onset of atrial burst pacing(Figure 2A).There is evidence of a slowly conducting accessory pathway with shortening of HVinterval and a long A-V interval.The His electrogram precedes the preexcited ventricular complex.Because only the distal electrode records a His electrogram, retrograde versus anterograde His activation cannot be discerned on this tracing.The H and V relationship changes further on the sixth paced beat when the His bundleelectrogramoccursaftertheventricular electrogram.Having established the presence of an atrio-fascicular(or nodo-fascicular)pathway,the only explanation for the late His is the development of retrograde block in the RB.Most likely,the His activation is still retrograde via transeptal activation of theleftbundlebranch.Thisisrepresented schematically Figure 2B.Less likely,retrograde block in the RB allows for antegrade conduction over an AV node slow pathway and anterograde His activation.
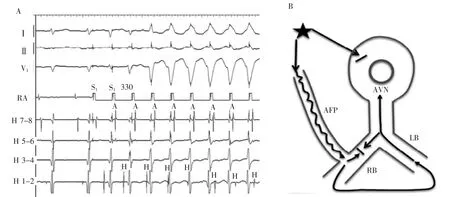
Figure 2A,Right atrial pacing at 330 ms.Note shortening of HV interval and fusion of QRS on the third-paced beat,retrograde His activation via right bundle branch on the fourth-and fifth-paced beats and sudden jump to a V-H relationship on the sixth-paced beat indicating retrograde block in the right bundle branch and conduction via left bundle branch.B,Schematic diagram of the mechanism of changes A,H,and V relationships seen in the last 3 beats in A.Atrial pacing results in anterograde block in AV node,anterograde conduction via atriofascicular pathway,and retrograde RB bock.His activation is retrograde via LB.A indicates atrial,AFP,atriofascicular pathway;AVN,AV node;H,His,LB,left bundle branch;RB,right bundle branch;and V,ventricular.
At termination of the atrial pacing,a left bundle branch block morphology wide QRS tachycardia is initiated(Figure 3).The tachycardia morphology is identical to the pre-excited QRS complex.The His activation is not seen on the first beat of tachycardia but occurs just before the onset of the QRS for the second and third beats.The His recording is seen after the ventricular signal for the last 2 complexes.Changes in the H-H intervals precede changes in the V-V intervals as well as the subsequent A-A intervals,although the relationship is not precise(Figure 4A).The H-A interval shortens on the last 2 beats and accounts for the inexact relationship.
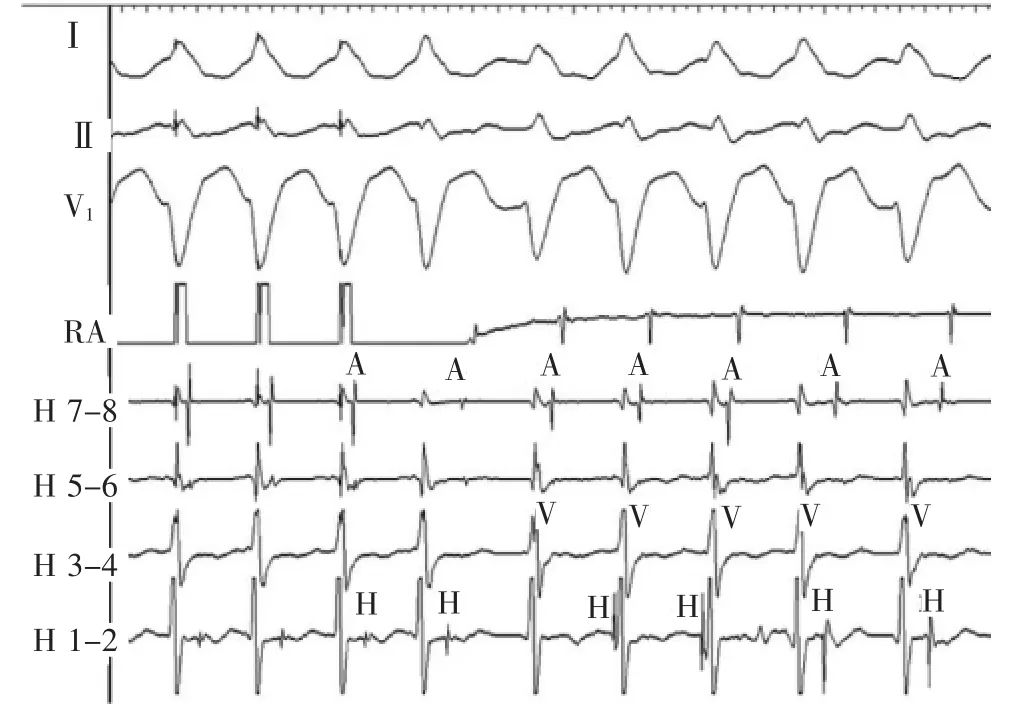
Figure 3Atrial pacing initiates tachycardia with left bundle branch block morphology,variable cycle lengths and variable relationships between the A,H,and V signals.A indicates atrial;H,His;RA,right atrium;and V,ventricular.
The differential diagnosis for the wide QRS complex tachycardia includes ventricular tachycardia,supraventriculartachycardia(SVT)withaberrant conduction,SVT with bystander accessory pathway conduction and preexcited(antidromic)tachycardia. Bundle branch reentry ventricular tachycardia and SVT with aberration are excluded by the short HV interval. Myocardial ventricular tachycardia with retrograde His activation is also excluded by the variable H and V relationshipswithchangesintheH-Hinterval predicting changes in the V-V intervals.
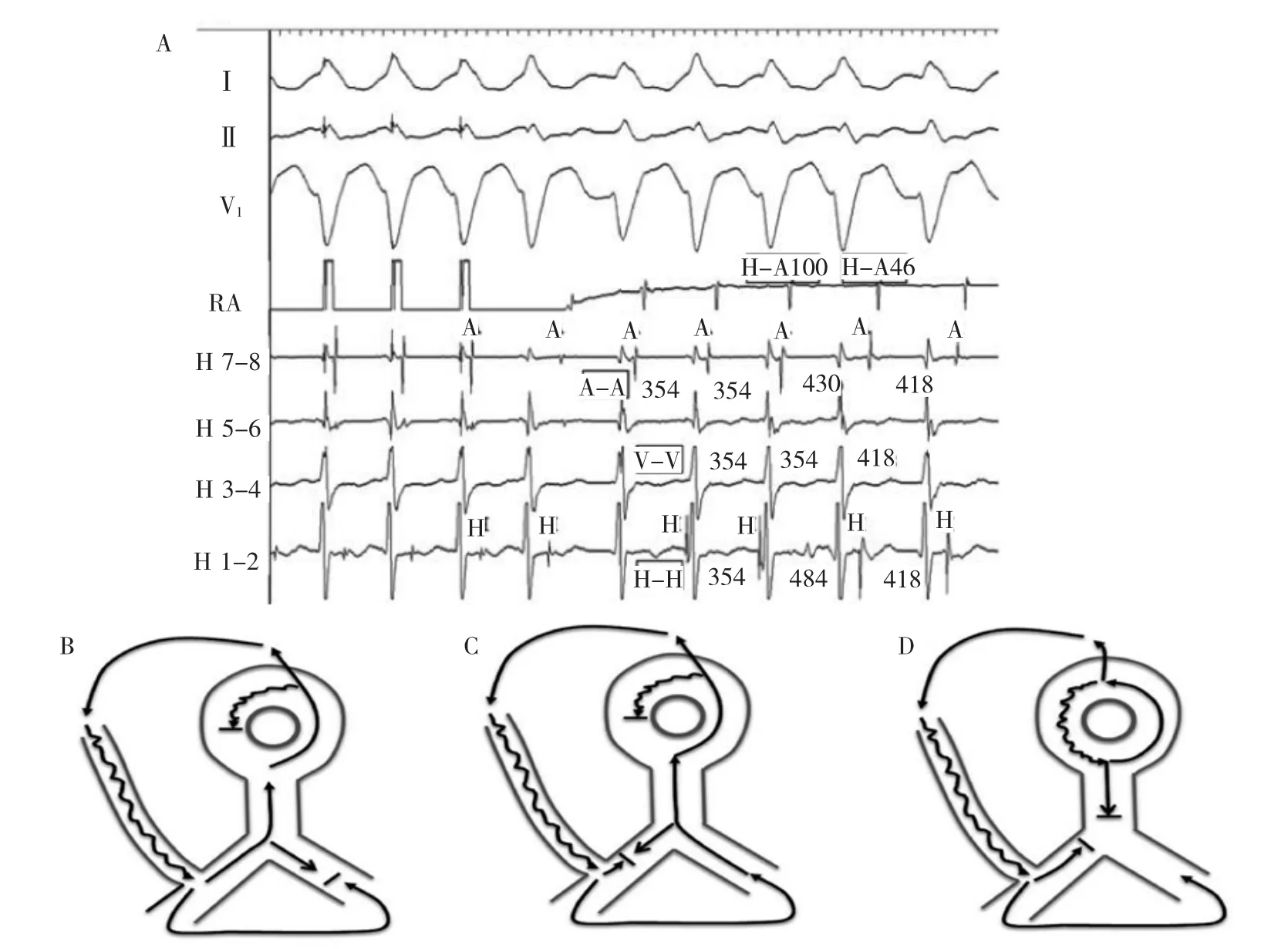
Figure 4A,Electrogram intervals of tachycardia in Figure 4.All intervals are in milliseconds.B-D,Schematic representation of tachycardia circuit changes in A.B,First 3 beats of the tachycardia.With cessation of atrial pacing anterograde conduction continues down the atriofascicular pathway with retrograde conduction via right bundle branch completing the circuit.C,Fourth and fifth tachycardia beats where retrograde block occurs in the right bundle branch,and transseptal conduction and left bundle branch activation forms the retrograde tachycardia limb.D,Alternative possibility of dual AVN physiology1during AVNRT with atriofascicular pathway conduction as a bystander.A indicates atrial;AFP,atriofascicular pathway;AVN,atrioventricular node;AVNRT,AV node reentry tachycardia;H,His;LB,left bundle branch;RA,right atrium;RB,right bundle branch;and V,ventricular.
Two possibilities remain:antidromic tachycardia and SVT(AV node reentry tachycardia or atrial tachycardia)withbystanderaccessorypathway conduction.The most probable mechanism is antidromic atrioventricularreentrytachycardia(AVRT). Development of retrograde block in the RB and transeptal conduction enlarges the circuit;increases the tachycardia cycle length;and-because of simultaneous conduction down the RB and up the His and AV node-shortens the RB-A interval(Figure 4B and 4C). Thetachycardiacyclelengthprolongationduring retrograde RB block is diagnostic of antidromic AVRT as it provides evidence for participation of the ventricle in the tachycardia.However,the possibility of SVT occurring simultaneously with antidromic AVRT is not excluded.During the first 2 beats of tachycardia, antidromic AVRT with its faster cycle length could entrain SVT.In the third and fourth beat,retrograde block in the RB prolongs the AVRT cycle length beyond that of an underlying SVT,allowing it to manifest (Figure 4D).In this scenario,the H-A interval(or RB-A interval)is longer during the first 2 AVRT beats because of the sequential retrograde conduction systemactivation and the HA interval shortens as expected for the third and fourth SVT(AV node reentry tachycardia)beats because of simultaneous conduction down the His bundle and up the AV node fast pathway.
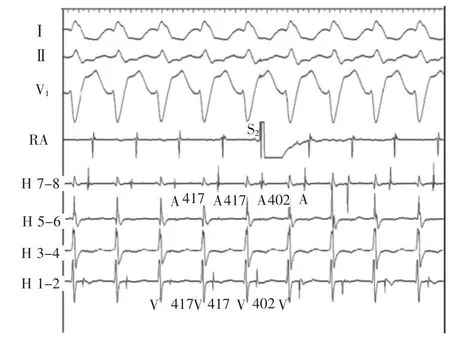
Figure 5A premature atrial complex(S2)introduced at the time of atrial septal refractoriness during the long V-H tachycardia advances the ventricular and atrial activation.A indicates atrial;H,His;RA,right atrium;and V,ventricular.
Proofofanatriofascicularasopposedto nodofascicular accessory pathway mediated antidromic tachycardia was confirmed by delivery of a paced premature atrial complex at a time of atrial septal refractoriness during the long V-H tachycardia.This advanced ventricular and subsequent atrial activation equally(Figure 5).A discrete accessory pathway potential was identified at the lateral tricuspid valve annulus(Figure 6)and a single 40 W radiofrequency energy application eliminated preexcitation and the tachycardia.Thiscaseillustratesthediagnostic opportunitiesandchallengeswithsimultaneous variations in multiple limbs of a reentrant rhythm.
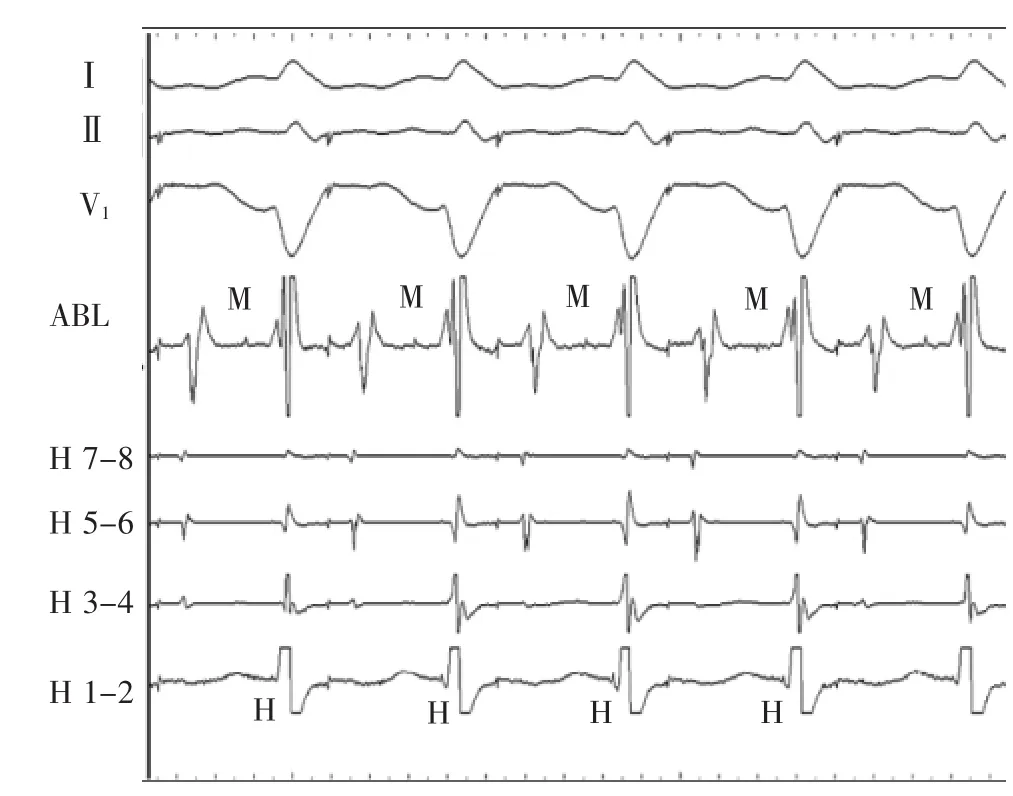
Figure 6Atriofascicular pathway potential(M)is recorded from the ablation catheter(ABL)placed at the lateral tricuspid annulus.Ablation here eliminated preexcitation.Note the short HV intervals on H1-2 electrodes.H indicates His.
词汇
octapolar adj.八极的
inference n.推论,推断结果
discern v.辨明,看出,辨识,认识,识别
schematically adv.在图表上地,按照图式,计划性地
inexact adj.不精确的,不严格的
bystander n.旁观者,看热闹的人
antidromic adj.逆向的,逆行的
participation n.参与,分享,参股
entrain v.&n.上火车,拖,产生,带走;热情
scenario n.剧本,概要,设想
proof n.&v.&adj.证明,证据,校稿;检验,校对;不能穿透的Delivery n.投递,分娩,交付,送货,讲话,交出
Discrete adj.&n.分离的,转折的;独立部件
注释
1.dual AVN physiology指双重的房室结生理机能,即房室结双径路功能
参考译文
第70课心房、希氏束和心室关系多变的宽QRS心动过速的机制
患者男性,57岁,心脏结构和基础心电图均正常,因心房颤动而接受肺静脉隔离手术。电生理检查中八极电极置于希氏束,消融电极置于中位右心房。
基础状态下,窦性心律周长890ms,A-H间期48ms,H-V间期80ms,QRS时间80ms。期外心房起搏刺激时,H-V间期缩短而QRS波群增宽呈左束支传导阻滞图形(图1)。以周长330ms心房猝发刺激证实进行性H-V间期缩短和QRS波群增宽,并呈左束支传导阻滞图形(图2A和2B)。图中最后3搏显示,希氏束电图似乎位于QRS波群后。起搏停止后,记录到同一形态的宽QRS波群心动过速(图3)。心动过速的机制是什么?
讨论
虽然基础心电图正常,通过期外心房起搏证实H-V间期缩短、QRS波群增宽及长A-V间期(图1),显然存在传导缓慢的旁道,尚可对旁道性质作进一步推论。逆传的右束支和希氏束激动早于呈左束支传导阻滞图形的预激心室波与旁道直接连接右束支(房束旁道或结束旁道)极为一致。
类似的变化见于心房猝发起搏开始时(图2A)。存在缓慢传导旁道伴随H-V间期缩短和长A-V间期的迹象。希氏束电图早于预激的心室波。由于只有远端电极记录到希氏束电图,在此图中不能辨识逆传或顺传希氏束激动。在第6个起搏波上,希氏束电图位于心室电图后,H与V的关系变化愈加明显。确定存在房-束(或结-束)旁道后,对于滞后的希氏束电位的唯一解释是在右束支中发生逆传阻滞。最有可能的是希氏束激动系跨间隔后左束支激动逆传所致。图2B为图示。不太可能的是右束支逆传阻滞容许经房室结慢径路顺传和前向激动希氏束。
心房起搏停止后,引发左束支传导阻滞图形的宽QRS波群心动过速(图3)。心动过速图形与预激的QRS波群一致。心动过速的第1个波上未见到希氏束激动,但出现在第2和第3个QRS波群开始前。最后两个心室波后见到希氏束波。H-H间期变化先于V-V间期及随后的A-A间期变化,虽然这种关系并不精确(图4A)。最后2搏的H-A间期缩短,解释了这种不确定关系。
宽QRS心动过速的鉴别诊断包括室性心动过速、室上性心动过速(SVT)伴差异性传导,室上性心动过速伴旁观旁道传导和预激的心动过速。短H-V间期排除了束支折返性室性心动过速和室上性心动过速伴差异性传导。易变的H-V关系和预示V-V间期变化的H-H间期变化也排除了室性心动过速伴逆传希氏束激动。
剩下2种可能性:逆传心动过速和室上性心动过速(房室结折返心动过速或房性心动过速)合并旁观旁道传导。最有可能的机制是逆传房室折返心动过速(AVRT)。右束支逆传阻滞的发生和跨间隔传导扩大了环路,增加了心动过速的周长;因同时顺传右束支和逆传希氏束及房室结,缩短了右束支-A间期(图4B、C)。逆向右束支传导阻滞时心动过速周长延长对于逆传AVRT具有诊断意义,因为这提供了心室参与心动过速的依据。然而,不能排除SVT与逆传AVRT同时发生的可能性。在心动过速的最初2个搏动中,周长较短的逆传AVRT可拖带SVT。在第3和第4个搏动中,右束支的逆传阻滞延长了AVRT周长并超出基础SVT的周长,使得SVT得以呈现(图4D)。在此情况下,因为相继的逆传系统激动,最初2个AVRT搏动的H-A间期(或右束支-A间期)较长,而第3个和第4个SVT(房室结折返心动过速)搏动,因为同时顺传希氏束和逆传房室结快径路,H-A间期缩短,正如预期的。
长V-H心动过速发作期间,于房间隔不应期作期前心房起搏,证实是房束而非结束旁道介导的逆传心动过速。这使心室激动提前,也使随后的心房激动提前(图5)。在三尖瓣环侧壁发现离散的旁道电位(图6),40W射频放电一次中止预激及心动过速。该病例阐述了折返节律多条径路同时变化时的诊断机遇和挑战。
图1心房期外起搏。S2刺激后,QRS波群增宽,H-V间期缩短,希氏束和右束支激动顺序逆转。虚线和箭标为顺传和逆传激动变化提供基准。预激伴长A-V间期、左束支传导阻滞图形及右束支激动早于心室激动提示房束旁道。希氏束电极记录从7-8到1-2是从近端到远端;起搏间期单位ms;H.希氏束;RA.右心房;S1.连续刺激;S2.期外刺激。
图2A.右心房300ms起搏。注意第3个起搏波H-V间期缩短和QRS波群融合,第4和第5个起搏波经右束支逆传希氏束激动及第6个起搏波突发跳跃至V-H关系表明右束支逆传阻滞并经左束支传导。B.A图上最后3搏A、H和V关系变化机制原理图。心房起搏引起房室结顺传阻滞,经房束旁路顺传,右束支逆传阻滞。希氏束是经左束支逆传激动。A.心房;AFP.房束旁路;AVN.房室结;H.希氏束;LB.左束支;RB.右束支;V.心室。
图3心房起搏引发左束支传导阻滞图形心动过速,周长不定,A、H和V之间关系不定。A.心房;H.希氏束;RA.右心房;V.心室。
图4A.图4心动过速电图间期。所有间期单位为ms。B-D.A图中心动过速环路变化原理图。B.心动过速最初3个搏动。心房起搏停止后,继续向下沿房束旁路顺传而经右束支逆传完成环路。C.第4和第5个心动过速搏动右束支逆传发生阻滞,跨间隔传导和左束支激动形成心动过速的逆传支。D.房室结折返合并房束旁路传导作为旁观者时,AVN双径路的交替可能性。A.心房;AFP.心房束支旁路;AVN.房室结;AVNRT.房室结折返心动过速;H.希氏束;LB.左束支;RB.右束支;V.心室。
图5长V-H心动过速时于心房间隔不应期引入的期前心房搏动使心室和心房激动提前。A.心房;H.希氏束;RA.右心房;V.心室。
图6置于三尖瓣环侧壁上的消融导管记录到房束旁路电位(M)。此处消融中止预激。注意希氏束1-2电极上的短H-V间期。H指希氏束。
参考文献
[1]Foreman J R,Steinberg L A,Prystowsky E N,etal.Mechanism of a wide QRS complex tachycardia with variable atrial,his,and ventricular relationships[J].Circ Arrhythm Electrophysiol,2015,8 (4):981-984.
(童鸿)
Lesson Seventy
Mechanism of a wide QRS complex tachycardia with variable atrial,his,and ventricular relationships
